Abstract
The geochemical signatures of a 12-year-old experimental bioreactor at a California landfill are used to identify elemental concentrations and ratios that characterize the landfill and relate it to the age and state of technology of the deposited waste. The bioreactor was constructed and sealed with a synthetic liner during 2001–2002 and operated and monitored as an anaerobic digester to enhance methane production. In 2013, the bioreactor was sampled and trace element concentrations of the extracted fine fractions were determined. The concentrations normalized to a regional soil composition, reveal systematic peaks for transition metals, alkali metals, heavy metals, and various metalloids and non-metals. A group of potential solder elements (Cu, Zn, Cd, In, Sn, Pb, Bi, and Sb) shows moderate to strong co-variations and is largely attributed to household electronic components and other similar products, while elements that correlated well with rare-earth and other elements are related to the diluting effect of a soil component used as cover. Batteries show modest to little effects on the overall concentrations. Circulating fluids (recycled leachate) in the controlled reactor did not completely redistribute and homogenize the elemental signatures within the time frame of the bioreactor. It is concluded that the present experimental landfill defines an Anthropocene marker identifiable by building material (plaster), PVC plastic, and household electronic components (Pb–Sn solder). These marker elements and ratios are variably diluted by soil components identified by alkali metals, rare-earths, and high field-strength elements (Hf, Zr, Nb, and Ta).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The geological times in which we live have profoundly been shaped by our own agricultural and industrial activities. This has happened to the extent that technological signatures have been imprinted on contemporaneous human deposits (Hogland et al. 2004; Krook et al. 2012; Jones et al. 2013; Zalasiewicz et al. 2014, 2016; Waters et al. 2014; Worm et al. 2017) as well as on our life-supporting hydrosphere, biosphere, and global climate (Vitousek et al. 1997; Peters and Meybeck 2000). This human effect on the environment has been so impactful that the present geological times have been proposed to be identified by a new stratigraphical epoch, following the Holocene, and informally referred to as the Anthropocene (Crutzen 2002; Erlandson and Braje 2013; Smith and Zeder 2013; Malhi 2017).
Here we examine the geochemical and technological signatures of a 12-year-old, experimental bioreactor in a California landfill (Seiser and Yazdani 2015) in light of stratigraphic indicators for the Anthropocene. The bioreactor cell was constructed during 2001–2002 and sealed with a synthetic liner and operated and monitored as an anaerobic digester to enhance methane production. The reactor thus provides a test-bed for formation of an “event layer with long-term preservation potential” (Zalasiewicz et al. 2016).
2 Study Site
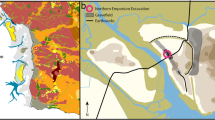
Twelve-year-old partially degraded wastes were collected in 2013 from the experimental bioreactor cell at the Yolo County Central Landfill in Woodland, California (West Cell; Yazdani et al. 2003; Seiser and Yazdani 2015). The anaerobic cell occupies six acres (2.4 ha) and was filled to a depth of 50 ft (15 m) with 193,000 tons waste during 18 months in 2001–2002. The fill was typical residential, commercial, and industrial waste (Table 1) as received at the landfill from throughout the county. The typical daily addition received at the reactor was about 500 tons originating from four cities and unincorporated portions of the county that had residential curbside recycling to remove some fractions of paper and cardboard, metals as cans and similar products, and polyethylene plastics. Additional sorting of waste was not in use at the landfill during the reactor construction. The cell also includes about 14–15% green waste used as temporary covers during waste filling. When soil was used as a temporary cover, it was mostly removed before the next filling event. The reactor leachate was collected by a network of pipes and recycled if needed with additional water distributed in the upper section to control moisture content.
3 Materials and Methods
3.1 Sampling and Sample Preparation
A total of three locations (Holes 1–3) in the cell were selected and sampled at 2–4 depth intervals giving a total of 9 depth samples of the digested material for further size separation. The holes were located on top of the cell and away from internally buried pipes and instrumentation wires (Fig. 1A). Representative sampling of the digestate was done by digging using a backhoe into the main body to a depth of a maximum of 97 inches (2.4 m), after removing the soil cover and breaking the synthetic sealing. At each specific depth, large metal, wood, and rock pieces were removed and the remaining material was air-dried and repeatedly divided into quarters until fitting into a large 50-gallon trash bag. This material was manually sorted by size and type (paper, cardboard, wood, textile, plastic, Styrofoam, metal, soil components, glass, and a fine fraction) as summarized in Table 1. The fine fractions, defined by below a few centimeters, in turn were further reduced using a riffle splitter into managable sizes. Following sampling, the holes were refilled with the remaining waste and the cell was restored to its original condition.
The bioreactor cell and sampling locations after Seiser and Yazdani (2015). A Anaerobic bioreactor cell (West Cell reactor of Yolo County landfill) scaled to 1:100, with surface contours in inches, and with the locations of the three sampled holes. B Depth of surface locations in the 50 feet deep (15 m) reactor and sampling intervals in inches together the maximum particle sizes in mm used for preparing the studied powders
The summary of the make-up of the solid landfill components in Table 1 shows that by weight, paper products are the most common component, followed by plastic, wood, and metal. The fine fractions are the subject of the present study. They were oven-dried at 105 °C to determine the remaining moisture content. It is assumed that the fine fractions contain many of the waste components that were also contained in the larger-sized fractions given in Table 1. The fines were sieved into mm-size fractions (2.4, 4.8, 6.3, 9.5, 12.5, 19 mm) and riffle split into workable volumes for analysis. Based on practical considerations, a total of two samples were retrieved from Hole 1, seven samples from Hole 2, and seven samples from Hole 3 ranging from 2.4 to 19 mm (Fig. 1).
Air- and water-flotation procedures were used in order further to remove heavy components (glass, silicates, and metals) and to obtain material that potentially is reactive and prone to impact groundwater in an unforeseeable leachate release. The smaller fractions (2.4, 4.8 mm) were separated in a fluidized bed operated by air. The larger fractions (≥ 6.3 mm) were separated using a water bath. The separated light fractions were re-dried and used for all subsequent analytical work.
Subsequent milling was performed to obtain a powder passing a 40-mesh sieve (0.42 mm) appropriate to further analysis. Mechanical processing of the powder was undertaken with a lapidary-style rotary rubber mill with 9/16″ (14 mm) 440C hardened stainless chrome steel balls for at least 50 h. Further, hand mortar and pestle or coffee grinder was used as needed to further reduce the powder size. A total of 16 powders were prepared for chemical analyses, spanning the lateral and vertical depth as well as grain size variation, of a single anaerobic bioreactor. Further details concerning sampling and sample preparation are given in Seiser and Yazdani (2015).
3.2 Analytical Techniques
The milled, fine-ground, light fractions were analyzed for proximate (ASTM D4442, E1755, and E872) and ultimate compositions (ASTM D3176). Some trace elements for selected samples were also measured on the crude powders in order check for elemental losses during ashing. These were Cl (ASTM D2361), Se (SW846‐7742), and Hg (EPA 7343 and ASTM D6722). Crude powder analyses were renormalized on a dry ash basis for direct comparison with dry ash-based trace element analyses.
Chlorine was analyzed by titration with silver nitrate and selenium by the atomic absorption method. The ultimate and elemental Cl and Se analyses were performed by Hazen Research Inc. (Golden, Colorado). Mercury concentrations were determined on crude powders using gold amalgamator and atomic absorption methods (LECO AMA254 Mercury Analyzer) that conform to requirements by Environmental Protection Agency Method 7343 (Mercury in Solids and Solutions) and ASTM D6722 (Total Mercury in Coal and Combustion Residues). Calibration was done using NIST 1633b fly ash (certified value for Hg is 143 ppb). The analytical precision is within 2 ppb with a lower limit of detection of 5 ppb (Thy and Jenkins 2010).
Selected thermogravimetric analyses (TGA) were likewise performed on the crude powders using a PerkinElmer Diamond TG/DTA Analyzer. About 20 mg powder was placed in an open alumina crucible and heated in purge air with a 200 ml/min flow rate and a constant heating rate of 10 °C/min from ambient temperature up to a final temperature of 1000 °C. Temperature was calibrated against the melting points of indium and gold. The mineralogy of the powders was characterized by X-ray diffraction with a PANalytical instrument with an anode of Cu and operated in continuous mode.
The ash and volatile contents of sample powders as well as for NIST standards SRM 1645 (river sediment) and SRM 2709a (San Joaquin soil) were determined by weight loss after firing in air at 575 °C. The elemental analyzes were done on these ash powders and are reported on a dry ash basis. Silica and other major elements were determined with micro-X-ray fluorescence (µ-XRF) using a standard-less method normalizing to 100%.
The dry ash powders were prepared for analysis by inductively coupled plasma mass spectrometry (ICP-MS) by dissolving 40 mg powder in a 1:9 HNO3/HF mixture, respectively. The dried residuals were re-dissolved in 3% HNO3, diluted by a factor of 50 and analyzed on an Agilent 7900 quadrupole ICP-MS in helium gas mode to minimize interferences. Calibration curves were constructed using a series of elemental dilutions (ranging from 10 ppb to 2 ppm) prepared from TraceCert Periodic Table mix 1, TraceCert Periodic Table mix 2, SPEX CertiPrep ICP-MS Calibration Standard 3, Agilent technologies Multi-element Calibration standard 1 as well as PlasmaCAL U single element solution. To monitor and correct for drift, a solution containing 100 ppb Rh (rhodium; PlasmaCAL) was introduced as the internal standard during analysis by mixing it with the sample solutions via a peristaltic pump. All reagents used throughout were distilled acids mixed with 18.2 MΩ water. Standards and blanks were run interspersed with sample solutions and the final analyses reduced using the Agilent software package. The certified NIST standards SRM 1643f (trace elements in freshwater) and SRM 1645 (river sediment) were run in the beginning and end of the run for assessing reproducibility (n = 2). These standards showed reproducibility in the order of 6% for most elements. Exceptions include terbium (Tb), holmium (Ho), thulium (Tm), tungsten (W) and tantalum (Ta) with reproducibility above 10% (NIST SRM 1645). Certified and known NIST standards run for assessing accuracy (SRM 1643f, SRM 1645, SRM 2709a, as well as USGS basalt standards BCR-2 and BHVO-2 typically show accuracies below 6%, except for B, Al, Fe, Mo, As, Cd, In, Sb, Cs, Ta, and Bi with accuracies above 10%. Palladium (Pd), rhenium (Re), iridium (Ir), platinum (Pt), and gold (Au) were not detected in any of the ash powders. The analytical ICP-MS results for the standards are given in Supplementary Table S1 as measured values and standard deviations compared to certified or recommended values. The results for the bioreactor powders are given in Supplementary Table S2, including preparation methods, moisture, and the ash content.
4 Results
4.1 Proximate and Ultimate Analyses
There are significant differences between the proximate analyses for the extracted material based on the two flotation methods (Table 2). The ash content for the air-floated powders (77 ± 6%) is more than double that of the water-floated powders (31 ± 10%) with corresponding lower combustible or volatile contents. This is further reflected in the ultimate results giving C/O ratios, respectively, of 0.8 and 1.5 on a weight basis. This is attributed to the preferential separation of a higher amount of plastic material during air-floated versus water-floated. The separated components are estimated to be 63.7 wt% C, 7.4 wt% H, 1.3 wt% N, and 23.1 wt% O and closely matches high-oxygen plastic types such as PETE (polyethylene terephthalate or polyester) (Arter 2011; Roosen et al. 2020) widely used in bottles, containers, bags, and synthetic clothing products and not recycled through the curbside recycling program. The relatively low ash content and low metal content of such plastic explain why the two groups of flotated powders are compositionally similar (Table 2).
4.2 Thermal Gravimetric/Differential Thermal Analyses
The mass loss (%) and heat flow (mW) for selected powders are shown in Fig. 2 as obtained using a differential thermal analyzer (DTA) with air as the carrier gas and temperatures from ambient to 1000 °C. The DTA results (Fig. 2A) illustrate that the two groups of flotated powders are identified by their ash contents. The mass loss for the two groups shows similarities despite highly variable ash contents with an initial dehydration to 115–129 °C, followed by a slightly declining plateau from 224–236 °C, after which decomposition is initiated. A stable plateau is reached at about 629–668 °C, after which the mass loss reaches near-constant ash content. This conforms well to values obtained using conventional box furnace methods at 600 °C. Results for fibrous cellulose are shown for comparison (Fig. 2A) and illustrate an initiated decomposition from 291 °C and fully reached the ash stage at 517 °C, presumably support the observation of the high plastic and low cellulose contents of the fine powders.
Thermal gravimetrical and heat flow analysis of raw landfill powders (air-dried) from ambient to 1000 °C in air. A Mass loss in wt. % of the original volume. B Corresponding heat flow (mW). The red curve is for standard cellulose, gray curves are the individual powders, and black curves are averages as marked. See text for details
Most of the analyzed powders show marked effects of intermittent ignitions only lasting a few minutes (Fig. 2A). The air-flotated powders ignited between 285–292 and 317–327 °C, lasting for 2–3 min before the instrument was able to resume ramp control. The water-flotated powders suffered from an additional short ignition interval between 374–380 and 394–400 °C (1–2 min). The fibrous cellulose also saw an intermittent ignition interval between 331 and 363 °C (3 min). When the same DTA treatment was performed using argon as the carrier gas, none of the powders as expected exhibited detectable ignitions.
The heat flux measured in milliwatts (mW) is shown in Fig. 2B with major positive exothermic excursions at 311–320 °C and minor excursions at 350–400 °C. The total energy release from the air-flotated powders ranges from 12.2 to 25.8 MJ/kg, while the water-floated powders are higher by a magnitude of about two from 35.4 to 53.4 MJ/kg. The lower plastic content of the air-floated powders can explain these heat flux differences. The heat value obtained from the fibrous cellulose is 15.5 MJ/kg, which is similar to values reported by e.g., ASME (1978) and Kim et al. (2017). High heating value (HHV) analyses using constant volume, adiabatic calorimetry of homogenized municipal solid waste (Hlaba et al. 2016), synthetic waste (Arter 2011), or calculated from source-segregated components (Farrell and Jones 2009; Jones, 2010; Götze et al. 2016), all suggest values of 15–22 MJ/kg in the low range of what we obtained. This is attributed to the fact that inert components (e.g., metal and glass) have been partially removed during the early phases of sampling.
The probable combustible components of the reactor powders can be identified by their decomposition characteristics and energy releases. The decomposition initiated at about 224 °C with a heat release peak of about 310–320 °C is attributed to the decomposition of cellulose-rich materials, such as paper, wood and plant material, and food waste, while the minor heat release peek at 350–400 °C is attributed to breakdown of the dominant polyester plastic components, consistent with previous studies of municipal solid waste (Thipse et al. 2002; Chen et al. 2015; Tang et al. 2017; Eriksen and Astrup 2019).
4.3 Inorganic Mineralogy
The mineralogy determined by XRD of water-floated powder (LF 443) after combustion is shown in Fig. 3. The dominant crystalline phases are quartz (32.1 wt%), plagioclase (20.3 wt%) and pyroxene (42.0 wt%), while anhydrite is found in minor amounts (5.5 wt%). Other phases may be present at below 3%, but cannot be detected by our powder diffraction method. Furthermore, significant, but not quantified, amount of amorphous material is likely to be present as indicated by the shape of the intensity curve (Fig. 3). Of these minerals, anhydrite (or gypsum, Ca and S), possibly from wallboard or sheetrock construction materials, is the only phase that can be associated with to human activity (Zalasiewicz et al. 2014; Hazen et al. 2017).
XRD pattern (Cu Kα radiation) for LF 443 fired at 575 °C. Measured d values used to identify specific peaks are in Ångstrom. Diffraction patterns for low temperature quartz, plagioclase (labradorite), pyroxene (augite), and anhydrite are identified. Intensity is counts per second and 2-Theta (2θ) is in degrees
4.4 Elemental Concentrations
After firing at 575 °C, the residual powders were analyzed for major, minor, and trace elements using mostly ICP-MS. The results for individual powders are given in Supplementary Table S2. Because variations with location and depth in the reactor are not observed (see a subsequent section), the results in Table 2 are reported as averages for the two flotation methods. These averages are further illustrated in Fig. 4 normalized to a standard Central California San Joaquin Valley soil (NIST SRM 2709; Supplementary Table S1) composition and arranged by increasing atomic number. Elements with more than double the concentration of the San Joaquin Valley soil include the transition metals Cr, Cu, Mn, Fe, Co, Ni, Zn, Ag, Cd, and Mo, alkalic metals Li and Ca, other metals In, Sn, Pb, and Bi, and various metalloids and non-metals B, P, Cl, and Sb (Fig. 4). The intra-variation for these elements is illustrated by the Pearson’s correlation coefficients (Supplementary Table S3). Strong positive correlations for which complete or near complete data sets (N ≥ 14) are available for the alkali and alkaline metals Li–B (0.66), Li–Sr (0.84), Be–Sc (0.90), B–Ca (0.82), and B–Sr (0.78). Of the remaining metals, strong positive correlations are seen for Mg–K (0.79), Mg–Ti (0.76), Mg–Sr (0.92), K–Ni (0.77), Ca–Ti (0.77), Ca–Sr (0.92), and Rb–Cs (0.81). Only few of the transition elements show strong positive correlation with other elements: Sc–Rb (0.93), Sc–Cs (0.91), Fe–Ag (0.87), Zn–Mo (0.78), Zr–Hf (0.99), Mo–Cd (0.78), and Nb–Ta (0.77). Among the remaining elements, only In and Sn show strong correlations with Bi: In–Bi (0.89) and Sn–Bi (0.82). There are additional notable correlations for several elements with and among the rare-earth elements (REE) that also appear to correlate with Si, although a consistent data set (N = 8) for the latter element is not available. Some of these element pairs of particular interest are illustrated in Fig. 5.
Averages and upper standard deviations of elemental concentrations normalized to San Joaquin Valley soil (NIST SRM 2709) plotted on a log10 scale against atomic number (Z). A few elements (Be, Tm, and Ta) used for the normalization are from average continental crust (Rudnick and Gao 2003). Black results (N = 12) are the water-floated powders and the gray results are the air-floated powders (N = 4)
4.5 Contamination from Milling
Because prolonged grinding was required to homogenize and reduce the original particles to a manageable grain size for analysis, contamination needs to be considered (Ammerman et al. 1970; Hickson and Juras 1986; Iwansson and Landström 2000; Waterlot et al. 2012; Bich et al. 2019). A lapidary-style rotary rubber mill with 9/16″ (14 mm) with 440C stainless steel balls was used for grinding the powder to pass through a 40-mesh sieve (0.42 mm). Because of these stainless steel balls, several elements may potentially have contaminated the final analyzed powders, including Fe, Cr, Ni, Mn, and Mo; however of these, only Cr and Mo show prominent high values above twice the standard soil composition (Fig. 4). Chromium has modest intra-element correlations (Table S3) (0.40–0.51) with other transitional metals (V, Mn, Co, Ni, and Cu), but correlates poorly with Mo (0.12). These correlations may result from either intrinsic landfill make-up or from steel contamination. The stainless steel balls are dominantly made up of Fe (76.7–82.7 wt. %), Cr (16–18%), Ni (0–0.8%), Mn (0–1%), and Mo (0.4–0.8%). Thus, contamination yield Cr/Fe slopes of 0.19–0.23, while other elements would be less affected given their low abundances (Mn/Cr slopes of 0.06; Ni/Cr slopes of < 0.06, and Mo/Cr of 0.03–0.05). The observed slopes for these elements are Mn/Cr of 0.09 (R2 = 0.186), Ni/Cr of 0.02 (R2 = 0.153), and Mo/Cr of 0.001 (R2 = 0.015), while all these elements correlate negatively with Fe. The lack of correlation with Fe can be attributed to the small surface areas analyzed by μ-XRF and heterogeneous distribution of steel particles in the powder (e.g., Bich et al. 2019).
Figure 6 shows the interpretation of the Ni–Cr relations and the effects of milling on powder compositions. The Cr content of the landfill powders shows a strong variation from about 250 ppm to high contents of 3,500 ppm, relatively independent of a restricted Ni content of 150–250 ppm. This is attributed to contamination from a low-Ni stainless steel. A maximum Cr steel assimilation of about 2% will result in 3,500 ppm Cr, < 160 ppm Ni, < 200 Mn, and 80–160 ppm Mo, which is reasonable given our observations for Cr and Ni (Table 2). The observations thus suggest an intrinsic reactor fine fraction content of ~ 250 ppm Cr and ~ 150 ppm Ni (Fig. 6). These values exceed the contents of typical biomass (Fig. 6; Thy et al. 2013a, b), but are consistent with maximum values observed in municipal solid waste (MSW) and from other similar landfill composts and fines (Farrell and Jones 2009; Arter 2011; Götze et al. 2016).
Illustration of the effect of stainless steel milling on the Ni versus Cr concentrations with comparisons of biomass, Yolo bioreactor material, and municipal solid waste (MSW) (Farrell and Jones 2009; Arter 2011; Thy et al. 2013a,b; Vassilev et al. 2014; Götze et al. 2016). The two stainless steel compositions are standard industrial steels with the Cr steel used in the present study shown for its maximum Ni content of 0.8 (McMaster-Carr, Hardened Bearing-Quality 440C Stainless Steel Ball). The hexagonal symbol is the inferred intrinsic composition of the landfill waste
4.6 Effects of Sampling Depth, Particle Size, and Flotation Method
The fine fractions of the residual material of the bioreactor were sampled as far as possible both laterally and horizontally (Fig. 1) with the intent to examine the compositional variation and possible elemental migrations during hydrothermal digestion processes. Where sufficient material was available, the elemental distribution controlled by grain size up to 19 mm in Holes 2 and 3 were additionally examined. There is a small, but notable, positive correlation between the combustible components and grain size and consequently negative correlation between ash content and grain size (Seiser and Yazdani 2015). Likewise, the combustible component is markedly lower for the air-floated compared to the water-floated fractions and vice versa for the ash content. The lower Cl content (Fig. 4) reflects the preferential removal of Cl-bearing PVC plastic components by the air-flotated powders. However, despite the considerable sampling and separation efforts, there are no detectable correlations among sampling depth, grain size, and elemental concentrations. As a consequence, the compositions are for the purpose of the present study summarized as averages for the two flotation methods in Table 1 and illustrated in Fig. 4. It should be noted, however, that sampling was restricted to the upper 16% (8 ft) of the total bioreactor.
5 Discussion
5.1 Background Soil Composition
To evaluate the Yolo landfill reactor (and other landfill deposits), the background soil composition is an essential, although an abstract and elusive component. For this reason, the analytical results are normalized to a reasonable regional soil composition (Fig. 4). The composition used is the only existing high quality soil analysis from the Central Valley of California that includes most of the trace elements of relevance in the present context (NIST SRM 2709a, San Joaquin soil; Supplementary Table S1). This is an approximation, considering the variable local soil types and their compositions (Shacklette and Boerngen 1984; Bradford et al. 1996; Gustavsson et al. 2001) and the few if any detailed chemical analyses available. However, the soil composition used appears to function reasonably well in pinpointing the elements in concentrations exceeding background.
5.2 Intrinsic Concentrations and Ratios
There are two groups of elements believed to have been unaffected by sampling and preparation methods. The first records the intrinsic values of soil, biomass and other organic wastes. This group includes several elements only moderately enriched (Fig. 4; < 2 times) relatively to average soil (Bradford et al. 1996; Gustavsson et al. 2001) and continental crust (Rudnick and Gao 2003). These elements most prominently include the alkali and alkali earth metals (Be, Na, Mg, K, Rb, Sr, Cs, and Ba), the rare-earth group (La–Lu) with addition of Y, some transition elements (Sc and V), and high field-strength elements (Hf, Zr, Nb and Ta). Most of these elements show moderate to strong intra-correlations (Table S3). This is seen, in particular, for the transition metals Sc and V as well as for most of the rare-earth elements (Fig. 5) and suggests that the concentrations of these elements are related to dilution of a soil component by one or several components with rather similar concentration or ratios of concentrations.
The second group records the effects of anthropogenic materials advertently or inadvertent admitted with the landfill waste (Fig. 7). Most solid components of landfills (paper, food waste, biomass, MSW) are all characterized by very low, but detectable, concentrations of high field-strength elements (e.g., Zr) and the rare-earth elements. By comparison, groundwater and plastic components have lower concentrations (Fig. 7) that are commonly below the detection limit of our analytical methods. This is expected since biomass (and food by-products) together with general food waste, generally lack Zr and rare-earth elements given their low nutritional value for plant growth (Gough et al. 1979; Marschner 2012). In contrast, these elements are concentrated in the soil where they originate from weathering and denudation of silicate rocks (Taylor and McLennan 1985). A consequence is that the abundance of elements, such as Zr and Sm, in the landfill material can be used to assess the relative proportion of soil to decomposed organic material.
Selected elements from Fig. 4 occurring in low normalized concentrations (< 2) in the Yolo bioreactor fine fractions compared to: certified values for fresh water standard NIST SRM 1643f; average compositions for biomass from Thy et al. (2013a); and municipal solid waste (MSW) as well as average of paper products and food waste represented by dog food from Arter (2011), analyzed and prepared in a similar fashion as Thy et al. (2013a)
Composition of degraded biomass as measured by the corresponding ash content (Thy et al. 2013a) suggests a dominating raw organic component initially admitted to the digester and a soil component of largely 10–11%, but depends on the type of biomass and the elements used in the estimation. This soil component is likely to have originated from temporary soil cover during construction of the reactor, as was a required daily procedure at the landfill. The soil component added to the landfill has no implications for fingerprinting of an Anthropocene context.
5.3 Solder Alloys
Several elements that appear in concentrations above twice the normalizing soil can be associated with solder (Humpston and Jacobson 2004; Jacobson and Humpston 2005) widely used in mechanical and electronic hardware devices to bind together metal and ceramic components due to their low melting points as pure metals or as alloys. These are notably Pb, Zn, Sn, Sb, Cd, Bi, and In (Fig. 4). Because solder metals often are alloys made up of a dominating metal with one or several subordinate metals (Bi, In, Pb, and Sn), elemental correlations are expected among common solder metals. The most commonly used solder is Pb-based, often in its pure or near pure form, but with variable amounts of Sn (12–63%), Sb (11–12%), and In (25–80%) (Humpston and Jacobson 2004, their Table 2.2). The observed correlation coefficients for some of these pairs correlate modestly: Pb–Sn (0.42), Pb–Sb (0.53), and Pb–In (0.32) (Supplementary Table S3). The covariation for Pb–Sn is shown in Fig. 8A. With the exception for two analyses (LF 345 and 387), the analyzed fines have elevated Sn and Pb contents compared to typical biomass compositions (Thy et al. 2013a). Most prominently, Pb shows strong enrichment, independent of Sn, toward very high values (> 260 ppm Pb), similar to an average landfill composition based on the literature review by Götze et al. (2016; 192 ppm Pb). This supports the presence of a strong Pb-based solder signature in the reactor cell. Three analyses stand out by showing concurrent enrichment in Sn (LF 442, 443, and 450 with 200–415 ppm Sn) suggesting a strong component of Pb–Sn solder in some of the fines (Fig. 8A). Pb and Sn solders, or their mixed recipes, dominate by far the solder signatures in the landfill fines (~ 95%), although other solder metals may also be indicated by moderate to strong correlation coefficients (Pb–Sb 0.53; Pb–In 0.32; Sn–Bi 0.82; and Sn–In 0.49).
Summary of solder interpretation of the fines from the Yolo bioreactor with comparisons. A Pb–Sn. Not shown is LF 383 (51 ppm Sn, 1460 ppm Pb). B In–Sn. C Bi–In. Comparisons are shown where analyses are available and further shown at the lower limit of detection from Thy et al. (2013a, b), Vassilev et al. (2014), and Götze et al. (2016). See text for discussion
The variations of In with Sn and with Bi are shown in Fig. 8B and C, respectively. The concentrations of these elements are also higher than typical organic and soil components despite the restricted data for in Figs. 4 and 8. There are, however, three fines that show elevated concentrations and positive linear correlations for all solder elements (LF 442, 443, and 450; Fig. 8). These fractions were obtained from the same depth level in hole 3 and are consistently high for the three separated grain sizes (Supplementary Table S2). These powders are convincing evidence that solder has affected the concentrations of Pb, Sn, In, and Bi and that traces of other solder recipes have left an imprint on the composition of all the bioreactor powders, as opposed to other source components like lead in paint and ceramic products.
Two other potential solder elements (Sb and Cd) also occur in elevated concentrations compared to typical soil and other organic sources (Fig. 4). Antimony has properties rather similar to Sn and is thus often added in small amounts to reduce cost as reflected by positive correlations with Sn and Pb (Pb–Sb 0.53; Sn–Sb 0.24; Supplementary Table S3). Cadmium on the other hand is (or has been) used in Sn, In, and Zn solders, but its beneficial effect in solder mixtures was unclear and has been phased out due to health concerns (Humpston and Jacobson 2004). Despite this, there is a reasonable correlation for Sb–Cd (0.54), Pb–Cd (0.25), and Sn–Cd (0.19) (Supplementary Table S3).
Zinc is the final metal that appears in relatively high concentrations (Fig. 4). There are moderate correlations between Zn with other common solders (Cd 0.69, Sb 0.46, In 0.44, Bi 0.41, Sn 0.36, and Pb 0.29). This element, together with Cu, is often used as a high-temperature solder to bind together aluminum components in electrical and hardware components. However, the widespread applications of Zn for other purposes, such as for galvanizing steel to prevent rusting, makes it uncertain if these correlations reflect solder alloy mixtures or other components used in individual hardware products.
5.4 Batteries
It was expected that batteries would have affected the compositions of fines in the bioreactor, despite the fact that programs to divert batteries from landfills in California have been in effect for many household hazardous wastes, including batteries, since 2006. A group of elements with low atomic numbers (Cl, Li, and B) shows elevated concentrations (Fig. 4); however, because of their multiple applications, it is not possible to pinpoint specific sources. Although chlorine often is elevated in biomass particularly in an arid climate like the Central Valley of California with crops produced under irrigation (Thy et al. 2013b), it is perhaps more likely that the main concentrations of Cl are, as already noted, caused by the large amount of PVC plastic in the reactor (Table 1). Lithium is observed in slightly elevated concentrations (Fig. 4) and shows very low or negative correlations with other potential battery elements (Supplementary Table S3). Both Li and B are used in multiple industrial products from batteries in portable electronic devices, to ceramics and specialty glasses, fiberglass, and additionally cleaning products. Because there are multiple uses of these elements, it is not possible to identify either unique or combined sources. The correlations between Ni–Zn (0.46), Ni–Cd (0.61), and Zn–Cd (0.69) (Supplementary Table S3) are moderately high and may signal mixed Zn and Ni–Cd batteries, although the later Zn–Cd correlation can also be related to solder metals as noted above.
The concentrations of Pb show low correlations with other battery components (Pb–Ni 0.19; Pb–Zn 0.29) and are within the ranges observed by Götze et al. (2016) and Farrell and Jones (2009) for landfills. It is therefore difficult to specifically attribute Pb to lead–acid batteries or to other sources including plumbing, ammunition and paint pigmentation. The observation of high Pb concentrations and strong correlations between Sn and Pb at least at one level in the landfill leads us to conclude that the Pb content is due to solder and not to batteries.
The lack of strong evidence for battery components in the landfill was surprising and may attest to the effectiveness of programs to divert batteries from landfills in California.
5.5 Hydrothermal Reactions and Migration
Degradation of the organic components in the Yolo bioreactor was probably controlled by recirculating leachate and the addition of water as needed to maintain the moisture content at about 50% capacity (Yazdani et al. 2003; Seiser and Yazdani 2015). For this reason, when the bioreactor was sampled after 12 years the decomposition of the organic component, perhaps with the exception of wood, was likely to have reached anaerobic maturity and to have seen a decrease in microbial activity and the production of H2O vapor, as well as CO2 and CH4 gases. Another expectation is that compaction because of the restricted size and overall shape of the digester must have occurred unevenly in the digester. At the time of sampling, the leachate was probably neutral to basic and contained various organic components, inorganic macro- and heavy metals, and xenobiotic, human-derived components (Kjeldsen et al. 2002). Temperature was just below 50 °C as is typical for landfills (Yazdani et al. 2003; Kjeldsen et al. 2002; Zekkos et al. 2010).
The solder components bearing heavy metals were discretely distributed in the waste and may have been mobilized by reaction with the circulating fluids (Fig. 8). It is therefore possible that corrosive reactions may have resulted in transport of free ions or complexes by the leachate (Holm et al. 1995). The solder components may have been associated with copper and brass wires and tubing or with laminated electronic circuit boards where the main components often were resin and other non-conductive materials. In the later case, the circuit boards were prone to corrosion and breakdown, particularly in high moisture environments, with the result that larger components could have contributed to the fine fractions (< 20 mm) extracted from the bioreactor. This could explain the presence of solder components in the analyzed fine fractions and is also supported by the observation that their highest concentrations independent of grain size are observed at one horizon (51–60 in. or 1.3–1.5 m depth) in hole 3 only. The solder elements thus originated locally and did not migrate and redistribute widely with the circulating and replenishing leachate within the time frame for the reactor. Additionally, we find marked lateral variations in concentration within the reactor presumably due to the uneven distribution of waste components.
5.6 Time Scale
Although the artisan use of lead dates back to antiquity (Aitchison 1960; Tylecote 1992), its use has escalated during the industrial post-war period as chemical additives, batteries, plumbing, and other consumer products have become more prominent (National Academy of Science 2017). Because of health issues (Mielke 1999), several recent legislative initiatives have been passed in an effort to eliminate lead addition to the environment. The use of lead additives in gasoline and other fuels declined in the US in the 1970s after the US Environmental Protection Agency (EPA) mandated that new cars should run on unleaded gasoline and further declined after 1986 when the sale of leaded gasoline was banned. The use of lead-based white paint has for the same reasons also markedly declined in the US since the 1930s (Mielke 1999; National Academy of Science 2017) and is today virtually eliminated as a source of lead in landfills. Likewise, since the early 1990s, there have been greater efforts to recycle lead–acid batteries that eliminated batteries as a source of lead in landfills (Wilburn 2014; Turner 2015; Zhang et al. 2016). The subsequent years have seen increasingly stricter US federally imposed limits for lead in disease prevention, occupational safety, and health standards that appear to have culminated in 2012 with the Center for Disease Control’s (CDC) upper value for the lead level in child blood (ATSDR 2020).
Cadmium is another toxic element that can lead to severe kidney dysfunction. Cadmium has mainly been used in batteries and in corrosion resistant coatings in addition to many other technological applications. Because Cd has a high geochemical mobility in acidic surface water and has chemical properties similar to Zn, Cd may substitute for Zn in organic material where Zn, in contrast to Cd, is an essential micronutrient. This may eventually lead to accumulation of Cd in the human food chain (ATSDR 2012; Genchi et al. 2020). It is thus possible that the observed correlation between Zn and Cd (0.69) can be attributed to substitution effects in plant and animal tissues. Like Pb, Cd has been regulated due to its human toxicity. Around 2007–9, EPA and Occupational Safety and Health Administration (OSHA) designated Cd as a hazardous substance and placed limits on the daily human intake and workplace exposure (ATSDR 2012). It has been estimated that about 27% of the industrial consumption of Cd was recycled in 2007, including Ni–Cd batteries (USGS 2021).
Because of the positive correlations between common solder elements, Pb and Cd in the Yolo reactor are primary attributed to solder mixtures like Pb–Sb–Sn, Sb–Zn–Cd, and Zn–Cd (Humpston and Jacobson 2004; Jacobson and Humpston 2005). Concurrently with the regulatory attempts to restrict the release of Pb and Cd to the environment, efforts have been made to develop Pb- and Cd-free solder alloys composed of Sn–Ag–Cu with minor and variable amounts of Zn, Bi, Sb, or In (Ogunseitan 2007; Baskin 2007). This effort is motivated by the 2006 banning of the use of lead solder in consumer products in the European Union, California, and China (Ogunseitan 2007). With the progressive elimination of the prominent lead and cadmium sources, such as batteries and paint, from landfills, the contributions from solder lead alloys and other minor sources would be expected to become relatively more prominent in the elemental signals from analyses of the fine fractions of landfills.
As noted, the Yolo landfill bioreactor was constructed during 2001–02. During that time period, attempts to divert batteries and consumer electronics from the landfill had already been effective in California for about a decade for lead and other early types of Zn-based batteries. It is estimated that by the early 2000s, 75% of lead batteries were being recycled (Wilburn 2014). Rechargeable Ni–Cd batteries were commercially introduced on the consumer marked in the 1960’s (power tools and cordless telephones) and by 2001 about 15% of the Cd metal used in batteries is estimated to have been recovered (Wilburn 2009), leaving substantial amounts either retained by the consumer or to be deposited in landfills. The use of Ni–Cd batteries peaked in around 1992 and subsequently declined rapidly with the appearance in the early 1990s of commercial Li–Fe batteries.
The construction of the reactor thus falls into a transitional period where major battery types utilizing Pb and Ni–Cd alloys were increasing being recycled or technologically phased out in consumer products. In the following period, Li–Fe and other lithium-ion type batteries usage in electronic consumer products increased and thus also their potential disposal in landfills. Considering this, from a total of 16 fine fractions from the reactor, perhaps contrary to expectations, none show strong indications of typical battery or combinations of battery characteristics. The low concentrations of Li are entirely consistent with their late introduction and by their longer life expectancy relative to the construction of the reactor. On the other hand, it is possible that Zn and Ni–Cd batteries may have had some impact on the reactor chemistry since these types of batteries peaked around the time of the construction. However, lead batteries were largely diverted from landfill materials because of recycling at the time of the reactor construction. We conclude that solder from small consumer products is the chief source of the heavy element signals found in the fine fractions of the Yolo landfill reactor.
6 Implications for the Anthropocene
The Anthropocene epoch of the geological time scale is proposed to cap the Holocene epoch from a point in time when significant human impact on the Earth's surface strata and ecosystems has become detectable. The human impact on landscape and environment is, however, not synchronous over large areas, but varies temporally and spatially as functions of population growth, population density and technological advancement. The concentrations of heavy trace elements of surface strata have steadily seen a strong increase since World War 2, particularly in proximity to major urban centers. Landfill deposits, however, constitute often only minor volumetric strata that presently may reach about 10% surface area in some highly industrialized regions (e.g., Dijkstra et al. 2019); including household, industrial, mining, and construction waste sites. Although often a minor component of landfills, municipal solid waste and household deposits are nevertheless important traces of human activity because they are able to magnify the effects specifically for heavy and toxic elements and consequently related industrial factors allowing geochemical and technological signatures to be more easily detected than in the regional soil surface.
Our study of a 12-year-old experimental landfill bioreactor has suggested geochemical variables that identify human technology and its impact on the environment that thus have long-term preservation potential as stratigraphic marker (Zalasiewicz et al. 2016). This offers suggestions for mineralogical and geochemical markers of an Anthropocene Epoch. The components that stand out from the soil background are identifiable as building material (plaster), PVC plastic, and household electronic components (Pb–Sn soldering). The early forms of batteries (Zn–Pb–Cd) and the use of lead in paint and plumbing products are less prominent in the fine fraction of the landfill waste. These observations constrain the digester material to be dated from the late 1990s and within a few years to a decade prior the reactor construction.
The geochemical and technological signatures identified in the experimental Yolo bioreactor have limited temporal and spatial extension. The reactor volume is 376,000 m3 (30 hectares) with a depth of 15 m extending over 2.5 ha and thus constitutes only a small fraction of the total Yolo landfill that is estimated to cover 190 hectares to an average depth of 42 m (a total of 37,000,000 m3). Further, the reactor was constructed during a 1–2 year period and was only about a decade old when sampled. In contrast, the Yolo landfill at large has been operated since 1974 and annually receives today about 200,000 metric tons of waste. The annual intake to the bioreactor over a period of 2 years was, however, about 60–70% of the total amount received at the landfill and can be taken to be representative of the waste intake during the period of construction. In contrast, the bioreactor only represents the landfill during a recent decade. The landfill at large may thus include other detectable geochemical and technological Anthropocene markers that were not detected in the bioreactor.
The elemental concentrations of the inorganic fractions of the solid waste will increase as a function of age and the breakdown of the organic components and resulting mechanical compaction. Cellulose and hemicellulose (plant, wood, paper, and cardboard) are degradable within the normal lifetime of a landfill although wood in particular may persist for extended durations. Plastics, aside from those specifically designed to biodegrade under anaerobic conditions, are persistent and will take longer to decompose, although in a deep Earth timeframe they will eventually also degrade. Compaction from increased overburden, creep, and biodegradation is another uncertainty that may also eventually increase the resulting concentrations of the trace elements. Another unknown factor is the possibility that penetrating fluids and groundwater may preferentially leach some elements from the landfilled material. Despite such uncertainty, the collective impact on the trace element concentrations may be restricted and in particular may not significantly affect trace elements ratios, unless selective leaching occurred. Deep geological time is also likely to homogenize the geochemical signatures to a higher extend that observed today in the reactor. Overall, deposits like those in the Yolo bioreactor may offer detectable Anthropocene signatures for future geologists, if we will still exist in the deep geological time frame.
7 Conclusion
The fine fractions extracted from a 12-year-old, experimental, anaerobic bioreactor in a landfill of the Central Valley of California shows that these are mainly composed of wood, paper, and plastic products in addition to a soil component. A multi-element spectroscopic study of the homogenized and fired fine fractions reveals, when normalized to a regional soil composition, systematic concentration peaks for transition metals (Cr, Cu, Mn, Fe, Co, Ni, Zn, Ag, Cd, and Mo), alkali metals (Li and Ca), heavy metals (In, Sn, Pb, and Bi), and various metalloids and non-metals (B, P, Cl, and Sb). Elements that correlate well with rare-earth elements are related to the diluting effect of a soil component used as temporary and permanent covers. These elements include the alkali and alkali earth metals (Be, Na, Mg, K, Rb, Sr, Cs, and Ba), the rare-earth group (La–Lu), some transition elements (Sc and V), and high field-strength elements (Hf, Zr, Nb and Ta). A group of potential solder elements (Cu, Zn, Cd, In, Sn, Pb, Bi, and Sb) is largely attributed to household electronic components and other similar products. Batteries show modest to little effects on the overall concentrations. The age of the reactor was insufficient for the circulating fluids to completely redistribute and homogenize the elemental signatures. It is concluded that the present experimental landfill defines an Anthropocene marker identifiable by building material (plaster), PVC plastic, and household electronic components (Pb–Sn solder).
References
Aitchison L (1960) A history of metals, 2 volumes. Macdonald and Evans, London
Ammerman CB, Martin FG, Arrington LR (1970) Mineral contamination of feed samples by grinding. J Dairy Sci 53:1514–1515. https://doi.org/10.3168/jds.S0022-0302(70)86425-6
Arter D (2011) Torrefaction of simulated separated municipal solid waste. MS Thesis, Mechanical Engineering, University of California Davis (unpublished)
ASME (1978) Thermodynamic data for waste incineration. NBSIR 78-1479. American Society for Mechanical Engineers, Research Committee on Industrial and Municipal Wastes, U.S. National Bureau of Standards, Washington, DC
ATSDR (2012) Toxicological Profile for Cadmium. US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, 2012. https://www.atsdr.cdc.gov/ToxProfiles/tp5.pdf. Accessed Aug 2021
ATSDR (2020) Toxicological Profile for Lead. US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, 2020. https://www.atsdr.cdc.gov/ToxProfiles/tp13.pdf. Accessed Aug 2021
Baskin P (2007) Brazing and soldering today: challenges in attaining lead-free solders. Weld J 2007:58–61
Bich LB, My HPT, Dinh RP, Minh TP, Trong TD, Harper S, Wuhrer R, Huang Q, George L, Holford P, Zhao CC, Mitchell C, Milham P (2019) Trace metal contamination during grinding of plant samples. Commun Soil Sci Plant Anal 50:102–107. https://doi.org/10.1080/00103624.2018.1554671
Bradford, GB, Change AC, Page AL, Bakhtar D, Frampton JA, Wright H (1996) Background concentrations of trace and major elements in California Soils. Kearney Foundation of Soil Science. Division of Agriculture and Natural Resources, University of California. Special Report. https://www.waterboards.ca.gov/water_issues/programs/compost/docs/kearney1996.pdf. Acessed May 2022
Byers HL, McHenry LJ, Grundl TJ (2016) Forty-nine major and trace element concentrations measured in soil reference materials NIST SRM 2386, 2587, 2709a, 2710a and 2711a using ICP-MS and Wavelength Dispersive-XRF. Geostand Geoanal Res 40:433–445. https://doi.org/10.1111/j.1751-908X.2016.00376.x
Chen S, Meng A, Long Y, Zhou H, Li Q, Zhang Y (2015) TGA pyrolysis and gasification of combustible municipal solid waste. J Energy Inst 88:332–343. https://doi.org/10.1016/j.joei.2014.07.007
Crutzen PJ (2002) Geology of mankind. Nature 415:23. https://doi.org/10.1038/415023a
Dijkstra JJ, Comans RNJ, Schokker J, van der Meulen MJ (2019) The geological significance of novel Anthopogenic materials: deposits of industrial waste and by-products. Anthropocene 28:100229. https://doi.org/10.1016/j.ancene.2019.100229
Eriksen MK, Astrup TF (2019) Characterisation of source-separated, rigid plastic waste and evaluation of recycling initiatives: effects of product design and source-separation system. Waste Manag 87:161–172. https://doi.org/10.1016/j.wasman.2019.02.006
Erlandson JM, Braje TJ (2013) Archeology and the anthropocene. Anthropocene 4:1–7. https://doi.org/10.1016/j.ancene.2014.05.003
Farrell M, Jones DL (2009) Critical evaluation of municipal solid waste composting and potential compost markets. Biores Technol 100:4301–4310. https://doi.org/10.1016/j.biortech.2009.04.029
Genchi G, Sinicropo MS, Luria G, Carocci A, Catalona A (2020) The effects of cadmium toxicity. Environ Res Public Health 17:3782. https://doi.org/10.3390/ijerph17113782
GeoRem, Geological and Environmental Reference Materials; MPI für Chemie, Mainz, Germany, http://georem.mpch-mainz.gwdg.de. Accessed Mar 2021.
Götze R, Pivnenko K, Boldrin A, Scheutz C, Astrup TF (2016) Physico-chemical characterisation of material fractions in residual and source-segregated household waste in Denmark. Waste Manag 54:13–26. https://doi.org/10.1016/j.wasman.2016.05.009
Gough LP, Shacklette HT, Case AC (1979) Element concentrations toxic to plants, animals, and man. Geological Survey Bulletin 1466, United States Printing Office, Washington DC
Govindaraju K (1994) 1994 Compilation of working values and sample description for 383 geostandards. Geostand Geoanal Res 18:1–158. https://doi.org/10.1046/j.1365-2494.1998.53202081.x-i1
Gustavsson N, Bølvikes B, Smith DB, Severson RC (2001) Geochemical landscapes of the conterminous United States—new map presentations for 22 elements. U.S. Geological Survey Professional Paper 1648. http://pubs.usgs.gov/pp/p1648/. https://doi.org/10.3133/pp1648
Hazen RM, Grew ES, Origlieri MJ, Downs RT (2017) Outlooks in earth and planetary materials. On the mineralogy of the “Anthropocene Epoch.” Am Miner 102:595–611. https://doi.org/10.2138/am-2017-5875
Hickson C, Juras S (1986) Sample contamination by grinding. Can Mineral 24:585–589
Hlaba A, Rabiu A, Osibote OA (2016) Thermochemical conversion of municipal solid waste—an energy potential and thermal degradation behavior study. Int J Environ Sci Dev 7:661–667. http://www.ijesd.org/vol7/858-S0014.pdf. https://doi.org/10.18178/ijesd.2016.7.9.858
Hogland W, Marques M, Nimmermark S (2004) Landfill mining and waste characterization: a strategy for remediation of contaminated areas. J Mater Cycles Waste Manag 6:119–124. https://doi.org/10.1007/s10163-003-0110-x
Holm PE, Andersen S, Christiansen TH (1995) Speciation of dissolved cadmium: interpretation of dialysis, ion exchange and computer (GEOCHEM) methods. Water Res 29:803–809. https://doi.org/10.1016/0043-1354(94)00205-L
Humpston G, Jacobson DM (2004) Principles of Soldering. ASM International, Ohio. https://doi.org/10.31399/asm.tb.ps.9781627083522
Iwansson K, Landström O (2000) Contamination of rock samples by laboratory grinding mills. J Radioanal Nucl Chem 244:609–614. https://doi.org/10.1023/A:1006769401251
Jacobson DM, Humpston G (2005) Principles of Brazing. ASM International, Ohio. https://doi.org/10.31399/asm.tb.pb.9781627083515
Jones PT, Geysen D, Tielemans Y, Van Passel S, Pontikes Y, Blanpain B, Quaghebeur M, Hoekstra N (2013) Enhanced landfill mining in view of multiple resource recovery: a critical review. J Clean Prod 55:45–55. https://doi.org/10.1016/j.jclepro.2012.05.021
Jones JC (2010) Thermal processing of waste. Ventus Publishing ApS. ISBN 978-87-7681-590-5
Kim D, Park KY, Yoshikawa K (2017) Conversion of municipal solid wastes into biochar through hydrothermal carbonization. In: Huang WJ (ed) Engineering applications of biochar, Chapter 3. IntechOpen. https://doi.org/10.5772/intechopen.68221.
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christinsen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336. https://doi.org/10.1080/10643380290813462
Krook J, Svensson N, Eklund M (2012) Landfill mining: a critical review of two decades of research. Waste Manag 32:513–520. https://doi.org/10.1016/j.wasman.2011.10.015
Malhi Y (2017) The concept of the Anthropocene. Annu Rev Environ Resour 42:77–104. https://doi.org/10.1146/annurev-environ-102016-060854
Marschner P (ed) (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, p 651
Mielke HW (1999) Lead in the inner cities. Am Sci 87:62–73. https://www.jstor.org/stable/27857784
National Academy of Science (2017) Investigative strategies for lead-source attribution at superfund sites associated with mining activities. National Academies of Sciences, Engineering and Medicine, Washington DC. https://doi.org/10.17226/24898 or http://nap.edu/24898
Ogunseitan OA (2007) Public health and environmental benefits of adopting lead-free solders. JOM July 2007, 12–17. https://doi.org/10.1007/s11837-007-0082-8
Peters NE, Meybeck M (2000) Water quality degradation effects on freshwater availability: impacts of human activities. Int Water Resour Assoc Water Int 25:185–193. https://doi.org/10.1080/02508060008686817
Roosen M, Mys N, Kusenberg M, Billen P, Dumoulin A, Dewulf J, Van Geem KM, Ragaert K, De Meester S (2020) Detailed analysis of the composition of selected plastic packaging waste products and its implications for mechanical and thermochemical recycling. Environ Sci Technol 54:13282–13293. https://doi.org/10.1021/acs.est.0c03371
Rudnick RL, Gao S (2003) Composition of the continental crust. In: Rudnick RL (ed) Treatise on geochemistry, the crust, vol 3. Elsevier and Pergamon, Amsterdam, pp 1–90. https://doi.org/10.1016/B0-08-043751-6/03016-4
Seiser R, Yazdani R (2015) Bioreactor recycling system for producing energy and SNG. Energy Innovations Small Grant Natural Gas Program, California Energy Commission, Final Report 11–04G. http://www.yolocounty.org/home/showdocument?id=31490
Shacklette HT, Boerngen JG (1984) Element concentrations in soils and other surficial materials of the conterminous United States. US Geological Survey Professional Paper 1270. https://doi.org/10.3133/pp1270
Smith BD, Zeder MA (2013) The onset of the anthropocene. Anthropocene 4:8–13. https://doi.org/10.1016/j.ancene.2013.05.001
Tang YT, Ma XQ, Wang ZH, Wu Z, Yu QH (2017) A study of the thermal degradation of six typical municipal waste components in CO2 and N2 atmospheres using TGA-FTIR. Thermochim Acta 657:12–19. https://doi.org/10.1016/j.tca.2017.09.009
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell Scientific Publications, Oxford
Thipse SS, Sheng C, Booty MR, Magee RS, Bozzelli JW (2002) Chemical makeup and physical characterization of a synthetic fuel and methods of heat content evaluation for studies on MSW incineration. Fuel 81:211–217. https://doi.org/10.1016/S0016-2361(01)00133-8
Thy P, Jenkins BM (2010) Mercury in biomass feedstock and combustion residuals. Water Air Soil Pollut 209:429–437. https://doi.org/10.1007/s11270-009-0211-9
Thy P, Yu C, Blunk SL, Jenkins BM (2013a) Inorganic composition of saline-irrigated biomass. Water Air Soil Pollution 224(1617):1–17. https://doi.org/10.1007/s11270-013-1617-y
Thy P, Yu C, Jenkins BM, Lesher CE (2013b) Inorganic composition and environmental impact of biomass feedstock. Energy Fuels 27:3969–3987. https://doi.org/10.1021/ef400660u
Turner JM (2015) Following the Pb: an envirotechnical approach to lead-acid batteries in the United States. Environ Hist 20:29–56. https://doi.org/10.1093/envhis/emu128
Tylecote RF (1992) A history of metallurgy, 2nd edn. Institute of Materials, London
USGS (2021) National Minerals Information Center, https://www.usgs.gov/centers/nmic/cadmium-statistics-and-information. Accessed Aug 2021.
Vassilev S, Vassileva C, Baxter D (2014) Trace element concentrations and associations in some biomass ashes. Fuel 129:292–313. https://doi.org/10.1016/j.fuel.2014.04.001
Vitousek PM, Moony HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499. https://doi.org/10.1126/science.277.5325.494
Waterlot C, Bidar G, Pruvot C, Douay F (2012) Effects of grinding and shaking on Cd, Pd and Zn distribution in anthropogenically impacted soils. Talanta 98:185–196. https://doi.org/10.1016/j.talanta.2012.06.068
Waters CN, Zalasiewicz JA, Williams M, Ellis MA, Snelling AM (2014) A stratigraphical basis for the Anthropocene? In: Waters CN, Zalasiewicz JA, Williams M, Ellis MA, Snelling AM (eds) A stratigraphical basis for the anthropocene, special publications, vol 395. Geological Society, London, pp 1–21. https://doi.org/10.1144/SP395.18
Wilburn DR (2009) Flow of cadmium from rechargeable batteries in the United States, 1996–2007 (Ver. 2). Scientific Investigation Reports 2007–5198. U.S. Geological Survey, Reston, VA. https://pubs.usgs.gov/sir/2007/5198. Accessed Aug 2021. https://doi.org/10.3133/sir20075198
Wilburn DR (2014) Comparison of the US Lead Recycling Industry in 1998 and 2011. US Geological Survey, Scientific Investigations Report 2014-5086. US Geological Survey, Reston, VA. https://pubs.usgs.gov/sir/2014/5086/pdf/sir2014-5086.pdf. Accessed Aug 2021. https://doi.org/10.3133/sir20145086
Worm B, Lotze HK, Jubinville I, Wilcox C, Jambeck J (2017) Plastic as a persistent marine pollutant. Annu Rev Environ Resour 42:1–26. https://doi.org/10.1146/annurev-environ-102016-060700
Yazdani R, Kieffer J, Akau H (2003) Full scale bioreactor landfill for carbon sequestration and greeenhouse emission control. DOE Quarterly Technical Progress Report. Yolo County, Plannimg and Public Works Department. https://www.osti.gov/servlets/purl/886513. https://doi.org/10.2172/794170
Zalasiewicz J, Kryza R, Williams M (2014) The mineral signature of the anthropocene in its deep-time context. In: Waters CN, Zalasiewicz JA, Williams M, Ellis MA, Snelling AM (eds) A stratigraphical basis for the anthropocene, special publications, vol 395. Geological Society, London, pp 109–117. https://doi.org/10.1144/SP395.2
Zalasiewicz J, Waters CN, Ivar do Sul JA, Corcoran PL, Barnosky AD, Cearreta A, Edgeworth M, Gałuszka A, Jeandel C, Leinfelder R, McNeill JR, Steffen W, Summerhayes C, Wagreich M, Williams M, Alexander P, Wolfe AP, Yonan Y (2016) The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 13:4–17. https://doi.org/10.1016/j.ancene.2016.01.002
Zekkos D, Kavazanjian E, Bray JD, Matasovic N, Riemer MF (2010) Physical characterization of municipal solid waste for geotechnical purposes. J Geotech Geoenviron Eng 136:1231–1241. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000326
Zhang W, Yand J, Wu X, Hu Y, Yu W, Wang J, Dong J, Li M, Liang S, Hu J, Kumar RV (2016) A critical review on secondary lead recycling technology and its prospect. Renew Sustain Energy Rev 61:108–122. https://doi.org/10.1016/j.rser.2016.03.046
Acknowledgements
Sven Erik Rasmussen kindly interpreted the XRD pattern. The study was supported by the California Energy Commission to RS and RY from a PIER Energy Innovation Small Grant (EISG) 11-04G. The analytical work by GHB and CEL at Aarhus University was in part supported by the Danish National Research Foundation (Danmarks Grundforskningsfond) under Grant 26 123/8 (Niels Bohr Professorship in Geoscience to CEL). The supports of the USDA and the California Agricultural Experiment Station are further appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barfod, G.H., Seiser, R., Yazdani, R. et al. Anthropocene Geochemical and Technological Signatures of an Experimental Landfill Bioreactor in the Central Valley of California. Anthr. Sci. 1, 246–263 (2022). https://doi.org/10.1007/s44177-022-00020-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44177-022-00020-6