Abstract
The degradation in the oil quality used by street food vendors during frying operations is an important health concern due to the toxic compounds produced in it. An effective strategy is to detect the oil quality by determining its physicochemical and antioxidant properties to prevent the use of potentially harmful oil. Therefore, this research aimed to investigate the physicochemical and antioxidant properties of frying oils used by local fried food vendors of Lucknow city, India. Oil samples as fresh, after 5th batch and 10th batch of repeated frying (total 15 samples), were collected from five randomly selected street food vendors consumed regularly by the local people. All the samples were analyzed by evaluating physicochemical properties such as specific gravity (SG), viscosity, free fatty acid (FFA), iodine value (IV), peroxide value (PV) and saponification value (SV) using standard methods. The results revealed that almost all repeated frying oils collected were above the permissible limit for FFA, IV, and PV. It was noted that the highest values were obtained in 10th batch of repeated frying oils. On the other hand, slight variation was noted for the SG and SV in all oil samples. Increased usage of the repeated frying oils also resulted in a decrease in total phenolic content and total flavonoid content as compared to values obtained in fresh oil samples. In conclusion, the analysis shows that the repeated frying of vegetable cooking oil degrades its physicochemical and antioxidant qualities, thus should not be consumed.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Deep fat frying, which involves food immersed in hot oil in the temperature between 140 and 200 °C, is a widely used cooking method due to its ease and distinctive effects on food flavor and texture [1, 2]. A few amount of oil is absorbed by the food during this process, which results in the certain amount of breakdown products from the oil are obtained by the food being fried [3]. During deep frying, a number of changes like oxidation, hydrolysis, isomerization, and polymerizations occur which alter the flavor and spoil the compounds present in cooking oil, affecting both the quality of oil and fried food. Food with different proximate composition, such as vegetables, chicken, potatoes, fish, etc., tends to transfer various substances like fat, water, salt, sugar, antioxidants, etc.) into frying oil, changing the rate and type of reactions that occur in the frying oil [4, 5]. As per Codex Alimentarius Commission, FAO and WHO Food Standards, repetitive deep fat frying lowers the amount of unsaturated fatty acids in cooking oil while increasing the number of free fatty acids, foaming, color darkening, viscosity, density, and specific gravity [6]. Due to harmful substances present in the oil as well as in fried food, the degraded frying oil might lead to negative impact on human health [7]. According to Good [8], using vegetable oil for deep frying repeatedly reduces the smoke point causing oil to smoke when heated at low temperature. Further Kaleem et al. [9] reported that the poor quality cooking oil indicator is the change in color, lower smoke point and iodine level, increased free fatty acid, peroxide value, viscosity, total polar material and high foam qualities. The effect of repetitive deep fat frying on the different brands of cooking oil’s physicochemical properties was also examined by Omara et al. [10]. Finding shows that the repeated use of oil for frying potatoes can be done just for 7 times on an average for hard oils (solid at room temperature) and 6 times for soft oils (liquid at room temperature), at 140 °C for 10–20 min. In another study, frying capacity of palm olein oil and its blend containing soybean oil, canola oil and sunflower oil was investigated by Siddique et al. [11]. Involving the frying of fish, whole process took place for > 60 min in 3 stages lasting 20 min for each frying at 200 °C. Among the frying oils tested in this study, palm olein oil was shown to be better against decomposition and oxidation. Mariana et al. [12] explained that frying at high temperatures (over 180 °C) and exposure to air and water caused a variety of degradation reactions in oil that gave rise to a number of reaction chemicals.
Meanwhile, a number of researches revealed that, consuming vegetable oil that has been heated repeatedly while being used for frying has been associated with atherosclerosis risk, low density lipoprotein (LDL) levels, and total blood lipid [13,14,15,16,17]. Repeatedly used frying oil causes lipid peroxidation, resulting in malondialdehyde (a genotoxic harmful degradative byproduct of lipid peroxidation) which shows both mutagenic and carcinogenic effects in body by changing cell membrane properties [18,19,20,21]. In this concern, some in vivo findings reported by Srivastava et al. [22] investigated that after 12 weeks of feeding Wistar mice a repeatedly heated cooking oil diet regularly, there were gene alterations and pre-cancerous lesions found in their liver cells. This result is strongly supported by the research of Sutejo et al. [23] stating that feeding repeatedly heated frying oil to experimental mice for 14 days and overall 27 times caused increased cholesterol levels and liver cell damage. Furthermore Farag et al. [24] demonstrated that organ damage was inversely correlated with the length of oil heating. The longer and more frequently the frying oil was heated, the damage to the kidneys, liver and heart of the subjects also got worsted.
In India, various food items that are cooked by deep frying include Kachori, Poori, Wada, Samosa, Bread pakoda, french fries, patties, etc. The aim of this study is to ascertain how the physicochemical and antioxidant qualities of cooking oil used by local fried food sellers, changed with repeated heating, whose consumption increases the risk of various diseases such as atherosclerosis, cardiovascular disease, diabetes, cancer, etc. For this purpose, fresh, after 5th batch, and 10th batch of repeated frying, total 15 samples (200 mL each) were collected from five randomly selected street fried food vendors located at different areas of Lucknow city. Table 1 shows the maximum allowable limit for Refined Soybean Oil for all physicochemical characteristic that has been analyzed for this study [25].
2 Materials and methods
2.1 Sample collection
A total number of fifteen oil samples (200 mL each) were collected from five different areas namely Indira Nagar, Gomtinagar, Hazratganj, Alambagh and Telibagh, all situated in Lucknow, Uttar Pradesh. Oil samples were collected from these areas at different stages of frying (after 5th batch and the 10th batch), as well as fresh oil sample before heating (0 batch). Almost all shops were chosen as same in terms of oil type [mixture of refined soyabean oil (RSO) of Fortune brand and hydrogenated vegetable oil commonly known as dalda vanaspati ghee (10% of RSO)], food items being fried, the type of fryers, and frying conditions. The amount of break time between two successive frying cycles was 5–10 min. The duration of sample collection took 5 days. The collected oils were transferred to dark colored glass bottles. The oil samples were filtered through a filter paper and stored at 4 °C to prevent further degradation. All the analysis was performed within 5 days. This research work was conducted in the laboratory of the Department of Food & Nutrition and Department of Chemistry of Babasaheb Bhimrao Ambedkar University, Lucknow, India. All the solvents and chemicals used in this investigation were of analytical grade purchased from SRL Pvt. Ltd. Mumbai (India).
2.2 Physicochemical analysis
2.2.1 Specific gravity measurement
Using a pycnometer with a 50 mL capacity, the densities of oil samples before and after frying were determined. Oil sample was added (W1) after the empty bottle had been weighed (W0). The contents were removed, filled with water, and reweighed as W2 again. In order to calculate the specific gravity, following equation was used [1].
2.2.2 Viscosity measurement
An Ostwald Viscometer with a technical constant of 0.05 Cs/c, ASTMAD 445 England, was used to evaluate the viscosity of oil samples both before and after frying. Oil sample flow time (measured in seconds) was noted using a stopwatch. Following equation was used to compute viscosity [1].
where t is the flow time in seconds.
2.2.3 Saponification value measurement
The saponification value was obtained according to ASTMD464 [26]. 2 g of oil sample weighed in a conical flask, 25 mL of 0.5 N ethanolic KOH was added into the flask, and vigorously mixed. The mixture was heated at 70 °C for an hour with a condenser attached to the conical flask. Two drops of 1% phenolphthalein indicator were used to titrate the heated mixture with 0.5 N HCl, until a colorless solution was achieved. The same method was applied to determine a blank. Following equation was used to obtain the SV [1].
where A is the HCl (mL) for blank, B is the HCl (mL) for sample, W is the weight of sample (g), N is the normality of HCl.
2.2.4 Iodine value measurement
Hubl method was used to determine the iodine value (IV) in oil samples. The Hubl’s iodine was filled in a 50 mL burette and the initial reading was noted. 5 mL of chloroform and 2–3 drops of Hubl’s iodine were mixed thoroughly. This serves as a control for color comparison. Then 5 mL of chloroform was taken in another beaker. 0.5 mL of oil sample was mixed and gently swirled to dissolve. Hubl’s iodine was added slowly from the burette until the color of iodine (matching with control) appears in the solution. The burette reading was noted. Following equation was used to calculate the iodine value [1].
where x is the volume of Hubl’s iodine in mL.
2.2.5 Peroxide value measurement
Oil samples were examined for peroxide value using AOCS Cd 8b-90 method [2]. In 250 mL conical flask 5 g oil sample was weighed firstly. The flask was then filled with 30 mL solution of glacial acetic acid: chloroform in a ratio of 3:2 and rapidly shaken. Then 0.5 mL of potassium iodide was added into the flask and shaken for 1 min. After adding 30 mL of distilled water, the solution was titrated with 0.1 M sodium thiosulphate. The flask was shaken vigorously until the yellow color of the solution got disappeared. The color changed to blue after a few drops of 1% starch solution were added. The mixture was again shaken until the blue color got disappeared. Blank titration was done twice using the same method. The PV was calculated using the formula below:
where Vsample is the titration volume of sample (mL); Vblank is the titration volume of blank (mL); N is the normality of potassium thiosulphate; W is the weight of the sample (g).
2.2.6 Free fatty acid measurement
Oil samples were analyzed for free fatty acids using AOCS Ca 5a-40 [2]. 10 g of oil sample was weighed in a 250 mL beaker. 60 mL of neutralized ethanol was added into it and shaken vigorously. The solution was titrated with standardized 0.025 M Sodium hydroxide using phenolphthalein indicator until the solution just turned pink. FFA was expressed as a percentage of oleic acid in the sample.
2.3 Antioxidant analysis
2.3.1 Total phenolic content
Total phenolic content of the oil samples was measured using the Folin–Ciocalteu reagent (FCR). In a conical flask 0.3 mL of oil sample was weighed firstly. Then 3 mL of Folin–Ciocalteu reagent was added into it. 3 mL of 6% sodium carbonate was added after 5 min, and the mixture was left to stand at room temperature for 90 min. A UV/VIS Spectrophotometer was used to measure the mixture’s absorbance at 725 nm. According to the calibration curve, the total phenolic content was determined, and the results were depicted in Fig. 3a as mg of gallic acid equivalent per gram of oil (mgGAE/g oil) [17, 27].
2.3.2 Total flavonoid content
To determine total flavonoid content, the oil sample (0.5 mL) was weighed into a conical flask. 20 mL of 80% aqueous methanol was added into it. The mixture was shaken for 3 h using an orbital shaker. Then it was filtered using Whatman filter paper. After that, the filterate was evaporated for 1 h at 105 °C, followed by cooling and weighing again [17]. Results are shown in Fig. 3b as Total Flavonoid Content (%).
2.4 Statistical analysis
All physicochemical analysis was performed in three replications from each batch and the obtained data were subjected to Mean and Standard Deviation (SD) values using Microsoft Excel 2007.
3 Results and discussion
3.1 Physicochemical properties of the oils
One of the physical characteristics that govern how quickly oil or fat breaks down is color [28, 29]. In this study, as the amount of time for frying grew the color of the oils continued to become darker as shown in Fig. 1.
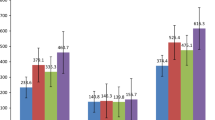
One important parameter is specific gravity that affects the heat transmission through natural convection. Bhavsar et al. [30] suggested that specific gravity measurements are extremely helpful for determining oil contamination or adulteration, as the SG of oil is influenced by chemical composition present in the oil. An increase in the amount of aromatic compounds present in oil results in an increase in the specific gravity. Table 2, Fig. 2 show the result of the specific gravity of fresh oils, oils after fifth and tenth batches of fry that were collected and examined. Oils obtained after the tenth batch of deep frying have the highest specific gravity values, ranging from 0.916 to 0.923. While fresh oils had values in the range of 0.911 to 0.920, and values after fifth round of frying ranged from 0.914 to 0.922. Here we can observe how the SG of each oil sample changed as the frying time increased.
Another parameter used to evaluate physical changes in oil sample is viscosity. It depends on the specific gravity, molecular weight, temperature and the degree of unsaturation [1]. The measured value of all samples is presented in Table 2; Fig. 2. Before heating (in fresh oil) the viscosity was lower than after repeated heating in all oil samples. After ten batches of repeated frying the oil’s viscosity peaked between 690.32 and 740.32. On the other hand, after five batches the viscosity was calculated between 670.23 and 720.12. The least viscous oil was fresh oil with values ranging from 638.26 to 648.32 in all oil samples. The increase in viscosity is probably because of saturation of the triglyceride chain by the process of hydrogenation while the decrease may be likely due to unsaturation of oil. The more oil is in unsaturated form (liquid at room temp.) the less its viscosity would be [31].
An indicator of the molecular weights of the triglycerides in the oil is saponification value (S.V.). It is inversely correlated with the average molecular weight or chain length of the fatty acids [32]. A low saponification value denotes longer fatty acid chain and a higher molecular weight in a sample; while a high SV indicates triacylglycerols with shorter fatty acid chains [33]. Table 2, Fig. 2 depict the S.V measured for all oil samples. It shows the fresh oil values ranging from 60.78 to 64.32 mg/KOH/g, the oil collected after the fifth batch of frying ranges from 60.82 to 64.43 mg/KOH/g, and the highest values from tenth batch of frying oils ranging from 61.92 to 65.24 mg/KOH/g. This conclusion is consistent with that of Alajtal et al. [34] who found that after frying, the saponification value of sunflower, olive oil and corn oil increased from 21.09 to 22.44 mg KOH/g, 21.54 to 22.27 mg KOH/g, and 19.41 to 21.15 mg KOH/g respectively. The increase in the SV shows that the oil has degraded and should be dumped or used to make soap instead.
Another crucial indicator of the degree of unsaturation in fatty acids is the iodine value. The potential of oil to oxidise while heating at higher temperature as in deep frying, increases with the decrease in iodine value. According to Cheplogoi et al. [35] a fall in iodine value is another sign that vegetable oils are degrading. Table 2, Fig. 2 depict the IV measured for all oil samples. It shows the values for fresh oil samples ranging from 1.199 to 1.207 g, the values for oils collected after fifth batch of frying ranging from 1.072 to 1.189 g, and the highest values for the frying oils collected after tenth batch ranging from 0.787 to 0.839 g. The observed reduction in iodine value is consistent with the reduction in double bonds that has been reported by Cheplogoi et al. and is attributed to oxidation and heat degradation. When considering the oxidative stability of cooking oils after frying food items, the iodine value is of particular importance.
Another frequent measure used to find out the oxidative stability of oils is the peroxide value (PV) [36]. The oxidation of carbon atoms adjacent to the double bonds in the triacylglyceride structure resulting in the formation of hydroperoxides, is what causes the increase in PV in oil samples after repeated frying. Therefore, the oil oxidation happens more quickly the higher the PV. The reason of decrease can be due to the peroxides produced during heating becoming unstable substances at high temperatures as well as during frying, where they turn into carbonyl and aldehyde molecules. A study done by Obeng et al. [37] suggested that continuous contact to light, oxygen, and high temperatures reacts with the oil to generate peroxides. According to Ramadan et al. [38] a good quality vegetable oil for frying should have a peroxide value of < 2 meq/kg. In our investigation, all fresh oil samples before frying have the PV below 2 meq/kg whereas all samples collected during frying operations after 5th batch and 10th batch showed the PV range from 3.6 to 17.3 meq/kg depicts in Table 2; Fig. 2. Decker et al. [39] suggests that adding antioxidants to oils may result in reduced peroxide value. Antioxidants are included to extend shelf life and stop rancidity.
Increases in free fatty acid (FFA) concentration and decreases in unsaturation of oil are indicators of the oxidation and chemical changes in oils during repeated heating [40]. A decrease in FFA may result from the loss of low molecular weight acids from the oil through volatilization process [41]. According to Gunstone [42] oils should have an FFA concentration of less than 0.1%. Table 2, Fig. 2 show that all fresh oil samples had the lowest FFA values between 0.05 and 0.08%, while oil samples from tenth batch of fry had the highest values ranging between 1.20 and 2.10%. Melton et al. [43] reported that the typical FFA concentration of used frying oil is around 4.95%, while Gupta [44] reported that the FFA concentration of absorbed frying oil ranged from 0.25 to 0.4% in the majority of snack foods. On the other hand, FFA levels in commercial fryers can go up to 0.5%, while the ideal range for fresh frying oil is between 0.03 and 0.5%. Tseng et al. [45] also suggests that if the FFA levels are greater than 1%, frying oil should be discarded. After 5 days of frying, a study done by Obeng et al. [37] likewise revealed comparable findings, with% FFA ranging from 0.62 to 2.41%. The FFA values of the frying oils differ as a result of new oil being added throughout the frying process, which reduces these values. According to Mcwilliams [46] the release of free fatty acid causes oil to smoke and develop acreolin. The acreolin can be seen as bluish and acrid smoke and its vapour can cause eye, nasal and respiratory tract irritations in low level exposure. Further Thomas et al. [47] explained that free fatty acid has a significant impact on the smoke point of oils. The faster the oil breaks down and begins to smoke, the more FFA it contains.
3.2 Antioxidant value
3.2.1 Total phenolic content (TPC)
Phenolics are known for their role in the oxidative stability of oils. Due to the frying oil's prolonged exposure to high heat during deep frying, the total phenolic content of the oil decreases during frying operations. Through quenching the radical processes that cause rancidity, their presence may prevent the deterioration of oils [48, 49]. Figure 3a displays the result of TPC of all collected oils. For fresh oil, the total phenolic content was 0.37, 0.38, 0.37, 0.35 and 0.32 mg GAE/g respectively. For fifth batch of repeated frying oil, values were 0.14, 0.15, 0.16, 0.11, and 0.09 mg GAE/g respectively. While for the tenth batch, values reported were 0.09, 0.09, 0.10, 0.08 and 0.07 mg GAE/g respectively. Result shows that all five fresh oils contained a good quantity of TPC. On the other hand, the TPC of the frying oils decreased after fifth and tenth batch of frying significantly. The findings here are consistent with Alonso et al. [4] study, where the number of deep-frying cycles and the decline in olive oil’s antioxidant activity were positively correlated.
3.2.2 Total flavonoid content (TFC)
Antioxidant activity is the most well-known property of flavonoids (flavonols and flavanols). Therefore, consumers and food manufacturers have developed an interest as the anti-inflammatory, anti-atherosclerotic, antioxidant, and anti-carcinogenic effects of flavonoids are well known. They are among the antioxidant defence mechanisms defending vegetable oils against oxidative deterioration [50, 51]. Figure 3b illustrates the finding of the flavonoid content of measured frying oils. Flavonoid content of all fresh oils obtained were 2.58, 2.81, 2.35, 2.79 and 2.17 respectively. After multiple batches of frying, the flavonoid content reported were 1.42, 1.96, 1.51, 1.86, 1.32 and 0.64, 0.63, 0.59, 0.79, 0.60 for 5th batch and 10th batch respectively. This finding is supported by Mala and Kurian [50] where the total flavonoid content of the oil samples used for general frying was significantly reduced, while the concentration of flavonoid increased in oil samples used for frying meat and fish. This could be due to flavonoids leaching into the frying oils from other food materials, particularly onions. Onion is a good source of quercetin, and flavonoids like quercetin are known for their in vitro stability even at high temperatures.
4 Conclusion
The result of this investigation demonstrated that repeated frying of vegetable cooking oil degraded its physicochemical and antioxidant qualities. All the oil samples that were taken when they first gathered from various streets were below acceptable limits, but as the heating time went on, they were typically beyond the allowable limit, specifically after 10th batch of frying. Additionally, in comparison to fresh oil samples, the oils' total phenolic and flavonoid contents were considerably low after repeated frying. This study thus demonstrates the degree to which the quality of the oils used by street vendors to fry their food has degraded. Therefore, safety precautions should be put in place because these declines in oil quality could have a negative impact on health due to the harmful compounds they create. Although degraded oils should not be consumed by humans, they can be used in alternative processes to make biodiesel and soap. We suggest that restaurants and food processing facilities perform oil quality checks and create regulatory requirements for frying oil quality.
Data availability
The data collected and measured are available with the corresponding author and may be provided upon reasonable request.
Code availability
Not applicable.
References
Nduka J, Omozuwa P, Imanah O. Effect of heating time on the physicochemical properties of selected vegetable oils. Arab J Chem. 2021. https://doi.org/10.1016/j.arabjc.2021.103063.
Sebastian A, Ghazani S, Marangoni A. Quality and safety of frying oils used in restaurants. Food Res Int. 2014. https://doi.org/10.1016/j.foodres.2014.07.033.
Ngozi E, Giwa O, Adeoye B, Ani I, Ajuzie N, Olutayo T. Quality effect of repetitive use of frying oil by street food vendors on quality of the oil. Niger J Nutr Sci. 2019;40:1.
Gómez A, Fregapane G, Salvador M, Gordon M. Changes in phenolic composition and antioxidant activity of virgin olive oil during frying. J Agric Food Chem. 2003. https://doi.org/10.1021/jf025932w.
Juárez MD, Osawa CC, Acuña ME, Sammán N, Gonçalves LA. Degradation in soybean oil, sunflower oil and partially hydrogenated fats after food frying, monitored by conventional and unconventional methods. Food Control. 2011. https://doi.org/10.1016/j.foodcont.2011.05.004.
Codex Alimentarius Commission/FAO/WHO Food Standards, Codex standards for named vegetable oils: CODEX STAN 210. CXSTAN 210-1999. 2000.
Tena N, Aparicio R, Garcia-Gonzalez D. Thermal deterioration of virgin olive oil monitored by ATR-FTIR analysis of trans content. J Agric Food Chem. 2009. https://doi.org/10.1021/jf9012828.
Good J. Healthiest cooking oil chart with smoke points, baseline of health foundation. 2012. https://www.jonbarron.org/article/healthiest-cooking-oil-chartsmokepoints.
Kaleem A, Aziz S, Iqtedar M. Investigating changes and effect of peroxide values in cooking oils subject to light and heat. FUUAST J Biol. 2015;5:191–6.
Omara T, Kigenyi E. Effects of continuous deep fat frying on the physical and chemical properties of assorted brands of edible cooking oils sold in metropolitan Kampala. Asian J Appl Chem Res. 2019. https://doi.org/10.9734/ajacr/2019/v3i230086.
Siddique B, Muhamad II, Ahmad A, Ayob A, Ibrahim MH, Ak M. Effect of frying on the rheological and chemical properties of palm oil and its blends. J Food Sci Technol. 2015. https://doi.org/10.1007/s13197-013-1124-6.
Mariana R, Susanti E, Hidayati L, Wahab R. Analysis of peroxide value, free fatty acid, and water content changes in used cooking oil from street vendors in Malang. AIP Conf Proc. 2020. https://doi.org/10.1063/5.0002656.
Firdaus MA, Mishra S. Industrial trans-fatty acid intake associated with coronary heart disease risk: a review. J Nutr Food Sci. 2021;11:806.
Venkata R, Subramanyam R. Evaluation of the deleterious health effects of consumption of repeatedly heated vegetable oil. Toxicol Rep. 2016. https://doi.org/10.1016/j.toxrep.2016.08.003.
Yee T, Loganathan R, Tiu T. Oxidative changes in repeatedly heated vegetable oils. J Oil Palm Res. 2018. https://doi.org/10.21894/jopr.2018.0051.
Firdaus MA, Sunita M. Nutritional recommendations for management of coronary heart disease during viral infections with an emphasis on COVID-19. J Cardiovasc Dis Res. 2021;36:982–91.
Emelike N, Ujong A, Achinewu S. Physicochemical and antioxidant properties of oils used by local fried food vendors in D/line-Port Harcourt, Rivers State. Agric Food Sci Res. 2020. https://doi.org/10.20448/journal.512.2020.71.89.96.
El-Sayed M, Elsanhoty R, Ramadan M. Impact of dietary oils and fats on lipid peroxidation in liver and blood of albino rats. Asian Pac J Trop Biomed. 2014. https://doi.org/10.1016/S2221-1691(14)60208-2.
Diggs D, Huderson A, Harris K, Myers J, Banks L, Rekhadevi P, Niaz S, Ramesh A. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J Environ Sci Health. 2011. https://doi.org/10.1080/10590501.2011.629974.
Obembe A, Owu D, Okwari O, Antai A, Osim E. Intestinal fluid and glucose transport in wistar rats following chronic consumption of fresh or oxidised palm oil diet. Int Sch Res Notices. 2011. https://doi.org/10.5402/2011/972838.
Bayraktar N, Karagözler A, Bayraktar M, Titretir S, Gözükara EM. Investigation of the blood biochemical status of gas station workers. Toxicol Environ Chem. 2006. https://doi.org/10.1080/02772240600842383.
Srivastava S, Singh M, George J, Bhui K, Shukla Y. Genotoxic and carcinogenic risks associated with the consumption of repeatedly boiled sunflower oil. J Agric Food Chem. 2010. https://doi.org/10.1021/jf102651n.
Sutejo I. Kerusakan sel hati dan peningkatkan Kolesterol Serum Mencit Akibat Pemberian Minyak Goreng Bekas Pakai. IKESMA. 2012;8(1). https://jurnal.unej.ac.id/index.php/ikesma/article/view/1109.
Farag R, Abdel M, Basuny A, Hakeem B. Effect of non-fried and fried oils of varied fatty acid composition on rat organs. Am J Agric Biol Sci. 2010;1:501–9.
CODEX ALIMENTARIUS International Food Standards. FAO/WHO. CXS 210–1999 adopted in 1999. Revised in 2001, 2003, 2009, 2017, 2019. Amended in 2005, 2011, 2013, 2015, 2019, 2021, 2022, last updated 01/02/2023. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf.
ASTM D464-15. Standard test methods for saponification number of pine chemical products including tall oil and other related products. 2020.
Emelike N, Akusu M, Ujong A. Antioxidant and physicochemical properties of oil extracted from cashew (Anacardium occidentale L.) kernels. Int J Food Sci Nutr. 2017;2:122–8.
Totani N, Yamaguchi A, Takada M, Moriya M. Color deterioration of oil during frying. J Oleo Sci. 2006;55:51–7.
And T, Shahidi F. Antioxidant potential of pea beans (Phaseolus vulgaris L.). J Food Sci. 2005;70:S85–90. https://doi.org/10.1111/j.1365-2621.2005.tb09071.x.
Bhavsar G, Syed H, Andhale R. Characterization and quality assessment of mechanically and solvent extracted Niger (Guizotia abyssinica) seed oil. J Pharmacogn Phytochem. 2017;6:17–21.
Santos R, Loh W, Bannwart A, Trevisan O. An overview of heavy oil properties and its recovery and transportation methods. Braz J Chem Eng. 2014. https://doi.org/10.1590/0104-6632.20140313s00001853.
Muhammad N, Bamishaiye E, Bamishaiye O, Usman L, Salawu M, Nafiu M, Oloyede O. Physicochemical properties and fatty acid composition of cyperus esculentus (Tiger Nut) Tuber Oil. Biores Bull. 2011;5:51–4.
Ivanova M, Hanganu A, Dumitriu R, Tociu M, Ivanov G, Stavarache C, Chira N. Saponification value of fats and oils as determined from 1H-NMR data: the case of dairy fats. 2022. Foods. https://doi.org/10.3390/foods11101466.
Alajtal A, Sherami F, Elbagermi M. Acid, peroxide, ester and saponification values for some vegetable oils before and after frying. AASCIT J Mater. 2018;4:43–7.
Chebet J, Kinyanjui T, Cheplogoi P. Impact of frying on iodine value of vegetable oils before and after deep frying in different types of food in Kenya. J Sci Innov Res. 2016;5:193–6.
Parzhanova A, Dimitrov D, Ivanova S, Perifanova M, Uzunova G. Study on the composition and oxidation stability of sunflower oil–oleic type during heat treatment. Annals. Food Science and Technology. 2021. https://afst.valahia.ro/wp-content/uploads/2022/10/III.1_Dimitrov.pdf.
Idun-Acquah N, Obeng G, Mensah E. Repetitive use of vegetable cooking oil and effects on physico-chemical properties—case of frying with redfish (Lutjanus fulgens). Sci Technol. 2016;6:8–14. https://doi.org/10.5923/j.scit.20160601.02.
Suleiman A, El-Makhzangy A, Ramadan M. Antiradical performance and physicochemical characteristics of vegetable oils upon frying of French fries: a preliminary comparative study. J Food Lipids. 2006. https://doi.org/10.1111/j.1745-4522.2006.00050.x.
Decker EA, Elias RJ, McClements DJ. Oxidation in foods and beverages and antioxidant applications: management in different industry sectors, vol. 2. Cambridge: Woodhead Publishing; 2010.
Perkins EG. Effect of lipid oxidation on oil and food quality in deep frying. ACS Publ. 1992. https://doi.org/10.1021/bk-1992-0500.ch018.
Hau LB, Young PK, Hwang LS. Quality assessment of oils during heating and frying. J Chin Agric Chem Soc. 1986;64:397–405.
Gunstone FD. Oils and fats in the food industry. 1st ed. Oxford: Wiley-Blackwell Publishing Ltd; 2008. ISBN 978-1-4051-7121–2.
Melton S, Jafar S, Sykes D, Trigiano M. Review of stability measurements for frying oils and fried food flavor. J Am Oil Chem Soc. 1994. https://doi.org/10.1007/BF02541345.
Gupta M. Frying oils. In: Shahidi F, editor. Bailey’s industrial oil and fat products. 6th ed. New Jersey: Wiley; 2005. p. 1–31.
Tseng Y, Moreira R, Sun X. Total frying-use time effects on soybean-oil deterioration and on tortilla chip quality. Int J Food Sci Technol. 1996. https://doi.org/10.1046/j.1365-2621.1996.00338.x.
Mcwilliams M. Nutrition and dietetics. 8th ed. Manila: Rex Bookstore; 2007. p. 177–84.
Thomas A, Matthäus B, Fiebig H. Fats and fatty oils. Ullmann’s Encycl Ind Chem. 2000. https://doi.org/10.1002/14356007.a10_173.pub2.
Siger A, Nogala-kalucka M, Lampart-Szczapa E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipids. 2008. https://doi.org/10.1111/j.1745-4522.2007.00107.x.
Koski A, Pekkarinen S, Hopia A, Wähälä K, Heinonen M. Processing of rapeseed oil: effects on sinapic acid derivative content and oxidative stability. Eur Food Res Technol. 2003. https://doi.org/10.1007/s00217-003-0721-4.
Mala K, Kurian A. Nutritional composition and antioxidant activity of pumpkin wastes. Int J Pharm Chem Biol Sci. 2016;6:336–44.
Adu O, Fajana O, Ogunrinola O, Okonkwo U, Evuarherhe P, Elemo B. Effect of continuous usage on the natural antioxidants of vegetable oils during deep-fat frying. Sci Afr. 2019. https://doi.org/10.1016/j.sciaf.2019.e00144.
Acknowledgements
The authors would like to thank the Research Laboratory of the Department of Food & Nutrition, and Department of Chemistry, Babasaheb Bhimrao Ambedkar University, Lucknow for providing required chemicals and equipments for the successful analysis.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
MAF done all the analysis and wrote the main manuscript. GP and SM analyzed the data. MP prepared the figures and table. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mishra, S., Firdaus, M.A., Patel, M. et al. A study on the effect of repeated heating on the physicochemical and antioxidant properties of cooking oils used by fried food vendors of Lucknow city. Discov Food 3, 7 (2023). https://doi.org/10.1007/s44187-023-00046-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-023-00046-8