Abstract
Purpose
The well-known traditional Chinese formula Guizhi Fuling capsule (GFC) has been reported to reverse ovarian cancer drug resistance. Extrachromosomal DNA (ecDNA) plays an important role in tumour metastasis and resistance. The purpose of this study was to investigate the potential mechanisms by which GFC blocks tumour metastasis and reverses drug resistance by targeting ecDNA.
Methods
CNKI and PubMed were used to obtain pharmacokinetic research data on GFC in rats, and the bioactive ingredients detected in rat serum or plasma were collected. Network databases were used to screen the abnormally expressed genes in ecDNA, tumour metastasis genes, resistance genes, and the active ingredient targets of GFC. The KOBAS3.0 database was used to enrich the KEGG pathways and GO functions; the STRING platform was used to construct the core protein interaction network; and the molecular docking online tool SwissDock was used to analyse the binding activity of the core targets and the active ingredients. RT-qPCR, Western blotting and laser confocal microscopy were used to verify the effect of the sera containing GFC on ecDNA, mRNA and protein expression of key targets.
Results
Twenty-three bioactive ingredients of GFC were retrieved from PubMed and CNKI. Nine shared targets were simultaneously involved in abnormal genes in ecDNA, tumour metastasis and resistance and the active ingredient targets of GFC. GO functional analysis indicated that the cotargets involved cell proliferation, apoptotic regulation, nuclear functions, etc. The potential pathways involved in the reversal of tumour metastasis and drug resistance of GFC were the PI3K-Akt signalling, cancer, and platinum drug resistance pathways. Three shared proteins targeting ecDNA (AKT1, EGFR and MYC) stand out from the top 20 PPI targets, and all of the bioactive ingredients of GFC have strong binding affinity to the three proteins. The active ingredients can reduce the expression of MYC, EGFR and AKT1 mRNA and protein and the amount of ecDNA in drug-resistant OC cells.
Conclusions
GFC targeting ecDNA to reverse tumour metastasis and drug resistance has the characteristics of multiple ingredients, multiple targets, and multiple pathways, which provides a new perspective for the development of new drugs targeting ecDNA to benefit tumour treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Tumours are systemic diseases involving multitarget and multipath cell signaling networks. Multitarget therapy plays an important role in the treatment of complex diseases such as tumours and should be considered during the development of antitumour drugs [1]. Multidrug resistance (MDR) to chemotherapy and tumour metastasis are the main causes of death among tumour patients.
Recent research has shown that extrachromosomal DNA (ecDNA) plays an important role in tumour occurrence, progression, heterogeneity and genomic instability and is closely related to tumour metastasis and resistance [2,3,4]. ecDNA is a circular DNA molecule shed from a chromosome during the middle of mitosis. It is freed from the chromosome genome and participates in physiological or pathological processes in a unique way. ecDNA participates in tumour metastasis and MDR through a large number of oncogenes, such as MYC, EGFR, MYCN, and AKT1 [5]. Therefore, the development of anticancer drugs that regulate the process of tumour metastasis and drug resistance by targeting ecDNA will have great significance in improving tumour treatment.
Guizhi Fuling capsule (GFC) was developed from a well-known formula, Guizhi Fuling Wan (GFW; also known as Cinnamon Twig and Poria Pill), which traditionally is said to invigorate the blood and dispel blood stasis. It first appeared in Essentials from the Golden Cabinet (Jin Gui Yao Lue), a classic clinical book of traditional Chinese medicine written by the eminent Chinese physician Zhang Zhongjing (Zhang Ji) (150–219 CE) in the Eastern Han dynasty. There are 5 substances in this formula: Ramulus cinnamomi Cassiae (Gui Zhi), Scierotium Poriae Cocos (Fu Ling), Radix albus Paeoniae Lactiflorae (Bai Shao), Cortex Radicis Moutan (Mu Dan Pi) and Semen Pruni Persicae (Tao Ren). Pharmacological studies found that it contains several bioactive ingredients that could reverse MDR in rats, such as gallic acid, paeonol, paeoniflorin, and dehydroturonic acid [6, 7]. Among these ingredients, paeonol and paeoniflorin can inhibit the expression of interstitial phenotype N-cadherin and other EMT-related genes in a variety of tumours [8, 9]. It is suggested that GFC may inhibit tumour metastasis and resistance. However, the mechanism by which GFC regulates tumour metastasis and resistance, its effects on complex signaling networks and whether it acts on ecDNA have not been reported.
Previous studies by our team demonstrated that GFC reversed cisplatin resistance by inhibiting the metadherin (MTDH) and PI3K/AKT signalling pathways and inducing phosphatase and tensin homologue (PTEN) in a cisplatin-resistant SKOV3/DDP cell line and the corresponding tumour-bearing mouse model [10, 11]. In this study, the bioactive ingredients in the blood of rats given GFC were retrieved from public databases. A comprehensive network pharmacology approach was conducted to screen their targets in ecDNA, tumour metastasis and drug resistance and to analyse the GO functions and KEGG pathways and the protein–protein interaction (PPI) network. In addition, molecular docking and molecular biological methods were performed to assess the effect of the active ingredients on the core targets. This study provides a valuable reference for exploring the active ingredients of GFC and clarifying the mechanism by which GFC reverses tumour metastasis and resistance by targeting ecDNA.
2 Methods
2.1 Collection of blood bioactive ingredients and targeted prediction of GFW
The China Integrated Knowledge Resources Database (CNKI) and PubMed were used to obtain pharmacokinetic research data on GFC in rats, and the bioactive ingredients detected in rat serum or plasma were collected. The known targets of the ingredients were obtained from the Integrative Pharmacology-based Research Platform of Traditional Chinese Medicine (TCMIP; www.tcmip.cn) [12] and STITCH database (www.stitch-db.org). PharmMapper (www.lilab-ecust.cn/pharmmapper) and SwisstargetPredict (www.swisstargetprediction.ch) were used to predict the potential targets of the ingredients, and the targets with a score above 0.9 were retained. All of the obtained targets were combined, and duplicates were removed.
2.2 Construction of the bioactive ingredient-target network
The bioactive ingredients and potential targets of GFC were imported into Cytoscape v3.8.0 software to visualize the network of active ingredient-potential targets. The Analyse Network plug-in in Cytoscape was used to analyse the topological parameters of the drug-potential target network, and the interaction network between the active ingredients and the targets was constructed according to the degrees of freedom of the nodes.
2.3 Screening of cotargets for MDR, EMT and ecDNA
The following keywords were used to search for genes associated with MDR in the OMIM (Online Mendelian Inheritance in Man) database (https://www.omim.org): "cancer resistance", "cancer resistance gene", and "multidrug resistance", and the results were merged after duplicates were removed. Tumour metastasis genes were collected from the HCMDB (Human Cancer Metastasis) database (https://hcmdb.i-sanger.com). The abnormally amplified genes in ecDNA were obtained from published articles in PubMed and other literature databases. All of the above genes and the targets of the active ingredients were input into FunRich v3.1.3 software (www.funrich.org) to screen for cotargets.
2.4 GO function and KEGG pathway enrichment
The gene function online enrichment database KOBAS v3.0 (http://kobas.cbi.pku.edu.cn) was used to analyse the above targets [13], selecting the species "Homo sapiens (Human)" in the online tool and performing KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis and GO (Gene Ontology) functional enrichment analysis. The pathways and biological functions with statistical significance were screened using the threshold P < 0.05.
2.5 Construction of PPI networks
The above cotargets were entered into the STRING plug-in of Cytoscape v3.8.0 software to inquire about the corresponding hub proteins. The core proteins were sorted based on the degrees of freedom of the nodes in Cytoscape.
2.6 Molecular docking
The online molecular docking tool SwissDock (www.swissdock.ch) [14] was used to conduct molecular docking between the core proteins and the bioactive ingredients. A commercially available inhibitor of the core proteins was used as a positive control to compare the binding activities of the ingredients.
2.7 Drugs and animals
GFC (batch no. 19101920883) was purchased from Kanion Pharmaceutical Co., Ltd. (Jiangsu, China). The capsules were prepared as a crude solution at a concentration of 290.625 mg·mL−1 in warm deionized water. Thirty healthy Wistar female rats, aged between 6 and 8 weeks old and weighing 220–250 g, were obtained from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. (animal licence number: SCXK (ZHE) 2019–0001). The rats were randomly divided into two groups with 15 rats per group: the control group was administered normal saline by gavage, and the GFC group was administered the crude solution by gavage at dosages of 4 g·kg−1·d−1 based on the clinical dosage of GFC [15]. All of the procedures in this study were conducted in accordance with the accepted standards of humane animal care as outlined in the ethical guidelines on the care and use of laboratory animals and approved by the Ethics Committee of Nanyang Institute of Technology (Nanyang, China). After five days of administration, the rats were anaesthetized with 1.5% isoflurane in an anaesthesia mask, blood was collected, and sera were separated as previously described [11].
2.8 RT-qPCR analysis
The human cisplatin-resistant SKOV3/DDP cell line was obtained from Zhejiang Meisen Cell Technology Co., Ltd. (Zhejiang, China). The cells were treated as previously described [11]. To verify the mRNA expression of the core targets, SKOV3/DDP cells were seeded into 12-well plates at a density of 2.0 × 10 [5] cells per well and treated with the medicated rat control sera or the sera containing GFC or MYCi975 (10 μmol·L−1; Selleck Chemicals, USA) for 48 h. Total RNA was extracted from the cells using the SPARKeasy Kit (Shandong Sparkjade Biotechnology Co., Ltd., China), and the RNA was reverse transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., USA). RT-qPCR was performed using the QuantiNova SYBR Green PCR Kit (QIAGEN, USA) on an Applied Biosystems ViiA7 Real-Time PCR System with the following primers: MYC: (F)5′-GTCAAGAGGCGAACACACAAC-3′ and (R)5′-TTGGACGGACAGGATGTATGC -3′; EGFR: (F)5′-TTGCCGCAAAGTGTGTAACG-3′ and (R)5′-GTCACCCCTAAATGCCACCG-3′; AKT1: (F)5′-AGCGACGTGGCTATTGTGAAG-3′ and (R)5′-GCCATCATTCTTGAGGAGGAAGT -3′; GAPDH: (F)5′-ACAACTTTGGTATCGTGGAAGG-3′ and (R)5′-GCCATCACGCCACAGTTTC-3′. The relative gene expression analysis was performed using the 2−ΔΔCt method as described previously [16].
2.9 Western blot analysis
Cell lysates from SKOV3/DDP cells treated as described above were separated on 10% sodium dodecyl sulfate–polyacrylamide gels (SDS–PAGE), transferred onto PVDF membranes (Millipore, USA) and incubated with MYC (Abcam, clone number Y69), EGFR (Abcam, clone number EP38Y), AKT1 (Abcam, clone number 9A4) and GAPDH (Proteintech, clone number 1E6D9) antibodies. Antibody binding was detected using Pierce ECL Western Blotting Substrate (Thermo Fisher, USA).
2.10 Confocal laser microscopy and ecDNA analysis
SKOV3/DDP cells were seeded in 20-mm-diameter NEST confocal glass-bottom cell culture dishes at 37 ℃ and 5% CO2. The cells were treated with the blank control sera or the sera containing GFC or hydroxyurea (20 μM; MedChemExpress, USA), an ecDNA inhibitor [17]. After 48 h, the cells were treated with Karyomax (0.01 μg·mL; Thermo Fisher, USA) for 2 h, fixed with Carnoy fixative (methanol: ethanoic acid = 3:1) and stained with ProLong® Gold Antifade Reagent with DAPI (Thermo Fisher, USA). The cells were observed with a Zeiss LSM800 laser scanning confocal microscope (Carl Zeiss AG, Germany). Images were analysed for ecDNA by ecSeg ( https://github.com/UCRajkumar/ecSeg) [18].
2.11 Statistics
Data are expressed as the mean ± SD of at least three independent experiments. Statistical evaluation of the data was performed using a t test for comparisons between groups. Differences were considered to be statistically significant at P < 0.05.
3 Results
3.1 Bioactive ingredients and target network of GFC
The retrieved results from CNKI and PubMed showed that there were 23 bioactive ingredients in the blood collected from rats given GFC, as detected by UHPLC-MS/MS, UPLC/Q-TOF–MS/MS, LC–MS and other methods [7, 19, 20]. A total of 530 targets of these ingredients were obtained from the TCMIP, Pharm Mapper, STITCH and Swiss target Predict databases. According to the target number sorted by Cytoscape, G4 (3β,4β,23-trihydroxy-24,30-dinorolean-12,20(29)-dien-28-oic acid) had the highest number of targets, followed by G1 (16α-hydroxy trametenolic acid), G16 (mudanpioside F), and G2 (3-dehydrotrametenolic acid), suggesting that they may be imbalanced among the bioactive ingredients (Table 1).
3.2 Targets of GFC bioactive ingredients-tumour metastasis and resistance gene network
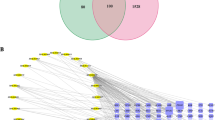
A total of 1,285 tumour drug resistance-related genes, 1,938 tumour metastasis-related genes and 162 abnormal genes in ecDNA were obtained from OMIM, HCMDB and PubMed, respectively. There were 394 shared genes for tumour drug resistance and metastasis, indicating that there was a close relationship between these two processes. There were 65 shared genes among the abnormal genes in ecDNA, tumour metastasis and drug resistance, suggesting that they may be the most important genes involved in tumour metastasis and resistance.
Further analysis of the 65 shared targets against the active ingredient targets of GFC revealed 9 shared targets (MDM2, MCL1, EGFR, MTDH, ABL1, FHIT, AKT1, ATM and MYC) (Fig. 1A). After constructing an active ingredient-shared target network of GFC and sorting by Cytoscape, the ingredients with higher degrees of freedom were 16α-hydroxytrametenolic acid, G4 (3β,4β,23-trihydroxy-24,30-dinorolean-12,20 (29)-dien-28-oic acid), catechin, and paeoniflorin, and they all targeted the 9 shared genes, suggesting that they may be important ingredients targeting ecDNA (Fig. 1B and 1C).
Bioactive ingredients and potential targets in GFC for tumour metastasis and drug resistance. A The Venn diagram results of potential targets of GFC-tumour metastasis-drug resistance-abnormal genes in ecDNA; B. the degrees of freedom of bioactive ingredients for the screened 65 targets where the degrees of freedom represent the targets of the bioactive ingredients; C. the bioactive ingredient-genes in ecDNA-tumour metastasis-drug resistance shared target networks of GFC. A larger shape and a deeper colour indicate a higher degree of freedom
3.3 GO function and KEGG pathway
GO functional and KEGG pathway enrichment analyses were conducted for the above 65 shared targets and 9 shared cotargets in the KOBAS 3.0 database; the threshold was set as P < 0.05. The results showed that the same GO functions of the 65 and 9 target groups were involved in cell proliferation, apoptotic regulation, and nuclear functions, etc. (Fig. 2A). The KEGG pathways included PI3K-Akt signalling pathways, platinum drug resistance, pathways in cancer, and proteoglycans in cancer (Fig. 2B). AKT1 is the highest frequency target of the pathways MYC, PTEN, EGFR, GSK3B and BCL2, which are involved in at least three pathways. These results indicate that the abovementioned GO functions and KEGG pathways may be important for the function of GFC.
3.4 PPI network of core targets
The 65 shared targets were analysed in the STRING database and sorted by Cytoscape, and the top 20 PPI targets were screened. The proteins listed in order of frequency from high to low were AKT1, EGFR, MYC, TP53, VEGFA, SRC, MAPK1, CASP3, PTEN, PTGS2, and MMP9, indicating that these proteins may play an important role in tumour metastasis and resistance. After alignment with the abnormally amplified genes in ecDNA, 6 of the 9 shared cotargets, AKT1, EGFR, MYC, MDM2, MCL1 and ATM, remained outstanding. Among them, AKT1, EGFR and MYC were the top 3 PPI targets, suggesting that they may be the core targets for GFC in tumour metastasis and resistance involving ecDNA (Fig. 3).
3.5 Molecular docking
The results of molecular docking analysis showed that, compared with the commercially available inhibitors, all of the bioactive ingredients of GFC have strong binding activity to MYC (6G6J), EGFR (3I2T) and AKT1 (6HHF) proteins (Table 2). It was considered that a compound has remarkable binding activity when the binding energy is less than -6 kcal/mol [21]. For the highest binding activity, the binding sites for the MYC inhibitor MYCi975 and paeoniflorin with the MYC protein were at the LYS939 and ALA237 residues of the protein involved in hydrogen bond (HB) interactions, respectively. Similarly, the binding sites for the EGFR inhibitor EGFR-IN-3 and mudanpioside E with the EGFR protein were at GLN 397, PRO 301 and MET 287 involved in the HB interaction. The binding sites for the AKT1 inhibitors Akti-1/2 and paeoniflorin to the AKT1 protein were at LYS297 and GLY294, respectively, and contributed to the HB interactions (Fig. 4). In view of the fact that the amino acid binding sites of the key ingredients and the inhibitors in the same protein were different, this study suggests that one protein may have multiple active binding sites and that the test ingredients could be potent inhibitors of MYC, EGFR, and AKT1.
3.6 Sera containing GFC inhibit the expression of the core targets
To investigate whether the core targets were regulated by sera containing GFC, we evaluated their expression by RT-qPCR and Western blotting. The expression of MYC, EGFR and AKT1 was significantly inhibited by sera containing GFC at the mRNA and protein levels compared with that of the control sera. The MYCi975 group showed a stronger inhibition of MYC, EGFR and AKT1 (Fig. 5A and 5B) than the sera containing GFC.
3.7 Sera containing GFC inhibit the amount of ecDNA
To further investigate the effect of sera containing GFC on ecDNA, metaphase cells were obtained by treating the cells with Karyomax. ecDNA from DAPI-stained metaphases was considered in an unbiased, semiautomated manner [3]. The ecSeg analysis results found that sera containing GFC could decrease the amount of ecDNA compared with that of the control sera, suggesting that GFC protects against tumour metastasis and resistance by targeting ecDNA (Fig. 6A, 6B and 6C).
4 Discussion
MDR or chemoresistance is a phenomenon that appears with the clinical application of chemotherapy drugs. Tumour evolution is due to the successive acquisition of mutations, resulting in heterogeneity and drug resistance of tumour cells [22]. It was demonstrated that MDR and tumour metastasis are deeply correlated and mutually promoted by the 384 shared genes between them screening by network pharmacology approach. The shared genes between ecDNA, MDR and tumour metastasis screened by this study further identified a link between them, in accordance with those of previous studies [5].
Through the construction of the network of the bioactive ingredient targets and PPIs of GFC targets, it was found that the average number of targets of each ingredient was 23, reflecting the multitarget nature of TCM. The active ingredients, such as gallic acid, catechin, 16α-hydroxy trametenolic acid, and paeoniflorin, regulate 65 shared targets of tumour metastasis and drug resistance. Gallic acid can induce PTEN expression and inhibit the proliferation of OVCAR-3 and A2780/CP70 ovarian cancer cells by diminishing the expression of VEGF, HIF-1α and AKT phosphorylation [23]. Catechin inhibited MCF-7 breast cancer cell proliferation and induced apoptosis by augmenting the expression of CASP3 and TP53 [24]. Paeonol has been shown to inhibit tumour metastasis and resistance in animal and cell models by regulating the NF-κB and STAT3 signalling pathways [25]. These findings, together with our previous studies, demonstrated that GFC has multitarget effects to reverse tumour metastasis and resistance. The PPI network shows that the bioactive ingredients of GFC target AKT1, EGFR, MYC, VEGFA, SRC, TP53, MAPK1, CASP3, PTEN, PTGS2 and MMP9, and they are the core targets of protein interactions. These targets are all important regulatory targets of the PI3K/AKT, EGFR and cisplatin resistance-related signalling pathways, which are involved in the occurrence and development of tumour metastasis and drug resistance [26,27,28].
Platelet adhesion is an important event in thrombosis. Anti-platelet adhesion and activation of platelets are often used as action indicators for the effect of TCM in promoting blood circulation and resolving blood stasis [29]. Studies have demonstrated that the enhanced activity of coagulation factor F2 is related to the degree of tumour malignancy. F2 can activate TGF-β1 by promoting the hydrolysis of the repetitive sequence of glycoprotein A that is bound to platelets and promotes tumour metastasis and resistance. These results provide a theoretical basis for GFC as a compound promoting blood circulation and resolving blood stasis, and it also has the effect of blocking metastasis and reversing the drug resistance of ovarian cancer.
The large number of amplified oncogenes carried by ecDNA accounts for approximately 1% of the total number of genes expressed in the tumour genome. Among them, MYC, EGFR, and AKT1 are the oncogenes with the highest amplification frequency in ecDNA. The overexpression of MYC promotes PTEN polyubiquitination, thereby inhibiting the dimerization, membrane recruitment and tumour suppressor functions of PTEN, further activating the PI3K/AKT pathway and promoting tumour metastasis and resistance [30]. AKT1 is an important gene in the PIK3-AKT pathway, and its primary target gene is vimentin. The binding of AKT1 to vimentin can promote EMT and induce tumour drug resistance, migration and invasion [31]. In addition, PTEN is an important modulator of controlling EGFR signalling [32]. The literature identifies MYC as a key upstream factor for EGFR and AKT1. The molecular docking and Western blotting results also proved that MYCi975 endowed with the images of inhibitors of MYC, EGFR and AKT1. The current study revealed that most of the bioactive ingredients in GFC have strong binding activity to MYC, EGFR and AKT1, and the sera containing GFC are also an inhibitor of the 3 proteins.
5 Conclusions
This study revealed the bioactive ingredients, targets and mechanisms of GFC in blocking tumour metastasis and reversing drug resistance by targeting ecDNA. The results provide a valuable reference for more in-depth research on GFC’s applications for the treatment of tumours. However, in vivo and in vitro experiments are required to further verify its effects on related targets and pathways.
Availability of data and materials
The datasets and supporting materials during the current study are available from the corresponding authors on reasonable request.
References
Ramsay RR, Popovic-Nikolic MR, Nikolic K, et al. A perspective on multi-target drug discovery and design for complex diseases. Clin Transl Med. 2018;7(1):3.
Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Sci. 2013;339(6119):580–4.
Nathanson DA, Gini B, Mottahedeh J, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Sci. 2014;343(6166):72–6.
Verhaak R, Bafna V, Mischel PS. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution. Nat Rev Cancer. 2019;19(5):283–8.
Turner KM, Deshpande V, Beyter D, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nat. 2017;543(7643):122–5.
Cai J, Chen S, Zhang W, et al. Paeonol reverses paclitaxel resistance in human breast cancer cells by regulating the expression of transgelin 2. Phytomedicine. 2014;21(7):984–91.
Zhao L, Xiong Z, Sui Y, et al. Simultaneous determination of six bioactive constituents of Guizhi Fuling Capsule in rat plasma by UHPLC-MS/MS: Application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1001(1):49–57.
Wang Z, Liu Z, Yu G, et al. Paeoniflorin inhibits migration and invasion of human glioblastoma cells via suppression transforming growth factor β-induced epithelial-mesenchymal transition. Neurochem Res. 2018;43(3):760–74.
Yang L, Xing S, Wang K, et al. Paeonol attenuates aging MRC-5 cells and inhibits epithelial-mesenchymal transition of premalignant HaCaT cells induced by aging MRC-5 cell-conditioned medium. Mol Cell Biochem. 2018;439(1–2):117–29.
Han L, Guo X, Bian H, et al. Guizhi Fuling Wan, a traditional Chinese herbal formula, sensitizes cisplatin-resistant human ovarian cancer cells through inactivation of the PI3K/AKT/mTOR pathway. Evid Based Complement Alternat Med. 2016;16(1):1–11.
Han L, Cao X, Chen Z, et al. Overcoming cisplatin resistance by targeting the MTDH-PTEN interaction in ovarian cancer with sera derived from rats exposed to Guizhi Fuling wan extract. BMC Complement Med Ther. 2020;20(1):57.
Xu HY, Zhang YQ, Liu ZM, et al. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47(D1):D976–82.
Bu D, Luo H, Huo P, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49(W1):W317–25.
Bitencourt-Ferreira G, de Azevedo WF Jr. Docking with SwissDock. Methods Mol Biol. 2019;2053(1):189–202.
Cheng P. Pharmacology of traditional Chinese medicine. Beijing: China Press Tradit Chin Med Co., LTD.; 2016. p. 479.
Li H, Hua B, Jingfeng O, et al. Wenyang Huazhuo Tongluo formula, a Chinese herbal decoction, improves skin fibrosis by promoting apoptosis and inhibiting proliferation through down-regulation of survivin and cyclin D1 in systemic sclerosis. BMC Complement Altern Med. 2016;16(1):69.
Von Hoff DD, McGill JR, Forseth BJ, et al. Elimination of extrachromosomally amplified MYC genes from human tumor cells reduces their tumorigenicity. Proc Natl Acad Sci U S A. 1992;89(17):8165–9.
Rajkumar U, Turner K, Luebeck J, et al. EcSeg: semantic segmentation of metaphase images containing extrachromosomal DNA. iScience. 2019;21:428–35.
Zhang Y, Cheng Y, Liu Z, et al. Systematic screening and characterization of multiple constituents in Guizhi Fuling capsule and metabolic profiling of bioactive components in rats using ultra-high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1061–1062(1):474–86.
Cheng Y, Chu Y, Su X, et al. Pharmacokinetic-pharmacodynamic modeling to study the anti-dysmenorrhea effect of Guizhi Fuling capsule on primary dysmenorrhea rats. Phytomedicine. 2018;48(1):141–51.
Cotterill JV, Palazzolo L, Ridgway C, et al. Predicting estrogen receptor binding of chemicals using a suite of in silico methods - Complementary approaches of (Q)SAR, molecular docking and molecular dynamics. Toxicol Appl Pharmacol. 2019;378:114630.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94.
He Z, Chen AY, Rojanasakul Y, et al. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling pathway in ovarian cancer cells. Oncol Rep. 2016;35(1):291–7.
Alshatwi AA. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J Exp Clin Cancer Res. 2010;29(1):167.
Zhang L, Tao L, Shi T, et al. Paeonol inhibits B16F10 melanoma metastasis in vitro and in vivo via disrupting proinflammatory cytokines-mediated NF-κB and STAT3 pathways. IUBMB Life. 2015;67(10):778–88.
Suda K, Tomizawa K, Fujii M, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6(7):1152–61.
Xu J, Liu D, Niu H, et al. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2017;36(1):19.
Liang F, Ren C, Wang J, et al. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncog. 2019;8(10):59.
Chen C, Wang F, Xiao W, et al. Effect on platelet aggregation activity: extracts from 31 traditional Chinese medicines with the property of activating blood and resolving stasis. J Tradit Chin Med. 2017;37(1):64–75.
Lee YR, Chen M, Lee JD, et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Sci. 2019;364(6441):eaau0159.
Zhu QS, Rosenblatt K, Huang KL, et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene. 2011;30(4):457–70.
Shinde SR, Maddika S. PTEN modulates EGFR late endocytic trafficking and degradation by dephosphorylating Rab7. Nat Commun. 2016;7(1):10689.
Acknowledgements
The authors would like to thank all members of this work for their advice and technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82074076), the Natural Science Foundation of Henan Province (No.202300410022).
Author information
Authors and Affiliations
Contributions
H.L. and B.H. conceived and designed the experiments. H.L., G.K. and L.Q. performed the experiments. G.X. and D.R. analyzed the data. L.F. and Z.X. contributed reagents/materials/analysis tools. H.L. and L.Q. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with the accepted standards of humane animal care as outlined in ethical guidelines on the care and use of laboratory animals (National Institutes of Health Guide for the Care and Use of Laboratory Animals). All animal procedures were conducted with protocol approval from the Ethics Committee of Nanyang Institute of Technology (NYIT-2020–3), and all efforts were made to minimize animal suffering.
Competing interests
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, L., Lv, Q., Guo, X. et al. Prediction and verification of the key ingredients and molecular targets of Guizhi Fuling capsule against tumour metastasis and resistance. Holist Integ Oncol 1, 12 (2022). https://doi.org/10.1007/s44178-022-00013-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-022-00013-w