Abstract
Purpose
Chemotherapy-induced nail pigmentation is a common adverse effect, but prospective studies focussing on its onset, recovery, and severity are few. We aim to evaluate the pattern of chemotherapy-induced nail pigmentation in early-stage breast cancer patients by calculating the comprehensive score based on hyperpigmentation area and color depth of the nail plate.
Methods
This prospective, observational study was conducted between February 2019 and December 2019. Early-stage breast cancer patients scheduled to receive anthracyclines combined with cyclophosphamide or taxane-containing regimens were enrolled. The clinicopathologic characteristics and treatment protocols were collected. The onset, patterns, and duration of nail changes were photographed and recorded regularly.
Results
A total of 90 patients were enrolled. The most common nail change was nail pigmentation (n = 81, 90.0%), followed by onycholysis (n = 39, 43.3%), Beau’s lines (n = 19, 21.1%), Mees’ lines (n = 16, 17.8%), Muehrcke’s lines (n = 7, 7.8%), and hemorrhage (n = 1, 1.1%). Forty-four (48.9%) patients developed severe nail pigmentation. The median onset time of nail pigmentation was 37 days after the initiation of chemotherapy. At the latest follow-up, 55(67.9%) patients achieved remission of melanonychia with the median recovery time of 118 days. The median duration of nail pigmentation was 214 days.
Conclusion
Our study revealed the specific pattern of chemotherapy-induced nail pigmentation, which onsets early and recovers slowly with a high incidence of severe nail pigmentation, in early-stage breast cancer patients. The results provide reference for further intervention studies.
Trial Registration
ClinicalTrials.gov Identifier: NCT04215744. Registered 30 December 2019—Retrospectively registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The morbidity and mortality of breast cancer rank first among female malignant tumors [1]. Anthracyclines combined with cyclophosphamide or taxane-containing regimens are commonly used for adjuvant or neoadjuvant chemotherapy in early-stage breast cancer patients. However, these chemotherapeutic drugs may cause nail toxicity, including nail pigmentation, onycholysis, Beau’s lines, Mees’ lines, Muehrcke’s lines, and hemorrhage [2,3,4]. Nail pigmentation, which is defined as pigmentation of the nail plate due to activation or proliferation of nail matrix melanocytes and induced by anthracyclines, cyclophosphamide, and taxanes [2, 5,6,7,8,9,10,11], affects the appearance of the hands and reduces the quality of life of patients [12].
The vast majority of patients with breast cancer are women, and female patients pay more attention to the appearance of their hands and take notice of nail pigmentation. However, previous studies focused on cutaneous or nail toxicity instead of melanonychia and results describing chemotherapy-induced nail pigmentation were mostly included under ‘cutaneous toxicities’ or ‘nail changes’ [10, 13, 14]. Data especially focus on chemotherapy-induced melanonychia in breast cancer patients was limited. There is no National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) standard for nail pigmentation and previous investigations did not assess melanonychia specifically. This prospective observational study was conducted to evaluate the patterns of nail pigmentation and other types of nail toxicity due to anthracyclines combined with cyclophosphamide or taxane-containing regimens in early-stage breast cancer patients and to provide reference for further intervention studies. It’s the first study to assess the nail pigmentation by calculating the comprehensive score based on hyperpigmentation area and color depth of the nail plate.
2 Patients and methods
2.1 Patients
This prospective observational study (ClinicalTrials.gov ID: NCT04215744) was carried out at Sun Yat-sen University Cancer Center between February 2019 and December 2019. The study was approved by Sun Yat-sen Cancer Center Institution Review Board, and the patients signed a written informed consent before enrollment.
Inclusion criteria were as follows: (1) early-stage breast cancer patients who were scheduled to receive anthracyclines combined with cyclophosphamide or taxane-containing regimens for adjuvant/neoadjuvant chemotherapy; (2) no previous nail or skin abnormalities; (3) have not received any antitumor treatment before. Patients who were unable to understand the content of this study or provide the required information were excluded from the study.
Ninety-one newly diagnosed early-stage breast cancer patients scheduled to receive anthracyclines combined with cyclophosphamide or taxane-containing regimens consented to participate. A patient receiving imatinib were excluded. A total of 90 patients were finally enrolled in the study and were evaluated and followed up monthly to assess nail pigmentation and other types of nail toxicity. Out of 90 patients, 3 patients were lost to follow-up (Fig. 1).
For each patient, we collected demographic and clinicopathologic data, details of systemic treatment (chemotherapy, target therapy, and endocrine therapy) and local treatment (surgery and radiotherapy), and details of nail pigmentation and other types of nail toxicity. Nails were examined in daylight and serial photographs were taken. The nail changes were recorded at regular intervals, including onset, patterns, and duration. The onset time of nail toxicity was defined as the time from the initiation of chemotherapy to the onset of nail toxicity. The duration of nail toxicity was defined as the time from the initiation of nail toxicity to the end of nail toxicity or last available follow-up. The recovery time of nail toxicity was defined as the time from the initiation to the end of nail toxicity in patients with disappeared manifestations of nail toxicity.
2.2 Evaluation criteria
There is no CTCAE standard for melanonychia at present, and previous studies have not specifically evaluated nail pigmentation. Therefore, we calculated the comprehensive score based on the area of the nail plate with hyperpigmentation and the color depth of the nail plate, including: (1) area score: 0 (no change), 1 (0–1/3 area of nail plate), 2 (1/3–2/3 area), and 3 (2/3–3/3 area) (Fig. 2); (2) color score according to the color card (Fig. 3): 0 (no change), 1 (light gray), 2 (dark gray), and 3 (black). The most severe affected finger was selected for the scoring. Nail pigmentation was defined as severe if the sum of the area score and the color score were greater than or equal to 5 points. Two independent medical staff (an associate consultant and an attending physician) examined the nails of patients and scored the most severe nail. Other types of toxicity were graded according to CTCAE v5.0.
2.3 Hematoxylin and eosin staining of the nails
The pigmented and normal nails of two breast cancer patients who received anthracyclines combined with cyclophosphamide or taxane-containing regimens were cut and decalcified using Osteosoft® solution for 3 weeks and then stained routinely with hematoxylin and eosin (H&E).
2.4 Statistical analysis
Assuming that the incidence of nail pigmentation induced by chemotherapy is 0.3, the confidence interval is 0.2 to 0.4, and the α value is 0.05, at least 81 patients should be enrolled in this study. Continuous variables were reported as medians with interquartile range (IQR), and categorical variables were described as whole numbers and percentages. The chi-square test of independence was used to evaluate the correlation between variables and development of nail pigmentation, if present, by SPSS version 24.0 (IBM, New York, USA). Statistical significance was set at P ≤ 0.05.
3 Results
3.1 Patient characteristics
Between February 2019 and December 2019, a total of 90 early-stage breast cancer patients were enrolled in this prospective noninterventional study. The median follow-up was 10.2 months (IQR: 8.6–12.2). The median patient age was 48 years (IQR 40–54) and 52 (57.8%) patients were younger than 50 years of age. All patients were female and 58.9%(n = 53) were premenopausal. The skin phototype was type IV in 47 (52.2%) patients and type III in other patients. Chemotherapy regimens included EC/AC × 4 cycles-T × 4 cycles (n = 80, 88.9%), TC × 4 cycles (n = 6, 6.7%), TCb × 6 cycles (n = 2, 2.2%), EC × 4 cycles-TCb × 4 cycles (n = 1, 1.1%), and TEC × 6 cycles (n = 1, 1.1%) (E: epirubicin; A: liposomal doxorubicin; C: cyclophosphamide; T: taxanes; and Cb: carboplatin). Targeted agents included trastuzumab (n = 18) and trastuzumab combined with pertuzumab (n = 29). Endocrine therapy included selective estrogen receptor modulator (SERM) (n = 7), aromatase inhibitor (AI) (n = 16), ovarian function suppression (OFS) + SERM (n = 8), and OFS + AI (n = 23). Ten patients received metronomic capecitabine maintenance, and 66 patients accepted radiotherapy. The characteristics of patients are summarized in Table 1. We also recorded comanagement with other medications. Most patients received a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist, such as palonosetron or ondansetron, for prophylaxis of emesis and nausea. Premedication with dexamethasone, neurokinin-1 (NK-1) receptor antagonists and proton pump inhibitors was routinely given before administration of anthracyclines and carboplatin. Dexamethasone, diphenhydramine, and cimetidine were routinely given before administration of taxanes.
3.2 Nail pigmentation and other types of nail toxicity
The patients (n = 90) presented various types of nail changes during chemotherapy. Nail pigmentation was the most common nail change (n = 81, 90.0%), followed by onycholysis (n = 39, 43.3%), Beau’s lines (n = 19, 21.1%), Mees’ lines (n = 16, 17.8%), Muehrcke’s lines (n = 7, 7.8%), and hemorrhage (n = 1, 1.1%) (Table 2). The consistency of the area and color scoring by two medical staff was 92.0% and 93.1%, respectively. The maximum sum of area score and color score of 44 patients was greater than or equal to 5 points, indicating that 48.9% of patients showed severe nail pigmentation. The median onset time of nail pigmentation was 37 days (IQR: 25–50) after the initiation of chemotherapy. At the latest follow-up, 55(67.9%) patients achieved remission of melanonychia with the median recovery time of 118 days (IQR 88–158). The median duration of nail pigmentation was 214 days (IQR: 191–248). Thirty-one patients presented with grade 1 onycholysis, and 8 patients suffered grade 2 onycholysis. The median onset time of onycholysis was 103 days (IQR: 65–142) after the initiation of chemotherapy. At the latest follow-up, 11 (28.2%) patients achieved remission of onycholysis with the median recovery time of 141 days (IQR 113–176). The median duration of onycholysis was 203 days (IQR: 138–272). The details of nail changes and other types of nail toxicity during chemotherapy are depicted in Table 2. We further analyzed the frequency distribution of nail toxicity under various chemotherapy regimens and found that patients receiving one of these five chemotherapy regimens can present nail pigmentation. Various nail changes corresponding to chemotherapy regimens and their frequency distribution are shown in S1.
3.3 Melanin granules in the nails
We performed the H&E staining of the normal and pigmented nails of two patients who received EC/AC × 4 cycles-T × 4 cycles. The color score of the pigmented nails of the two patients were both 2, and that of the normal nails were both 0. It’s observed that the melanin granules in the pigmented nails were more than that in the normal nails of the same patient (S2).
4 Discussion
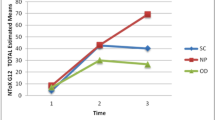
Nail pigmentation may be caused by the activation of nail matrix melanocytes induced by anticancer drugs, including anthracyclines, cyclophosphamide, and taxanes [2, 5,6,7,8,9,10,11]. This prospective observational study was the first to evaluate the onset, pattern, and duration of nail pigmentation induced by anthracyclines combined with cyclophosphamide or taxane-containing regimens in early-stage breast cancer patients. Our study demonstrated that nail pigmentation, whose incidence was up to 90%, was the most common nail change in early breast cancer patients receiving anthracyclines combined with cyclophosphamide or taxane-containing chemotherapy (Fig. 4). Forty-six percent of the patients suffered from severe nail pigmentation. A study of 55 patients with metastatic breast cancer treated with docetaxel indicated that the incidence of nail toxicity can reach 58% after 4 cycles of chemotherapy and further increase to 88% after 3 additional cycles [15]. Yorulmaz A et al. found that 4 (30.8%) of 13 patients receiving anthracyclines-containing chemotherapy presented nail pigmentation and 10 (35.7%) patients had nail pigmentation in 28 patients treated with taxanes (docetaxel or paclitaxel) [16]. A recent prospective observational study included 42 breast cancer patients, and the most commonly used chemotherapy agents were taxanes and cyclophosphamide. The data demonstrated that 71.3% of patients had nail changes, and chromonychia was the most common nail toxicity (54.26%) [10]. The incidence of nail pigmentation in our study was higher than that in previous studies. The difference may be due to the following reasons. The chemotherapy agents used in patients in this study were anthracyclines, cyclophosphamide, and taxanes; each of these agents can cause nail pigmentation. In addition, over 90% of the patients received these three drugs sequentially or in concurrent combination, and some of the patients were treated by dose-dense scheduled chemotherapy. The combination of the drugs and the intensive dose may eventually increase the incidence of nail pigmentation.
There is no CTCAE standard for nail pigmentation at present and previous studies did not specifically evaluate melanonychia. Therefore, this research is the first to calculate the comprehensive and simple score based on hyperpigmentation area and color depth of the nail plate. The consistency of area and color scoring by two medical staff was higher than 90%, indicating that the method was relatively accurate. The typical pictures of score of 1 + 1, 2 + 2, and 3 + 3 were shown in Fig. 5. The proportion of patients with severe hyperpigmentation, which corresponds to the comprehensive score more than or equal to five, was relatively high. The median duration of severe nail pigmentation was 230 days (IQR: 204–257), significantly longer than that of mild nail pigmentation (comprehensive score less than five) (198 days, IQR: 178–223) (P = 0.01). This supports the rationality of the evaluation method. Previous research indicated that chemotherapy-related nail pigmentation is usually slowly reversible and may persist throughout life in some patients [17]. Our data revealed that the median onset time of nail pigmentation was 37 days after initiation of chemotherapy. Only 55 patients achieved remission of melanonychia with the median recovery time of 204 days, whereas 26 patients (32.1%) with melanonychia had not recovered at the last follow-up. These results demonstrate that chemotherapy-induced nail pigmentation is usually severe, has an early onset, and persists for a long time.
In addition to melanonychia, other types of chemotherapy-induced nail toxicity were evaluated. Onycholysis was the second common nail toxicity, which may be caused by anthracyclines and taxanes. In this study, 39 (43.3%) patients treated with anthracyclines and taxanes suffered onycholysis (S3). Transient arrest of the mitotic activity of the keratinocytes of the nail matrix causes Beau’s lines, which appear as transverse depressions of the nail surface. All chemotherapeutic agents can result in Beau’s lines, which are more common after combination chemotherapy [2]. Beau’s lines (S4) were noted in 19 patients receiving combination chemotherapy in our study. Mees’ lines appear as one or more very thin transverse white bands parallel to the contour of the lunula due to retention of matrix keratinocyte nuclei in the nail plate [18]. These white lines have been reported with chemotherapeutic agents such as anthracyclines and cyclophosphamide [2]. We observed Mees’ lines (S5) in 16 patients who were treated with anthracyclines combined with cyclophosphamide. Muehrcke’s lines refer to apparent blanch-able and paired leukonychia and were initially occurred in patients with hypoalbuminemia. Chemotherapeutic agents and particularly cytotoxic drugs are also common causes of Muehrcke’s lines [2, 19]. In our study, 5 out of 7 patients with Muehrcke’s lines (S6) had hypoalbuminemia and all 7 patients received cyclophosphamide-containing chemotherapy. Vascular abnormalities and thrombocytopenia induced by taxanes may cause subungual hemorrhage [20]. Subungual hemorrhage was noted in 1 patient treated with 4 cycles of epirubicin + cyclophosphamide followed by 4 cycles of docetaxel; however, the patient didn’t have thrombocytopenia (S7).
Nail toxicity, though not life-threatening and easily overlooked by oncologists, could be so severe that it has a significantly impact on daily life. The nail changes are emotionally disturbing and cosmetically undesirable to female cancer patients, limit manual activities, reduce the quality of life, and may even lead to discontinuation of treatment [3, 4]. Some nail changes disappear with nail regrowth after drug withdrawal, whereas others may leave with persisting deformities and sequelae [21]. Nail pigmentation seriously affects the appearance of the hands of the early-stage breast cancer patients who are expected to return to normal life. Besides, the vast majority of breast cancer patients are women who are more concerned about the beauty of nails. Therefore, oncologists should pay more attention to nail pigmentation, and it is crucial to find effective intervention measures to prevent melanonychia. Reported preventive measures include avoidance of pressure and ultraviolet light, maintenance of nail integrity, nail moisturization, nail polish, and proper manicure [4, 22]. Cryotherapy with frozen gloves and socks and use of hydrating nail solution have shown promising results in previous trials [21, 23,24,25,26,27]. However, previous investigations mainly focused on onycholysis instead of melanonychia and have not yet explored the preventive measures for melanonychia.
The limitation of our study was that we failed to establish a correlation between the onset of nail pigmentation and a certain chemotherapeutic agent, chemotherapy drug, age, menopausal status, and skin phototype.
Our study revealed the specific pattern of chemotherapy-induced nail pigmentation, which occurs early and recovers slowly with a high incidence of severe nail pigmentation, in early-stage breast cancer patients. We additionally evaluated other types of common chemotherapy-related nail toxicity. Nail pigmentation and other types of nail toxicity, which can affect the instrumental activities, impact the quality of life, or even interrupt the therapy, deserve more attention from oncologists. Intervention studies are required to explore effective preventive measures against nail pigmentation, and we have further conducted a prospective trial to evaluate the frozen glove therapy to prevent chemotherapy-induced nail pigmentation.
Availability of data and materials
The data that support the findings of this study are available in the Research Data Deposit (http://www.researchdata.org.cn).
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618–26. https://doi.org/10.1016/j.clindermatol.2013.06.013.
Ferreira MN, Ramseier JY, Leventhal JS. Dermatologic conditions in women receiving systemic cancer therapy. Int J Women’s Dermatol. 2019;5:285–307. https://doi.org/10.1016/j.ijwd.2019.10.003.
Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract. 2009;15:143–55. https://doi.org/10.1177/1078155208100450.
Yang ST, Cheng M, Lee NR, Chang WH, Lee YL, Wang PH. Paclitaxel-related nail toxicity. Taiwan J Obstet Gynecol. 2019;58:709–11. https://doi.org/10.1016/j.tjog.2019.07.023.
Sudhir M, Vikas M, P. M S, Suman S, Simran K. Cyclophosphamide-induced melanonychia in a patient with steroid dependent nephrotic syndrome: a rare presentation. Saudi J Kidney Dis Transpl 2019;30:978–981. https://doi.org/10.4103/1319-2442.265478
Maiti A, Bhattacharya S. A patient with cancer and nail pigmentation. BMJ. 2016;353:i2346. https://doi.org/10.1136/bmj.i2346.
Lopes M, Jordao C, Grynszpan R, Sodre C, Ramos ESM. Chromonychia secondary to chemotherapy. Case Rep Dermatol. 2013;5:163–7. https://doi.org/10.1159/000351874.
Kumar S, Dixit R, Karmakar S, Paul S. Unusual nail pigmentation following cyclophosphamide-containing chemotherapy regimen. Indian J Pharmacol. 2010;42:243–4. https://doi.org/10.4103/0253-7613.68433.
Zawar V, Bondarde S, Pawar M, Sankalecha S. Nail changes due to chemotherapy: a prospective observational study of 129 patients. J Eur Acad Dermatol Venereol. 2019;33:1398–404. https://doi.org/10.1111/jdv.15508.
Yorulmaz A, Dogan M, Artuz F, Zengin N. Comparison of pigmentary side effects of taxanes and anthracyclines: an onychoscopic evaluation. J Toxicol Cutan Ocular Toxicol. 2016;36:135–9. https://doi.org/10.3109/15569527.2016.1173698.
Lee J, Lim J, Park JS, et al. The impact of skin problems on the quality of life in patients treated with anticancer. Cancer Res Treat. 2018;50:1186–93. https://doi.org/10.4143/crt.2017.435.
Saini K, Sutaria A, Shah B, Brahmbhatt V, Parmar K. Cutaneous adverse drug reactions to targeted chemotherapeutic drugs: a clinico-epidemiological study. Indian J Dermatol. 2019;64:471–5. https://doi.org/10.4103/ijd.IJD_491_18.
Biswal SG, Mehta RD. Cutaneous adverse reactions of chemotherapy in cancer patients: a clinicoepidemiological study. Indian J Dermatol. 2018;63:41–6. https://doi.org/10.4103/ijd.IJD_65_17.
Winther D, Saunte DM, Knap M, Haahr V, Jensen AB. Nail changes due to docetaxel–a neglected side effect and nuisance for the patient. Support Care Cancer. 2007;15:1191–7. https://doi.org/10.1007/s00520-007-0232-0.
Yorulmaz A, Dogan M, Artuz F, Zengin N. Comparison of pigmentary side effects of taxanes and anthracyclines: an onychoscopic evaluation. Cutan Ocul Toxicol. 2016;36:135–9. https://doi.org/10.3109/15569527.2016.1173698.
André J, Lateur N. Pigmented nail disorders. Dermatol Clin. 2006;24:329–39. https://doi.org/10.1016/j.det.2006.03.012.
Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin. 2008;26:59–68. https://doi.org/10.1016/j.det.2007.08.003.
Huang TC, Chao TY. Mees lines and Beau lines after chemotherapy. CMAJ. 2010;182:E149. https://doi.org/10.1503/cmaj.090501.
Wasner G, Hilpert F, Baron R, Pfisterer J. Clinical picture: nail changes secondary to docetaxel. Lancet. 2001;357:910. https://doi.org/10.1016/s0140-6736(00)04210-0.
Huang KL, Lin KY, Huang TW, et al. Prophylactic management for taxane-induced nail toxicity: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2019;28:e13118. https://doi.org/10.1111/ecc.13118.
Hussain S, Anderson DN, Salvatti ME, Adamson B, Mcmanus M, Braverman AS. Onycholysis as a complication of systemic chemotherapy: report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88:2367–71. https://doi.org/10.1002/(sici)1097-0142(20000515)88:10%3c2367::aid-cncr22%3e3.0.co;2-#.
Sakurai M, Todaka K, Takada N, et al. Multicenter phase II study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity for the breast cancer patients (Kinki Multidisciplinary Breast Oncology Group: KMBOG-0605). Cancer Res 2009;69. https://doi.org/10.1158/0008-5472.SABCS-4093
Scotté F, Banu E, Medioni J, et al. Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer. 2010;112:1625–31. https://doi.org/10.1002/cncr.23333.
Scotte F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23:4424–9. https://doi.org/10.1200/JCO.2005.15.651.
Thomas R, Williams M, Cauchi M, Berkovitz S, Smith SA. A double-blind, randomised trial of a polyphenolic-rich nail bed balm for chemotherapy-induced onycholysis: the UK polybalm study. Breast Cancer Res Treat. 2018;171:103–10. https://doi.org/10.1007/s10549-018-4788-9.
Kim J-Y, Ok ON, Seo J-j, et al. A prospective randomized controlled trial of hydrating nail solution for prevention or treatment of onycholysis in breast cancer patients who received neoadjuvant/adjuvant docetaxel chemotherapy. Breast Cancer Res Treat. 2017;164:617–25. https://doi.org/10.1007/s10549-017-4268-7.
Acknowledgements
The authors would like to thank all the women participating in this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data acquisition were performed by Kuikui Jiang, Simei Shi, Qiulian Lin, Peng Sun, Luan Zhang, Xia Liu, Jingmin Zhang, Jiajia Huang, Xiwen Bi, and Wen Xia. Data analysis and interpretation were performed by Kuikui Jiang, Simei Shi, Qiulian Lin, Zhongyu Yuan, Ruoxi Hong, Yanxia Shi, Qianyi Lu, Qiufan Zheng, Shusen Wang, and Fei Xu.The first draft of the manuscript was written by Kuikui Jiang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by Sun Yat-sen University Cancer Center Institution Review Board (No. B2020-002–02). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Competing interests
The authors declare no potential conflicts of interest.
Supplementary Information
Additional file 1:
S1. Frequency distribution of nail changes to various chemotherapy regimens.
Additional file 2:
S2. Hematoxylin and eosin (H&E) staining of the nails of two breast cancer patients. (A) The normal nail of Patient 1 (magnification: ×20). (B) The normal nail of Patient 1 (magnification: ×40). (C) The pigmented nail of Patient 1 (magnification: ×20). (D) The pigmented nail of Patient 1 (magnification: ×40). (E) The normal nail of Patient 2 (magnification: ×20). (F) The normal nail of Patient 2 (magnification: ×40). (G) The pigmented nail of Patient 2 (magnification: ×20). (H) The pigmented nail of Patient 2 (magnification: ×40).
Additional file 3:
S3. Onycholysis induced by chemotherapy.
Additional file 4:
S4. Beau’s lines developed after chemotherapy.
Additional file 5:
S5. Mees’ lines followed by chemotherapy.
Additional file 6:
S6. Muehrcke’s lines observed in a patient who received 4 cycles of epirubicin + cyclophosphamide (EC) without hypoalbuminemia.
Additional file 7:
S7. Subungual hemorrhage after 4 cycles of epirubicin + cyclophosphamide (EC) followed by 4 cycles of docetaxel.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, K., Shi, S., Lin, Q. et al. Nail pigmentation induced by chemotherapy: an observational study of patients with early-stage breast cancer. Holist Integ Oncol 1, 10 (2022). https://doi.org/10.1007/s44178-022-00008-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-022-00008-7