Abstract
Jellies are usually preserved by artificial preservatives where they have harmful side effects and health hazards especially to infants. Honey and fresh lemon extract juice is a natural preservative and is considered a good substitute for artificial preservative. Due to increasing level of demand regarding health concern issue safe food, an attempt has undertaken to formulate the artificial preservative free guava–pineapple jelly and their marketability, physicochemical, bioactive compounds, microbial and antioxidant properties analysis during storage. In this study, the jelly was formulated using artificial preservative potassium metabisulphite (KMS) (T1), natural preservative honey and fresh lemon extract juice (T2 and T3), without artificial and natural preservative (T4) using different proportion of guava–pineapple extracted juice. Results of different treated jelly showed that T2 and T3 jelly was nutritionally superior. Bioactive compounds and antioxidant properties was highly present in T2 and T3 jelly. The brightest color (L* and C*) and highest hue angle (H*) value was noted in honey and fresh lemon extract juice treated jelly T2 and T3 upto 8 months of storage but after 9 months, darkest color was noted gradually. The consumer acceptance was extended to honey and lemon extract juice treated jelly (T2 and T3) for their impressive color and flavor. High incidence of microbial load was recorded in preservative free (both natural and artificial) jelly (T4). Low incidence of microbial load obtained from this study gives an indication that T2 and T3 jelly is microbiologically safe as natural jelly. Shelf life of natural preservative treated jelly (T2 and T3) could be extended upto 8 months and artificial preservative treated jelly could be upto 9 months (T1) at ambient condition without any quality deterioration. Overall, honey and fresh lemon extract juice are valuable source of natural preservative for formulation of fruit jelly. The processors could process and preserve the fruit jelly with decent aroma by applying this technology as an alternative to artificial food additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Guava (Psidium guajava) is known throughout every tropical region worldwide. The fruit is eaten as a dessert, but the numerous minute seeds give it an unpleasant “sandy” texture. Its juice is very acidic and is too tart and strongly flavored for direct use and consumption due to its hard shell. According to the Food and Agriculture Organization (FAO) statistics world guava production increased from 4.92 million metric tonnes (mMT) in 2010 to 54.83 mMT in 2021 [1]. Ten countries namely India, China, Thailand, Pakistan, Mexico, Indonesia, Brazil, Bangladesh, Philippines and Nigeria are the top guava producing countries around the world with a production of 21.80, 4.80, 3.80, 2.30, 1.63, 1.31, 1.18, 1.05, 0.83 and 0.79 mMT whereas Bangladesh ranks 8th in production of 1.05 mMT (FAO STAT, 2021) [1]. In Bangladesh, it is a seasonal and perishable fruit. It is mainly cultivated in the hill areas but recently its cultivation is tremendously increasing year by year all over the country [2]. Its leading growing areas are Chattragram, Dhaka, Barishal, Rajshahi, Khulna, and Pirojpur. The fruit contains 250 IU/100 g of vitamin A, 75–265 mg/100 g of vitamin C, 17.80–30 mg/100 g of thiamin, riboflavin, niacin, and phosphorus [3]. Short storage life (6–8 days) is the major problem that does not allow to keep the fresh fruit in shelf life for a long time [4]. Therefore, processing of guava into jelly may be one of the way to make value added process products [5].
The pineapple (Ananas cocomus Merr.) is a large fruit composed of number six-sided berries arranged spirally and embedded in the juicy pulp of the swollen stem. The socalled fruit carries a crown of spiny leaves and may grow to a weight of 1–3 kg per fruit. The fruit is found in almost all the tropical and sub-tropical areas of the world [6] and its productin is increasing year to year [1]. The total world production of pineapple is estimated as 27.82 mMT [1]. In the world, top pineapple producer countries are Philippines, Costa Rica, Brazil, Indonesia, China, India, Thailand, Nigeria, Mexico, Colombia, Vietnam, Ghana, Angola, Peru, Dominican Republic, Venezuela, Benin, Taiwan, Tanzania, Guatemala, Kenya, Cameroon, Malawi, Malaysia, Bangladesh, Democratic Republic of Congo and United Sates with a production of 2.70, 2.62, 2.45, 2.44, 2.22, 1.79, 1.53, 1.51, 1.20, 0.88, 0.70, 0.67, 0.59, 0.58, 0.50, 0.48, 0.44, 0.42, 0.37, 0.36, 0.33, 0.31, 0.30, 0.29, 0.22, 0.19 and 0.19 mMT respectively [1]. Pineapple is the source of bromelain, used as a meat-tenderizing enzyme, and high quality fiber [7]. It contains considerable amount of calcium, potassium, fiber, vitamin C, low fat and cholesterol. The fruit is a good source of vitamin B1, vitamin B6, copper and dietary fiber. It is a digestive aid and a natural anti-Inflammatory fruit [8]. In Bangladesh, it is a major fruit and its predominant growing areas are concentrated to Modhupur, Sreemongal, Rangamati, Khagrachari, and Chattagram region [2]. Every year the fruit goes to postharvest loss due to lack of proper processing and preservation techniques.
Various processed products are made from guava and pineapple fruits worldwide including jam, jelly, leather, nectar, squash, dried powder, toffee, ice-cream, candy, syrup, juice, concentrated puree, canned fruit segments, ready to serve drinks, etc. [9] but these are meager in the country. Artifical guava jelly can be found in the market made by a huge amount of pectin (E-440), citric acid (E-330), sodium benzoate (E-211), potassium metabisulphite (E-224), permitted food colors (Allura Red AC E-129 and Ponceau 4R E-124) [10]. Current research indicates that artificial chemicals and preservatives have various side effects. Their use could be the foundation of the long-term effects on health, especially the manifestation of kidney diseases, liver injury, type-2 diabetes, coronary heart disease, and stroke, which is tremendously increasing across the globe [11]. Most of the synthetic food contains a significant amount of heavy metals that accumulate in the human body. The buildup of heavy metals in the body is the leading cause of developing the above-mentioned diseases and this can also develop abnormality among children [12]. Recent studies have shown that the process of soaking, washing, boiling, grilling and cooking significantly decrease the the concentration of arsenic and heavy metal residue in processed food [12,13,14,15,16]. Organic citric acid is also found to be more effective to reduce the heavy metal residues due to its working ability as a chelating agent [12]. The acid is also used as a flavoring and acidifying agent [17].
Traditional methods of preservation includes boiling, freezing, pasteurizing, dehydrating, smoking, pickling, adding sugar, lemon juice, honey, etc. Jam and jelly are usually preserved using high sugar content. Lemon juice is a very effective method to preserve fruits and vegetables. Honey is a rich source of nutrients having antimicrobial properties that have been proven by the several researchers [18,19,20,21,22,23]. Almost all natural honey contains flavonoides (such as apigenin, pinocembrin, kaempferol, quercetin, galangin, chrysin and hesperetin), phenolic acids (such as ellagic, caffeic, p-coumaric and ferulic acids), ascorbic acid, tocopherols, catalase, superoxide dismutase (SOD), reduced glutathione (GSH), Millard reaction products and peptides. Most of those compound works together and subsidizes energy, immunity system and remedy for several ailments by providing a synergistic antioxidant and inhibitory effect [24,25,26,27,28,29]. Recently honey is considered as a new addition to the modernization and commercialization of agriculture in Bangladesh where the country received an export order for 400 MT of honey from Japan [30]. Considereing its present production trend, medicinal and antimicrobial activities, hence, an attempt has been taken to utilize the honey for processing of agricultural commodities as well as industrial utilization to make the process economically viable to the honey producers and utilizers.
Among natural substances obtained from the plants, sugar, lemon juice and honey efficiently decrease the growth of bacteria, increase flavor, and impart attractive color to food [10]. In recent years, peoples’ choice is increasing to find out alternative natural foods instead of artificial foods. Recently, there is concern by food scientists, medical scientists, and nutritionists to discover natural foods for the promotion of safe food production, processing, preservation, and distribution. The objective of the current work was to determine the influence of honey and lemon juice on the physicochemical, nutritional, microbial and antioxidant properties of guava–pineapple jelly during storage periods.
2 Materials and methods
2.1 Materials and chemicals
Matured local variety of guava was harvested from the Farm Division of Bangladesh Agricultural Research Institute (BARI), Gazipur-1701, Bangladesh. Ripe pineapple was collected from the farmer’s field of the hilly area of Chattagram, Bangladesh. After collection, the fruits were shifted to the laboratory of Postharvest Technology Division, BARI, Gazipur, Bangladesh for pre-processing. All chemicals and reagents used in this study were of analytical grade. The chemicals and reagents were collected from the Sigma Aldrich Co. Ltd. USA.
2.2 Processing of guava–pineapple jelly
After pre-processing i.e. cooling, sorting and grading, guava–pineapple jelly was formulated according to the following treatments, calculation and process flow chart (Fig. 1). Total weight of the product was 1758 g (1.758 kg). Percent of individual ingredient (i.e. juice, sugar, KMS, citric acid, honey, lemon extract juice) was calculated according to the following formula and total weight of the product.
Treatments:
During making of jelly, total soluble solids (TSS) content was checked by a digital hand refractometer and it was maintained from 67.03°B to 67.31°B in the experimental sample. Sheet/drop test was performed as an alternative of digital hand refractometer [4]. In this test, a little amount of jelly was allowed to the bottom of water filled transparent glass. When the jelly was falled to the bottom of the glass in the form of flake or sheet, the formation of jelly was determined.
2.3 Collection of fresh lemon and honey
The fresh lemon was collected from the farmers field of Marta, Gazipur, Bangladesh. Locally produced honey was collected from the Sundarban (enlisted as World Herritage by the UNESCO) of Khulna Division, Bangladesh. After collection all samples were transported to the laboratory of Postharvest Technology Division, Bangladesh Agricultural Research Institute (BARI), Gazipur, Bangladesh for laboratory procecures and experimentation. The lemon juice was directly extracted using locally made juicer from the collected fresh whole lemon instead of direct procurement of juice. The fresh lemon extract juice was used for formulation of jelly.
2.4 Sensory evaluation
The sensory evaluation was done on the day of preparation and after 9 months of storage following the procedure of Joshi [31] based on 9-point hedonic scale. Three judgment panel groups were formed comprising thirty expert members from the Bangladesh Agricultural Research Institute (BARI) inter-divisional Scientists to evaluate the sensory attributes i.e. color, flavor, texture, mouthfeel, spreadable capacity, and overall acceptability of the formulated jelly. As each judgement group contained 30 members, thus total 90 members (1 group 30 members × 3 groups) were requested to evaluate the sensory attributes. The score obtained by the panelists was statistically analyzed.
2.5 Color measurement
The color of experimental guava–pineapple jelly was assessed according to the method described by Dervisi et al. [23] with little modification using a Chroma Meter (Model CR-400, Minolta Corp, Japan). International Commission on Illumination (CIE) lightness (L*), Chroma (C*), and hue angle (H*) values were documented using D65 illuminates and a 10E standard viewer as an orientation method. The equipment was calibrated on a standard white tile. Then, it was adjusted to measure the values of L*, C*, and H* and was replicated three times for each treatment.
2.6 Texture analysis
Texture of different treated jelly was measured by a texture analyzer (Stable Micro System, Godalming, UK). The analyzer probe (p-5) was directly inserted into the jar of jelly by the back extrusion method. The instrument working parameters were determined by the test mode compression with test speed at 1 mm/s, and a distance of 2.50 cm. The analysis of the data was measured by Texture Exponent Lite version 6.1.14.0 software (Stable Micro System, Godalming, UK) to determine the rupture force (FR) and expressed in the unit newton (N).
2.7 Physicochemical and nutritional analysis
The physicochemical properties of the treated jelly concerning moisture, protein, ash, vitamin-C, total, and reducing sugar content were determined according to the procedure described by Ranganna [33]. pH was recorded by a digital pH meter (Delta 320, Mettler, Shanghai). total acidity (%) was measured using Auto Titrator (Metrohm 814, USB Sample Processor, Switzerland). total soluble solid (TSS; °Brix) was recorded using a digital hand refractometer (Model NR151). The water activity (aw) of the jelly was recorded using Lab Touch-aw (Novasina, AG, CH-8853, Switzerland).
2.8 Minerals analysis
The minerals analyzed in this study were: sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P), sulfur (S), boron (B), copper (Cu), manganese (Mn), iron (Fe), and zinc (Zn). Atomic absorption spectrophotometry (Model-AA-7000S, Shimadzu, Tokyo, Japan) was used to assess Na, Fe, Cu, Zn, B, Mn, Ca, and Mg. K was measured using flame photometry, while P and S were assessed with the spectrophotometric method. Individual minerals were quantified by comparing the corresponding protocol procured from the Sigma Chemical Co., USA.
2.9 Determination of bioactive compounds
2.9.1 Total phenolic content
The total phenolic content of jelly was determined according to the Folin–Ciocalteu method [34] with gallic acid (GAE) as the standard and expressed in the unit milligram (mg) as gallic acid equivalents (GAE)/g of the jelly. In this method, twenty milligrams (0.02 g) of jelly was dissolved in 1 mL of methanol to prepare a stock-solution for experiments. A volume of 0.5 mg of jelly (100 µg/mg) was mixed with 2 mL of the Folin-Ciocalteu reagent (diluted 1:10 with de-ionized water) and was neutralized with 4 mL of sodium carbonate solution (7.5%, w/v). The reaction mixture was incubated at room temperature for 30 min. with intermittent shaking for color development. The absorbance of the colored solution was measured at 765 nm using a double beam UV–VIS spectrophotometer. The total phenolic content was determined from the linear equation of a standard curve set with gallic acid.
2.9.2 Determination of total flavonoid content
The total flavonoid content (TFC) of jelly was measured by the aluminum chloride method [35] with slight modifications. The sample solution was prepared by mixing jelly in methanol at a concentration of 1 mg/mL. 0.5 mg of sample solution was then mixed with 1.5 mL of methanol. To this mixture, 0.1 mL of 10% aluminum chloride and 0.1 mL of 1 M potassium acetate were added. The final volume was made up to 5 mL by adding 2.8 mL distilled water and left to react for 30 min at room temperature. The absorbance of the reaction mixture solution was measured at 415 nm using a UV–VIS spectrophotometer. The TFC was calculated based on the R2 value of the calibration curve and expressed as mg quercetin equivalent per gram of jelly (mg QE/g of jelly).
2.9.3 Determination of total carotenoid content
The determination of total carotenoid content was performed according to the method by Thaipong et al. [36]. The measured jelly was dissolved in n-hexane pro analysis. The β-carotene solution in various concentrations was used as a standard of the carotenoid compound and as a standard curve. Absorbance was measured at 470 nm. The linear regression equation of the standard curve was used for calculating total carotenoid content. The results were expressed as beta-carotene equivalent mg per 100 g of jelly (mg/100 g).
2.9.4 Determination of β-carotene content
β-Carotene content of the jelly was measured accordinf to the method described by Holden et al. [37] and minor modification made by Molla et al. [38]. A 3 g of jelly was diluted with acetone (Fisher Scientific Ltd., UK) and petroleum ether. It was further purified with acetone, metabolic potassium hydroxide (KOH) and distilled water. The subsequent solution was filtered with anhydrous sodium sulphate and the absorbance was measured by UV–Vis Double Beam Spectrophotometer at 765 nm against petroleum ether as a blank. The value was noted as µg/100 g of jelly.
2.9.5 Determination of total anthocyanin
Total anthocyanin of jelly was measured based on the method described by Burgos et al. [39]. Accordingly, 0.2 g of jelly was mixed with 10 mL of methanol/1.0 M HCl (75:25, v/v) and sonicated for 10 min at room temperature. The mixture was centrifuged at 5000 rpm for 10 min. and the pellet was re-extracted. The combined supernatants were filtered and the volume was made up to 25 mL with the extraction solution. The absorbance of the extract was read at 545, 535, and 515 nm, and the concentration of TA was calculated using the molar extinction coefficient and molecular weight of malvidin-3-p-coumaroyl-glucoside for blue-violet pigments (545 nm, 3.02 × 104 L/mol/cm, 718.5 g/mol), pelargonidin-3-glucoside for red pigments (515 nm, 2.73 × 104 L/mol/cm, 486.5 g/mol), and cyanidin-3-glucoside for purple pigments (535 nm, 3.43 × 104 L/mol/cm, 449.2 g/mol). The results were expressed in mg/100 g of jelly.
2.10 Determination of antioxidant activity
2.10.1 Total antioxidant activity
The total antioxidant activity was evaluated by the phosphomolybdenum system based on the method described by Prieto et al. [40]. Briefly, 0.3 g of jelly was taken in a glass tube and 3 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) was added. The mixture was heated at 60 °C in a water bath shaker for 90 min. Then, the mixture was cooled at room temperature and the absorbance was read at 695 nm. The result was stated as microgram ascorbic acid (AA) per gram (µg AA/g) of jelly.
2.10.2 Reducing power assay
The reducing power of jelly was assessed using the approach of Guao et al. [41] and the result was expressed in the unit microgram ascorbic acid per gram (µg AA/g) of the jelly (µg AA/g). The jelly (0.2 g) was mixed with 0.5 mL phosphate buffer (0.2 M, pH 6.6) and 0.5 mL potassium ferricyanide (1% w/v) and mixed properly. The mixture was then incubated at 50 °C for 30 min, 0.5 mL of trichloroacetic acid (10%, w/v) was added, and subjected to centrifugation for 10 min. The upper portion of the solution (0.5 mL) was taken, mixed with 0.1 mL of 0.1% (w/v) FeCl3 and 0.5 mL of distilled water. The absorbance was noticed at 700 nm and ascorbic acid was used as the standard for the preparation of the calibration curve.
2.10.3 Ferric reducing antioxidant power (FRAP)
FRAP activity was measured following the scheme outlined by Benzie and Strain [42]. Briefly, 40 μg of jelly was taken in a glass tube and 3 mL of freshly prepared FRAP solution was added. The mixture was incubated at 37 °C for 4 min and the absorbance was taken at 593 nm against the blank (distilled water). A standard curve was made using the ferrous sulfate aqueous solution (1–10 mM) and the FRAP value was expressed as μM Fe (II) per 100 g of the jelly.
2.10.4 DPPH radical scavenging activity (DPPH-RSA) and IC50
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical quenching property was measured using procedure described by Brand-Williams et al. [43]. The result was expressed in percent radical scavenging activity. Exactly 0.1 mg of jelly was put in a falcon tube and 1.4 mL of methanolic solution of DPPH was added. The mixture was left to rest for 30 min. in dark and the absorbance at 517 nm was measured against the blank (0.1 mL methanol in 1.4 mL DPPH solution). The result was stated in terms of percent radical scavenging activity.
where Ao is the absorbance of the blank and As is the absorbance of jelly. Then, the inhibition curves were prepared and IC50 values were calculated [43]. BHT was considered a positive control.
2.10.5 Metals chelating capacity
The metal chelating capacity (MCC) was measured according to the method of Bahadori et al. [44] and the result was expressed in percent metal chelating capacity (%). Briefly, 2 mg of jelly was taken in a glass tube to which 0.05 mL of ferrous chloride (2 mM), 3.7 mL of distilled water, and 0.2 mL of ferrozine (5 mM) were added. After 20 min. of incubation at atmospheric conditions, the absorbance was read at 562 nm against the blank. The following formula was applied to calculate the metal-chelating capacity.
2.10.6 Assessment of phenolic acids by HPLC
Phenolic compounds were assessed based on the method described by Pandey and Negi [45] with some adjustment using high-performance liquid chromatography (Shimadzu SPD-M10A) coupled with a photodiode array detector and autosampler at 280 and 320 nm. The separation was achieved by the C18 column (250 mm × 4.6 mm) with 5 µm particle size at room temperature. The mobile phase was 1% acetic acid (A) and 80% acetonitrile in A (B). The following gradient was followed: 0.01–35 min, 0% of B; 35–40 min, 50% of B; 40–45 min, 100% of B; and 45–60 min, 0% of B. The flow rate was 1 mL/min and the injection capacity was 20 µL. A total of 60 min. was taken for chromatographic analysis. All solvents used for HPLC were degassed using a vacuum filter. Six phenolic standards (gallic acid, vanillic acid, caffeic acid, ferulic acid, + catchin acid, and syringic acid) were used for the identification of respective phenolics, and quantification was accomplished using a standard curve prepared by injecting the mixture of all the standards (0.1–0.7 mg/ 100 g).
2.10.7 Shelf life of the jelly
The prepared jelly was poured into a pre-sterilized auto lug cap glass jar and stored at room temperature up to 9 months for shelf life studies. The shelf life of jelly was determined based on microbiological studies. Data on shelf life was recorded at 0, 3, 6 and 9 months of storage which is considered as maximum shelf life limit.
2.10.8 Statistical analysis
All data was expressed in triplicate as means ± standard deviation. One-way analysis of variance (ANOVA) with post-hoc using Tukey’s Multiple Comparison Test was performed to analyze the data. The connotation was distinct at the 95% confidence level. SPSS 17.0 (IBM INC., New York) software was used for statistical analysis.
3 Results
3.1 Physicochemical, nutritional, bioactive compounds and antioxidant properties of fresh guava and pineapple
The physicochemical, nutritional, bioactive compounds and antioxidant properties of fresh experimental guava and pineapple are shown in Table 1. The findings revealed that bioactive compounds and antioxidant activities of the fresh guava and pineapple samples were significantly differed whereas total sugar, reducing and nor-reducing sugar, total soluble solid and moisture content were insignificantly differed. The variation might be due to individual nature of frui, different fruit orchard, classification, genus and family, soil type, and texture [46].
3.2 Physicochemical and nutritional properties of guava–pineapple jelly on the day of storage
Moisture, total sugar, reducing sugar, non-reducing sugar, TSS, acidity, pH, water activity, vitamin-C, β-carotene, crude protein, crude fat and energy of different treated jelly were analyzed on the day of storage and are shown in Table 2. The lowest moisture content was recorded in sample T1 (21.95%), T2 (21.75%) and T3 (21.85%), while the highest moisture content was recorded in sample T4 (31.62%). The analysis of variance for ash contents show that the differences were highly significant (Table 2). Lowest ash content was found in sample T4 while the highest was in sample T1, T2, and T3. In the case of sugar content, samples T1, T2 and T3 were found to have lower total sugar, reducing and non-reducing sugar whereas the sample T4 was found to be higher as 63.44%, 23.11% and 40.34% respectively (Table 2). TSS of the jelly ranged from 67.10 ± 0.10 to 68.70 ± 0.02°B. The lower TSS (°B) content was calculated in T1 (67.31°B), T2 (67.20°B) and T3 (67.10°B), while the highest was found in sample T4 (68.70°B).
The lowest aw was recorded in T1 (0.59), T2 (0.56) and T3 (0.58), while the highest aw (0.76) was recorded in T4 jelly. The lower value of pH (2.54) was recorded in T4 jelly whereas the higher value was recorded in all treated jellies (3.38–3.48) (Table 2). In this study, acidity varied significantly in treated jelly T1, T2 and T3 and T4.
The highest vitamin-C and β-carotene contents were observed in treated jelly T1, T2 and T3 than controlled jelly T4. The highest vitamin-C content of the treated jelly T1, T2 and T3 was recorded as 15.29 ± 0.02 mg/100 g, 34.13 ± 0.15 mg/100 g and 43.01 ± 0.10 mg/100 g respectively whereas the T4 jelly was calculated as 12.91 ± 0.15 mg/100 g (Table 2).
The β-carotene content of the treated jelly T1, T2 and T3 ranged from 6.31 ± 0.01 to 12.78 ± 0.05 µg/100 g whereas the T4 jelly was found 8.40 ± 0.40 µg/100 g. The results indicate that treated jelly T2 and T3 possessed higher value of β -carotene content than T4 jelly (Table 2). The significant difference of the crude protein was observed among the treated jelly T1, T2, T3 and T4. The treated jelly T2 and T3 possessed higher value of crude protein as compared to T1 and T4 (Table 2). The crude fat content of the different treated jelly was statistically significant on the day of storage (Table 2). The less amount of crude fat was found in all treated jelly T1, T2, T3 and T4. The energy content of the treated jelly T1, T2 and T3 ranged from 303.08 ± 0.01 to 374.29 ± 0.02 cal/g while T4 jelly possessed 300.53 ± 0.02 cal/g (Table 2). All the treated jelly was significantly differed and the lower energy content was found in jelly T1 and T4 than T2 and T3.
However, the above results conclude that jelly treated with honey and fresh lemon extract juice (T2 and T3) found nutritionally superior followed by traditionally treated jelly (T1) and control jelly (T4).
3.3 Sensory evaluation of guava–pineapple jelly on the day of preparation
The sensory evaluation of guava–pineapple jelly was done by forming three expert panel groups consisted 90 members (each group contained 30 members) following a 9-point hedonic scale and shown in Table 3. The highest overall acceptability score gained by the treatment T2 (8.19 ± 0.38) and T3 (7.84 ± 0.27) in terms of color, flavor, texture, mouthfeel, and overall acceptability. T1 and T4 treated jelly was less acceptable by the judgement group and obtained lowest score (6.30 ± 0.63 and 5.72 ± 0.66). Each member of the judgement group opined that treated jelly T2 and T3 was impressive due to their attractive color and flavor as honey and lemon juice was incorporated during making the jelly (Fig. 5). Hence, treatment T2 and T3 may be selected as best combination for formulation for guava–pineapple jelly.
3.4 Storage studies of guava–pineapple jelly
After standardization, the jelly was stored upto 9 months for its physicochemical, nutritional, color, texture, minerals, bioactive compounds, antioxidant properties, phenolic acids and microbial count studies during 3, 6 and 9 months.
3.5 Physicochemical and nutritional properties of guava–pineapple jelly during storage
Table 4 shows the physicochemical and nutritional properties of the standardized jelly during 3, 6 and 9 months of storage. The study indicates that total sugar, reducing, and non-reducing sugar of the stored jelly were non-significantly changed with the progression of storage periods (Table 4). During 9 months of storage, the highest total and reducing sugar content was recorded as 61.91–61.95% and 13.36–13.48% respectively in treated sample T3 than others, while the highest non-reducing sugar content was calculated as 48.55–48.54% in the sample T1 (Table 4). During storage, both total and reducing sugar content was increased slightlty and there was no significant difference among the treatments.
Initially the TSS content of the fresh guava fruit was recorded as 9.86°B, while it was recorded as 67.23°B to 67.57°B in the jelly during the stotage periods 3–9 months (Table 4). Higher amount of TSS was found in the treated jelly T3 (67.43–67.57ºB) as more water loss occurred throughout the storage periods (3–9 months) but, there was no significant difference (p ˂ 0.05) among the treatments (Table 4). The acidity of the fresh guava and pineapple was recorded as 0.31% and 0.63% respectively (Table 1), while it was found 0.42–1.01% on the day of preparation of the jelly (Table 2). After storage, the acidity ranged from 0.52 ± 0.02% to 0.83 ± 0.03% for all the stored jelly. Results revealed that higher amount of acidity was found in the treated jelly T4 but, the acidity was significantly varied with the advancement of storage periods (Table 4). The pH of the fresh guava was 4.02 (Table 1), while it was recorded as 3.38–3.48 on the day of preparation (Table 2). After storage, the pH ranged from 3.37 ± 0.01–3.09 ± 0.00 for all treatments but, it was decreased slightly throughout the storage periods. The higher amount of pH was found in the treared jelly T4 but, there was significant difference among the treatments (Table 4). It was noteworthy that there was an inverse relationship between the acidity and pH of the different treated jelly throughout the storage periods (Table 4).
Vitamin-C content of the fresh guava and pineapple was found 73.43 mg/100 g and 39.49 mg/100 g respectively (Table 1). After processing into jelly, the highest amount (42.94 mg/100 g) of vitamin-C was found in the treated jelly T3 whereas the lowest amount (41.00 mg/100 g) was found in the treated jelly T1 throughout the storage periods (3–9 months; Table 4). The vitamin-C content of the different treated and stored jelly significantly decreased with the progression of storage periods. The highest amount (42.94 mg/100 g) of vitamin-C preserved in the T3 treated jelly probably due to the variation of mixture and extracted fruit juice in different treatments. The reduction of vitamin-C could be explained through the increase in oxidative stress under different ingreadients used in the different treatments. It is well proven that vitamin-C reacts strongly with thermal treatments which reduces vitamin-C during storage [47].
Moisture content range of the treated jelly T1, T2, T3 and T4 was recorded as 21.95–31.62% respectively on the day of storage (Table 2) but after 9 months of storage, the moisture content decreased 21.78–20.01% (Table 4). The moisture content was significantly decreased as TSS increased throughout the storage periods (Table 4). Silvia et al. [48] cited in minimally process products it is common to increase TSS contents in response to moisture loss during storage.
The ash content of the treated jelly ranged from 0.4 to 0.6% but, there was no significant differences among the treated jelly. Ash content of the treated jelly was increased with the advancement of storage periods. Results obtained by the guava–pineapples jelly contain higher amount of ash than pineapple, jackfruit and mango jams (0.15–0.49 g/100 g) [49, 50]. The highest ash content was found in T2 and T3 treated jelly whereas the lowest was recorded in T1 and T4 jelly. The highest ash content in T2 and T3 jelly could be attributed to the rich source of minerals as the T2 and T3 jelly is prepared to the addition of honey.
The growth of microorganisms depends on water activity (aw). The cells of the microorganisms become dormant in the presence of low aw and osmotic stress conditions. It has been documented that the microorganisms could not be eliminated but the activities of the microorganisms can be stopped by limiting aw. On the day of preparation, the lowest aw was calculated as 0.56 and 0.58 (Table 2) but it was increased throughout the storage periods (3 to 9 months) from 0.56 to 0.60 for treated jelly T1, T2 and T3 (Table 4). Highe amount of aw (0.66) was found in the treated jelly T4 thus it activated boost Aspergillus, Shijella and E. coli to grow more in T4 jelly (Table 11). The aw in T1, T2 and T3 jelly ranged from 0.57 to 0.60 indicating that microorganisms were generally inhibited to grow within this range, suggesting that the stored jelly T1, T2 and T3 can be considered safe in terms of microbial stability and quality, and are shelf-stable up to 9 months. Herein, the study also confirmed that there was a highly significant relationship between the moisture content and aw (Fig. 2), i.e. the presence of low moisture and slightly decreased of moisture in jelly might be contributed to achieve lower aw within the range of limit (Table 11).
The crude protein content was slightly increased throughiut the storage periods but, the increases were observed non-significant difference among the treated jelly (Table 4). Higher amount of crude protein was found in treated jelly T3 (3.12 ± 0.01–3.21 ± 0.02) and lower amount of the protein was found in T4 jelly (3.07 ± 0.01–3.10 ± 0.02). The lower amount of T4 jelly might be due to microbiological activities and drastically loss in shelf life during storage periods (Table 4; Fig. 4).
The crude fat content of the treated jelly slightly increased throughout the storage periods (3–9 months) but, there was no significant difference among the treatments (Table 4). Higher amount of crude fat (0.02%) was found in T3 jelly whereas the lower amount of crude fat (0.01%) was observed in T4 jelly. The highest amount of crude fat obtained by the T3 jelly might be due to combination of extracted guava and pineapple jelly using honey and extracted fresh lemon juice. Likely the lower amount of fat content obtained by the T4 jelly might be due to free from honey and extracted fresh lemon juice. It is noteworthy that the less amount of crude fat was found in all treated jelly throughout the storage periods (3–9 months). The less amount of fat presence in the treated jelly might be due to slow or stop the breakdown of fat metabolism by the the presence of oxygen [10]. The lower amount of crude fat content had also reported by Naeem et al. [51], those reported that apricot, strawberry, blueberry and grape fruit jam have very low fat content (0.1–0.2 g/100 g).
The highest energy content was found in T3 jelly, ranged from 406.35 ± 0.12–406.94 ± 0.01 cal/g whereas the lowest was found in T4 jelly (374.30 ± 0.01–374.63 ± 0.05 cal/g). Results revealed that guava–pineapple jelly provides higher amount of energy value than the results obtained by Giamperi et al. [52], those reported that strawberry jam provides energy value only 48.9 cal/g which is much lesser than guava–pineapple jelly. The highest energy value obtained by the T3 jelly followed by other treatments could be attributed to the addition of guava–pineapple extracted juice, honey and fresh lemon juice during the jelly making process as they are great source of energy (Table 2).
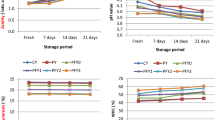
3.6 Color of guava–pineapple jelly during different storage periods
Appearance is the most common phenomenon used to measure the quality of any product whereas color and surface conditions performance a fundamental role in the appearance of the product. L* is an approximate quantity of lightness that can be well-thought-out as compared to the member of the greyscale, between black and white [53]. Chroma (C*) is a measurable characteristic of colorfulness used to measure the variance of a hue in contrast to a grey color by a similar lightness. The result obtained from the storage studies showed that the values of L* and C* were decreased with the advancement of storage periods and statistically the values were insignificant (Table 5). The decreased L* and C* values throughout the storage periods (0–9 months) indicate that color lightness and intensity of the different treated jelly T1, T2, T3 and T4 were lowered gradually. H* value of the treated T1, T2, T3 and T4 jelly was significantly increased 69.12 ± 8.53 to 72.11 ± 3.01 (T1), 110.23 ± 4.01 to 120.90 ± 5.59 (T2), 108.41 ± 2.07 to 119.11 ± 5.89 (T3) and 70.51 ± 3.19 to 71.91 ± 3.01 (T4) up to 6 months of storage, indicating that T2 and T3 jelly was within 180° and 270° region with attractive color (Fig. 5). But during 9 months of storage, the H* value of all the treated jelly had began to lose its attractive color gradually, indicating that the treated jelly started to lose their initial color. All the treated jelly T1, T2, T3 and T4 finally faded out and turned into dark after 9 months of storage. Here, it is noteworthy that higher amount of color value L*, C* and H* was found in honey and lemon juice treated T2 and T3 jelly followed by T1 and T4. The color variation between T1, T4 and T2, T3 jelly could be attributed due to addition and without addition of honey as its (honey) color is an essential indicator (Fig. 5) that reflects the presence of some components like terpenes, polyphenols and carotenoids [54].
3.7 Texture of guava–pineapple jelly after 9 months of storage
Figure 3 shows the textural properties as well as rupture forces (FR) of the guava–pineapple jelly after 9 months of storage. But it is difcult to determine accurately the mechanisms and the potential reasons for the diferences of textural properties of the treated jelly. Increase and decrease in jelly strength/hardness could be related to the internal degradation or formation of pectin, which under the infuence of temperature and pH, undergo depolymerization [55]. In this study, the initial FR of the four treated jelly T1, T2, T3 and T4 were found 0.15, 0.14 N, 0.13 and 0.101 N, respectively. But after 9 months of storage, the FR value was increased to 0.630 N, 0.620, 0.617 N and 0.606 N, respectively. The increase in FR value of the stored jelly partially contributed to the jelly hardness; hence, the jelly samples were slightly disliked by the panel of judges after 9 months of storage. In comparison among four treatments, T1 jelly was relatively harder than T2 and T3. The increase in hardness afer 9 months of storage could be a result of the polymerization of low-molecular-weight compounds or the interaction between jelly components [56, 57]. More softness was found in T4 jelly that was stored without any artificial (KMS) and natural preservative (honey). But it lost nutritional (Tables 4, 7, 8), sensory (Table 10), microbiological (Table 11) and shelf quality (Fig. 4). The decrease in frmness and consistency of T4 jelly might be due to internal mechanism of degree of high and low methoxyl pectin as it was belongs to the low methoxyl pectin due to its free of sufficient acid and preservative. T2 and T3 jelly was belongs to the high methoxyl pectin as sufficient citric acid (lemon juice) and preservative as well as sugar was present there. However, the decrease and increase of firmness of the treated jelly was associated with increasing storage periods that is strongly supported by Kopjar et al. [58], those found that the firmness of strawberry jam was decreased for two weeks of storage but after 12 months of storage, the firmness was increased.
3.8 Minerals of guava–pineapple jelly during different storage periods
Minerals are the inorganic components present in food and turn into ash when food is consumed to produce energy. Generally, two forms of minerals are present in foodstuff, macro and micro minerals, both playing important metabolic roles in the functions of our body [59] and contribute to our daily dietary requirements. In this study, eleven (11) minerals of the standardized jelly were assessed as shown in Table 6. All the minerals of the treated jelly decreased with the progression of storage periods but, there was no significant differences among the treatments throughout the storage periods (0–9 months). Results revealed that Ca, Mg, Na, and K were highly present in T3, while S, Fe, Mn, Zn, B, Cu, and P were impressively present in T2. On the other hand, T1 and T4 jelly analysed had very low content of Ca, Mg, Na, K, S, Fe, Mn, Zn, B, Cu, and P followed by T2 and T3 jelly. The lower content of Ca, Mg, Na, K, S, Fe, Mn, Zn, B, Cu, and P for T1 and T4 jelly found in the present study could be attributed to preparation of jelly without honey and extracted fresh lemon juice. Likely, the higher amount of Ca, Mg, Na, and K in T3 jelly found in the study could be attributed to the dilution of extracted fresh guava–pineapple fruit juice with honey and extracted fresh lemon juice during jelly making process. Similarly, T2 jelly had the highest amount of S, Fe, Mn, Zn, B, Cu, and P found in this study can be associated with the presence of extracted fresh lemon juice and honey during jelly making. The differences among the treatments T1, T2, T3 and T4 could be linked to the addition of honey as the trace minerals highly present in honey [60].
3.9 Bioactive compounds and antioxidant activity of guava–pineapple jelly during different storage periods
The bioactive compounds e.g. total phenolic content, flavonoid, carotenoid, β-carotene, and anthocyanin content present in guava–pineapple natural jelly are listed in Table 7, showing the values on the day of storage and after 9 months of storage at ambient condition. Higher amount of total phenolic content, flavonoid, carotenoid, β-carotene, and anthocyanin content in T3 jelly were observed in the range of 4.15 ± 0.26–4.01 ± 0.20 mg GAE/100 g, 0.91 ± 0.02–084 ± 0.01 mgQE/g, 0.94 ± 0.03–0.81 ± 0.04 mg/100 g, 12.44 ± 0.33–12.20 ± 0.20 mg/100 g, and 4.06 ± 0.19–4.01 ± 0.10 mg/100 g respectively. The lower amount of total phenolic content, flavonoid, carotenoid, β-carotene, and anthocyanin content in T1 jelly were recorded in the assortment of 3.90 ± 0.19–3.88 ± 0.10 mgGAE/100 g, 0.69 ± 0.02–0.66 ± 0.01 mgQE/g, 0.78 ± 0.05–0.74 ± 0.01 mg/100 g, 9.00 ± 0.05–8.95 ± 0.01 µg/100 g and 3.58 ± 0.03–3.53 ± 0.30 mg/100 g, respectively. Lower amount of phenol, flavonoid, carotenoid, β-carotenoid and anthocyanin found in T1 jelly might be due to biochemical reaction of potassium metabisulphite (KMS). Though KMS is antimicrobially active against yeasts, molds and bacteria but the sulphite reacts with a series of food constituents [17]. The results of bioactive compounds were found lower in T4 jelly, might be due to its oxidation, deterioration of microbiological quality (Table 11) and lower shelf life (Fig. 4). Higher amount of phenol, flavonoid, carotenoid, β-carotenoid and anthocyanin content possessed in T2 and T3 jelly could be attributed to use of honey and lemon juice during making jelly as the experimental honey contained high amount of phenolic, flavonoid, carotenoid, β-carotenoid and anthocyanin [60]. Higher amount of total phenol, flavonoid and β-caroten also recoded by this study in fresh guava and pineapple (Table 1).
In this investigation, the antioxidant properties of the standardized jelly were analyzed and shown in Table 8. It can be seen that the standardized storage jelly exhibited potent antioxidant properties. Total antioxidant capacity values of T1, T2, T3 and T4 samples have been found to be 104.10 ± 0.33 to 101.20, 105.84 ± 0.33 to 104.40 ± 0.51, 109.11 ± 0.22 to 107.88 ± 0.32 µg AA/g respectively. Results indicate that total antioxidant capacity were statistically significant and decreased with the advancement of storage periods. Lower amount of antioxidant capacity was found in T1 and T4 jelly whereas T2 and T3 jelly was rich source of antioxidant activities of phenolics are related to a number of different mechanisms i.e. free radical-scavenging, hydrogen-donation, singlet oxygen quenching, metal ion chelation, and acting as a substrate for radicals such as superoxide and hydroxyl [61]. On the day of storage and after 9 months of storage, the higher amount of DPPH was recorded in T2 and T3 sample followed by T1 and T4. The reducing power assay (RPA) of T2 and T3 showed values of 6.17 ± 0.06 to 6.05 ± 0.09 and 7.23 ± 0.08 to 7.06 ± 0.05 µgAA/mg, respectively throughout the storage periods, indicating that T2 and T3 are capable of reducing different metallic ions by making a stable chemical bond to scavenge free radicals.
The FRAP assay is frequently used to quantify the antioxidant potential of foodstuff and determine the capacity to convert ferric (Fe3+) into ferrous iron (Fe2+) in FRAP reagent [62]. As regards to the capacity of T2 and T3 to reduce Fe3+–Fe2+, the values were found as 29.51 ± 0.40 to 28.55 ± 0.54 and 27.99 ± 0.06 to 27.40 ± 0.52 µM Fe2SO4/100 g, respectively. The high radical scavenging activity found in T2 and T3 jelly followed by T1 and T4 could be due to its richness of ascorbic acid, phenols and flavonoids during making jelly using honey and extracted fresh lemon juice [63].
3.9.1 Phenolic acids of guava–pineapple jelly
Six key phenolic acids were observed and displayed in Table 9. All the phenolic acids were significantly differed (except syringic acids) throughout the storage periods (0–9 months). Findings depict jelly T2 and T3 had abundant phenolic acids followed by T1 and T4 jelly. Higher amount of gallic acid, vanilic acid, caffeic acid, ferulic acid, (+) catchin acid and syringic acid have been found in T2 and T3 jelly. Among the well-known phenolic acids, the (+) catchin acid was the leading phenolic compounds in both T2 and T3 jelly followed by T1 and T4. Significant differences were found in T1, T2, T3 and T4 jelly could be attributed by adding and without adding of honey and extracted lemon juice during making of jelly. Identified six phenolic acids were found higher in T2 and T3 jelly compared with T1 and T4 jelly as T2 and T3 jelly contain progressive amount of phenols, flavonoid, carotenoid, β-carotene, ascorbic acid and antioxidant activities (Tables 2, 4, 7, 8, 9). Six phenolic acids of T1, T2, T3 and T4 jelly were significantly and gradually decreased throughout the storage periods. The present reduction of phenolic acids are in accordance with [64, 65], those reported that phenolic compounds of guava-alovera pulp were significantly declined during 6 months of storage. Phenolic compounds are volatile in nature, thus it may reduce during long term storage [33].
3.9.2 Sensory evaluation of guava–pineapple jelly after 9 months of storage
Table 10 represents the evaluation of the sensory attributes of the standardized jelly after 9 months of storage. The results of sensory attributes were significantly affected by treatments T1, T2, T3 and T4. Results revealed that highest sensory score was secured by T2 (6.76 ± 0.41) and T3 (6.64 ± 0.21) jelly for its color, flavor, texture, mouthfeel, spreadability and overall acceptability. Addition of honey and fresh lemon juice significantly improved the color, flavor and mouthfeel of T2 and T3 (Fig. 5) compared to T1 and T4. Accordingly addition of honey had the ability to decrease the sourness and increase the consumer acceptability of T2 and T3 jelly. This results are strongly supported with findings of Metry and Owayss [66], those reported that addition of honey can improve the sensory quality of yoghurt without quality deterioration. T1 sample was less acceptable due to its weak flavor as it is made using artificial preservative (KMS). T4 jelly was unacceptable by the consumers due to its unpleasant flavor, color and mouthfeel (Fig. 5).
3.9.3 Microbial analysis of guava–pineapple jelly
Microbiological safety of processed food is the vital and safety issue to the consumers’ regarding health concern. Its compromise results food born diseases. Food born microbes are very dangerous that’s threaten the life risk. Microbiaload in the product gives an indication on the safety and hygiene during processing and storage. Table 11 shows the microbial load (Aspergillus, Shigella and E. coli) count of the jelly throughout the stotage periods (0–9 months). None of the microorganism was detected upto 6 months of storage except T4 jelly. These might be due to higher dilution used for the enumeration. After 6 months of storage, Aspergillus (14.58 × 1010), Shigella (15.69 × 109) and E. coli (5.50 × 107) was detected in T4 jelly only. But after 9 months of storage, Aspergillus (10.00 × 109, 10.03 × 109, 10.01 × 109 and 15.13 × 1010), Shigella (10.01 × 109, 11 × 109, 12.00 × 109 and 16.25 × 109) and E. coli (3.41 × 107, 4.10 × 107, 4.10 × 107 and 5.90 × 1010) was detected in all the treated jelly (T1, T2, T3 and T4) at ambient condition. Similar trends also were observed by Chaturvedi et al. [67] in intermediate moisture carrot shreds during storage. In comparison with different treated jelly, T4 jelly drastically changed its microbial quality and showed the highest growth of Aspergillus, Shigella and E. coli that was stored without any chemical preservative (Table 11). The lowest microbial count was found in T2 and T3 treated jelly. This could be attributed due to addition of hony, extracted fresh lemon juice, growth inhibitory effect [66] and antimicrobial effect of honey [68, 69] on the food spoilage organisms. Low aw and higher amount of polyphenolic compounds may have resulted in low microbial load in treate jelly T2 and T3. Lower amount of microbial load was also found in T1 jelly followed by others. This could be due to use of KMS as artificial preservative in T1 treated jelly which has been widely studied to inhibit the growth of microorganisms as well as mold, yeast, bacteria and fungi of different processed products [70, 71].
3.9.4 Shelf life of guava–pineapple jelly
The maximum shelf life (9 months) was recorded in jellies treated with synthetic preservative (T1) stored at ambient condition (Fig. 4). The highest shelf life was recorded by KMS (artificial preservative) treated jelly (T1). The optimum shelf life was recorded by honey and lemon extract juice treated jelly (T2 and T3) due to their low aw, moisture content and pH. The minimum shelf life (3 months) was recorded in jelly using guava:pineapple (50:50) without honey and lemon juice (T4). The shelf life of the treated jelly significantly decreased with increasing storage periods at ambient condition.
3.9.5 Processing effect and mechanism to produce guava–pineapple natural jelly
Free radicals are hazardous for human health and are produced by oxidation during processing, packaging, and storage of processed food products. Considering that processed food should be produced in a safe and healthy way that is beneficial for humans, free radicals must be prevented. The addition of antioxidants in processed food may be a useful way to prevent the production of dangerous free radical compounds. In this study, there was added total phenolic (201.78 mg GAE/100 g) and flavonoid (6.04 mg QE/g) enriched honey bearing antioxidant potential. Honey, used in the study, was obtained from the natural sources having antioxidant and antimicrobial activities, while it was used instead of synthetic chemicals and additives (Sodium benzoate, KMS, Acetic acid, etc.). Fresh lemon juice was used instead of synthetic citric acid (100 mL of fresh lemon juice contains 5–7 g of citric acid). Thus, a combination of natural honey and fresh lemon extract juice contributed to impart the jelly with improved attractive color, flavor, and internal metabolism (Fig. 5). Moreover, the natural ingredients honey and lemon extract juice may also act as energy and immunity system builder, and health beneficial for human to combat various illnesses including headache, allergy, asthma, and dermatitis, and even cancer [72].
4 Discussion
The shelf life of fresh and processed products be contingent on the moisture content, aw and pH. Higher the moisture content enhances the water activity of the products. Maximum moisture content was recorded on the day of preparation for all treated jelly. In this study, the moisture content was slightly decreased over the storage periods (0–9 months). Several similar findings have also been described by the Mehta and Bajaj [73] and Tripathi et al. [74] for candy preparation, those reported that the moisture content may decreased during the storage periods. The slight decrease in moisture content could be due to moisture loss by the process of evaporation, thus increasing the total solids of the jelly.
Herein, the study also confirmed that there was a highly significant relationship between moisture content and aw (Fig. 2), i.e. the presence of low moisture in jelly may contribute to achieve lower aw (0.56). The aw was found in lower throughout the storage periods where it ranged from 0.60 to 0.56 in natural and artificial preservative treated jelly (T1, T2 and T3). The results indicating that the formulated jelly was within the range of aw, thus it generally inhibited to grow microorganisms and shelfstable upto 8 months. Lower pH value after storage [75, 76], and honey acts as a prebiotic due to contained fructose and oligosaccharides which might be contributed to inhibition the growth of microorganisms [77]. Another reason, the diluted honey in jelly might be generated H2O2 by the process of oxidizes glucose to gluconic acid have been found to be more effective [18]. The antibacterial properties of honey was more effective due to its high sugar concentration, low moisture content, along with its acidic values that all the characteristics were present in the stored jelly to inhibit the growth of microbial loads. The antimicrobial properties of honey have been proven and fully agreement by the sevral researchers [18,19,20,21,22,23] those reported that honey acts against pathogenic bacteria, oral bacteria as well as food spoilage organisms.
Ash content of a foodstuff represents inorganic residue remaining after destruction of organic matter [78]. It represents minerals like calcium, phosphorus and iron. When the jelly was stored for storage studies up to 9 months, the ash content was found to be increased significantly over the storage periods. The increase in ash content during storage has also been found in pitanga jam [79], conventional and light blackberry jam [80], indicate that the products were stable during the storage periods.
Water activity (aw) is only of limited use as an indicator for the storage life of foods with low water content. Minor changes in water content lead to major changes in aw [17]. In this study, the effect of aw in processing of jelly influenced the food compositions that are shown in Tables 2 and 4. In Fig. 2, highly correlation was found between aw and moisture content where the aw decreased, the moisture content was also decreased. Foods with aw values between 0.60 and 0.90 are largely protected against microbial spoilage. In this study, aw values were found from 0.56 to 0.60 in honey and lemon extract juice treated jelly (T2 and T3) (Table 4), which was favorable to increase the shelf life of the jelly upto 8 months without quality deterioration. However, the results obtained from the study disclose that the decreased aw between the range of 0.60–0.63 retarded the growth of microbial activities (Table 11) through slowing down the enzymatic catalyzed reactions.
Total and reducing sugar significantly increased from fresh sample to processed sample even during entire storage periods. An increase in sugar content was reported by several researchers for the guava jelly, fruit bar, and different fruit candies [46, 81,82,83,84,85,86,87]. The increasing of total and reducing sugar content between the fresh and the processed samples might be due to variation of sugar content and the formulation variation among the treated samples. The increased total sugar content might be differenced in the samples because of insoluble polysaccharides and other starch converted into soluble sugars completely during the storage periods [4]. Another reason might be the increasing of total soluble solids (TSS) entire the storage periods (0–9 months) contributed to increase the sugar content in jelly sample [88].
In this study, TSS value of the treated jelly was recorded from 67.23 ± 0.03 to 68.57 ± 0.04°B (Table 4). Nurani et al. [89], reported that TSS of the prepared jam and jelly should be ranged from 50 to 7°B, indicating that TSS of T1, T2, T3 and T4 jelly was within the range of limit. The substantial changes (P < 0.01) of TSS during storage might be for the degradation of polysaccharides into soluble compounds [73, 90].
One important feature of storing jelly is the high acidity which usually prevents the growth of food poisoning bacteria and also helps maintain the color and flavor of jelly, jam and marmalade. In this study, the acidity of fresh guava and pineapple were 0.31 ± 0.01–0.63 ± 0.01% (Table1) but after processing into jelly the acidity was increased from 0.52 ± 0.02 to 0.83 ± 0.03% (Table 4). The increasing trend of the acidity was also observed throughout the storage periods (0–9 months). The results are fully agreement with the findings of Kumar et al. [82]. The increased acidity might be due to combination of guava and pineapple extract juice during making of jelly. The significant increase of acidity with the advancement of storage periods might be due to conversion of pectic constituents into soluble solids [3, 82]. There was inverse relationship between acidity and pH of different treated jelly. The variation of pH and acidity might be occured due to variation of formulation during processing of jelly [91]. pH of the treated jelly was gradually decreased with the advancement of storage periods. A decrease in pH thus may promote an inhibitory effect on the growth of microorganisms in jellies throughout the storage periods. Similar findings also have been made by Tobal and Rodrigues [79]; Nachtigall et al. [80], those reported that decrease in pH was observed in pitanga jams during 90–320 days of storage.
Vitamin-C is present in all animals and plant foods, mostly in free from, and it is probably bound to protein as well. It is fully absorbed and distributed throughout the body with the highest concentration in adrenal and pituitary glands. The daily requirement of the vitamin-C for an adult is 100 mg/day. The intake of the vitamin-C is essential to recover the scurvy disease and lower level in blood plasma but in the opposite the high intake of vitamin-C can increase the oxalic acid level that may interrupt the kidney functions [17]. In this study, it is well reported that vitamin-C content of fresh guava and pineapple fruits were recorded as 73.43 ± 1.64 mg/100 g and 39.49 ± 0.01 mg/100 g (Table 1) but after processing into jelly it was noted as 43.01 ± 0.00 and 34.13 ± 0.15 mg/100 g (Table 2) in T2 and T3 jelly. The highest vitamin-C content obtained in fresh guava than pineapple have been reported by the several researchers due to their fruit nature and environmental factors [4, 92, 93]. Results indicate that vitamin-C content of the treated jelly dramatically decreased throughout the storage priods (0–9 months). The decreased vitamin-C content entire the storage periods might be due to thermal destructions during making jelly through heat processing, leaching of vitamin-C into water and its subsequent oxidation during storage [83]. The loss of vitamin-C activates to reduce immediately after harvest and destroys steadily during storage and other processes. Similar results also has been made by Singh and Harshal [94] for processing of leafy vegetables where they reported that the loss of vitamin-C in green leafy vegetables might be due to the processing method employed in its preparation and subjected to boiling and microwave heating as well as blanching.
Vitamins are required for the normal growth, maintenance and functioning of human body. Hence, their preservation during processing and storage in jelly is of far reaching importance. Vitamin A not occurs in plant origin foods, it occurs in animal tissue. But in plant originated food it is found as β-carotene. β-carotene is the major dietary precursor of vitamin A. Food processing and storage can lead to 5–40% destruction of β-carotene [17]. In this study, β-carotene was drastically lost from fresh to processing into jelly even entire the study periods (0–9 months). The results obtained from this study are strongly supported with findings of Jane et al. [81], those reported that 40–53% loss of β-carotene might occur during the process of boiling lettuce and carrot. Hackett et al. [84] reported that the conversion of trans form into cis form could be the reason for the loss of β-carotene during processing. The loss might be occurred due to absence of oxygen and at higher temperature during cooking, boiling and sterilization of jelly.
The lower L* and C* values for color measurement indicate that the color lightness and intensity of all treated jelly (T1, T2, T3 and T4) were lowered gradually throughout the storage periods (0–9 months). The decrease in L* values might be due to reduction of anthocyanin content (Table 7) and the occurrence of Maillard reaction during storage of jelly. The findings are also supported by Maskan et al. [95], who showed that a* and b* values were improved and L* values were reduced during the processing and preservation of grape juice. L* and C* values for all stored jelly (T1, T2, T3 and T4) finally faded out and turned into dark after 9 months of storage (Table 5). This might be due to an increase in aw from 0.56–0.60 and 0.64–0.66 (Table 4), reduction of carotenoid and anthocyanin (Table 7), and the development of browning compounds. Similar results also have been obtained by Rhim and Hong [96], those reported that red color of the red pepper paled and tarnished black due to an increase in aw and temperature. H* value indicates Hue angel value of the stored jelly. The H* value was statistically significant and increased up to 6 months of storage. But after 9 months, H* value of T1, T2, T3 and T4 jelly was significantly decreased to 70.41 ± 3.01, 117.33 ± 6.13, 116.11 ± 5.60 and 70.63 ± 1.10 respectively (Table 5), indicates that the jelly started to lose its initial color. The decreased hue angle obtained by this study are also fully agreement with findings of Tijskenset et al. [97], those reported that the color change could be attributed to the air removal around the surface, the air expulsion between cells and its replacement with water and cell juice that was released from the deteriorated membranes that occurred during storage. Another thing is, the color change could be attributed to enzymatic or non-enzymatic browning (Maillard reactions) [98]. In fact, the presence of a higher amount of reducing sugars after inversion of sucrose during cooking, and/or higher pH, could contribute to these browning reactions.
Pectin is the main factor to determine the the jelly consistency and its content and type have an effect on gel hardness [32, 99]. Thus, in this study, the texture profile of the stored jelly was investigated to evaluate the softness and hardness entire the storage periods as most of the consumer preferences high spreadable jelly. The results showed that T1 jelly found slight harder than the T2 and T3 jelly, could be attributed due to use of guava extract juice only (T1), which contains solid pectin. In opposition, T2 and T3 jelly was found less hardness than T1, might be due to addition and dilution through honey and lemon extract juice to the guava and pineapple extract juice. The softness was found in T4 jelly due to dilution to the proportion of 50:50 guava–pineapple extract juice and their internal metabolism, enzymatic and non-enzymatic degradation of pectin [53, 58]. The hardness found by the T1 jelly are consisted to the findings of Raj et al. [99], those reported that papaya jam gained more hardness throughout the storage periods due to gell properties nature and the capability of water retention. These results are fully agreement with the findings of Morris et al. [55] and Korus et al. [100], those reported that the decreased gel strength could be due to decomposition of pectin compounds by the presence of acids in gooseberry jam.
All the minerals were found to be decreased with increasing of storage periods. The minerals value Na, K, Ca and Mg was found to be highest in jelly T3 whereas Fe, Mn, Zn, B, Cu, P and S was found to be highest in T2 jelly. The variation of T2 and T3 jelly could be due to their treatment effect using different concentration of the extracted juice. Similar variations had also been recorded by Mumtaz et al. [70] on different jams and jellies. The researchers determined Fe, Zn, Na, and K as 0.52–0.910 mg/100 g, 0.02–0.09 mg/100 g, 44.62–71.45 mg/100 g and 26.10–50.11 mg/100 g, respectively; however, no Mn was detected in the their jelly, while the guava–pineapple jelly contained Mn in the range of 0.56–0.69 ppm, respectively (Table 6). The results indicate that the values (Fe, Zn, Na, and K) obtained by Mumtaz et al. [70] were higher than treated jelly T1, T2, T3 and T4. It has been claimed that these variations could be due to the nature of the product, soil structure, soil fertility, orchard type, orchard geographical conditions, the method of processing and preservation (as their sample was collected from the local market), and experimental error. T3 jelly had the highest Na content followed by T2 sample. The results are similar to the apricot and buberry jam that was reported by Naeem et al. [51] and Pleasi et al. [101]. The differences in Na content between the samples might be associated with presence of sodium citrate during jelly preparation. Sodium citrate is the sodium salt for citric acid and functions as an acidity regulator in jelly. The average daily requirement of Na intake for the male 3.30 g and female 2.50 g. From a nutritional stand point, the daily Na intake should be limited to 2.30 g (equivalent to 6 g NaCl). As the Na absorption in the human body is rapid and starts within 3–6 min after intake and is completed within 3 h, therefore its too much intake can result in serious disorders [17]. K, Ca and Mg content had significantly higher in T3 jelly whereas lower was recorded by T2 jelly. The results are similar to the findings of Giampieri et al. [52]. The higher K, Ca and Mg content in T3 jelly found in the present study could be attributed to dilution of guava–pineapple extract juice during making of jelly [51]. The intake of K, Ca and Mg in normal diet to be ranged from 2.0–5.9 g/day (minimum 782 mg), 0.80–1.50 g/day and 0.30–0.50 g/day respectively [17]. The highest Fe content of T2 jelly have similar levels with blueberry and strawberry jams as reported by Naeem et al. [51]. The higher content of Zn in this study are similar to the grape and strawberry jams [51]. The higher Cu level of T2 jelly found similar to the apricot jam [51]. Other minerals Mn, B, P and S found to be higher in T2 jelly. The possible destruction of Fe, Cu, Mn, Zn, B, P and S found in T3 jelly might be caused by processing with different treatments, material separation, dilution and thermal heat treatment during processing [17]. The daily intake of Fe, Cu, Mn, Zn, B, S and P in normaldiet to be from 1.50–2.20 mg/ day, 1.00–1.50 mg/day, 2.00–5.00 mg/day, 5.00–10.00 mg/day, 1.30–4.30 mg/day, 0.80–1.00 mg/day and 0.80–1.20 mg/day respectively [17]. However, all minerals were present more or less all treated jelly (T1, T2, T3 and T4). The identified 11 minerals obtained by this study not have only nutritional and physiological imporatance but also contribute to increase the food flavor and activate or inhibit the enzyme-catalyzed and other reactions in the jelly [17].
Numerous bioactive compounds and antioxidant activities such as total phenolic, total flavonoid, total carotenoid, anthocyanin, antioxidant activity, DPPH free radical scavenging activity, ferric reducing antioxidant power, reducing power assay and IC50 have carriedout in T1, T2, T3 and T4 jelly on the day of preparation and after storage (Table 7). Results revealed that all bioactive compounds decreased with increasing of strage periods. In case of anthocyanin, it pigments are very sensitive to temperature and heat treatment during processing of jelly might be contributed to greatly reduce the content of pigments in the treated jelly. Storage temperature is another main factor for retention and destruction of anthocyanin content [58]. As the final jelly was stored at room temperature (25–30 °C) therefore, it might be contributed to decrease the anthocyanin content (Table 7).
Carotenoid has a crucial part in human nutrition and health, which can lessen the risks of cancer and heart diseases because of the activity of pro-vitamin A [102]. The carotenoids extremely present in the diet as β-carotene and α-carotene are involved in the reduction of the incidence of type 2 diabetes [103]. Here, the results obtained from this study indicates that T3 jelly have been found to higher β-carotene and total carotenoid content followed by others (Table 7). Almost similar observations were made by Dars et al. [104], those reported that mango juice contain 578 µg/100 g and 1.95 mg/100 g of total carotenoid and β-carotene content. The variation of total carotenoids and β-carotene content observed in all treated jelly might be affected by heat processing and storage temperature. The highest total phenolic, flavonoid, carotenoid, β-carotene, and anthocyanin content in T3 probably be due to combination of guava–pineapple extract juice during preparation. On the other hand, guava and pineapple contained different bioactive compounds [105]. Vukoja et al. [106] calculated total phenolic content as 1.69 gGAE/kg (dw) and total anthocyanin content as 98.48 mgcya-3-glu/kg in cherry jam, whereas these contents were higher in treated jelly T1, T2, T3 and T4. The presence of higher total phenolic and anthocyanin content in the treated jelly was probably because of adding natural honey and lemon extract juice during preparation of jelly. Besides, the experimental honey contained 201.78 mg GAE/100 g of total phenolic content and 6.04 mg QE/g of total flavonoid content. Therefore, this findings confirm that honey and lemon extract juice treated jelly (T2 and T3) was the ample source of bioactive compounds.
The maximum total antioxidant activity significantly present in T3 jelly might be due to abundance of phenolic components highly present in T3 compared with T1, T2 and T4. Among all treated jelly, T2 and T3 showed a sturdy capability to scavenge free radicals as their total antioxidant capacity values were found to be 105.84–104.40 µg AA/g and 109.11–107.88 µg AA/g respectively (Table 8). The determination of IC50 is a generally well established technique to judge the antioxidant activity of foodstuff and its lower value indicates higher free radical quenching ability [107]. Results revealed that both T2 and T3 showed potential antioxidant capacity due to their lesser assessment of IC50 (17.98–16.65 and 15.53–14.19 µg/g, respectively (Table 8). The less amount of IC50 present in the T2 and T3 jelly might be contributed to gain maximum amount of total antioxidant activity that could be accredited to the existence of significant quantities of phenolic compounds and flavonoids.
The presence of FRAP values in T2 and T3 jelly (Table 8) could donate an electron to decrease the yellow ferric complex to a blue ferrous complex. The high FRAP value in T2 and T3 indicate that phenolic composite is the leading provider of high antioxidant ability in the jelly.
Phenolic compounds are an important bioactive compounds that preserve against dissimilar lethal chemical responses and diseases, and their association in antioxidants rely on their structure [108]. The difference of phenolic acids in T1, T2, T3 and T4 (Table 8) depends on the food matrix and chemical structures, extraction techniques used, solvent used, and the solubility of individual phenolic acid [108]. However, the results indicate that honey and lemon extract juice treated jelly (T2 and T3) were a rich source of phenolic compounds which were decreased slightly with the advancement of storage periods. The slightly decreased phenolic acids still now remains unknown. But the possible reason might be due to fluctuation of room temperature throughout the storage periods (0–9 months). Another reason might be oxidisability of the studied phenolic acids with fluctuation of room temperature. Reblova [109] reported that acivity of phenolic acids for pork lard decreased with increasing temperature. They also found inverses linear correlation between the relative decrease in phenolic antioxidant activity with increasing temperature and the oxidisability of the studied phenolic acids.
5 Conclusion
This study first time exposed the details information regarding natural preservative honey and lemon extract juice treated jelly and their effect on physicochemical, nutritional, microbial, bioactive compounds and antioxidant properties. From the above explanation and results obtained in this study conclude that honey and lemon extract juice treated jelly (T2 and T3) is an abundant source of health beneficial different bioactive compounds and antioxidants. The jelly (T2 and T3) did not affect by the microorganisms up to 8 months but after 9 months, Aspergillus, Shigellus and E. coli were formed within the range of limit. Lower aw, moisture content and pH as well as high sugar content contributed to preserve the jelly upto 8 months without any significant quality deterioration (Though storage studies were conducted upto 9 months). The findings showed a very informative message regarding natural and artificial preservative to formulation of jelly. However, honey and lemon extract juice could be explored for processing into natural fruit jam, jelly, marmalade etc. especially those fruits pulp have off flavor. The limitation of the findings was to determine the glycemic index (GI) of the natural jelly. Therefore, future study could be continued to identify the GI of honey and lemon extract juice treated process products.
Data availability
The data generated during the study is available upon reasonable request from the corresponding author.
Code availability
Not applicable.
Abbreviations
- BBS:
-
Bangladesh Bureau of Statistics
- mMT:
-
Million Metric Ton
- BARI:
-
Bangladesh Agricultural Research Institute
- FAO STAT:
-
Food and Agriculture Organization Statistics
- KMS:
-
Potassium metabisulphite
- IU:
-
International unit
- TSS:
-
Total Soluble Solid
- L*:
-
Lightness
- C*:
-
Chroma
- H*:
-
Hue angle
- Na:
-
Sodium
- K:
-
Potassium
- Ca:
-
Calcium
- Mg:
-
Magnesium
- S:
-
Sulfur
- Cu:
-
Copper
- Mn:
-
Manganese
- Fe:
-
Iron
- Zn:
-
Zinc
- ANOVA:
-
Analysis of variance
- NS:
-
Non-Significant
- ND:
-
Not detected
- M:
-
Month
- D:
-
Day
- CFU:
-
Colony forming unit
- aw :
-
Water activity
- FRAP:
-
Ferric Reducing Antioxidant Power
- GAE:
-
Gallic Acid Equivalent
- AA:
-
Ascorbic Acid
- MCC:
-
Metal Chelating Capacity
- DPPH-RSA:
-
2,2-Diphenyl-1-picryl hydeazil-Reducing Scavenging Activity
- RPA:
-
Reducing Power Assay
- UV–Vis Spectrophotometer:
-
Ultra-Violet Visible Spectrophotometer
- HPLC:
-
High Pressure Liquid Chromatography
- AFACI:
-
Asian Food and Agriculture Cooperation Initiative
- RDA:
-
Rural Development Administration
- BGD:
-
Bangladesh
- APPT:
-
Agricultural Products Processing Technology
References
Food and Agriculture Organization (FAO). Statistical Yearbook. World Food and Agriculture. Food and Agriculture Organization of the United Nations, Rome, Italy. 2021; 86–368.
Bangladesh Bureau of Statistics (BBS). Yearbook of Agricultural Statistics of Bangladesh. Bangladesh Bureau of Statistics, Statistics and Information Division, Ministry of Planning, Government of the Peoples Republic of Bangladesh, Dhaka. 2022; 210–33.
Bhat SA, Singh ER. Extraction and characterization of pectin from guava fruit peel. Int J Adv Res Technol. 2014;2:1–7.
Kuchi VS, Gupta R, Tamang S. Standardization of recipe for preparation of guava jelly bar. J Crop Weed. 2014;10:77–81.
Jain PK, Asati VK. Evaluation of guava cultivars for pulp preparation. J Food Sci Technol. 2004;41:684–6.
Paull RE, Duarte O. Tropical Fruits, 2nd Ed. CAB International. 2011:327–65.
Coppens d’Eeckenbrugge G, Sanewski GM, Smith MK, Duval MF, Leal F. Ananas In: Kole C. (ed). Wild crop relatives: genomic and breeding resources: tropical and subtropical fruits. Springer. 2011:21–41.
Joy PP. Benefits and Uses of Pineapple, Pineapple Research Station (Kerala Agricultural University), Vazhakulam. 2010 (http://prsvkm.kau.in/book/importance).
Khan MA. Bitter truth: slow poisoning continues unabated. The Daily Star, 17 May, Bangladesh. 2004.
Anand SP, Sati N. Artificial preservatives and their harmful effects: looking toward nature for safer alternatives. Int J Pharm Sci Res. 2013;4:2496–501.
Molla MM. Effect of foxtail millet diet on liver injury and blood lopid profile induced by D-galactoseamine in mice. PhD dissertation, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing-100083, China. 2016;113p.
Amir RM, Randhawa MA, Sajid MW, Nadeem M, Ahmad A, Watto FM. Evaluation of various soaking agents a novel tool for heavy metal residues mitigation from spinach. Food Sci Technol. 2020;39:176–80.
Wang Z, Jackson LS, Jablonski JE. Factors affecting the level of heavy metals in juices processed with filter aids. J Food Prot. 2017;80:892–902.
Abu-Almaaly RA. Effect of cooking method on the content of heavy metals in rice that available in local market. Plant Arch. 2020;20:2976–81.
Inobeme A, Ajai AI, Eziukwu C, Obigwa PA, Okonkwo S, Ekwoba LM. Effect of cooking methods on heavy metals content of food. J Xidian Univ. 2020;14:704–14.
Shinta YC, Zaman B, Sumiyati S. Citric acid and EDTA as chelating agents in phytoremediation of heavy metal in polluted soil: a review. IOP Conf Series: Earth Environ Sci. 2021;896:1–8.
Belitz H-D, Grosch W, Schieberle P. Food Chemistry. In: Burghagen M (3rd ed.) Translation from the fifth German edition 3rd ed. Springer, Germany. 2004.
Bang LM, Buntting C, Molan PC. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J Altern Complement Med. 2003;9:267–73.
Badawy OFH, Shafii SSA, Tharwat EE, Kamal AM. Antibacterial activity of bee honey and its therapeutic usefulness against Escherichia coli O157:H7 and Salmonella typhimurium infection. Rev Sci Technol Int Epiz. 2004;23:1011–22.
Mundo MA, Padilla-Zakour OI, Worobo RW. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int J Food Microbiol. 2004;97:1–8.
Lusby PE, Coombes AL, Wilkinson JM. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res. 2005;36:464–7.
Adeleke OE, Olaitan JO, Okepekpe EI. Comparative antibacterial activity of honey and gentamicin against Escherichia coli and Pseudomonas aeruginosa. Ann Burn Fire Disasters. 2006;19:201–4.
Basualdo C, Sgroy V, Finola MS, Juam M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet Microbiol. 2007;124:375–81.
Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr Res. 2002;22:1041–7.
Alvarez-Suarez JM, Tulipani S, Romandini S, Bertoli E, Battino M. Contribution of honey in nutrition and human health: a review. Mediterr J Nutr Metab. 2010;3:15–23.
Johnston JE, Sepe HA, Miano CL, Brannan RG, Alderton AL. Honey inhibits lipid oxidation in readyto-eat ground beef patties. Meat Sci. 2005;70:627–31.
Turkmen N, Sari F, Poyrazoglu ES, Velioglu YS. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006;95:653–7.
Rakha MK, Nabil ZI, Hussein AA. Cardioactive and vasoactive effects of natural wild honey against cardiac malperformance induced by hyperadrenergic activity. J Med Food. 2008;11:91–8.
Eteraf-OskoueiT NM. Traditional and modern uses of natural honey in human diseases: A Review. Iran J Basic Med Sci. 2013;16:731–42.
Daily Bangladesh. Good news to honey farmers. Daily Bangladesh news 17 February. 2020.
Joshi VK. Sensory science: principles and application in food evaluation. Jaipur (India): Agrotech Publish Academy; 2006.
Dervisi P, Lamb J, Zabetakis I. High pressure processing in jam manufacture: effects on textural and colour properties. Food Chem. 2001;73:85–91.
Ranganna S. Hand book of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Publishing Co. Ltd. New Delhi; 1995.
Ough CS, Amerine MA. Phenolic compounds. In: Methods for analysis of musts and wines. Wiley, New York; 1988.
Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82.
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins BD. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–75.
Holden JM, Eldridge AL, Beecher GR, Marilyn BI, Bhagwat S, Davis S, Schakel CS. Carotenoid content of U.S. foods: an update of the database. J Food Compos Anal. 1999;12:169–96.
Molla MM, Rahman E, Khatun A, Islam MF, Uddin MZ, Ullah MA, Saha MG, Miaruddin M. Color retention and extension of shelf life of litchi fruit in response to storage and packaging technique. Am J Food Technol. 2017;12:322–31.
Burgos G, Amoros W, Muñoa L, Sosa P, Cayhualla E, Sanchez C, Díaz C, Bonierbale M. Total phenolic, total anthocyanin and phenolic acid concentrations and antioxidant activity of purple-fleshed potatoes as affected by boiling. J Food Compos Anal. 2013;12(30):6–12.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41.
Guo H, Saravanakumar K, Wang M. Total phenoli Stachys affinis. Biocatal Agric Biotechnol. 2018;15:235–9.
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6.
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25.
Bahadori MB, Zengin G, Bahadori S, Dinparast L, Movahhedin N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int J Food Prop. 2018; 21:198–8.
Pandey A, Negi PS. Bioactive compounds composition, in vitro antioxidant activity and antibacterial mechanisms of Neolamarckia cadamba fruits extracts. Nat Prod Res. 2018;32:1189–92.
Sharma SK, Chaudhary SP, Rao VK, Yadav VK, Bisht TS. Standardization of technology for preparation and storage of wild apricot fruit bar. J Food Sci Technol. 2013;50:784.
Paul R, Ghosh U. Effcet of thermal treatments on ascorbic content of pomegranate juice. Indian J Biotechnol. 2012;11:309–13.
Silvia AVC, Oliveria DSN, Yaguiu P, Carnelossi MAG, Muniz EN, Narain N. Temperature e embalagem para abobora minimamente processada. Cienc Technol Aliment. 2009;29(2):391–4.
Eke-Ejiofor J, Owuno F. The physico–chemical and sensory properties of jackfruit (Artocarpus heterophilus) jam. Int J Nutr Food Sci. 2013;2:149–52.
Kansci G, Koubala BB, Lape IM. Effect of ripening on the composition and the suitability for jam processing of different varieties of mango (Mangifera indica). Afr J Biotechnol. 2003;2:301–6.
Naeem MNM, Fairulnizal MNM, Norhayati MK, Zaiton A, Norliza AH, Syuriahti WZW, Azerulazree JM, Aswir AR, Rusidah S. The nutritional composition of fruit jams in the Malaysian market. J Saud Soc Agric Sci. 2017;16(1):89–96.
Giampieri F, Tulipani S, Alvarez-Suarez JM, Quiles JL, Mezzetti B, Battino M. The strawberry: composition, nutritional quality, and impact on human health. Nutr. 2012;28:9–19.
Sila DN, Duvetter T, De Roeck A, Verlent I, Smout C, Moates GK, Hillsm BP, Waldron KW, Hendrickx M, Va LA. Texture changes of processed fruits and vegetables: potential use of high pressure processing. Trends Food Sci Technol. 2008;19:309–19.
Naab OA, Tamame MA, Caccavari MA. Palynological and physicochemical characteristics of three unifloral honey types from central Argentina. Span J Agric Res. 2008;6(4):566–76.
Morris GA, Castile J, Smith A, Adams GG, Harding SE. The effect of different storage temperatures on the physical properties of pectin solutions and gels. Polym Degrad Stab. 2010;95(12):2670–3.
Buchweitz M, Speth M, Kammerer DR, Carle R. Impact of pectin type on the storage stability of black currant (Ribes nigrum L.) anthocyanins in pectic model solutions. Food Chem. 2013; 39(1–4): 1168–78.
Poiana M-A, Munteanu M-F, Bordean D-M, Gligor R, Alexa E. Assessing the effects of different pectins addition on color quality and antioxidant properties of blackberry jam. Chem Cent J. 2013;7(1):121–33.
Kopjar M, Piliˇzota V, Tiban NN, Šubarić D, Babić J, Ačkar Đ, Sajdl M. Strawberry jams: Influence of different pectins on colour and textural properties. Czech J Food Sci. 2009;27:20–8.
Reilly C, Minerals CJK, In Henry, Chapman C (ed.). The nutrition handbook for food processors. Woodhead Publishing Limited, Abington Hall, Abington Cambridge CB1 6AH, England. 2002; 97–6.
Laaroussi H, Bouddine T, Bakour M, Ousaaid D, Lyoussi B. Physicochemical Properties, Mineral Content, Antioxidant Activities, and Microbiological Quality of Bupleurum spinosum Gouan Honey from the Middle Atlas in Morocco. J Food Qual. 2020.
Küçük M, Kolayl S, Karaoğlu Ş, Ulusoy E, Baltacı C, Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007;100:526–34.
Garzon GA, Narváez CE, Riedl KM, Schwartz SJ. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010;122:980–6.
Chua LS, Rahaman NLA, Adnan NA, EddieTan TT. Antioxidant activity of three honey samples in relation with their biochemical components. J Anal Method Chem. 2013; 8.
Cansino NC, Carrera GP, Rojas QZ, Olivares LD, García EA, Moreno ER. Ultrasound processing on green cactus pear (Opuntia ficus Indica) juice: physical, microbiological and antioxidant properties. J Food Process Technol. 2013;1:4–9.
Kapoor S, Ranote PS. Antioxidant components and physico-chemical characteristics of jamun powder supplemented pear juice. J Food Sci Technol. 2016;53(5):2307–16.
Metry WA, Owayss AA. Influence of incorporating honey and royal jelly on the quality of yoghurt during storage. Egypt J Food Sci. 2009;37:115–31.
Chaturvedi A, Sujatha V, Ramesh C, Dilip BJ. Development of shelf stable intermediate carrot (Daucus carota) shreds. Int Food Res J. 2013;20(2):775–81.
Wang R, Starkey M, Hazan R, Rahme LG. Honey’s ability to counter bacterial infections arises from both bactericidal compounds and QS inhibition. Front Microbiol. 2012;3:1–7.
Combarros-Fuertes P, Fresno JM, Estevinho MM, Sousa-Pimenta M, Tornadijo ME, Estevinho LM. Honey: another alternative in the fight against antibiotic-resistant bacteria? Antibiotic. 2020;9:774.
Mumtaz B, Mozakkin MJI, Motalab M, Jahan S, Ferdous T, Saha BK. Nutritional and microbiological evaluation on jams and jellies available in Bangladesh. Food Nutr Res. 2019;7:113–9.
Varo MA, Martin-Gomez J, Serratosa MP, Merida J. Effect of potassium metabisulphite and potassium bicarbonate on color, phenolic compounds, vitamin C and antioxidant activity of blueberry wine. LWT-Food Sci Technol. 2022;163: 113585.
Bondi MA, Lauková Niederhausern Sde, Messi P, Papadopoulou C. Review on natural preservatives to improve food quality and safety. J Food Qual. 2017. https://doi.org/10.1155/2017/1090932
Mehta V, Bajajm S. Effect of storage and methods of preservation on the physic-chemical characteristics of citrus juices. Indian Food Pack. 1969;37:42–51.
Tripathi VK, Singh MB, Singh S. Studies on comparative compositional changes in different preseved products of Aonla (Emblica officinalis Gaertn.) var. Banarasi. Indian Food Pack. 1988; 42:60–6.
Lankaputhra WEV, Shah NP, Britz ML. Survival of bifidobacteria during refrigerated storage in the presence of acid and hydrogen peroxide. Milchwissenshaft. 1996;51:65.
Saccaro DM, Tamime AY, Pillegg AOS, Oliveira MN. The viability of three probiotic organisms grown with yoghurt starter cultures during storage for 21 days at 4°C. Int J Dairy Technol. 2009;62:387–96.
Roumyan N, Zapryanov P, Kondareva S. On some aspects of a new fermented milk product medina. Biotechnol Biotechnol Equip. 1996;10:86–9.
Vidhya R, Narain A. Formulation and evaluation of preserved products utilizing under exploited fruit, wood apple (Limonia acidissima). Am-Euras J Agric Environ Sci. 2011;10(1):112–8.
Tobal TM, Rodrigues LV. Effect of storage on the bioactive compounds, nutritional composition and sensory acceptability of pitanga jams. Food Sci Technol. 2019;39(2):581–7.
Nachtigall AM, Souza EL, Malgarim MB, Zambiazi RC. Light blackberry jellies. Boletim Centr de Pesquisa Process Aliment. 2004;22(2):337–44.
Jane M, Sachi JD, John J. Reversed phase HPLC analysis of alpha and beta-carotene from selected raw and cooked vegetables. J Nutr Sci. 1988;38:333–41.
Kumar S, Singh IS. Storage studies of aonla fruit products at ambient temperature. Horticult Train Prog. 2001;33:169–73.
Brock VD, Ludikhuyze L, Weemaes CL, Van A, Hendrickx M. Kinetics forisoberic isothermal degradation of l-ascorbic acid. J Agric Food Chem. 2001;46:2001–6.
Hackett M, Lee J, Schwartz S. Thermal stability and isomerization of lycopene in tomato oleoresins from different varieties. J of Food Sci. 2002;69:536–41.
Paul SE, Chakrabarty S, Jana SC, Hasan MA, Mandal KK, Sarkar S, Mazumdar D. A multivariate approach to study the sensory parameters of guava jelly on the basis of the physico-chemical parameters of guava fruit. Acta Hortic. 2007;35:561–8.
Nayak P, Tandon DK, Bhatt DK. Study on changes of nutritional and organoleptic quality of flavored candy prepared from aonla (Emblica officinalis G.) during storage. Int J Nutr Metab. 2012;4:100–6.
Mondal SC, Kamal MM, Mumin MIA, Hosain MM, Ali MR. Effect of sucrose on the physicochemical properties, organoleptic qualities and shelf-life stability of aonla (Emblica Officinalis) candy. IOSR J Environ Sci Toxicol Food Technol. 2017;11:85–94.
Uribe-Wandurraga ZN, Bravo-Villar M, Igual1 M, Savall C, García-SegoviaP, Martínez-Monzó J. Sugar and no sugar added fruit microalgae-enriched jams: a study about their physicochemical, rheological, and textural properties. Eur Food Res Technol. 2021; 247:2665–78.
Nurani FP, Sulistyoningsih EKB. Physio-chemical Characteristic of Red Dragon Fruit and Pineapple Jam. J Phys Conf Ser. 2021; 012056.
Ghosh SN, Chattopadhyay N. Performance of some guava cultivar under rainfed semi-arid region of West Bengal. J Hort. 1996;9:121–7.
Selvamuthukumaran M, Khanum F, Singh BA. Development of sea buckthorn mixed fruit jelly. Int J Food Sci. 2007;42:403.
Khatun R. Studies on storage stability of guava juice and jelly. Masters of Science (MS) Thesis, Department of Food Technology and Rural Industries, Bangladesh Agricultural University, Mymensingh-2202. 2011;78p.
Naseer S, Hussain S, Naeem N, Pervaiz M, Rahman M. The phytochemistry and medicinal value of Psidium guajava (guava). Clin Phytosci. 2018;4:32.
Singh R, Harshal A. Effects of cooking on content of vitamin-C in green leafy vegetables. Sch J Agric Vet Sci. 2016;3(6):416–23.
Maskan A, Kaya S, Maskan M. Effect of concentration and drying processes on color change of grape juice and leather (pestil). J Food Eng. 2002;54:75.
Rhim JW, Hong SI. Effect of water activity and temperature on the color change of red pepper (Capsicum annuum L.) powder. Food Sci Biotechnol. 2011; 20:215–2.
Tijskens LMM, Barringer SA, Biekman ESA. Modeling the effect of pH on the colour degradation of blanched broccoli. Innov Food Sci Emer Technol. 2001;2:315–22.
Granato D, Masson ML. Instrumental color and sensory acceptance of soy-based emulsions: a response surface approach. Ciência Tecnol Aliment. 2010;30:1090–6.
Raj A, Albert P, Radha K, Vijayalakshmi M, Pavulraj S, Anuradha P. Study on the utilization of paneer whey as functional ingredient for papaya jam. Ital J Food Sci. 2017;29:171–4.
Korus A, Jaworska G, Bernas E, Juszczak L. Characteristics of physico-chemical properties of bilberry (Vaccinium myrtillus L.) jams with added herbs. J Food Sci Technol. 2015; 52:2815–3.
Plessi M, Bertelli D, Albasini A. Distribution of metals and phenolic compounds as a criterion to evaluate variety of berries and related jams. Food Chem. 2007;100:419–27.
Tiburski JH, Rosenthal A, Deliza R, de Oliveira Godoy RL, Pacheco S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res Int. 2011; 44:2326–1.
Sluijs I, Cadier E, Beulens JWJ, Van-der ADL, Spijkerman AM, Van-der SYT. Dietary intake of Carotenoids and risk of type 2 diabetes. Nutr Metabol C cardiovasc Dis. 2015;25:376–81.
Dars AG, Hu K, Abbas A, Chen Y, Khaskheli AA, Liu Q, Li X, Homaida MA, Lakho ABJ, Bijun Xie B, Sun Z. Comparative analysis of antioxidant activities of different varieties of mangos with some selected fruits. Afr J Agric Res. 2018;13:1633.
Chiari-Andréo BG, Trovatti E, Marto J, de Almeida-Cincotto MGJ, Melero A, Corrêa MA, Chiavacci LA, Ribeiro H, Garrigues T, Isaac VLB. Guava: bioactive compounds composition of a potential source of antioxidants for cosmetic and/or dermatological applications. Braz J Pharm. 2017;53: e16141.
Vukoja J, Pichler A, Kopjar M. Stability of anthocyanins, phenolics and color of tart cherry jams. Foods. 2019;8:255.
Sathyanarayanan S, Chandran R, Thankarajan S, Abrahamse H, Thangaraj P. Bioactive compounds composition, antioxidant and anti-bacterial activity of Syzygium calophyllifolium Walp fruit. J Food SciTechnol. 2018;55:341.
Mahmood T, Anwar F, Abbas M, Saari N. Effect of maturity on phenolics (Phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from Pakistan. Int J Mol Sci. 2012;13:4591–607.
Rebolva Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech J Food Sci. 2012;30:171–5.
Acknowledgements
The authors wish to acknowledge Dr. Talukder Humayun Kabir, Proprietor, South Asian Agro-products, Naga, Gazipur-1706, Bangladesh for application this technology and present marketing of these product to local and city market of the country.
Funding
This study was supported by the grant of Asian Food and Agriculture Cooperation Initiative (AFACI), Rural Development Administration (RDA), Korea under the project entitled ‘Development of Agricultural Products Processing Technology (BGD-APPT-01).
Author information
Authors and Affiliations
Contributions
MMM collected experimental raw material resources; Conceptualization; Experiment design; Experiment performer; Data record, Data analysis and was a major contributor in writing the manuscript. AAS Conducted field experiment on pre and post-harvest management of guava and pineapple and was a contributor for analysis of minerals. MHHK performed analysis of phenolic acids of the sample, release fund and advisory work. MGFC assisted to collect chemicals and reagent and contributed to analysis total carotenoid of the sample. MM supervised the whole research activities and contributed to interprete the patient data of the storage sample. MA contributed to analysis of sample color and format the manuscript. AK contributed to analyze crude protein, total fat and microbial count of the sample. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The authors conducted the study following ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Molla, M.M., Sabuz, A.A., Khan, M.H.H. et al. Effect of honey and lemon juice on the physicochemical, nutritional, microbial and antioxidant properties of guava–pineapple jelly during storage periods. Discov Food 2, 31 (2022). https://doi.org/10.1007/s44187-022-00033-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-022-00033-5