Abstract
Introduction
Locally advanced thyroid cancer refers to thyroid cancer that invades important structures of the neck, with poor prognosis. Neoadjuvant targeted therapy has the potential to increase the R0/1 resection rate in locally advanced thyroid cancer and improve the outcome in these patients.
Methods
We conducted a systematic review of studies that reported neoadjuvant targeted therapy in locally advanced thyroid cancer. Individual patient data was extracted from eligible studies. Objective response rate (ORR) and R0/1 resection rate were calculated.
Results
Sixteen studies and 32 patients were included into analysis, including 18 differentiated thyroid cancer (DTC), 3 medullary thyroid cancer (MTC), 8 anaplastic thyroid cancer (ATC) and 3 poor-differentiated thyroid cancer (PDTC). Most patients were stage T4a (53.1%) and T4b (28.1%). 81.3% patients had regional lymph node metastasis and 37.5% had distant metastasis. RET mutated MTC and BRAF mutated ATC were treated with selective RET inhibitor and selective BRAF/MEK inhibitors. Other treatment regimens were multitarget tyrosine kinase inhibitors (mTKIs). The average duration of treatment was 4.3 months (SD = 4.1). The overall ORR was 78.1% (95%CI: 60.0%–90.7%), and the R0/1 resection rate for the intention to treat population was 78.1% (95%CI: 60.0%–90.7%). With a median follow-up time of 12.1 months, 1 DTC patient and 3 ATC patients died of the disease.
Conclusions
Neoadjuvant targeted therapy was a new treatment option for locally advanced thyroid cancer and might improve the R0/1 resection rate in selective cases. However, more clinical trials with longer follow-up time are awaited to confirm the clinical benefit of neoadjuvant targeted treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thyroid cancer is the most common endocrine malignancy. Most early-stage thyroid cancers have good prognosis and long-term survival, while 5–10% are locally advanced cases [1, 2]. Locally advanced thyroid cancer refers to thyroid cancer that invades important structures of the neck, including the recurrent laryngeal nerve, trachea, esophagus, larynx, extensive skin and muscle invasion, and encasement of great vessels of the neck or superior mediastinum. Locally advanced thyroid cancer patients have poor prognosis, and the estimated 10-year disease specific mortality is as high as 41–42% [3].
The treatment strategy for locally advanced thyroid cancer is surgery-based comprehensive treatment. Whether R0/1 resection can be achieved during surgery (no tumor residue or only microscopic residue) is essential to prognosis. Compared with R2 resection (gross residual tumor), R0/1 resection can significantly prolong the survival of patients and reduce the local recurrence rate [4]. Nevertheless, locally advanced thyroid cancer usually requires extensive surgery, involving the resection and reconstruction of vital organs. Tumor residual and recurrence are the main causes of death in these patients [5]. Therefore, increasing the R0/1 resection rate of locally advanced thyroid cancer is the key to improving the efficacy of treatment.
Due to the insensitivity of thyroid cancer to chemoradiotherapy, the application of neoadjuvant therapy in locally advanced thyroid cancer is also limited. Up to today, multiple phase III clinical studies have laid the foundation for targeted therapy in the treatment of advanced thyroid cancer [6,7,8,9]. In 2010, Cleary et al. first reported a case of neoadjuvant treatment of medullary thyroid cancer (MTC) with sunitinib [10], which opened the prelude to the neoadjuvant targeted therapy in locally advanced thyroid cancer. However, despite one phase II clinical trial [11], the neoadjuvant targeted therapy of locally advanced thyroid cancer is refined to case reports and case series.
In this review, we aimed to include studies that reported neoadjuvant targeted therapy in locally advanced thyroid cancer, and to analyze the efficacy of the treatment.
2 Material and methods
2.1 Literature search and study selection
We used the search term “thyroid cancer” in combination with “thyroid carcinoma” and “neoadjuvant” on Pubmed (up to June 19, 2022). The inclusion criteria were: (1) original reports, including case reports, case series, cohort studies and clinical trials; (2) locally advanced thyroid cancer with or without distant metastasis; (3) patients who received neoadjuvant targeted therapy; (4) locally advanced thyroid cancer, including differentiated, medullary, poor-differentiated, and anaplastic thyroid cancer confirmed by pathology. Locally advanced thyroid cancer was defined as (1) Unresectable thyroid cancer with extensive disease and multiple-organ invasion; or (2) thyroid cancer and/or local-regional metastatic lymph node invaded at least one of the structures (trachea, esophagus, larynx, anterior vertebral fascia, brachial plexus) or encased at least one of the vessels (common carotid artery, mediastinal vessels), and complete resection might not be achieved based on pre-operative assessment.
The authors screened the titles and abstracts of all articles and identified potentially eligible studies. Then the full texts of the articles were acquired and evaluated. Studies which did not provide individual patient data were excluded.
2.2 Data extraction
The authors extracted data from each manuscript. The data included: (1) patient baseline features: age of diagnosis, sex; (2) disease information: pathology, TNM stage; (3) neoadjuvant treatment: drug, duration, and response of treatment; (4) surgery performed and the status of resection (R0/R1/R2 resection); (5) treatment after surgery and follow-up information. If TNM stage was not available in the original text, the authors re-staged the patients according to the text description of the disease, imaging and pathology.
Response of neoadjuvant treatment was evaluated according to RECIST v1.1 criteria. In case series that reported cases with targeted therapies, only cases that treated with neoadjuvant targeted regimens were included. The information from the phase II clinical trial was directly extracted from Fudan University Shanghai Cancer Center [11].
2.3 Statistics
Objective response rate (ORR) was defined as proportion of patients who had a complete remission (CR) or partial remission (PR). R0/1 resection rate was defined as the proportion of patients who had complete resection of all tumor cells or only microscopic residue was present. ORR, and R0/1 resection rate were reported with 95% confident intervals (CIs). Summary statistics were provided for baseline features.
3 Results
3.1 Characteristics of included studies
A total of 65 published studies were identified by initial pubmed search, and finally 16 studies met the inclusion criteria were included into analysis. Among these 16 studies, 15 were case studies/ series, 1 was a phase II clinical trial. The clinical data of 32 patients were extracted, among whom 18 were differentiated thyroid cancer (DTC), 3 were MTC, 3 were poor-differentiated thyroid cancer (PDTC), and 8 were anaplastic thyroid cancer (ATC) (Table 1). The study selection flow chart was demonstrated in Fig. 1.
3.2 Baseline features of patients
The mean age of included patients was 59.1 ± 14.8 years old, with 22 (68.8%) female patients. In 18 DTC patients, 17 were papillary thyroid cancer (PTC), 1 was follicular thyroid cancer (FTC). In 3 PDTC patients, 2 were the combination of PDTC and PTC. In 8 ATC patients, 5 were the combination of ATC and PTC, and 1 was the combination of ATC, PDTC and PTC. The majority of the patients were stage T4a (53.1%) and T4b (28.1%). 81.3% patients had regional lymph node metastasis and 37.5% had distant metastasis. The most common distant metastasis sites were lung (61.5%), distant lymph nodes (30.8%) and liver (23.1%).
Of all patients, 18 (56.3%) were treatment-naive, 10 (31.3%) had at least one previous surgery; 3 (9.4%) had previous radioactive iodine (RAI) treatment; 3 (9.4%) had previous chemotherapy, including cisplatin, doxorubicin, carboplatin, paclitaxel for MTC, paclitaxel and carboplatin for ATC, paclitaxel, carboplatin, and doxorubicin for PDTC. Radiotherapy was delivered in 2 PDTC and 1 MTC cases. Additionally, 1 PDTC case had sorafenib 400 mg for 1 week after chemotherapy and radiotherapy; 1 PTC case who developed liver metastasis had yttrium-90 microsphere radioembolization (Table 2).
3.3 Gene mutations and Neoadjuvant regimens
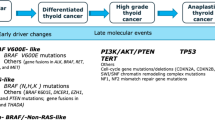
Twenty-four out of 32 patients had gene test results according to institutional protocols. All MTC cases reported RET gene mutation status, and only one patient had RET mutation. 12 DTC (66.7%) cases had gene test, and the most common mutation site was BRAF V600E, which was identified in 8 cases. BRAF mutation was accompanied by TERT promoter mutation in 4 cases, accompanied by TERT promoter mutation and HRAS mutation in 1 case. In 4 cases without BRAF mutation, 1 patient had NRAS mutation and 1 had TERT promoter mutation. All ATC patients had gene test and 7 out of 8 cases had BRAF V600E mutations. Other identified mutations included TP53 (50.0%), EGFR (12.5%), CDKN2A (12.5%), ATM (12.5%), and MCL1 (12.5%). Only 1 PDTC case had gene test and BRAF V600E, TERT promoter and PIK3CA mutations were present (Fig. 2).
Since RET mutated MTC and BRAF mutated ATC had well-established treatment regimens in advanced thyroid cancer, they were treated with selective RET inhibitor and selective BRAF/MEK inhibitors. Other treatment regimens were multitarget tyrosine kinase inhibitors (mTKIs), including sunitinib, lenvatinib, sorafenib, apatinib and anlotinib. In 2 BRAF mutated ATC cases, patients also received pembrolizumab despite dabrafenib and trametinib [22] (Fig. 3).
The duration of neoadjuvant treatment was reported in 29 patients, and the average duration of treatment was 4.3 months (SD = 4.1, range: 0.8–19.0). However, the duration of treatment varied among different pathology types. The average duration times of treatment in DTC, MTC, ATC and PDTC were 3.8 months (SD = 3.0, range: 1.5–14.0), 9.0 months (SD = 8.7, range: 4.0–19.0), 4.5 months (SD = 4.3, range: 0.8–12.0), and 1.8 months (SD = 0.3, range: 1.5–2.0).
3.4 Efficacy of neoadjuvant targeted therapy
In 32 patients, 25 had PR, 5 had stable disease (SD), and 2 had progressive disease (PD). The overall ORR was 78.1% (95%CI: 60.0%–90.7%). For 18 DTC cases, 15 had PR, 3 had SD, and the ORR for DTC was 83.3% (95%CI: 58.6%–96.4%). For 3 MTC cases, all patients had PR, and the ORR for MTC was 100.0%. For 8 ATC cases, 7 had PR, 1 had PD due to occipital metastasis, and the ORR for ATC was 87.5% (95% CI: 47.3%–99.7%). For 3 PDTC cases, 2 had SD, 1 had PD, and the ORR for PDTC was 0%.
In 23 out of 32 cases, the best change in sum of lesion diameters could be evaluated, including17 DTC cases, 3 MTC cases and 3 PDTC cases (Fig. 4). The average change of lesion diameters was − 36.5% (SD = 22.6%, range: − 84.0%- 22.5%). In 17 PR cases, the average reduction of lesion diameters was 45.5% (SD = 16.0%, range: 30.9%–84.0%).
The best change in sum of lesion diameters in 23 patients. This figure showed a theoretical optimistic response of neoadjuvant targeted therapy in patients with locally advanced thyroid cancer. DTC, differentiated thyroid cancer; MTC, medullary thyroid cancer; PDTC, poor-differentiated thyroid cancer
Nineteen cases reported the time from drug discontinuation to surgery. The average time interval was 16.7 days (SD = 10.8, range: 1–42). Adverse events (AEs) of neoadjuvant therapies could not be evaluated because case reports reported AEs with inconsistent standards. Only Huang et al. demonstrated the AEs of neoadjuvant anlotnib according to CTC AE criteria [11]. The most common AEs were hypertension, fatigue, hand-foot syndrome, and proteinuria (supplementary Table 1).
3.5 Surgeries
In 32 patients, 4 did not receive surgery after neoadjuvant treatment, all of whom were from the phase II clinical trial (2 cases were not resectable, 2 cases refused to surgery). 1 patient received surgery however the status of the margins was not provided. In 27 patients, the proportions or R0, R1, and R2 resection were 59.3%, 33.3% and 7.4%, respectively. The R0/1 resection rate for the intention to treat population was 78.1% (95%CI: 60.0%–90.7%), and for the per protocol population was 92.6% (95%CI: 75.7%–99.1%).
In 6 ATC patients, although the size of tumor reduction was not reported, Wang et al. [22] provided the pathological findings after tumor excision. All cases demonstrated complete resection of the ATC component, and up to 50–100% ATC cells lost viability in the specimens. Although 2 cases were R1 resections, the unresected component of the tumor was PTC.
3.6 Postoperative treatment and follow-up
Twenty-seven (84.4%) cases reported postoperative treatment. 14 DTC patients all received RAI treatment. In 3 MTC patients, only 1 continued selpercatinib treatment since the patient had metastatic disease involving lungs, liver, and skeleton [16]. The other 2 MTC patients did not receive postoperative treatment. In 8 ATC patients, 6 received chemoradiation, 7 continued targeted therapy (1 case with lenvatinib, 6 cases with dabrafenib and trametinib), 4 cases also resumed pembrolizumab after surgery.
Twenty-six cases reported the follow-up data after neoadjuvant targeted therapy and surgery with a median follow-up time of 12.1 months (range: 1.4–33 months). In 14 DTC cases, only Riker AI et al. reported a PTC patient who developed soft tissue and bone metastasis at 19 months after surgery. The patient then developed multiple bone metastasis at 33 months and died. The full clinical course and treatment was approximately 39 months [13]. All 3 MTC patients remained recurrence-free at follow-up. In 2 PDTC patients who received definite surgery, 1 had mild progression at the lung lesions with a stable bone lesion on RAI imaging at 6 month of follow-up and the other 1 was only followed-up for 1.4 months. In 7 ATC patients, 4 had recurrences and 3 patients subsequently died (Table 3).
4 Discussion
By reviewing 16 studies of 32 locally advanced thyroid cancer that received neoadjuvant targeted therapy, we summarized the treatment course and outcome of these patients. The overall ORR of neoadjuvant treatment was 78.1% (95%CI: 60.0%–90.7%) and the R0/1 resection rate for the intention to treat population was 78.1% (95%CI: 60.0%–90.7%). Up to our knowledge, this was the first systematic review to discuss the efficacy of neoadjuvant targeted therapy in locally advanced thyroid cancer.
The concept of neoadjuvant therapy was proposed by Haagensen and Stout in 1970 to shrink tumors through systemic chemotherapy, allowing patients with locally advanced, inoperable breast cancer to receive curative surgery [26]. Similarly, based on the current review and institutional experience, we proposed that neoadjuvant targeted therapy could be applied in the following scenarios: (1) to downstage the tumor, making inoperable cases operable; (2) to increase the R0/1 resection rate in cases with potential R2 resection; (3) to preserve the function of vital organs and improve the quality of life after tumor shrinkage; (4) for DTC with distant metastases, local surgery after neoadjuvant therapy can provide patients with the opportunity to receive RAI treatment; (5) to observe the drug sensitivity and guide the systemic treatment plan after surgery.
Nevertheless, neoadjuvant targeted therapy is not a standard treatment for locally advanced thyroid cancer so far, and its application is limited to the scope of clinical trials and case reports. Some guidelines recommend targeted therapy in advanced, initially inoperable BRAF-mutant ATC [27], followed by surgical resection if satisfactory tumor regression were achieved. In a recent cohort study of ATC, those who underwent surgery following neoadjuvant BRAF-directed therapy had statistically significant improvement in median OS compared with those who did not (hazard ratio = 0.29, P = 0.02), indicating the clinical benefit of anti-BRAF neoadjuvant treatment in combination with surgery [28]. Moreover, although the selection of targeted drugs is based on BRAF status in ATC and RET status in MTC, there is still a lack of molecular markers to predict the efficacy of neoadjuvant treatment, especially for cases treated with TKIs. Kim et al. reported a cohort of 8 advanced ATC patients treated with neoadjuvant lenvatinib (regardless of BRAF status) and concluded that lenvatinib could effectively reduce the tumor burden but showed doubtful survival benefit [29].
As discussed in this review, the efficacy of neoadjuvant targeted therapy was dependent of pathology and molecular features. PDTC showed unfavorable response with neoadjuvant TKI treatment, while BRAF mutated ATC and RET mutated MTC demonstrated satisfactory responses. Generally speaking, the efficacy of neoadjuvant treatment might refer to the results of phase III clinical trials in advanced thyroid cancer. The duration of treatment also varied from case to case. In most cases, the duration of treatment was 4 to 6 months. Prolonged neoadjuvant therapy may lead to the accumulation of drug-related AEs while shortened course of treatment may decrease the chance for optimal tumor regression. Therefore, tumor pathology, molecular features and AEs should be taken into consideration when making treatment plans.
There is no consensus on the time to stop targeted drugs before surgery, either. Most authors made the decisions based on drug AEs, drug half-lie, complications of surgery, etc. Interestingly, although most TKIs targeted VEGF/VEGFR pathways, no authors reported increased hemorrhage risk during surgery.
Another key dispute regarding neoadjuvant treatment is the maintenance of systematic therapy. For DTC and MTC patient with complete surgical resection, RAI treatment or regular follow-up should be performed according to guidelines. There is no evidence that cured patients will benefit from targeted drug maintenance therapy. In ATC patients, on the other hand, the maintenance of systematic treatment should be considered despite R0/1 resections. As summarized in Table 3, patients treated with DT + PD1 showed favorable outcome compared with those did not.
There are several limitations of the current study. Firstly, the included studies were mainly case reports and case series, which would over-estimate the efficacy of neoadjuvant treatment due to selection bias and publication bias, and we could only report a theoretical optimistic contribution for neoadjuvant targeted therapy. Secondly, there was no census on the choice of treatment regimens and cycles of treatment. The diversity of treatments made it difficult to evaluate the efficacy of a specific protocol and to compare between different protocols. Lastly but not least, the follow-up time of the reports was insufficient. We were unable to conclude that whether the benefit of neoadjuvant treatment would convert into survival benefits.
5 Conclusion
In this systematic review, we included 32 locally advanced thyroid cancer cases that received neoadjuvant targeted therapy. The choice of treatment regimens was dependent on gene mutations and the duration of treatment was subject to treatment response. Neoadjuvant targeted therapy was a new treatment option for locally advanced thyroid cancer and might improve the R0/1 resection rate in selective cases. However, more clinical trials with longer follow-up time are awaited to confirm the clinical benefit of neoadjuvant targeted treatment.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
References
Wang LY, Nixon IJ, Patel SG, et al. Operative management of locally advanced, differentiated thyroid cancer. Surgery. 2016;160:738–46. https://doi.org/10.1016/j.surg.2016.04.027.
Nixon IJ, Simo R, Newbold K, et al. Management of Invasive Differentiated Thyroid Cancer. Thyroid. 2016;26:1156–66. https://doi.org/10.1089/thy.2016.0064.
Shindo ML, Caruana SM, Kandil E, et al (2014) Management of invasive well-differentiated thyroid cancer: An American head and neck society consensus statement: AHNS consensus statement: AHNS Consensus Statement. Head Neck 36:n/a-n/a. https://doi.org/10.1002/hed.23619.
Hartl DM, Zago S, Leboulleux S, et al. Resection margins and prognosis in locally invasive thyroid cancer. Head Neck. 2014;36:1034–8. https://doi.org/10.1002/hed.23406.
Kitamura Y, Shimizu K, Nagahama M, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metabolism. 1999;84:4043–9. https://doi.org/10.1210/jcem.84.11.6115.
Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol Official J Am Soc Clin Oncol. 2011;30:134–41. https://doi.org/10.1200/jco.2011.35.5040.
Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol Official J Am Soc Clin Oncol. 2013;31:3639–46. https://doi.org/10.1200/jco.2012.48.4659.
Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28. https://doi.org/10.1016/s0140-6736(14)60421-9.
Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. New Engl J Medicine. 2015;372:621–30. https://doi.org/10.1056/nejmoa1406470.
Cleary JM, Sadow PM, Randolph GW, et al. Neoadjuvant treatment of unresectable medullary thyroid cancer with sunitinib. J Clin Oncol. 2010;28:e390–2. https://doi.org/10.1200/jco.2009.27.4225.
Huang N, Wei W, Xiang J, et al. The efficacy and safety of anlotinib in neoadjuvant treatment of locally advanced thyroid cancer: a single-arm phase II clinical trial. Thyroid. 2021;31:1808–13. https://doi.org/10.1089/thy.2021.0307.
McCrary HC, Aoki J, Huang Y, et al. Mutation based approaches to the treatment of anaplastic thyroid cancer. Clin Endocrinol. 2022;96:734–42. https://doi.org/10.1111/cen.14679.
Riker AI, Hodgdon IA, Dewenter TA, et al. Metastatic papillary thyroid cancer to the liver: the central role of a multidisciplinary approach to treatment. Ochsner J. 2021;21:224–9. https://doi.org/10.31486/toj.20.0067.
Alshehri K, Alqurashi Y, Merdad M, et al. Neoadjuvant lenvatinib for inoperable thyroid cancer: A case report and literature review. Cancer Rep. 2021:e1466. https://doi.org/10.1002/cnr2.1466.
Zhang Y, Deng X, Ding Z, et al. Preoperative neoadjuvant targeted therapy with apatinib for inoperable differentiated thyroid cancer: A case report. Medicine. 2021;100:e25191. https://doi.org/10.1097/md.0000000000025191.
Jozaghi Y, Zafereo M, Williams MD, et al. Neoadjuvant selpercatinib for advanced medullary thyroid cancer. Head Neck. 2021;43:E7–E12. https://doi.org/10.1002/hed.26527.
Iwasaki H, Toda S, Ito H, et al. A Case of Unresectable Papillary Thyroid Carcinoma Treated with Lenvatinib as Neoadjuvant Chemotherapy. Case Reports Endocrinol. 2020;2020:6438352. https://doi.org/10.1155/2020/6438352.
Barbaro D, Lapi P, Viacava P, Torregrossa L. Low-intermediate dose of lenvatinib in anaplastic thyroid cancer is highly effective and safe. Bmj Case Reports. 2020;13:e236934. https://doi.org/10.1136/bcr-2020-236934.
Golingan H, Hunis B, Golding AC, et al. Neoadjuvant Lenvatinib in advanced unresectable medullary thyroid carcinoma: a case report. Aace Clin Case Reports. 2020;6:e73–8. https://doi.org/10.4158/accr-2019-0365.
Nava CF, Scheffel RS, Cristo AP, et al. Neoadjuvant Multikinase Inhibitor in Patients With Locally Advanced Unresectable Thyroid Carcinoma. Front Endocrinol. 2019;10:712. https://doi.org/10.3389/fendo.2019.00712.
Gay S, Monti E, Antonelli CT, et al. Case report: lenvatinib in neoadjuvant setting in a patient affected by invasive poorly differentiated thyroid carcinoma. Future Oncol. 2019;15:13–9. https://doi.org/10.2217/fon-2019-0099.
Wang JR, Zafereo ME, Dadu R, et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid. 2019;29:1036–43. https://doi.org/10.1089/thy.2019.0133.
Stewart KE, Strachan MWJ, Srinivasan D, et al. Tyrosine Kinase Inhibitor Therapy in Locally Advanced Differentiated Thyroid Cancer: A Case Report. European Thyroid J. 2019;8:102–7. https://doi.org/10.1159/000494880.
Cabanillas ME, Ferrarotto R, Garden AS, et al. Neoadjuvant BRAF- and immune-directed therapy for anaplastic thyroid carcinoma. Thyroid. 2018;28:945–51. https://doi.org/10.1089/thy.2018.0060.
Tsuboi M, Takizawa H, Aoyama M, Tangoku A. Surgical treatment of locally advanced papillary thyroid carcinoma after response to lenvatinib: A case report. Int J Surg Case Rep. 2017;41:89–92. https://doi.org/10.1016/j.ijscr.2017.10.010.
Rubens RD, Sexton S, Tong D, et al. (1980) Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer. 1965;16:351–6. https://doi.org/10.1016/0014-2964(80)90352-7.
Bible KC, Kebebew E, Brierley J, et al. 2021 american thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2021;31:337–86. https://doi.org/10.1089/thy.2020.0944.
Maniakas A, Dadu R, Busaidy NL, et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000-2019. Jama Oncol. 2020;6:1397–404. https://doi.org/10.1001/jamaoncol.2020.3362.
Kim M, Ahn J, Song DE, et al. Real-world experience of lenvatinib in patients with advanced anaplastic thyroid cancer. Endocrine. 2021;71:427–33. https://doi.org/10.1007/s12020-020-02425-y.
Acknowledgements
None.
Funding
The research was supported by the National Natural Science Foundation of China (81902721 to Nai-si Huang, 82072951 to Yu Wang), the Science and Technology commission of Shanghai Municipality (19411966600 to Yu Wang), Zhuhai Fudan Innovation Institute (20665 to Yu Wang) and Shanghai Hospital Development Center Clinical Research (SHDC2020CR6003 to Yu Wang).
Author information
Authors and Affiliations
Contributions
Conception of the work: Nai-si Huang, Yu Wang, Qing-hai Ji. Acquisition, analysis, and interpretation of data: Wen-jun Wei, Jun Xiang, Jia-ying Chen, Qing Guan, Yun-jun Wang, Zhong-wu Lu. Drafted the work and substantively revised it: Nai-si Huang, Yu Wang, Wen-jun Wei, Jun Xiang, Jia-ying Chen, Qing Guan, Yun-jun Wang, Zhong-wu Lu, Ben Ma, Jia-qian Hu, Yu-long Wang. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Detailed information for neoadjuvant treatment and major adverse events.s
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Ns., Wang, Y., Wei, Wj. et al. A systematic review of neoadjuvant targeted therapy in locally advanced thyroid cancer. Holist Integ Oncol 1, 16 (2022). https://doi.org/10.1007/s44178-022-00016-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-022-00016-7