Abstract
Spirulina powder contains vitamins A, E and all B vitamins, high-quality proteins, 18 of the 20 known amino acids, Ca and K as well as many essential minerals and enzymes. The current study was conducted to make healthy Spirulina probiotic labneh (SPL) by investigating the effect of adding Spirulina (Spirulina platensis) powder on physicochemical, microbiological properties, antioxidant activity and sensorial characteristics beside nutritional value compared to control. SPL was inoculated by Lactobacillus acidophilus and Streptococcus thermophilus. Added of microalgae to concentrated fermented buffalo’s milk by (0, 0.2, 0.4, 0.7 and 1% w/v) Spirulina individually. Spirulina probiotic labneh samples exhibited significantly (p ≤ 0.05) increased the viability of probiotic, higher levels of protein, dietary fiber and antioxidant activity, while lower syneresis than the control labneh. Vitamins B1, B9, and B12, as well as minerals like Fe, Zn, K, and Mg, were found to be higher in the SPL samples than in the control. The phytopigments increased with increasing levels of Spirulina with values ranging from 0.16 to 0.61 for chlorophyll a and from 3.10 to 4.89 for Phycocyanin. (Chlorophyll a, carotenoids) were increased with increasing the added levels from Spirulina comparing with control. Subsequently, it is recommended manufacture probiotic labneh with forficate by Spirulina as a potential source for phenolic and flavonoid compounds, phytopigments, vitamins, fiber and a high content of minerals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spirulina, now called Arthrospira, is a microorganism and filamentous cyanobacterium (blue-green alga). Microalgae, such as cyanobacteria and eukaryotic algae, are photoautotrophic microorganisms that use cheap and widely available natural resources [1]. Arthrospira platensis and A. maxima under the term Spirulina, are arranged in multicellular filaments (50–500 μm in length and 3–12 μm in diameter) and are found in tropical and subtropical waters of alkaline lakes, they are used as food supplements. Due to its have high protein content and antioxidant properties, its role as a stimulant of cell renewal, and its positive impact on kidney and memory problems. S. platensis (dried biomass) contains A, E and all B vitamins, 18 of the 20 known amino acids, high-quality proteins, Ca and K as well as many essential minerals and enzymes [2] showed that yogurt added with SP is a good medium for lactic acid bacteria. The limited amino acids of Spirulina are methionine and cysteine and Spirulina has a higher lysine content than all vegetables except legumes, and is a vegetable protein supplement. Only 36 g of Spirulina provides all daily essential amino acid requirements for an adult male [3]. However, Spirulina is good source of pigments, fatty acids, and mainly (ω-6) omega-6 representatives such as γ- linolenic acid and gamma-linolenic acid (GLNA), a precursor to the hormones prostaglandins in the body. El-Sattar et al. [4] revealed that yoghurt supplemented with Spirulina platensis powder provides a new technological approach to manufacture a functional food that shows cardiovascular protective effects and improves liver, kidney and heart functions.
Labneh, is an old fermented buffalo milk product with longer keeping quality than yogurt. That is common throughout the Middle East countries traditionally made from yogurt after some of the whey has been removed. Labneh is a creamy to white paste that has smooth and soft texture, a good spread ability with a hint of syneresis, clean flavor and a little acidic concentrated fermented milk to around 22% total solids contents [5]. Whereas, according to El-Sayed et al. [6] Ultra Filtration (UF) labneh was made as concentrated fermented buffalo milk, yoghurt or especially probiotic fermented milk, provides natural nutrients and supports the intestines with LAB and probiotic cultures. Lactic acid bacteria has a variety of a healthy effects such as regulating the intestines, lowering the level of cholesterol in the blood, strengthening the immune system, improving the mouth diseases immune [7]. Besides of prolonging the shelf life, improves flavor and texture and removes undesirable compounds of primary food substrates enhances nutritional and sensory qualities [8]. L. acidophilus is known to have a health promoting effect and antiviral activity against foodborne pathogens [3].
The aim of this study is fortifying functional labneh by different ratio of Spirulina platensis powder (SPP), then investigate some chemical, organoleptic properties, antioxidants, vitamin, minerals, phytopigments and assessment the viability of the probiotic bacteria for the SFL and probiotic control Labneh during refrigerated storage.
2 Materials and methods
2.1 Materials
UF retentate were obtained from a pilot dairy plant, Faculty of Agriculture., Fayoum University, Egypt. Lactobacillus acidophilus (La 5) was obtained from Chr. Hansen's laboratories (Copenhagen, Denmark). Streptococcus salivarius subsp. thermophilus was obtained from EZAL, Groupe Rhone poulenc, France. Spirulina platensis Biomass was obtained from Imtenan Company (Cairo, Egypt). The chemical composition of Spirulina (protein 65.4%, fat 14.3%, ash 8.8%, moisture 8.1%, total solids 91.9%). All chemical used in this study were obtained from Merck (Germany) and were of analytical grade.
2.2 Methods
2.2.1 Labneh manufacturing
Preliminary experiments were carried out to obtain acceptable labneh with different addition percentages of SPP (ranging from 0.2 to 2.5%). The sensory acceptance test found that labneh with SPP less than 1.2% (g/100 g) was accepted. Thus, for labneh production, SPP of 0.2%, 0.4%, 0.7% and 1% were selected.
The Labneh was manufactured using the buffalo’s milk retentate at concentration ratio (2.1×) (6.4 pH, 10.35% protein, 33% total solids, 17.5% fat and 2.20% as kk h) was heat treated for 10 min at 90 °C. Then cooled to about 42 °C and divided to 5 parts. Four of the parts were supplemented with Spirulina Biomass at a rate of 0.2–0.4–0.7–1% (these ratios were selected, based on the initial experiments) and stirring in the blender for 10 min and the last part used for making the probiotic control and inoculated with 5% L. acidophilus and S. thermophilus (1:1) at 37 °C and stirred to distribute starter through the retentate. The SFL samples and control were poured into plastic containers and incubated at 37 °C, for about 5 h. Then they were kept in a refrigerator at 5 °C overnight, then kept at 5 °C for 28 days. Sensory evaluation, chemical, biochemical and microbiological analyses were performed in replicate (three times) for each treatment on each measurement day (fresh, 7, 14, 21 and 28).
2.2.2 Determination of kinetic parameters of the fermentation
Sterile base formulation samples were cooled and inoculated with 10% L. acidophilus activated culture. The inoculated milk was incubated at 37 °C for 14 h. Kinetic parameters, pH and titratable acidity were evaluated every 2 h, until pH 4.6 or an equivalent value was reached [9].
2.2.3 Microbiological analysis
Under aseptic condition, 10 g of SFL samples was transferred in to sterile Hun of Chinese porcelain and homogenized in 10 mL of sterile trisodium citrate solution 2%. Dilutions were made by adding (80 mL) sterilized saline for making the suitable dilutions required for the microbiological tests. L. acidophilus was counted on MRS agar. The plates were incubated at 37 °C for 48 h and the submerged or shallow colonies may be compact or feathery and are small, opaque and colonies were taken as L. acidophilus. Total viable counts (TVC) of SFL samples were enumerated with nutrient agar medium the plates were incubated at 35 °C for 48 h. To detect and enumerate coliform bacteria group and staphylococci counts labneh samples were plated with MacConkey agar medium and mannitol salt agar and incubated at 37 °C for 40–43 h and 24 h, respectively. Potato dextrose agar medium was used in counting yeasts and molds and the plates were incubated at 25 °C for 5 days.
2.2.4 Physiochemical analysis:
pH values of fresh milk and SFL samples were measured by using laboratory pH-meter (ORION 420A, USA). Syneresis (%) was determined according to Awad et al. [10] next: 100 g of SFL was added in a plastic cup and cut into four sections and converted to a funnel with a 120-mesh plastic screen. Whey was drained and measured after 120 min at room temperature.
2.2.5 Chemical composition
The labneh samples were then analyzed for their titratable acidity expressed as lactic acid (%), protein, moisture, fat, ash contents according to AOAC [11].
2.2.5.1 Determination of phenolic content and vitamins
The phenolic compound and vitamin B content were determined by HPLC analysis as follows. Lyophilized biomass of each sample was extracted in 70% ethanol (10 mg of biomass added to 1 mL of ethanol) for 24 h and then cell walls were broken by sonication in an ultrasonic cleaning bath (Ultrasons, J.P. Selecta, S.A., Spain) at room temperature. After that, the cell fragments were separated by centrifugation at 10,000×g for 10 min. The supernatants were collected for the analysis of phenolic compounds and vitamin B. HPLC analysis was performed using an Agilent 1260 series. Separation was performed using an Eclipse C18 column (4.6 mm × 250 mm ID, 5 μm). The mobile phase consisted of water (a) and 0.05% trifluoroacetic acid in acetonitrile (b) at a flow rate of 1 mL/min. The mobile phase was programmed successively in a linear gradient as follows: 0 min (82% a); 0–5 min (80% A); 5–8 min (60% A); 8–12 min (60% A); 12–15 min (85% A) and 15–16 min (82% A). A multi-wavelength detector was observed at 280 nm. The injection volume was 10 µL for each of the sample solutions. The column temperature was maintained at 35 °C.
2.2.5.2 Determination of mineral content and heavy metals:
Samples were ground and approximately 0.2–0.3 g of sample was weighed and added to a polytetrafluoroethylene digestion vessel along with 5 mL of concentrated HNO3 and 2 mL of H2O2. Subsequently, the samples were digested using a two-step temperature program. During the first step, the temperature was increased linearly to 190 °C with 10 min coverage; the maximum power of the rotating magnetron was 1000 watts. During the second step, the temperature was maintained at 190 °C for 30 min. After digestion and cooling, each solution was evaporated to 2 mL and diluted with deionized water in a 50 mL volumetric flask for the Atomic Absorbtion Spectroscopy (AAS) analysis. The results were reported as an mean of three repeated measurements, and all digestions were performed in triplicate. The mineral concentration was determined by ICP-OES [12].
2.2.6 Sensory evaluation
The different treatments of SFL were examined at (fresh, 7, 14, 21, 28 days) by 10 panelists of staff members of dairy department (faculty of agriculture—Fayoum University) are trained prior to sensory evaluation, who are familiar with such Middle Eastern products. The following properties have been evaluated: the score points were 60 for flavor, 30 for body and texture and 10 for appearance and colour according to Ayyad et al. [13]. The coagulum (curd) was broken with a spoon and visually evaluated for the consistency properties of SFL samples, all ratings are specified in duplicate, in individual cabinets equipped with daylight.
2.2.7 Statistical analysis
All the experiments were performed in triplicate and the results obtained were analyzed statistically. General Linear Models (GLM) were performed using Statistical Package for Social Sciences (SPSS) (1999) for windows, version 26 software package according to the following model:
Yijk: observation of the ith treatment within the jth storage period, µ: grande mean, Ti: treatments, Pj: storage period, TPij: interaction of treatment by storage period and eijk: random error term.
Significant differences among treatments, storage period and the interaction means between them were compared at P ≤ 0.001 level of significance using Duncan’s multiple range test.
3 Results and discussion
3.1 Microbiological properties
3.1.1 Viability of Lactobacillus acidophilus
The viability of L. acidophilus in SFL during storage at 5 ± 1 °C summarized in Table 1. Log counts of L. acidophilus is varied from 7.84 to 8.26 cfu/g in the fresh samples. The highest value was obtained from PSL fortified with SP (S4) samples. Table 1 shows that the counts L. acidophilus were significant affected by the changes in different concentrations of SP as well as the storage period. The highest count of L. acidophilus in S4 treatment was recorded at 28th day of storage (log 9.83 cfu/g), the highest value was 9.16 cfu/g obtained from S3 but control sample was recorded the lowest count 8.74 cfu/g; on the other hand, the highest period effect was 9.63 log cfu/g obtained at 28th days of cold storage. Bhowmik et al. [14] also found that SP enhanced the growth of L. acidophilus in the product. SP may be a unique source of nutrients for probiotic bacteria due to redox potential reduction, prebiotic effect, having O2 scavengers (vitamin C, β-carotene, carotenoids) and nutritional enrichment of media (free amino acids, short-chain peptides, minerals, vitamins) nucleic acid precursors, mineral and, etc., among (Guldas and Irkin) [2] according to Varga et al. [15] The presence of nitrogenous substances such as free amino acids, peptone, adenine and hypoxanthin in these algae is able to stimulate the growth and production of probiotic acid bacteria significantly.
Incorporation of SP into Lactobacillus stimulated the growth of LAB. L. acidophilus count was increased in all treatments during the storage period may be attributed to the higher buffering capacity due to its high protein content of SP and concentrated milk. There were significant differences in L. acidophilus viability between SFL samples compared with control (p < 0.05). Which are consistent with results mentioned by Guldas and Irkin [2].
3.1.2 Total viable counts
The results in Table 1 illustrate the changes occurred in total counts (TC) in SFL samples during storage at 5 ± 1 °C. These results show that, TC varied from 6.28 to 6.85 log cfu/g TC which is fresh. The highest count was obtained from the SFL fortified with SP S4 sample. This means that the SP caused a significant (p ≤ 0.01) increase in total viable count, whereas the lowest counts was in control treatment. However, during storage the TC significant increased during the storage period. TC in all SFL samples increased. As well as either SP increase and as cold storage period. As shown in results, the effect of SP as additive is less than the effect of storage period (8–8.45) and (6.48–9.50) respectively. The gradual of TC increase may be due to the presence of nitrogenous substances such as free amino acids, peptone, vitamins, nucleic acid etc. derived from Spirulina biomass. The obtained results agree with results mentioned by Guldas and Irkin [2].
3.1.3 Determination of coliforms, Staphylococcus aureus, molds and yeasts
Staph. aureus, coliform, molds and yeasts were neither detected in control and all treatments of SFL, whether when fresh or during storage at 5 ± 1 °C during 28 days. This may be due to the effective heat treatment of milk which destroy the vegetative cells, also high sanitation and hygienic condition during manufacture and storage in addition to the effect of acidity of SFL which plays an important role in reducing rate of growth Staph. aureus and coliform. These results are in agreement with those reported by Martelli et al. [16] who reported, SP has antimicrobial activity against major foodborne pathogens such as Salmonella spp. and L. monocytogenes, E. coli, S. aureus, and B. cereus.
3.2 Kinetic Parameters of the Fermentation
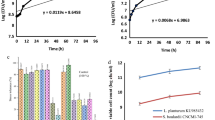
Table 2 investigate the effect of milk samples enriched with SP at different concentrations (0%, 0.2%, 0.4%, 0.7% or 1%) inoculated at a rate of 1% with the L. acidophilus and S. thermophilus strains tested. The pH and TA value were measured at regular intervals (every 2 h). Showed that SP levels were able to effectively stimulate acid production from starter strain. SP, used at 0.2% to 1%, was found to have a significant (P < 0.05) increase in acid rate and pH value. Regarding the pH variable estimated values equivalent 4.80 to 4.50 at 14 h of incubation were obtained for the control and sample content 0.7% SP, respectively with a compatibility to TA, the estimated values of control and samples with (0.2, 0.4, 0.7, 1%) of SP were equivalent to 0.70, 0.71, 0.82, 0.85 and 0.85% of lactic acid at 14 h, respectively. Which is in agreement with results reported by Vogadoe et al. [9]. The presence of some nitrogenous substances such as free amino acids, adenine, peptone and hypoxanthine in these algae is able to stimulate the growth and production of lactic acid for probiotic bacteria [17].
3.3 Titratable acidity % and pH values
The results given in Tables 3 that the TA values ranged from 0.88 to 1.81% in SFL. The symmetrical pH values varied from 5.03 to 6.11 at the beginning of storage, the highest value was of (S4) On the other hand, the control samples had lower TA values and higher pH values than other treatments. TA values were gradually increased with simultaneous a decrease trend of the pH values during storage at 5 ± 1 °C for 28 days. At the end of storage the TA varied from 1.48 to 1.82% and pH values varied from 5.17 to 5.26, this increase in the pH of SFL may be attributed to the higher buffering capacity of SP due to its high protein content. The partially lowest value of TA and the highest value of pH were shown in control compared to the samples supplemented with SP. Presence of SP increased TA at the same time it decreased the values of pH. However, there is a positive relation between the addition SP and TA, the opposite with pH values in a computability, similar findings by Guldas and Irkin [2]. This was probably caused from the addition of SP which promoted the growth of starter and production of lactic acid designated by Akalin et al. [17].
3.4 Syneresis properties of Spirulina functional labneh
Syneresis or release of whey is main defect that could limit the shelf life and acceptability due to undesirable appearances in labneh. Table 3 illustrated the effects of SP on the release of whey in SFL treatments during cold storage period. The incorporation of SP of probiotic led to decreased significantly (p ≤ 0.05) syneresis compared with control labneh and significantly decreased by increasing SP level addition. This decrease may be due to the related increase in the total solid content of labneh when SP incorporation that can bind the released whey and thus prevent the whey from being drained. Syneresis in all labneh samples showed an increasing trend during storage. As labneh, probably due to the fact that SP can bind free water in samples and consequently decrease the syneresis. This may be useful because labneh may maintain its stability during storage. These results are in accordance with those reported by El-Sattar et al. [4].
3.5 Chemical composition
The results given in Table 4 illustrate that, the changes in total solid (TS), protein and fat contents of SFL either between control treatments or during cold storage at 5 ± 1 °C for fresh time. The increase in the percentage of TS is due to the increase in the percentage of protein, fiber and carbohydrates present in the additive SP. Concerning ash content, S4 had the highest ash content (Table 4). As well as increasing the addition of Spirulina platensis powder, the protein content gradually increased in SFL samples. The highest level of protein was seen in S4 but control recorded the lowest value. SFL supplemented with SP had higher protein content than labneh without SP. This was due to the high protein content of the SP these results were agreement with Varga et al. [18]. However, as it is known that S. platensis is a superior source of proteins (60–70% of its dry weight). As shown in Table 4, fiber content significantly increased on SFL samples. The content of fortified labneh with 0.2, 0.4, 0.7 and 1% of SP gave 1.41, 1.77, 2.04 and 2.57% of the fiber, respectively. These results were agreement with Barkallah et al. [19] found that significant increase of total dietary fiber content in SP-fortified yogurts. However, fiber play an important role in gut health.
3.6 Minerals and trace elements content
SP fortified labneh had higher amount of total Zn, Fe, K, and Mg than control labneh (Table 4). The increased level of SP in SFL resulted in significant improvement in mineral contents and compensation for iron deficiency in milk.
These results are in agreement with those reported by Rahim et al. [20] explained that SP contains high amounts of minerals particularly K, Mg and Fe. Also, SP is rich in iron bioavailability and proves very beneficial in improving iron deficiency-anemia [21].
Among the complementary labneh, 1% SP had higher amount of minerals compared with 0.4 and 0.7 percent complementary labneh and control. It may be due to the addition of SP which has many high fold mineral contents [22]. These results are also in agreement with those reported by Ghaly et al. [23] in SP supplemented bread, muffins and biscuits. The availability of iron, potassium, magnesium and zinc were also found higher in treatments of SFL than control labneh (Table 4). [24] SP does not contain phytic acid which is known to bind divalent cations thus reducing their bioavailability.Heavy metals were not detected in control and all samples of Spirulina Functional Labneh SFL. Cadmium, lead, nickel heavy metals are not detected in all labneh treatments, while mercury appear in trace amount 1.92 μg/kg in all treatments including control labneh.
3.7 Phytopigments in Spirulina functional labneh
The results cleared that when the concentration of the SP addition increase, the proportion of the pigments content increase and the highest concentrations were in sample S4. Also the results in Table 4 referred that S4 contains 4.89 mg/mL of phycocyanin. These results are in agreement with those founded by Rahim et al. [20]. However, chlorophyll-a, chlorophyll-b and β- carotene contents of SFL were 0.61, 0.06, 0.07 mg/mL, respectively in Labneh fortified with SP S4 may be due to higher content of β-carotene and total carotenoids in S. platensis as indicated by Domínguez-Bocanegra et al. [25], [28] Found that SP contains 4.69 mg/g DW total carotenoids. SP contains a higher levels of phycobiliproteins (phycocyanins, allophycocyanins, and phycoerythrins), on the other hand, it contains a low level of carotenoids [20]. The levels of pigments in SP can be influenced by several factors such as pH, temperature, salinity, and light [26] Konícková et al. [27] reported that phytopigments such as chlorophyll A, phycocyanin and carotenoids play an important role as antioxidants and anti-carcinogens. The increase in the free radical scavenging might be attributed to increased content chlorophylls, carotenoids and phycocyanin.
3.8 Phenolic and flavonoid profile
The phenolic profile of the SFL samples is shown in Table 5 and seven different phenolic compounds and two flavonoids were revealed. Most abundant phenolic compounds in the S2 are gallic acid 459.41, caffeic acid 198.08, taxifolin 83.97, and ellagic acid 40.70 mg/100 g. The results show low amounts of chlorogenic acid, cinnamic acid and ferulic acid 22.77, 11.04 and 3.11 mg/100 g, respectively. On the other hand the S2 content flavonoid compounds as kaempferol 78.54 and naringenin 47.77. These results have been supported by study of Gabr et al. [28] they recorded gallic, chlorogenic, caffeine, and other phenols, Flavonoids were naringenin, kaempferol and other flavonoids have identified in SP. The phenolics in the S. platensis have antioxidant effects. Also, presence of few numbers of flavonoids and large number of phenolic acids.
Colla et al. [29] mentioned that phenolic compounds in SP such as organic acids (chlorogenic, synaptic, quimic, salicylic, transcinnamic and caffeic) can act as antioxidants synergistically or individually. Phenolic compounds in S. platensis such as organic acids (chlorogenic, synaptic, cinnamic and caffeic) can act as synergistic antioxidants or individually. Guldas et al. [30] found that honey with SP causing increases in amounts of kaempferol, taxifolin and caffeic acid versus the control honey [31].
3.9 The vitamin contents of SFL
Results in Table 5 show high values of vitamin contents in SFL contained high amounts of folic acid (B9), thiamine (B1) and cobalamin (B12) were recorded 0.08, 213.70 and 11.59 µg/g, respectively. S. platensis is a potential source of some water-soluble vitamins (B1, B2, B9, B12), which act as coenzymes for mitochondrial enzymes, playing essential roles in cell metabolism and energy production Rahim et al. [20] reported that the SP had content high amount of water-soluble vitamins. Also the results show that the added initiator (L. acidophilus, S. thermophilus) had an effect on increasing the content of the vitamins B1 and B12 in the control sample which is in agreement with results reported by Neimat and Wafaa [32]. LeBlanc et al. [33] explained the most lactic acid bacteria (LAB) are auxotrophic for many vitamins, it is now known that certain strains have the ability to synthesize water-soluble vitamins such as those of group B (riboflavin, folates and vitamin B12 amongst others).
3.10 Sensory evaluation of Spirulina functional labneh
The sensory evaluation scores of labneh samples are illustrated in Table 6. The results revealed significant (p ≤ 0.05) differences between the control sample and treatments containing 0.2%, 0.4%, 0.7% and 1% of SP. As shown, samples with higher SP concentrations (0.7 and 1%) exhibited lower sensory acceptability scores as compared to the control and the samples containing 0.2 and 0.4% of S. platensis powder. Based on the above, we recommend adding SP to the probiotic labneh at ratio 0.2 and 0.4%.
The addition of S. platensis powder slightly changed the labneh colour to blue-green because of the microalgae used, which directed the panelists to consider it as unusually appearance. Shimamatsu [34], the unpleasant flavor resulted from SP supplementation might attributed to the compounds produced from lipids oxidation in addition to the metallic off-flavors produced by minerals. Also, Barkallah et al. [19] concluded that using 0.25% of SP did not cause substantial differences in the organoleptic properties of labneh.
4 Conclusion
Production SFL with Lactobacillus acidophilus and Streptococcus thermophilus and fortified by Spirulina powder with different concentrations. As a result, found that SPF fortified by SP increased probiotic viability, protein and fiber levels, antioxidant activity when compared with the labneh without the fortification by SP. Especially, the SFL treatments increased vitamins level such as B1, B9, B12 and enhanced other minerals and phytopigment contents as well. Based on these results, that fortification of labneh with Spirulina are recommended for increased phenolic, flavonoid compounds, phytopigment, vitamins, fiber and minerals contents in SFL. Application of edible spirulina to labneh and enhancement of nutritional value and pre- and probiotic properties.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- UF:
-
Ultrafiltration
- HPLC:
-
High-performance liquid chromatography
- LAB:
-
Lactic acid bacteria
- TA:
-
Titratable acidity %
- SFL:
-
Spirulina functional Labneh
- TVC:
-
Total viable counts
- h:
-
Hours
- min:
-
Minutes
- SP:
-
Spirulina powder
References
Sánchez-Bayo A, López-Chicharro D, Morales V, Espada JJ, Puyol D, Martínez F, Rodríguez R. Biodiesel and biogas production from isochrysis galbana using dry and wet lipid extraction: a biorefinery approach. Renew Energy. 2020;146:188–95.
Guldas M, Irkin R. Influence of Spirulina platensis powder on the microflora of yoghurt and acidophilus milk. Mljekarstvo. 2010;60:273–243.
Mazinani S, Fadaei V, Khosravi-Darani K. Impact of Spirulina platensis on physicochemical properties and viability of Lactobacillus acidophilus of probiotic UF feta cheese. J Food Process Preserv. 2016;40(6):1318–24.
El-Sattar A, Ghafar NA, Ali AH. Impact of functional stirred low fat yoghurt supplementation with Spirulina platensis powder on some quality characteristics and therapeutic effects in vivo. J Food Dairy Sci Mansoura Univ. 2021;12(4):99–110.
Tamime AY, Kalab M, Davies G. The effect of processing temperatures on the microstructure and firmness of Labneh made from cow’s milk by the traditional method or by ultrafiltration. Food Struct. 1991;10(4):8.
El-Sayed SM, El-Sayed HS, Salama HH, El-Nor SA. Improving the nutritional value and extending shelf life of labneh by adding Moringa oleifera oil. Int J Dairy Sci. 2017;12(2):81–92.
Hashemi SMB, Mousavi KA, Kontominas MG, Sant’Ana AS, Martinez RR, Drider D. Fermentation of sarshir (kaymak) by lactic acid bacteria: antibacterial activity, antioxidant properties, lipid and protein oxidation and fatty acid profile. J Sci Food Agric. 2017;97(13):4595–603.
Kaprasob R, Kerdchoechuen O, Laohakunjit N, Sarkar D, Shetty K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017;59:141–9.
Vogado CDO, Leandro EDS, Zandonadi RP, De Alencar ER, Ginani VC, Nakano EY, Aguiar PA. Enrichment of probiotic fermented milk with green banana pulp: characterization microbiological, physicochemical and sensory. Nutrients. 2018;10(4):427.
Awad RA, Farahat AM, Salama WM. Production and in vivo nutritional evaluation of functional soft cheese supplemented with broccoli. World J Dairy Food Sci. 2012;7(2):150–9.
Horwitz W, Association of Official Agricultural Chemists. AOAC International. 19th ed. Gaithersburg: AOAC; 2010.
Tel-Çayan G, Öztürk M, Duru ME, Yabanlı M, Türkoğlu A. Content of minerals and trace elements determined by ICP-MS in eleven mushroom species from Anatolia. Turkey Chiang Mai J Sci. 2017;44(3):939–45.
Ayyad KM, Abdul Ghaffar I, Aida SS, Ismail MM. Production of probiotic low fat labneh by using exopolysaccacharide enhancing strains. Egyptian J Dairy Sci Sci. 2015;43(1):41–51.
Bhowmik D, Dubey J, Mehra S. Probiotic efficiency of Spirulina platensis- stimulating growth of lactic acid bacteria. World J Dairy Food Sci. 2009;4(2):160–3.
Varga L, Szigeti J, Ördög V. Effect of a Spirulina platensis biomass and that of its active components on single strains of dairy starter cultures. Milchwissenschaft. 1999;54(4):187–90.
Martelli F, Cirlini M, Lazzi C, Neviani E, Bernini V. Edible seaweeds and Spirulina extracts for food application: In vitro and in situ evaluation of antimicrobial activity towards foodborne pathogenic bacteria. Foods. 2020;9(10):1442.
Akalin AS, Unal G, Dalay MC. Influence of Spirulina platensis biomass on microbiological viability in traditional and probiotic yogurts during refrigerated storage. Ital J Food Sci. 2009;21(3):357–64.
Varga L, Szigeti J, Kovács R, Földes T, Buti S. Influence of a Spirulina platensis biomass on the microflora of fermented ABT milks during storage (R1). J Dairy Sci. 2002;85(5):1031–8.
Barkallah M, Dammak M, Louati I, Hentati F, Hadrich B, Mechichi T, Ayadi MA, Fendri I, Attia H, Abdelkafi S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT. 2017;84:323–30.
Rahim A, Çakir C, Ozturk M, Bihterahin S, Soulaimani A, Sibaoueih M, Nasser B, Eddoha R, Essamadi A, El Amiri B. Nutritional value of Spirulina platensis cultivated in natural conditions of Chichaoua-Morocco and comparison of natural well water containing wood ash medium with previous mediums. S Afr J Botany. 2021;141:235–42.
Bensehaila S, Doumandj A, Boutekrabt L, Manafikhi H, Peluso I, Bensehaila K, Kouache A, Bensehaila A. The nutritional quality of Spirulina platensis of Tamenrasset, Algeria. Afr J Biotechnol. 2015;14(19):1649–54.
Vijayarani D, Ponnalaghu S, Rajathivya J. Development of value added extruded product using Spirulina. Int J Res Health Sci. 2012;2(4):42–7.
Ghaly A, Hammouda A, Hattab M. Development and sensory evaluation of Spirulina chocolate chip oatmeal cookies. Int J Bioprocess Biotechnol. 2015;1(2):63–73.
Saharan V, Jood S. Vitamins, minerals, protein digestibility and antioxidant activity of bread enriched with Spirulina platensis powder. Int J Agric Sci. 2017;9(9):3917–9.
Domínguez-Bocanegra AR, Torres-Muñoz JA, Aguilar López RA. Biosorption of cadmium (II), lead (II) and nickel (II) by Spirulina Maxima. Int J Sci. 2013;2(10):45–55.
Kumar M, Kulshreshtha J, Singh GP. Growth and biopigment accumulation of cyanobacterium Spirulina platensis at different light intensities and temperature. Braz J Microbiol. 2011;42:1128–35.
Konícková R, Vanková K, Vaníková J, Vánová K, Muchová L, Subhanová I, Vítek L. Anti-cancer effects of blue-green alga Spirulina platensis: a natural source of bilirubin-like tetrapyrrolic compounds. Ann Hepatol. 2014;13(2):273–83.
Gabr GA, El-Sayed SM, Hikal MS. Antioxidant activities of phycocyanin: a bioactive compound from Spirulina platensis. J Pharm Res Int. 2020;32:73–85.
Colla LM, Reinehr CO, Reichert CJ, Costa AV. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Biores Technol. 2007;98:1489–93.
Guldas M, Gurbuz O, Cakmak I, Yildiz E, Sen H. Effects of honey enrichment with Spirulina platensis on phenolics, bioaccessibility, antioxidant capacity and fatty acids. LWT. 2022;153:112461.
Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy-a review. Nutrients. 2016;8(2):68.
Neimat AH, Wafaa MS. Evaluation of certain probiotic bacteria for biosynthesis of vitamin B complex in cultured milk Egypt. J Food Sci. 2011. https://doi.org/10.5772/63117.
LeBlanc JG, Laiño JE, del Valle MJ, Vannini VV, van Sinderen D, Taranto MP, Sesma F. B-Group vitamin production by lactic acid bacteria–current knowledge and potential applications. J Appl Microbiol. 2011;111(6):1297–309.
Shimamatsu H. Mass production of Spirulina, an edible microalga. Hydrobiologia. 2004;512(1):39–44.
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Eُbid: Participated in the idea, plan development, laboratory experiments, analysis of results, writing the manuscript and published it. Ali: Participate in the idea, plan development, laboratory experiments, analysis of results and writing the manuscript. Elewa: participated the idea, plan development, review the results, and revision the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ebid, W.M.A., Ali, G.S. & Elewa, N.A.H. Impact of Spirulina platensis on physicochemical, antioxidant, microbiological and sensory properties of functional labneh. Discov Food 2, 29 (2022). https://doi.org/10.1007/s44187-022-00031-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-022-00031-7