Abstract
Introduction
The purpose of this study was to evaluate the use of a cadaveric teaching model for needle compression of pneumothorax simulated by thoracoscopy.
Materials and methods
A standardized didactic instruction was provided to medical personnel participants at a single institution tertiary medical center. A thoracoscope was inserted into cadavers and insufflated to 20 mm Hg for simulation of a tension pneumothorax. The study participants performed six needle decompressions (3 anterior, 3 lateral), which were all directly observed thoracoscopically. Demographic data and post-surveys were obtained. The primary endpoint was participant assessment of this teaching model for a simulated pneumothorax. Secondary endpoints were successful decompression of the pneumothorax, perceived success of each attempt, and injury to intrathoracic structures.
Results
Forty participants completed 240 attempted decompressions. Participants reported that 43% had taken ATLS, and 63% had performed a needle decompression on a simulated patient prior to this study. The rate of successful decompressions was 85.86%. Participants reported a perceived successful completion rate of 82%. 73.7% performed this safely, while 88.5% perceived that they performed it safely. 85.7% stated that they felt more confident and capable after the study.
Conclusion
A simulated model is essential for adequate teaching. Our use of a thoracoscopic cadaveric model provides a realistic simulation of the pneumothorax used for training. Participants had similar rates of actual completion and perceived completion including higher rates of perceived safety. Further use of this model as a teaching tool will potentially improve the success of this life-saving procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tension pneumothorax (tPTX) is a potentially life-threatening condition seen in both civilian and military trauma. In military settings, tPTX is the third most common potentially survivable physiological cause of death [1]. The diagnosis requires prompt recognition, and immediate needle decompression is recommended in the prehospital setting as the initial step to temporize circulatory collapse. Although rare, the true incidence of tPTX varies widely. Military databases from Vietnam report that 3–4% of combat fatalities were secondary to tPTX [2]. Since then, the increasingly prevalent use of effective body armor has reduced the number of fatal penetrating chest injuries [3]. Despite this, trauma patients who sustained a thoracic injury requiring needle thoracostomy in civilian and military settings based on clinical recognition have been reported as 1.7% and 2.5%, respectively [4, 5].
The Advanced Trauma Life Support (ATLS) program® developed by the American College of Surgeons, Pre-hospital Trauma Life Support (PHTLS) course sponsored by the National Association of Emergency Medical Technicians, and the Tactical Combat Casualty Care (TCCC) course administered by the military are various trauma education programs that recommend emergent decompression of a tPTX with needle thoracostomy. Current recommendations by ATLS and TCCC to perform needle thoracostomy are using an 8 cm (cm) 14-gauge (g) angiocatheter at the 4th or 5th intercostal space (ICS) along the anterior axillary line in adult patients as the initial location [6, 7].
Prior recommendations for performing needle thoracostomy included using a 5 cm 14-g angiocatheter at the 2nd ICS along the midclavicular line as the initial location. Failure rates of needle thoracostomy were seen as high as 41% in autopsies of service members killed in combat and up to 65% in civilian trauma centers [8, 9]. The change in recommendation was prompted by multiple cadaveric and radiologic studies demonstrating improved success rates, decrease chest wall thickness and no difference in complication rates at the 5th ICS [10,11,12,13].
Due to the rarity of this diagnosis and the variable methods in treating this condition, the ideal training model remains unknown. High-fidelity training models have shown significant advantages to low-fidelity training models [14]. However, most tPTX needle decompression training models are low fidelity and lack capabilities in measuring the success of performing a needle decompression [15]. We utilized a thoracoscopic cadaveric model, which would allow real-time evaluation of needle decompression. Although there is a debate on the use of cadaver models from an ethical standpoint, it remains a potential option in the military training setting [16]. With this teaching model, we assessed numerous variables using both approaches. The primary goal is to evaluate the utility of a thoracoscopic cadaver model in teaching healthcare providers to perform needle decompression for a simulated tPTX. Second, we looked to validate the success and safety of needle decompression at the 4/5th ICS using this teaching model.
Materials and methods
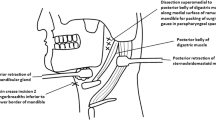
At a single military institution, 30 adult cadavers were used for this research study. All procedures were completed in the Biomedical Teaching facility at Naval Medical Center San Diego (NMCSD). This facility is equipped with standard operating tables to include full laparoscopic capabilities. Fresh frozen cadavers were used exclusively. Each of our thoracoscopic models was of secondary use to allow for cost savings, multiple dissections, and teaching sessions for each cadaver. All cadaveric chest cavities had not been violated and were free of any visible surgical procedures or external trauma. Cadavers were excluded if they had undergone previous thoracic procedures or if significant intrathoracic adhesions or excessive pleural fluid were present, which would limit visualization. The cadaver was placed in the supine position on a standard operating table during each needle decompression.
Participants in the study were volunteer residents, medical students, and Naval hospital corpsmen from a single military institution. Participants completed a pre-trial survey that included demographic data, trauma experience, and needle decompression procedural experience. Relative procedural experiences were evaluated by reviewing each participant’s years of medical training, prior experience with needle decompression, chest tube placement, and/or thoracotomy. Regardless of their experience, a standardized teaching module was administered to all participants consisting of a standardized ATLS video on the proper insertion of a needle for decompression of a tPTX as well as an educational handout on appropriate needle placement. All participants were randomized to perform the decompression first at the 2nd intercostal space along the midclavicular line or the 4th–5th intercostal space along the anterior axillary line. This was accomplished to prevent any learned procedural bias in performing one approach first before the other approach.
A standardized simulated tPTX was created on each cadaver. The tension pneumothorax was performed by inserting a 10 mm (mm) 30° thoracoscope at the 4–5th ICS along the nipple line. The chest was insufflated to 20 mmHg at a high flow rate of 20 Liters/minute with carbon dioxide. The pleural cavity was examined for any abnormalities that would limit compression of the lung, such as dense adhesions or large pleural effusions, which would limit the visualization of the needle during decompression. These cadavers were excluded from use. After the insufflation of our first cadaver, an anterior–posterior chest x-ray was obtained, which demonstrated findings consistent with a tPTX. Figure 1A depicts a chest radiograph with a significant shift of the trachea and mediastinal structures.
a Iatrogenic tension pneumothorax simulated with insufflation of carbon dioxide along the left chest. Chest radiograph demonstrates a mediastinal shift towards the right chest. b and c Thoracoscopic view of left hemithorax and corresponding images of external positioning during the completion of a needle decompression at the 2nd ICS mid-clavicular line. d and e Internal thoracoscopic view of needle decompression and corresponding external view at the 4/5th ICS anterior axillary line. ICS—intercostal space
Each participant was asked to attempt three successive needle decompressions using a 14-g 8-cm over-the-needle catheter or angiocatheter for each approach: the 2nd ICS anteriorly and the 4/5th ICS laterally. The participants were blinded to the video monitor, but only the proctor was able to visualize and evaluate the insertion of the needle. (Fig. 1B and D) The participant verbally indicated when they perceived they had successfully completed the needle decompression of the tension pneumothorax. Afterward, the needle was secured in place. The participants were given no feedback until they had completed all six needle decompressions. (Fig. 1C and E) Results recorded include successful placement of the needle, injury to intra-thoracic structures (puncture to the lung, heart, or great vessels), the proximity of the needle tip to the closest intra-thoracic structure, and chest wall thickness. The chest wall thickness was measured by observing direct depth upon initial needle insertion. The distance to the closest intra-thoracic structure was determined using a stiff guidewire. The total depth of insertion of the needle was also recorded.
Results
During the study period, 40 participants completed a total of 240 attempts for needle decompression. The average age of the participants was 27 years. Most of the participants were medical students (62.5%). Concerning prior experience, 43% had previously completed ATLS training, and 63% previously performed a needle decompression on a simulated patient. Despite this simulation experience, not one of the candidates had performed this procedure on an actual patient. Thirty percent of participants had previously placed a chest tube on either an actual patient or a simulated patient (Table 1).
Each participant completed three needle decompressions at both the 2nd ICS along the mid-clavicular line and 4/5 the ICS along the anterior axillary line for a total of 6 decompressions each. Although the success rate of needle decompression was higher (85.8%) for the 4/5th ICS lateral approach versus the anterior 2nd ICS approach, this was not considered statistically significant (p = 0.80). Intrathoracic structures were punctured during 31.6% of all decompression (27.5% vs. 35.8% for 2nd ICS anteriorly vs. 4/5th ICS laterally) (Fig. 2). Chest wall thickness was higher for the 4/5th ICS laterally approach (3.68 cm) than the 2nd ICS anterior approach (3.01 cm), but again this was not statistically significant (p = 0.18). When an intrathoracic structure was not injured, the average distance to the closest structure was 2.8 cm but not statistically significant. (Fig. 3).
With this model, the majority of candidates had successfully completed the needle decompression based on the proctor’s visual evaluation (82.9%). Each participant was asked after each attempt if they perceived a successful needle decompression. The overall reported perceived successful completion rate was very similar at 82%. However, the actual safety performance of needle decompression (no injury to intrathoracic structures and successful decompression) was 73.7% which is lower than the participants’ perceived performance (88.5%). In relation to our thoracoscopic cadaveric teaching model, most candidates (85.7%) stated that they felt more confident and capable after the study (Table 2).
In evaluating individuals based on prior thoracic trauma skills, 65% (N-26) of the candidates had prior simulated or actual experience in performing a needle decompression. A significantly higher success rate (84.6% vs. 69%) was seen in those providers with prior needle decompression skills. In comparison to more advanced trauma skills, there were fewer individuals who had performed a chest tube (32.5%, N-13) or thoracotomy (10%, N-4). Although candidates with prior thoracotomy experience had a markedly higher rate of successful attempts (91.7% vs. 74.1%), this observation was not seen in relation to chest tube placement (61.5% vs. 81.5%).
Discussion
Tension pneumothorax requires prompt, effective treatment whether it is recognized in the prehospital setting by emergency medical technicians, corpsmen, or medics or whether it is in the hospital by mid-level practitioners, residents of all training levels, or attendings. Given the recent changes by 10th edition of ATLS® on the recommendation of the length and location of needle decompression, an effective teaching model is required for all levels and backgrounds of training. Our cadaveric thoracoscopic model improved the confidence and capabilities of performing a needle decompression for tension pneumothorax among healthcare providers of different backgrounds. Secondly, with our high-fidelity thoracoscopy that allows skills training in real time, we demonstrated that needle decompression at the 4/5th ICS is equally effective and safe for the treatment of tPTX.
A thoracoscopic cadaver model is unique from the open approaches because it simulates a pneumothorax by insufflation of air into the pleural cavity. The use of video also allows real-time assessment of needle decompression by the examiner. Direct feedback can be provided to a trainee based on the trajectory of the needle, depth of insertion, and proximity to key vital structures. Video thoracoscopy can also be recorded to allow the candidate to review their skills after the procedure; however, we did not feel this was necessary for our study. The survey data revealed the value of such education and real-time feedback after each test. Based on our post-procedure survey, 85% of participants indicated that they would be more comfortable and capable of performing this vital procedure if they had experience with an actual patient.
We also learned that this thoracoscopic cadaveric model could be used in the future as a teaching simulation for healthcare providers of various levels of training. When we evaluated prior experience with trauma skills such as prior needle decompression, there was a higher success rate for experienced candidates versus non-experienced individuals. Interestingly, more advanced thoracic procedures did not consistently relate to the success rate of needle decompression in our cadaver model, emphasizing the need for continuous training. Simulations are increasingly used throughout training courses and residency programs to teach different procedures from central line insertions to complex operations. Cadaveric training for needle thoracostomy has been proven more effective than didactic teaching alone in U.S. Navy corpsmen enrolled in TCCC training [17]. Corpsmen assigned to cadaveric-based training correctly placed needle thoracostomies 75% of the time compared to 35% of corpsmen assigned to slide-based lectures. Numerous synthetic models and mannequins are currently being developed to teach these procedures; however, these models do not provide an ideal replacement compared to cadaveric models. Previous cadaveric models for needle decompression have verified correct needle or catheter placement by performing thoracotomies to assess the intrapleural space, which limits the use of the cadaver. Although the costs of purchasing a cadaver are significant, all our cadavers were of secondary use where other dissections, training procedures, or surgical procedures were performed (excluding any thoracic violation) prior to our needle decompressions. This secondary use kept costs to a minimum. Based on our experience with our candidates, utilizing a high fidelity cadaveric-based training with thoracoscopy is an effective teaching method for such uncommonly performed life-saving procedures such as needle decompression, finger thoracostomy, and chest tube insertion.
Several studies have examined the optimal location, needle length, and needle gauge required for successful completion. A study by Inaba et al. reviewed chest computed tomography (CT) in trauma patients and showed that decompression with a 5 cm needle would only be effective 66–80% of the time at either location assuming the optimal trajectory; however, effective needle decompression was achieved greater than 96% of the time at either location with the use of an 8 cm needle. Additionally, when assessing the ability of the needle to hit vital structures, the 5 cm needle would only be able to hit a vital structure in 1% of cases while an 8 cm needle could hit a vital structure in 0–32% of the time [11]. More recently, TCCC has changed their guidelines making a 10-g angiocatheter an alternative option to treat tPTX, and Norris et al. demonstrated that the use of a larger caliber 10-g needle was more successful in the treatment of tPTX compared with 14-g catheter [6, 18]. Our study also utilized a 14-g 8-cm over-the-needle catheter consistent with the current 10th edition ATLS recommendations. Although TCCC accepts both 14 g or 10 g catheter use for tPTX treatment, the current contents of Improved First Aid Kits (IFAK) issued to service members often contain the 14 g 8-cm angiocatheter needle. Our results indicate success rates at each location using the 14 g 8-cm angiocatheter with a trend toward increased success at the lateral location.
These data support the recommended change in length of the needle thoracostomy to a 14 g 8-cm needle as well as the location to the 4/5th ICS. With the two sites being effective in treatment, optimal placement of the needle thoracostomy should take into consideration the situation in which it is being employed. A lateral approach in a prehospital setting or during routine transport can be challenging with obstruction by adducted extremities on a gurney with limited space. In contrast to the military population, the anterior location is often completely blocked by body armor while in combat. Figure 4 shows the standard body armor worn by military personnel. To prevent leaving service members vulnerable to additional injury while in austere conditions, the lateral location would allow treatment of tPTX by medics or corpsmen without removal of essential protective gear, which would have occurred based on prior teachings using the 2nd midclavicular intercostal space. A corpsman or medic should comfortably and expeditiously treat a tPTX with the lateral location as the initial approach rather than as an alternative.
a Anterior and b lateral photo of standard body armor worn by service members in combat. The (red) circle indicates the location site for needle decompression at the 2nd ICS mid-clavicular line. The (blue) diamond indicates the location for needle decompression at the 4/5th ICS anterior axillary line. ICS—intercostal space
Our research does have limitations. The number of participants may have limited our ability to reach statistical significance. Increasing the number of participants may show one location to be superior and safer. We were unable to demonstrate correction of tension physiology because of the behavior of preserved cadaveric tissue. The use of a large animal model or cadaver-perfused model in which physiologic conditions could be more closely replicated would potentially answer this question. However, costs and administrative obstacles have made the use of animal models more difficult in many training institutions. Furthermore, our study did not include another training model, such as live tissue, to compare effectiveness in training and performance. A previous study performed by Hart et al. compared the effectiveness of simulation-based training methods to live tissue training methods for needle decompression and found that training on simulation-based models showed no difference in subsequent performance. Both simulation-based training methods and live tissue training methods produced an overall success rate of 90% [19]. In addition, safety was difficult to assess in this study using this cadaveric model, and our assessment of “injured” structures was presumed. We counted a structure injured if the needle punctured any structure in the chest. This most often was lung parenchyma, which was equally injured in 25% of attempts at both the 2nd ICS and 4/5th ICS. Most often, the needle pierced the lung to varying depths. In no attempt was a major vessel or the heart injured. The clinical significance of these injuries remains unknown and is likely minimal in the setting where a chest tube would be eventually placed. The use of a perfused cadaver model or animal study may help elucidate the clinical significance of these injuries [12]. Additionally, body mass index (BMI) can impact the depth required to insert a decompressive needle and, at times, make anatomic landmarks more difficult to ascertain. Due to the preservation process and desiccation of our cadaveric bodies, BMI was a variable we were unable to account for as it was altered and unreliable in our study. With each participant completing multiple trials at two different locations, the participants would have learned and improved with each subsequent attempt, possibly altering the final results.
There are several areas to expand this research for future studies. Suggested training to include a cadaveric model with thoracoscopy for real-time feedback may assist in improving the success rate of needle thoracostomy. For military providers in austere environments unable to remove essential protective gear, treatment of tPTX may rely on limited visualized landmarks or palpation alone . A thoracoscopic training model could be used with cadavers wearing tactical gear to train military providers facing these obstacles but provide confidence in their treatment with visual biofeedback. Simulation training can provide a valuable learning opportunity for trainees, especially when learning rare but life-saving procedures. This model is not limited to needle decompression but can be applied to train providers to perform chest tube insertions and finger thoracostomy, a necessary skill to have if needle decompression fails [6], thoracotomies, and cricothyrotomies. This training experience is especially important for residents and young surgeons with less experience. Finally, we see value in following these participants longitudinally over time and assessing their skill and knowledge retention, which could provide some suggestions for timing training refresher courses.
Conclusions
Decompression of a tPTX requires proper placement of a needle thoracostomy. Based on our cadaver model, placement at the 2nd ICS along the mid-clavicular line and 4/5th ICS along the anterior axillary line are equally effective in relieving the tension pneumothorax. Consideration of a cadaver model using thoracoscopic evaluation can be an ideal teaching tool to improve education, training, skills retention, and confidence in performing this life-saving procedure.
Disclaimer
The views expressed are solely those of the authors and do not reflect the official policy or position of the US Navy, US Army, US Air Force, the Department of Defense, or the US Government.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data were generated at Naval Medical Center San Diego. Derived data supporting the findings of this study are available from the corresponding author [RCI] on request.
References
Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6):S431–7.
McPherson JJ, Feigin DS, Bellamy RF. Prevalence of tension pneumothorax in fatally wounded combat casualties. J Trauma Injury Infect Critical Care. 2006;60(3):573–8.
Mabry RL, Holcomb JB, Baker AM, Cloonan CC, Uhorchak JM, Perkins DE, et al. United States Army Rangers in Somalia: an analysis of combat casualties on an Urban Battlefield. J Trauma Injury Infect Critical Care. 2000;49(3):515–29.
Eckstein M, Suyehara D. Needle thoracostomy in the prehospital setting. Prehosp Emerg Care. 1998;2(2):132–5.
Chen J, Nadler R, Schwartz D, Tien H, Cap A, Glassberg E. Needle thoracostomy for tension pneumothorax: the Israeli defense forces experience. Can J Surg. 2015;58(3):S118–24.
Butler FK, Holcomb JB, Shackelford S, Montgomery HR, Anderson S, Cain JS, et al. Management of suspected tension pneumothorax in tactical combat casualty care: TCCC guidelines change 17–02. J Spec Oper Med. 2018;18(2):19–35.
Student Course Manual ATLS ® Advanced Trauma Life Support ®. 2018.
Ball CG, Wyrzykowski AD, Kirkpatrick AW, Dente CJ, Nicholas JM, Salomone JP, et al. Thoracic needle decompression for tension pneumothorax: clinical correlation with catheter length. Can J Surg. 2010;53(3):184–8.
Harcke HT, Mabry RL, Mazuchowski EL. Needle thoracentesis decompression: observations from postmortem computed tomography and autopsy. J Spec Oper Med. 2013;13(4):53–8.
Inaba K, Branco BC, Eckstein M, Shatz DV, Martin MJ, Green DJ, et al. Optimal positioning for emergent needle thoracostomy: a cadaver-based study. J Trauma Injury Infect Crit Care. 2011;71(5):1099–103.
Inaba K, Ives C, McClure K, Branco BC, Eckstein M, Shatz D, et al. Radiologic evaluation of alternative sites for needle decompression of tension pneumothorax. Arch Surg. 2012. https://doi.org/10.1001/archsurg.2012.751.
Leatherman ML, Held JM, Fluke LM, McEvoy CS, Inaba K, Grabo D, et al. Relative device stability of anterior versus axillary needle decompression for tension pneumothorax during casualty movement: preliminary analysis of a human cadaver model. J Trauma Acute Care Surg. 2017;83(1):S136–41.
Inaba K, Karamanos E, Skiada D, Grabo D, Hammer P, Martin M, et al. Cadaveric comparison of the optimal site for needle decompression of tension pneumothorax by prehospital care providers. J Trauma Acute Care Surg. 2015;79(6):1044–8.
Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning: simulation fidelity. Med Educ. 2012;46(7):636–47.
Tenorio LEM, Devine KJ, Lee J, Kowalewski TM, Barocas VH. Biomechanics of human parietal pleura in uniaxial extension. J Mech Behav Biomed Mater. 2017;75:330–5.
Rubeis G, Steger F. Is live-tissue training ethically justified? An evidence-based ethical analysis. Altern Lab Anim. 2018;46(2):65–71.
Grabo D, Inaba K, Hammer P, Karamanos E, Skiada D, Martin M, et al. Optimal training for emergency needle thoracostomy placement by prehospital personnel: didactic teaching versus a cadaver-based training program. J Trauma Acute Care Surg. 2014;77(3):S109–13.
Norris EA, McEvoy CS, Leatherman ML, Boboc MR, Fitch JL, Jensen SD, et al. Comparison of 10- versus 14-gauge angiocatheter for treatment of tension pneumothorax and tension-induced pulseless electrical activity with hemorrhagic shock: bigger is still better. J Trauma Acute Care Surg. 2020;89(2S):S132–6.
Hart D, Rush R, Rule G, Clinton J, Beilman G, Anders S, et al. Training and assessing critical airway, breathing, and hemorrhage control procedures for trauma care: live tissue versus synthetic models. Acad Emerg Med. 2018;25(2):148–67.
Acknowledgements
We would like to thank the staff at Naval Medical Center San Diego Bioskills and Simulation Training Center for use of the facilities. The authors have no financial disclosures. The opinions or assertions contained within the manuscript are the private views of the authors and are not to be construed as official or as reflecting the views of the US Navy or Department of Defense.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parra, K.T., Gower, J.R., Floan, G.M. et al. A thoracoscopic cadaveric teaching model for needle decompression of a tension pneumothorax. Global Surg Educ 2, 54 (2023). https://doi.org/10.1007/s44186-023-00134-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44186-023-00134-4