Abstract
Purpose
To establish a competing-risks model and compare it with traditional survival analysis, aiming to identify more precise prognostic factors for angiosarcoma. The presence of competing risks suggests that prognostic factors derived from the conventional Cox regression model may exhibit bias.
Methods
Patient data pertaining to angiosarcoma cases diagnosed from 2000 to 2019 were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Multivariate analysis employed both the Cox regression model and the Fine-Gray model, while univariate analysis utilized the cumulative incidence function and Gray’s test.
Results
A total of 3,905 enrolled patients diagnosed with angiosarcoma were included, out of which 2,781 succumbed to their condition: 1,888 fatalities resulted from angiosarcoma itself, and 893 were attributed to other causes. The Fine-Gray model, through multivariable analysis, identified SEER stage, gender, race, surgical status, chemotherapy status, radiotherapy status, and marital status as independent prognostic factors for angiosarcoma. The Cox regression model, due to the occurrence of competing-risk events, could not accurately estimate the effect values and yielded false-negative outcomes. Clearly, when analyzing clinical survival data with multiple endpoints, the competing-risks model demonstrates superior performance.
Conclusion
This current investigation may enhance clinicians’ comprehension of angiosarcoma and furnish reference data for making clinical decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Angiosarcoma represents an uncommon malignant neoplasm characterized by aggressive invasiveness and an unfavorable prognosis [1, 2]. It originates from vascular endothelial cells and can manifest in various anatomical sites, encompassing the skin, subcutaneous soft tissues, internal organs, and bones [1, 2]. The initial clinical manifestations of angiosarcoma tend to be atypical, posing a risk of oversight and misdiagnosis [1]. Hence, clinicians must remain vigilant regarding this condition when elderly patients present with painless and non-pruritic ecchymotic or hematoma-like lesions in the craniofacial region [1]. Angiosarcoma can be categorized as primary or secondary, with the latter often arising following radiation therapy or in the context of chronic lymphedema [3]. Given the rarity of this malignancy and its genetic heterogeneity, most existing studies suffer from limited sample sizes, impeding the establishment of comprehensive treatment guidelines [3].
In order to ascertain the prognostic risk factors associated with various diseases, the application of Cox proportional-hazards models and Kaplan-Meier marginal regression models (KM) is widespread. To accurately assess the mortality risk faced by patients, it becomes imperative to estimate the impacts of both cancer-related and non-cancer-related factors in the era of personalized cancer therapies. Beyond cancer itself, additional ailments, traffic accidents, and instances of suicide may also contribute to the demise of cancer patients [4, 5]. Such non-cancer-related causes of death, as exemplified earlier, are recognized as competing-risk events [6]. The occurrence of a competing risk event serves to impede the transpiring of the primary event of interest [7]. It is noteworthy that traditional survival analysis methods fail to account for the influence of competing risk events, resulting in inherent biases within the ultimate findings [8]. Thus, to investigate the prognostic risk factors impacting angiosarcoma, this study employed a competing-risks model, thereby enabling a more precise evaluation of the genuine impact of each variable and pertinent hazard factors when compared to conventional survival analyses.
To our utmost understanding, this study represents a pioneering endeavor in formulating a competing-risks model for angiosarcoma, coupled with a comparative analysis against the conventional Cox proportional-hazards model—an established approach to survival analysis. The primary objective of this investigation was to foster a more profound comprehension of the prognostic factors that underlie angiosarcoma, thereby endowing clinicians and future research with invaluable insights. Statistical analyses were diligently conducted employing data sourced from the comprehensive Surveillance, Epidemiology, and End Results database (SEER), thereby effectively addressing the challenge of inadequate sample size [9].
2 Materials and methods
2.1 Database
The patient data pertaining to angiosarcoma in this study were procured from the SEER database, utilizing the SEER*Stat software (version 8.4.0.1). Notably, the SEER database embodies exceptional qualities, characterized by its high caliber and extensive scope [10]. This comprehensive resource, established in 1973, encompasses 18 registries across the United States, thereby encompassing approximately 30% of the nation’s population [11]. It furnishes a diverse array of information pertaining to patients afflicted with malignant tumors, encompassing even the most rarefied of neoplasms. This wealth of data encompasses cancer demographics, treatment modalities, and survival outcomes, thus conferring substantial value as a point of reference for clinical researchers [10,11,12].
2.2 Data collection
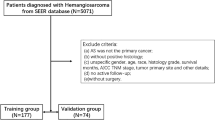
We conducted an extensive search within the SEER database, spanning the period from 2000 to 2019, to identify and retrieve all documented cases of angiosarcoma, using the tumor-site ICD-O-3: “Hist/behave, malignant” code 9120/3 (“Hemangiosarcoma”). In addition, a prerequisite for inclusion was the presence of microscopically confirmed angiosarcoma. Exclusion criteria encompassed the following: (1) incomplete data, (2) age below 18 years, or (3) reliance solely on autopsy findings. We extracted the following variables for the enrolled patients: age (age at diagnosis), gender (male or female), race (white, black, other), SEER stage (localized, regional, distant), surgery status (yes and no/unknown), radiotherapy status (yes and no/unknown), chemotherapy status (yes and no/unknown), marriage (marital status at diagnosis), regional lymph nodes positivity status (no, yes, unknown), vital status (live, dead), survival months, cause of death, and median household income.
Patients who succumbed to angiosarcoma were categorized as primary events of interest, while those who died from alternative causes were deemed competing events. Patients who remained alive throughout the study period were treated as censored events.
The SEER database, a publicly accessible resource, provides de-identified patient data, rendering individual informed consent and ethics committee approval unnecessary. Figure 1 illustrates the inclusion of 3,905 eligible angiosarcoma patients in the analysis.
2.3 Statistical analysis
Baseline information was presented in both numerical and percentage formats. The cumulative incidence function (CIF) was employed for univariate analysis, taking competing events into consideration while explaining the cumulative incidence of a specific event [13], and for group comparisons, Gray’s test was employed [14]. In the presence of competing risks, the conventional Kaplan-Meier (KM) method often yields higher cumulative incidence of a particular event compared to the CIF method [15]. For multivariate analysis and identification of variables associated with the cumulative incidence of angiosarcoma, the Fine-Gray model was utilized. Moreover, the Fine-Gray model is considered more suitable for developing clinical prediction models and assessing the risks and prognoses of diseases according to some researchers [14]. Additionally, we performed Cox regression analysis and compared the results with the Fine-Gray model, as the traditional Cox regression model can introduce biases in risk assessment when competing risks are present [6].
Data analysis and processing were conducted using the R software (version 4.2.1) and IBM SPSS software (version 27.0, SPSS, Chicago, IL, USA). Statistical significance was defined as a probability value (p) less than 0.05.
3 Results
3.1 Patient characteristics
Table 1 presents a comprehensive overview of the fundamental patient demographics. Among the 3,905 individuals enrolled in the study with a diagnosis of angiosarcoma, 2,781 (71.22%) experienced mortality, with 1,888 attributed to angiosarcoma itself and 893 to other causes. On the other hand, 1,124 (28.78%) patients were recorded as still living. The majority of the participants were over the age of 65 (n = 2,369, 60.67%), identified as female (n = 2,139, 54.78%), classified as white (n = 3,262, 83.53%), married (n = 2,071, 53.03%), presented with localized or regional metastasis (n = 2,963, 75.88%), underwent surgical procedures (n = 2,682, 68.68%), did not receive chemotherapy or had an unknown status regarding its administration (n = 2,622, 67.14%), did not receive radiotherapy or had an unknown status (n = 2,795, 71.57%), had an unknown status regarding regional lymph node positivity (n = 3,328, 85.22%), and possessed a medium or high household income (n = 3,206, 82.10%).
3.2 Univariate analysis
During the univariate analysis, Gray’s test was employed to examine ten potential prognostic variables, revealing significant impacts on the prognosis of angiosarcoma patients (p < 0.05). These variables include gender, race, marital status, SEER stage, surgery status, chemotherapy status, radiotherapy status, and regional lymph node positivity status. Across all variables, the cumulative incidence function (CIF) values exhibited an upward trend over 1, 3, and 6 years. Specifically, higher CIF values were observed in male patients, individuals of other racial backgrounds, unmarried patients, those with distant metastasis, patients who did not undergo surgery or had an unknown surgical status, patients who received chemotherapy and individuals with positive regional lymph nodes. These insightful details are presented in Table 2. Figure 2 showcases the Kaplan-Meier curves for cancer-specific survival of each parameter.
A Kaplan-Meier curves for cancer-specific survival according to age groups: 1- ≤ 65 years, 2- > 65 years. B Kaplan-Meier curves for cancer-specific survival according to gender groups: 1-Male, 2-Female. C Kaplan-Meier curves for cancer-specific survival according to race groups: 1-White, 2-Black, 3-Other. D Kaplan-Meier curves for cancer-specific survival according to marital status groups: 1-Married, 2-Unmarried, 3- divorced/separated/widowed (DSW), 4-Unknown. E Kaplan-Meier curves for cancer-specific survival according to SEER stage groups: 1-Localized, 2-Regional, 3-Distant. F Kaplan-Meier curves for cancer-specific survival according to surgery groups: 1-Yes, 2-No/Unknown. G Kaplan-Meier curves for cancer-specific survival according to chemotherapy groups: 1-Yes, 2-No/Unknown. H Kaplan-Meier curves for cancer-specific survival according to radiotherapy groups: 1-Yes, 2-No/Unknown. I Kaplan-Meier curves for cancer-specific survival according to regional lymph nodes positive (RNP) groups: 1-No, 2-Yes, 3-Unknown. J Kaplan-Meier curves for cancer-specific survival according to median household income groups: 1-Low, 2-Middle, 3-High
3.3 Multivariate analysis
Variables that exhibited statistical significance (p < 0.05) in the univariate analysis were selected for inclusion in the multivariate analysis. The multivariate analysis involved the application of both the Cox proportional-hazards regression model and the Fine-Gray model.
The Cox model identified several risk factors that independently influenced the prognosis of patients with angiosarcoma. These factors included SEER stage, gender, surgery status, chemotherapy status, radiotherapy status, and marital status. Notably, the hazard of a poor prognosis was found to be positively associated with SEER stage. Patients with distant metastasis had the worst prognosis compared to those with localized metastasis (hazard ratio [HR] = 3.283, 95% confidence interval [CI] = 2.957–3.644, p < 0.0001), followed by patients with regional metastasis (HR = 1.709, 95% CI = 1.554–1.881, p < 0.0001). Female patients exhibited a better prognosis compared to male patients (HR = 0.714, 95% CI = 0.659–0.773, p < 0.0001). The three common tumor treatments—surgery, chemotherapy, and radiotherapy—were associated with significant findings. Patients who did not undergo surgery or had unknown surgical status had a higher hazard compared to those who underwent surgery (HR = 2.333, 95% CI = 2.128–2.558, p < 0.0001). Similarly, patients who did not receive chemotherapy or had unknown chemotherapy status had a higher hazard compared to those who received chemotherapy (HR = 1.744, 95% CI = 1.597–1.904, p < 0.0001). Additionally, patients who did not undergo radiotherapy or had unknown radiotherapy status had a higher hazard compared to those who received radiotherapy (HR = 1.264, 95% CI = 1.161–1.377, p < 0.0001). Furthermore, patients who were divorced, separated, or widowed (DSW) exhibited a worse prognosis compared to married patients (HR = 1.331, 95% CI = 1.216–1.456, p < 0.0001).
The Fine-Gray model identified the following risk factors that independently influenced the prognosis of patients with angiosarcoma: SEER stage, gender, race, surgery status, chemotherapy status, radiotherapy status, and marital status. In the competing-risks model, patients with regional and distant metastases continued to exhibit a poorer prognosis. The results are as follows: regional metastasis (vs localized metastasis: HR = 1.795, 95% CI = 1.596–2.020, p < 0.0001), distant metastasis (vs localized metastasis: HR = 3.092, 95% CI = 2.713–3.525, p < 0.0001). Similarly, women showed a more favorable prognosis compared to men (vs men: HR = 0.712, 95% CI = 0.644–0.786, p < 0.0001). Contrasting with the Cox model, patients from other racial backgrounds experienced a less favorable prognosis (vs whites: HR = 1.193, 95% CI = 1.017–1.400, p = 0.0303). However, no statistically significant difference was observed among black patients. The Fine-Gray model also revealed that patients who received certain treatments had a more positive impact on the disease, such as those who did not undergo surgery or had unknown surgical status (vs surgery: HR = 1.937, 95% CI = 1.730–2.169, p < 0.0001), those who did not receive chemotherapy or had unknown chemotherapy status (vs chemotherapy: HR = 1.222, 95% CI = 1.098–1.361, p = 0.0003), and those who did not receive radiotherapy or had unknown radiotherapy status (vs radiotherapy: HR = 1.111, 95% CI = 1.002–1.232, p = 0.0456). Furthermore, being divorced, separated, or widowed (DSW) continued to have adverse effects on the prognosis of the disease (vs married patients: HR = 1.211, 95% CI = 1.082–1.357, p = 0.0009). The data are presented in detail in Table 3.
4 Discussion
Angiosarcoma, a rare malignant tumor originating from blood vessels, represents approximately 1–2% of all soft-tissue sarcomas [16]. The skin serves as the most frequent location for angiosarcoma, with around one-third of cases manifesting in this region [17]. Notably, cutaneous angiosarcoma often affects the head and neck, particularly the scalp [18]. Unfortunately, the prognosis for angiosarcoma is grim, as evidenced by a comprehensive study utilizing the SEER database, which revealed a mere 26.3% 5-year survival rate among patients [19]. Another investigation focusing on primary angiosarcoma of the bone reported a 5-year survival rate of 20%, with a stark 0% 5-year overall survival (OS) for patients who developed metastases [20]. The incidence of angiosarcoma has shown an upward trend over the past three decades, potentially linked to increased utilization of radiation therapy for breast cancer, heightened disease awareness, or enhanced precision in histopathological characterization [18]. Nevertheless, the precise etiology of angiosarcoma remains elusive, and substantial challenges persist in its treatment. Moreover, there is a paucity of studies exploring the prognosis and distinctive characteristics of individuals afflicted with angiosarcoma.
Hence, there exists a necessity to undertake a precise investigation of the prognostic factors associated with angiosarcoma. Traditional survival analyses, such as the Kaplan-Meier method and Cox model, often focus solely on a single endpoint [6]. Moreover, conventional survival analysis approaches can yield flawed estimations of outcomes. For instance, when competing risk events are present, multivariate Cox regression analysis may result in erroneous HR calculations [15].
Our objective was to identify prognostic factors relevant to specific death for angiosarcoma by employing a competing-risks model and analyzing data obtained from the SEER database. This study represents the first instance of establishing a competing-risks model for angiosarcoma and comparing it with traditional survival analysis methods. The choice of the competing-risks model stems from its consideration of not only deaths resulting from angiosarcoma but also those from other diseases or causes [6]. Through the analysis of the SD model, also known as the Fine-Gray model, we have obtained significant insights. The SD model accounts for competing endpoint events in the calculation of the event of interest, thereby yielding more realistic results [6]. Furthermore, the SD model is well-suited for constructing a clinical predictive model [14]. Consequently, the utilization of the competing-risks model enables us to better discern the independent factors that impact the prognosis of angiosarcoma [6].
We also aimed to evaluate the influence of different treatment strategies on the prognosis of angiosarcoma patients. In comparison to the Fine-Gray model, the Cox regression model overestimated the risks associated with no/unknown surgery, chemotherapy, and radiotherapy. Moreover, both models indicated that surgery reduces the risk of poor prognosis to a greater extent than chemotherapy and radiotherapy. Given the rarity of angiosarcoma, large-sample randomized trials that inform clinical management decisions are still lacking. Additionally, prospective studies investigating treatment regimens are limited in their scope [21]. For non-metastatic angiosarcoma, some experts argue that complete resection with negative margins is crucial whenever feasible [21, 22]. Both a meta-analysis of 11 publications [23] and a comprehensive study based on the SEER database [19] demonstrated a statistically significant detrimental effect on survival outcomes for patients who did not undergo surgery. However, certain studies have shown that even after complete surgical resection, the malignancy still carries a high risk of local recurrence. Factors such as the tumor’s aggressive and multifocal nature or its specific location (as in cardiac angiosarcoma) pose significant challenges in achieving negative margins. Therefore, adjuvant radiation therapy is recommended as it may improve survival rates [18, 22, 24]. It should be noted that previous studies have indicated that negative margins may not confer a survival benefit for patients [25, 26]. The Cox proportional hazards model, as demonstrated by Japanese researchers, indicated that only wide local surgical resection had an adverse impact on the prognosis [27]. Based on this observation, they speculated that the severe tissue injury post-surgery leads to the production of vascular endothelial growth factor (VEGF) by fibroblasts, which in turn promotes angiogenesis during the wound healing process [28,29,30]. Since angiosarcoma originates in the vascular endothelium and has shown responsiveness to anti-VEGF agents [31,32,33], it is plausible to speculate that VEGF in the surgical area may contribute to the progression of residual tumor cells. Furthermore, Kevin C et al., utilizing the SEER database, discovered that surgery did not provide a survival benefit for patients with angiosarcoma of the head and neck [34]. Findings from small-sample and single-center treatment regimens often yield disparate results. In their cohort, Darya et al. observed that patients with localized angiosarcoma experienced improved local control following surgical resection and radiotherapy [21]. Some studies have also demonstrated the protective effect of postoperative radiotherapy [24]. However, other Japanese scholars noted that patients in their series did not achieve prolonged survival regardless of the combined adjuvant therapy with surgery [27, 35]. Additionally, it has been observed by scholars that for patients with large tumors (> 5 cm in diameter) and persistently positive margins, the outcomes of surgery combined with radiotherapy are not optimistic, with a high rate of recurrence and limited survival time [24]. Furthermore, several previous studies have indicated that surgery alone improves the survival prognosis of patients with angiosarcoma, while chemotherapy and radiotherapy do not yield positive effects on patient survival outcomes [23, 36, 37]. Nevertheless, some scholars maintain that radiotherapy holds significance in preventing the progression of primary tumors. They recommend complete surgical resection for small enough primary or metastatic lesions [35]. Additionally, certain investigators have incorporated postoperative adjuvant radiotherapy into their current practices [36]. However, in the case of angiosarcomas resulting from therapeutic radiotherapy, further radiation therapy should be avoided [18]. Nonetheless, a review by A.L. Depla et al. suggests that combining irradiation with surgical treatment for radiation-associated angiosarcoma may enhance local control and prove beneficial [38]. Robin J et al. have stated that current evidence does not support the use of adjuvant chemotherapy for nonmetastatic angiosarcoma. However, for metastatic angiosarcoma, chemotherapy remains the primary treatment option, despite the limited evidence available [18]. When surgical resection is contraindicated or deemed inoperable, chemotherapy is typically administered with the aim of palliation or enabling resection [21]. A previous investigation revealed a trend towards an OS benefit with chemotherapy in angiosarcoma patients with metastasis within their cohort [21]. Furthermore, a comprehensive retrospective European study uncovered that (neo)adjuvant chemotherapy can significantly benefit patients with larger tumors or a higher risk of death [39]. In recent years, the use of taxanes in angiosarcoma treatment has gained considerable attention, owing to their remarkable anti-angiogenic properties [18]. Scholars have noted that taxane-based chemotherapy offers advantages in terms of local control and inhibition of distant tumor metastasis, thereby extending survival [27]. Some have even suggested that continuous use of taxanes in chemotherapy yields positive prognostic effects for patients [35]. Ren and colleagues have strongly recommended chemotherapy as the preferred treatment approach for patients with metastatic angiosarcoma [40]. However, it should be acknowledged that the majority of patients are elderly, with compromised overall health, multiple comorbidities, and difficulties in tolerating chemotherapy-related toxicities. These factors pose limitations on the use of chemotherapy [18]. A study by Rosalynn et al. highlighted the absence of an optimal treatment strategy for cutaneous angiosarcoma [41]. Given the wide variation in research findings regarding treatment options for angiosarcoma, most scholars emphasize the need for rigorous confirmation through larger sample sizes and multicenter studies. The management of angiosarcoma necessitates a multidisciplinary approach. In our case series, both models indicated that surgery, radiotherapy, and chemotherapy independently predicted prognosis in angiosarcoma. Considering the findings from previous related studies, we contend that the competing-risks model yields more accurate results.
Numerous scholars have encountered patients who developed metastases regardless of their prior treatment regimen, and once metastasis occurs, it carries a fatal prognosis. Previous articles have reported a median OS of less than 1 year for patients with concurrent metastases from angiosarcoma [21, 35, 42, 43]. Therese et al. observed that mortality rates were significantly lower among patients without initial metastases [44]. Ren et al. concluded that the presence of tumor metastasis is a major contributor to patient mortality, irrespective of the primary tumor site or the number of metastatic sites [40]. Both of our models demonstrated a statistically significant higher risk of death for patients with regional or distant metastases compared to those with localized disease. These findings align with the results of the aforementioned studies and a related investigation involving 4537 patients in the large SEER database [19]. However, when compared to the competing-risks model, the Cox proportional hazards model exhibited either overestimation or underestimation of the HR. Although the discrepancies were only in the point estimates, they still highlight the fact that the competing-risks model, considering the impact of competing events, yields more realistic results. Additionally, utilizing the SEER database, Lee et al. [34] discovered that angiosarcoma patients with distant metastases faced a significantly higher risk of overall and disease-specific mortality compared to those without metastases, which is consistent with our study’s findings. However, their Cox model revealed that regional metastasis was associated with a statistically significant lower risk of death compared to the absence of metastasis. They attributed this phenomenon to angiosarcoma being a multifocal disease, suggesting that patients initially classified as having no metastasis may have developed regional or distant metastasis that went undetected. Conversely, patients diagnosed with regional metastasis at the outset can benefit from early and appropriate treatment and management. We did not observe similar findings in our study, which may be attributed to factors such as sample size, analyzed subgroups, and the utilization of the competing-risks model. The reason we included the SEER stage as one of the variables in our paper is that the AJCC staging system, widely used to predict the behavior and prognosis of most soft-tissue sarcomas, is not applicable to angiosarcoma due to its differentiation level having no demonstrated correlation with clinical outcomes and prognosis [44].
Neither of our two models indicated that positive regional lymph nodes is an independent prognostic factor for angiosarcoma. This finding can be elucidated by previous literature. Greek scholars have highlighted that angiosarcoma tumor cells typically do not invade regional lymph nodes, rendering axillary lymph node dissection unnecessary for most patients [45]. Angiosarcoma primarily spreads through the bloodstream, with the lungs being the most frequent site of metastasis. Although angiosarcomas can potentially spread through the lymphatic system, the significance of sentinel lymph node biopsy remains uncertain [18].
Our model identified gender as an independent prognostic factor for angiosarcoma patients. Additionally, there was a disparity in point estimation between the two models. This aligns with the findings of two previous studies, one of which observed a significant association between gender and prognosis in non-metastatic angiosarcoma patients, while the other study reported that male sex might predict a poorer prognosis in post-operative angiosarcoma [46]. However, other studies have concluded that gender does not significantly correlate with OS [40, 47]. In a prior study, it was noted that the majority of female angiosarcoma patients in their case series had received radiation therapy for breast cancer, resulting in the tumor being predominantly located in the trunk region [41]. Jorge et al., utilizing the SEER database, found that patients with angiosarcoma in the trunk region exhibited better prognosis; nevertheless, the exact reason for the higher survival rate in trunk angiosarcoma patients remains unclear [48]. Primary angiosarcoma situated in the head and neck region has been associated with a worse survival prognosis [47], and literature suggests that the head and scalp are more common sites for male angiosarcoma patients [17, 24, 44].
The majority of our study cohort consisted of Caucasian individuals, which aligns with previous literature reports [18, 47]. It is worth noting that in our Fine-Gray model, individuals from other ethnic groups were found to be an independent risk factor when compared to the white race, whereas the Cox proportional hazards model did not yield these results. Hence, it becomes apparent that the Cox model, apart from differences in point estimation, may also lead to incorrect estimation of the direction of independent risk factors and associations with outcomes. Furthermore, neither of our two models identified black race as an independent risk factor, which is similar to the findings of Rosalynn et al. [41]. However, studies conducted by other scholars have indicated that black race is an independent risk factor for worse OS. The reasons behind why non-white races may experience poorer survival outcomes are not well understood, as race as a prognostic factor has been rarely studied. Whether this disparity is related to differences in socioeconomic status, insurance coverage, or even tumor biology remains a question that warrants further investigation in the future [47].
In a comprehensive SEER data study conducted by Zhang et al., it was discovered that the unmarried status exhibited a negative correlation with survival outcomes [19]. Both of the current models indicate that being unmarried may increase the risk of death in comparison to being married, but the results are not statistically significant. Among researchers, there is a general consensus that a stable partnership can contribute to the improved chances of survival for cancer patients [49, 50]. It is worth noting, however, that according to official U.S. data from 2010, approximately two-thirds of unmarried Americans live with their partners, even though their marital status is classified as single [49]. Those who cohabit with their partners may still receive emotional and, in some cases, financial support similar to that of married individuals. This support serves to enhance the patient’s adherence to management and treatment. Furthermore, for cancer patients who have never been married, they have yet to experience major life-altering events such as divorce, separation, or widowhood [50]. It is also plausible that unmarried patients tend to be younger and can receive support from parents, relatives, coworkers, or friends [51]. A previous study concluded that having the love and care of a partner stimulates the release of oxytocin, which inhibits the growth of cancer cells through various mechanisms [52]. Both models demonstrate that the marital status of divorced, separated, or widowed (DSW) individuals is an independent risk factor; however, there are differences in the estimation of the HR. It has been observed that cancer patients who are divorced or separated face a significantly higher risk of death compared to those who are married. Particularly for those who are widowed, the grief associated with the loss of a significant partner can have a detrimental impact on their health, resulting in reduced social interactions and a lack of emotional and financial support, ultimately affecting their treatment negatively. Additionally, widowed patients often exhibit a compromised immune response [50].
Studies investigating whether age is an independent prognostic factor for angiosarcoma patients exhibit variations among scholars. Yara et al., in their single-center study, did not discover any association between age and worse outcomes in patients with secondary breast angiosarcoma [53]. Similarly, a study on cutaneous angiosarcoma did not demonstrate statistically significant clinical improvements in patients under the age of 70 [22]. Furthermore, several other studies failed to identify a link between advanced age and poorer OS [25, 26, 54]. Conversely, Jorge et al., utilizing the SEER database, found that patients under the age of 50 had a more favorable prognosis [48]. Moreover, Therese et al. and Kevin et al. also observed that older patients (above the age of 70) exhibited worse survival outcomes [34, 44]. Sinnamon et al. emphasized the use of age as a prognostic factor since angiosarcoma commonly occurs in older individuals; however, this pertains specifically to cutaneous angiosarcoma [47]. In the case of primary angiosarcoma of the breast, it is frequently diagnosed in younger women, typically between the ages of 30 and 40 [55]. In our study, we did not find age to be an independent prognostic factor for angiosarcoma patients. Based on the aforementioned article, we speculate that this discrepancy may be attributed to the specific tumor subgroup and the size of the samples studied.
Undoubtedly, our study is not without limitations. Primarily, its retrospective design renders it susceptible to potential selection bias. Furthermore, we omitted variables that would have led to a significantly reduced sample size due to missing data, such as tumor size. Besides, our study did not include the variable of primary site. This decision was made because the primary site in our cases encompassed 48 subcategories, including breast, bones and joints, skin, soft tissue including heart, liver, intestines, kidney, esophagus, gum, larynx, hypopharynx, peritoneum, endocrine gland, urinary bladder, genital organs and so on. We considered that categorizing this variable into either too few or too many groups might lead to an inability to accurately determine the impact of primary site on patient prognosis. Therefore, we did not include this variable. However, the impact of tumor size and primary site on the prognosis of angiosarcoma deserves further exploration in our future research.
By utilizing pertinent data encompassing demographics, therapeutic options, and clinicopathological characteristics sourced from the SEER database, we successfully constructed a competing-risks model for angiosarcoma. In comparison to the conventional Cox regression model, the competing-risks model offers enhanced accuracy in estimating the effects by considering the influence of multiple endpoints on the outcome event. We found that relying solely on the Cox proportional-hazards model, which focuses on a single endpoint, was insufficient for accurately estimating effect values. This could potentially result in either overestimation or underestimation of the impacts of independent prognostic factors. However, it is important to note that sometimes these differences are only seen in point estimation. In this study, the Cox proportional-hazards model proved inadequate in accurately estimating effects and determining the direction of the association between risk factors and outcomes. This issue could effectively be addressed by utilizing the competing-risks model, which demonstrated superior performance in multivariate analysis, especially when dealing with multiple outcome endpoints. The findings of our study can furnish clinicians with a deeper comprehension of angiosarcoma and serve as a reference for clinical decision-making.
Availability of data and materials
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Surveillance, Epidemiology, and End Results (SEER) database. https://seer.cancer.gov/.
References
Shustef E, Kazlouskaya V, Prieto VG, Ivan D, Aung PP. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70(11):917–25.
Chan JY, Lim JQ, Yeong J, Ravi V, Guan P, Boot A, et al. Multiomic analysis and immunoprofiling reveal distinct subtypes of human angiosarcoma. J Clin Invest. 2020;130(11):5833–46.
Florou V, Wilky BA. Current and future directions for angiosarcoma therapy. Curr Treat Options Oncol. 2018;19(3):14.
Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann SM, Park HS, Chinchilli VM. Suicide among cancer patients. Nat Commun. 2019;10(1):207.
Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. 2017;28(2):400–7.
Wu W, Yang J, Li D, Huang Q, Zhao F, Feng X, et al. Competitive risk analysis of prognosis in patients with cecum cancer: a population-based study. Cancer Control. 2021;28:1073274821989316.
Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56.
Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48(6 Suppl):S96-105.
Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: a population-based analysis. Cancer Med. 2020;9(1):361–73.
Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13(1):57–69.
Su H, Xue X, Wang Y, Lu Y, Ma C, Ji Z, et al. Competitive risk model for specific mortality prediction in patients with bladder cancer: a population-based cohort study with machine learning. J Oncol. 2022;2022:9577904.
Wu WT, Li YJ, Feng AZ, Li L, Huang T, Xu AD, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. 2021;8(1):44.
Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–400.
Bian F, Li C, Han D, Xu F, Lyu J. Competing-risks model for predicting the postoperative prognosis of patients with papillary thyroid adenocarcinoma based on the Surveillance, Epidemiology, and End Results (SEER) database. Med Sci Monit. 2020;26:e924045.
Yu Z, Yang J, Gao L, Huang Q, Zi H, Li X. A competing risk analysis study of prognosis in patients with esophageal carcinoma 2006–2015 using data from the Surveillance, Epidemiology, and End Results (SEER) database. Med Sci Monit. 2020;26:e918686.
Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: a challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120–31.
Guadagnolo BA, Zagars GK, Araujo D, Ravi V, Shellenberger TD, Sturgis EM. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33(5):661–7.
Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11(10):983–91.
Zhang C, Xu G, Liu Z, Xu Y, Lin F, Baklaushev VP, et al. Epidemiology, tumor characteristics and survival in patients with angiosarcoma in the United States: a population-based study of 4537 cases. Jpn J Clin Oncol. 2019;49(12):1092–9.
Palmerini E, Maki RG, Staals EL, Alberghini M, Antonescu CR, Ferrari C, et al. Primary angiosarcoma of bone: a retrospective analysis of 60 patients from 2 institutions. Am J Clin Oncol. 2014;37(6):528–34.
Buehler D, Rice SR, Moody JS, Rush P, Hafez GR, Attia S, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37(5):473–9.
Perez MC, Padhya TA, Messina JL, Jackson RS, Gonzalez RJ, Bui MM, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20(11):3391–7.
Shin JY, Roh SG, Lee NH, Yang KM. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39(2):380–6.
Pawlik TM, Paulino AF, McGinn CJ, Baker LH, Cohen DS, Morris JS, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98(8):1716–26.
Bernstein JM, Irish JC, Brown DH, Goldstein D, Chung P, Razak ARA, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39(6):1205–11.
Patel SH, Hayden RE, Hinni ML, Wong WW, Foote RL, Milani S, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141(4):335–40.
Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25(5):419–23.
Ankoma-Sey V, Matli M, Chang KB, Lalazar A, Donner DB, Wong L, et al. Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene. 1998;17(1):115–21.
Arbiser JL, Larsson H, Claesson-Welsh L, Bai X, LaMontagne K, Weiss SW, Soker S, et al. Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR-1 and VEGFR-2 in vivo. Am J Pathol. 2000;156(4):1469–76.
Ozawa K, Kondo T, Hori O, Kitao Y, Stern DM, Eisenmenger W, et al. Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport. J Clin Invest. 2001;108(1):41–50.
Park MS, Ravi V, Araujo DM. Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor. Curr Opin Oncol. 2010;22(4):351–5.
Fuller CK, Charlson JA, Dankle SK, Russell TJ. Dramatic improvement of inoperable angiosarcoma with combination paclitaxel and bevacizumab chemotherapy. J Am Acad Dermatol. 2010;63(4):e83–4.
De Yao JT, Sun D, Powell AT, Rehmus EH. Scalp angiosarcoma remission with bevacizumab and radiotherapy without surgery: a case report and review of the literature. Sarcoma. 2011;2011:160369.
Lee KC, Chuang SK, Philipone EM, Peters SM. Characteristics and prognosis of primary head and neck angiosarcomas: a Surveillance, Epidemiology, and End Results program (SEER) analysis of 1250 cases. Head Neck Pathol. 2019;13(3):378–85.
Ito T, Uchi H, Nakahara T, Tsuji G, Oda Y, Hagihara A, Furue M. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142(6):1387–94.
Oashi K, Namikawa K, Tsutsumida A, Takahashi A, Itami J, Igaki H, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face -a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44(6):823–9.
Trofymenko O, Curiel-Lewandrowski C. Surgical treatment associated with improved survival in patients with cutaneous angiosarcoma. J Eur Acad Dermatol Venereol. 2018;32(1):e29–31.
Depla AL, Scharloo-Karels CH, de Jong MAA, Oldenborg S, Kolff MW, Oei SB, et al. Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: a systematic review. Eur J Cancer (Oxford, England: 1990). 2014;50(10):1779–88.
Conforti F, Gronchi A, Penel N, Jones RL, Broto JM, Sala I, et al. Chemotherapy in patients with localized angiosarcoma of any site: a retrospective European study. Eur J Cancer (Oxford, England: 1990). 2022;171:183–92.
Ren S, Wang Y, Wang Z, Shao J, Ye Z. Survival predictors of metastatic angiosarcomas: a surveillance, epidemiology, and end results program population-based retrospective study. BMC Cancer. 2020;20(1):778.
Conic RRZ, Damiani G, Frigerio A, Tsai S, Bragazzi NL, Chu TW, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. 2020;83(3):809–16.
Lahat G, Dhuka AR, Lahat S, Smith KD, Pollock RE, Hunt KK, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16(9):2502–9.
Italiano A, Cioffi A, Penel N, Levra MG, Delcambre C, Kalbacher E, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012;118(13):3330–6.
Dettenborn T, Wermker K, Schulze HJ, Klein M, Schwipper V, Hallermann C. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg. 2014;42(8):1623–8.
Kaklamanos IG, Birbas K, Syrigos KN, Vlachodimitropoulos D, Goutas N, Bonatsos G. Breast angiosarcoma that is not related to radiation exposure: a comprehensive review of the literature. Surg Today. 2011;41(2):163–8.
Jiang T, Ye Z, Shao T, Luo Y, Wang B. Prognostic nomograms for predicting overall survival and cancer-specific survival in patients with angiosarcoma, a SEER population-based study. Sci Rep. 2022;12(1):3479.
Sinnamon AJ, Neuwirth MG, McMillan MT, Ecker BL, Bartlett EK, Zhang PJ, et al. A prognostic model for resectable soft tissue and cutaneous angiosarcoma. J Surg Oncol. 2016;114(5):557–63.
Albores-Saavedra J, Schwartz AM, Henson DE, Kostun L, Hart A, Angeles-Albores D, et al. Cutaneous angiosarcoma. Analysis of 434 cases from the Surveillance, Epidemiology, and End Results Program, 1973–2007. Ann Diagn Pathol. 2011;15(2):93–7.
Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–76.
Krajc K, Miroševič Š, Sajovic J, KlemencKetiš Z, Spiegel D, Drevenšek G, et al. Marital status and survival in cancer patients: a systematic review and meta-analysis. Cancer Med. 2023;12(2):1685–708.
Cai W, Fan J, Shen T, Yu J. The influence of marital status on the survival of patients with uveal melanoma. J Ophthalmol. 2020;2020:7012940.
Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29(4):377–87.
Abdou Y, Elkhanany A, Attwood K, Ji W, Takabe K, Opyrchal M. Primary and secondary breast angiosarcoma: single center report and a meta-analysis. Breast Cancer Res Treat. 2019;178(3):523–33.
Ogawa K, Takahashi K, Asato Y, Yamamoto Y, Taira K, Matori S, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85(1019):e1127–33.
Torres KE, Ravi V, Kin K, Yi M, Guadagnolo BA, May CD, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267–74.
Acknowledgements
We thank Surveillance, Epidemiology, and End Results(SEER) database for providing us with easily accessible free data. Funding: this work was supported by Key Scientific Problems and Medical Technical Problems Research Project of China Medical Education Association [2022KTZ009]; and Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization [2021B1212040007].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Chaodi Huang, Jianguo Huang, Jun Lyu and Liehua Deng. The first draft of the manuscript was written by Chaodi Huang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, C., Huang, J., He, Y. et al. Competing-risks model for predicting the prognosis of patients with angiosarcoma based on the SEER database of 3905 cases. Holist Integ Oncol 3, 13 (2024). https://doi.org/10.1007/s44178-024-00080-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00080-1