Abstract
Microfluidic devices have been widely used for gene analysis, immunoassays, organ-on-chip technology, cell engineering, and disease modeling. Their integration into high throughput screening (HTS) platforms has led to large-scale testing of various biological and chemical agents. This brief review discusses existing microfluidic HTS modalities, including the droplet mode, the perfusion mode, and the array-based platforms in active or passive designs, by exploring their fabrication methods and key design features. The main compartments are discussed, and the future trajectories of microfluidic HTS platforms, particularly in drug screening, are explained in detail. This review aims to serve as a guide for bioengineers and clinicians, offering insights to advance the development of the microfluidic toolboxes utilized in drug screening efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A high-throughput screening (HTS) platform is commonly used to test for the presence of any desired chemical compounds or biological markers. An HTS platform integrates automated equipment for rapid simultaneous detection and/or analysis of thousands of biophysical and chemical compounds [1]. These platforms incorporate robotic modules for liquid and plate handling. The efficiency of HTS platforms mainly relies on automation [2]. Microfluidic chips process small volumes (10−9 to 10−18 L) of fluids in channels with dimensions around ten to hundreds of micrometers [3]. Microfluidic HTS [4, 5] can help handle biological and chemical compounds with a low-cost analysis, consumption of economic samples, and ease of handling for biological assays [6, 7]. From a design perspective, the main compartments are a propulsion system, a dedicated cell culture module, and a monitoring unit [8]. Biocompatible materials such as polydimethylsiloxane (PDMS) and polymethyl methacrylate (PMMA) can be used to form the parts in contact with the media and cells.
By integrating diverse components into a unified microfluidic device, microfluidic HTS systems are classified as droplet and perfusion flow modes and the conventional microarrays [1]. Droplet-based microfluidic HTS systems provide precise control over reactions with reduced reagent consumption, perfusion flow platforms enable continuous processing, and microarray modes facilitate parallelized screening. With this perspective, the current review aims to highlight the droplet and the perfusion flow modes in microfluidic HTS platforms, which have rarely been reviewed in the past few years [9]. Each droplet- and perfusion-based microfluidic HTS system can be categorized as continuous, digital, centrifugal, and acoustic-based microfluidics. The focus is on the continuous flow platforms, which are more promising to provide high throughput and automation. Centrifugal microfluidics are more challenging to design, acoustic-based microfluidics have scalability limitations, and digital microfluidics are also reviewed in the literature [10]. To date, the improvements in microfluidic HTS have allowed efficient incorporation of cells into the drug screening platforms [7, 11], transportation of samples and reagents [12, 13], and testing of toxicity and biological markers [14, 15].
A suitable strategy can be adapted for integrating microfluidic HTS and clinical studies to apply microfluidic devices for automated drug discovery, thereby reducing reliance on animal testing and potentially contributing to developing more effective and safer drugs. Nevertheless, practical challenges exist in implementing microfluidic HTS platforms in the pharmaceutical industry. For instance, the miniaturization of cell-based systems faces constraints due to limitations in the compound library, the unrealistic nature of a low number of cells, and challenges related to materials used for fabricating platforms [16, 17].
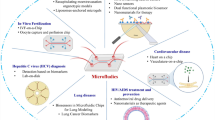
In this review, we concentrate on the microfluidic HTS modes, their fabrication methods, challenges, and integrations, aiming to establish a comprehensive understanding of microfluidics platforms in HTS systems. Table 1 summarizes some key studies related to cell-based HTS models. The discussed advancements in these platforms, dosing strategies, and streamlined workflows have created new possibilities for their commercialization. With ongoing efforts to address these challenges, we believe the field is poised for broader adoption and application in the next decade (Fig. 1).
Cell-based HTS platforms: A showing the intricacies of an array-based “Christmas tree”-shaped gradient generator within the realm of a brain cancer-on-chip microfluidic platform, utilizing 24 distinct culture chambers [18]; B Unveiling the sophistication of a microfluidic setup composed of a programmable membrane-valve-based chip to orchestrate a dynamic parallel drug screening for tumor organoids [19]
Droplet Mode Platforms
Droplet mode microfluidics have enhanced the scalability of chemical and biological experiments, surpassing the capabilities of traditional lab workflows. An economical alternative to robotic liquid handlers in high-throughput experimentation has been water-in-oil droplet-based emulsion systems. Microfluidic HTS achieves a notable 103 to 106-fold decrease in the volume of bioassays compared to traditional procedures [30]. Sample manipulations through droplet mode microfluidic HTS can exceed 500 Hz, demonstrating speeds significantly higher than those attained by robotic liquid handling, which operates below 5 Hz [30]. Droplet mode microfluidics enables ultrahigh-throughput for screening and sample manipulations (up to 105 samples per day) [9].
Droplet mode microfluidics feature a multi-input microchip integrated with aqueous reservoirs controlled by flow pressures and/or rates, collection and sensing elements, and other hardware components. The central element comprises a nozzle-like distributor that orchestrates uniform droplets by emulsifying dispersed and carrier phases at a very high rate and controlled composition [31]. For example, flow-focusing and T-channel microchips are commonly known as the nozzle design (Fig. 2). In the last case, dispersed and carrier phases create an intersection, where the carrier phase meets the dispersed phase to burst into droplets. In the T-channel configuration, the dispersed phase intersects with the carrier phase, causing the dispersed phase to shear into droplets. The droplets' size and formation rate are contingent upon the ratio between the flow rates of both phases. The target reagents influence the size and uniformity of droplets.
Surveying droplet mode platforms: A A comprehensive overview of active and passive droplet generation techniques [42]; B demonstration of the crossflow geometry: (i) illustration of a T-junction droplet platform designed for cell encapsulation; (ii) architecture of a Head-on configuration droplet generation platform; C Investigating co-flow microfluidic geometry: (i) components of a co-flow platform for droplet generation, exploring droplet generation mechanisms including (ii) dripping and (iii) jetting. D Architecture of flow-focusing microfluidics platforms: (i) detailed schematics of a flow-focusing platform for droplet generation, highlighting flow patterns in the flow-focusing microfluidic platform; (ii) threading; (iii) Jetting; (iv) dripping; (v) tubing, and (vi) viscous displacement
Droplets serve as miniaturized reaction compartments, generating volumes ranging from nano- to pico-liters. These droplets offer an ideal environment characterized by confined volume, limited dispersion, restricted cross-contamination, and a high surface area. Their versatility allows for encapsulating single cells within different areas [32] or multiple cells [33], material and drug carriers [34], miniaturized reaction chambers [32], nanomaterials, and building blocks for tissue engineering [35]. The droplet mode platform has demonstrated significant potential for high-throughput applications. It enables the integration of functional components within a single platform, finding applications in the chemical, medical, biological, and food industries [36]. It also benefits from low volume consumption, rapid mixing, high surface-area-to-volume ratio, and independent control of individual droplets [37, 38]. The droplet mode platforms are further summarized in the following section based on the mechanisms of droplet formation, droplet functionalization, and their application.
Droplet Formation
The droplet formation process engages the interplay of inertial, gravitational, frictional, and viscous forces, emphasizing the interfacial tension between two immiscible phases [39, 40]. The total energy within a microfluidic channel is the sum of surface energy (representing interfacial tension between liquids) and kinetic energy. The Weber number (We) is the kinetic-to-surface energy ratio, making it a useful metric for characterizing droplet formation and size. In the droplet mode microfluidic HTS platform, a nozzle and/or distributor injection mechanism manipulates uniform droplets through dispersed carrier phases [31, 41]. The droplets are formed when viscous shear forces are potent enough to break the dispersed phase into the carrier phase. The size of the droplet depends upon factors such as fluid viscosity, the dimensions of the microchannels, and the flow properties. The ratio between the flow rates of both phases can control the droplets' size and formation rate.
Droplet formation can be manipulated through either active or passive methods concerning flow control or actuation principles. Operational management employs external cues to stabilize the generation rate, size, and response time of droplets [42]. External influences may manifest as electric, magnetic, and centrifugal fields or alterations in intrinsic fluid properties like velocity, viscosity, interfacial tension, channel wettability, and density (Fig. 2Ai). Passive droplet control technology introduces dispersed fluid into a continuous fluid flow in a microfluidic channel by inducing flow instability, requiring no external factors [43]. The droplet mode high throughput platform can be adapted to produce polymerized shells, microgels, Janus particles, and crystals. For instance, polymerized shell droplets can be created through the double emulsion and crosslinked using ultraviolet light [44].
Droplet generation in a microfluidic channel is primarily governed by viscous shear force. However, the regulation of this shear force within the microfluidic channel can be achieved through alterations in geometry. The fundamental geometric modalities include crossflow, co-flow, and flow-focusing [42, 45]. In crossflow geometry (Fig. 2B), the dispersed and continuous phases meet at an angle higher than 0° and smaller than 180°. The crossflow geometry consists of a T-junction arrangement; however, based on the structure of the number of inlets and the angle of incidence of these inlets, these systems can be classified in a hybrid manner. This classification includes variations apart from classical T-junctions, such as head-on [46], Y-junction, K-junction [47], and V-junction systems [42, 48].
T-junction (Fig. 2B i) is a standard geometry used in the microfluidic platform to produce droplets, owing to its simple design and easy operation [49]. The high shear stress at the intersection of two phases breaks the interfacial tensions of the aqueous phase, inducing localized breaking that results in spherical droplets downstream. The size and formation of droplets in T-junction are commonly affected by the dimension of the channel, surface properties, flow rate, fluid viscosity, and use of surfactants [50]. Jamalabadi et al. demonstrated that when contact angle, slip length, and injection angles are near perpendicular and parallel, the diameter of the droplet increases [51].
In contrast, flow rate, density, viscosity, and other injection angles decrease. In a head-on system (Fig. 2B ii), two identical channels from the opposite direction are introduced for continuous and dispersed fluid flow. This system was applied for droplet generation in a low capillary number [46]. Additional modifications are K and V-junction systems introduced for high throughput droplet generation with the desired size [47, 48]. While T-junction can form droplets from less viscous fluids at lower velocities, Y-junction produces a smaller droplet with high viscous fluids [52]. This modification also helps clean the switching of samples and production, especially in the V-junction [48].
The co-flow geometry (Fig. 2C) utilizes a dispersed phase in a continuous phase channel in a parallel stream. In this geometry, droplets with sizes ranging from 2 to 200 µm [53] are generated by two processes (Fig. 2C ii–iii) [54] dripping and jetting. While dripping is the phenomenon where the jet breaks at the end of the nozzle due to dominant capillary force, in jetting, the dispersed phase extends out of the nozzle as a single jet. It happens since viscous and inertial forces become comparable to capillary force, and the droplet is formed from the tip due to destabilization. Other factors include applying flow ratio and electric control and controlling droplet size and frequency. For example, electric force suppresses interfacial tension and enhances the squeezing, necking, and breaking of the inner fluid jet, which favors droplet generation frequency, rate, and size [55].
The flow-focusing (Fig. 2D) involves elongating the dispersed phase through hydrodynamic force into a continuous fluid. The device produces a thin jet regulated by the phase's pressure gradient and flow rate, which eventually breaks downstream, driven by surface tension force. The droplet generation in the flow-focusing device has five flow regimes (Fig. 2D ii–vi): threading, jetting, dripping, tubing, and displacement [56]. The size of the droplets in flow-focusing ranges from 30 to 400 µm [57]. The droplet size can be tuned by altering the flow rate and channel aspect ratio: changing these factors resulted in liposomes with small diameters [58].
Droplet Functionalization
The droplet mode HTS platform enables mono-disperse droplet synthesis and encapsulation of biomolecules within droplets. It has widespread demand in chemical and biological applications due to its advantage of utilizing small samples, surface-to-volume ratio, effective control, and massive throughput generation of droplets. These droplets are generally functionalized and are applied in drug transportation, cell studies, material synthesis, and tissue engineering. The droplet mode high throughput platform can be modified to form polymerized shells, microgels, Janus particles, and crystals. Polymerized shell droplets can be fabricated using the double-emulsion technique (Fig. 3A), which may be crosslinked using ultraviolet light [44, 59]. It is usually used as cargo for delivery, for example, the drug for time or stimulus-triggered on-demand cell capsule [60]. Microgels (Fig. 3B) can also be used similarly via stimuli-response release of the drug or degradation [61]. Microgels are colloidal particles with a crosslinked polymer network where particle release occurs. Drug application such as ampicillin via direct encapsulation has been observed with a high throughput active control of droplet diameter (100–800 μm) in a droplet platform [62].
Advancing droplet functionalization techniques: A Double-emulsion microfluidic droplet generation technique using microfluidic hydrophobic and hydrophilic junction positioned serially in T-shaped microchannels provides high precision [59]; B Agarose (hydrogel) microfluidic droplet system for single cancer cell capsulation provides high biocompatibility and control over functionality [66]; C Janus particle fabrication using immiscible monomers emulsified in an aqueous solution of sodium dodecyl sulfate in a microfluidic device enables producing intricate formulations [64]; D Protein crystallization in Teflon capillary where the precipitation increases are transitioned to a single crystal and small microcrystal can be considered a precise technique for droplet functionalization [67]
Crystals are highly structured molecules formed by solidification in a microfluidic platform. The droplet platform provides a conviction-free and efficient environment for high-quality crystal-forming: it is applicable for protein analysis, which has a crucial role in the biological process. Additionally, a droplet platform may produce droplets with anisotropic properties, commonly known as Janus particles (Fig. 3C), which can be used in optics, sensors, and biomedicine [63, 64]. For example, a particle with anisotropic mechanical properties can be fabricated for drug delivery, detection in magnetic resonance imaging, and nucleic acid separation [65]. Among many applications, low-volume protein crystallization is also used (Fig. 3D).
Droplet Platform in High Throughput Applications
Current research on HTS is based on 2D cell culture models. However, a 3D culture environment that can mimic in vivo characteristics is crucial to interpreting near-exact conditions for drug discovery and screening. The droplet mode microfluidic platform can be used for droplet libraries and compound screening, cell growth and cell–cell interactions, bacterial persistence screens, plaque assay and virus-host interaction in drops, antibody screens, directed evolution of enzymes, and PCR-based analysis of single rare templates [68]. The droplet mode microfluidic platform has a superior high throughput capability compared to traditional microtiter plates because of its lower reagent consumption, faster screening time/rate, the minimum volume of each reactor, and lower consumable cost (1 × 106-fold less) [69]. Miniaturization is limited in the 2D cell culture model due to capillary action's evaporation and liquid draw. Additionally, various investigations and reactions in the droplet platform can be performed without increasing the device's size or complexities [37]. Thus, the droplet platform's distinct advantage is producing microdroplets that serve as an individual reaction chamber for HTS. Due to the small droplet volume, the system can analyze single cells. For example, single-cell miRNA was encapsulated in droplets at 80–150/s to demonstrate the high throughput single-cell encapsulation capability [70].
Droplet mode allows for breaking down a single bulk sample into millions of microdroplets, each acting as an isolated reaction chamber [71]. This has emerged as a popular platform for biomarker detection and screening because the significant reduction in volume facilitates an equivalent reduction in background noise and a drastic increase in the local concentration of the biomarkers. This further increases the signal-to-noise (SNR) ratio, limits of detection, and sensitivity of the assay [72]. The first seminal work using microdroplet HTS was described in the BEAMing protocol [73], in which PCR reagents, primer functionalized magnetic beads, and nucleic acid templates were manually stirred in an oil/detergent mixture to create microemulsion of droplets [74]. These droplet-based PCR platforms have been used to analyze liquid biopsies for the detection of circulating DNA in blood plasma for skin, lung, breast, and gastric cancer [75,76,77,78], as well as in cerebrospinal fluid for monitoring the progression of brain tumors [75]. Custom droplet PCR platforms have also been developed to detect viral nucleic acids, focusing on speed, quantification, and integration [76].
Droplet mode can provide an opportunity for low-abundance protein detection using ELISA, where proteins linked with reporter enzymes are conjugated to immobilized antibodies on the surface of a suspended microdroplet [77]. Commercial immunoassay platforms have focused on optically coding microdroplets to multiplex analyte detection [78]. Shim et al. developed a femtoliter droplet platform for single-molecule counting immunoassays where the platform was used to detect PSA, an important protein biomarker for prostate cancer, as low as 46 fM in concentration, approximately two orders of magnitude lower than conventional bulk ELISA [79]. In addition to ELISA, droplet technology has delivered platforms for sensitive enzyme kinetic analysis and quantification. Droplet platforms' improved control of sample reaction time offers more controlled and more accurate kinetic analysis. Droplets have been used to determine dilute concentrations of enzymes like β-glucosidase and β-galactosidase [80]. One such enzyme detection droplet platform investigated glucose oxidase reaction kinetics to determine glucose concentration in a sample [81]. Such platforms can expedite the detection of a vast array of analytes, including proteins, by incorporation into droplet-based ELISA workflows.
Microdroplets also provide a practical method for parallel immobilizing single cells at predefined positions on a surface. By using the method of limited dilution and varying cell density and seeding time, Zhu et al. optimized the distribution of single cells across the droplet microarray on superhydrophobic–super-hydrophilic patterned substrates. They built a droplet-based single-cell reverse transcription-quantitative polymerase chain reaction (qPCR) system to quantify gene expression in individual cells [82]. Li et al. reported a facile strategy to create single live cell arrays using the droplet-splitting method on a pre-patterned substrate [83]. These strategies have been used widely to develop biomarker encapsulation, incubation, and detection platforms. They have been successfully used for nucleic acid, protein, and single-cell detection and screening, applied to diseases ranging from cancer to bacterial and viral infections. Commercial platforms such as BioRad's ddPCR have become the industry standard for quantitatively detecting nucleic acid biomarkers. The potential of droplet microfluidic platforms has led to widespread interest and research, with the current focus on improving detection limits, sensitivity, multiplexing, automation, and integration of sample preparation.
Perfusion Flow Mode Platforms
Perfusion flow HTS platform requires continuous fluid or reagent flow to introduce reagents, transfer fluid in the microfluidic network, and combine/mix reagents [1] (Fig. 4). The flow is pressure-driven or electroosmotic-based [84]. The pressure-driven flow follows the Poiseuille flow theory [85] and the velocity distribution is parabolic across the channel. The electroosmotic flow applies the movement of liquid in a material due to potential differences. In electroosmotic flow, the surface charge on the channel wall produces a layer of counterions when an electric field is used. A layer of ions is drawn toward the oppositely charged electrode dragging the bulk solution [86]. This section includes the fabrication of the perfusion mode platform, mixing mechanism, and high throughput application.
Perfusion-mode HST platforms: A an integrated microfluidic device for high throughput screening, featuring an upstream drug concentration gradient generator and parallel downstream culture channel linked to a mass spectrometer for detection [96]; B a microfluidic platform utilizing electrospray ionization mass spectrometry for the quantitative and qualitative analysis of cell metabolism [97]; C a microfluidic device designed to generate diverse gradient drug and sensitizer concentrations, facilitating perfusion through distinct cell-cultured microchambers downstream [98]; D the schematic of a high throughput PDMS 280-chamber perfusion chip. Channels are segmented into individual chambers by valves, allowing for the cultivation of 600 cells in each chamber [99]
Fabrication of Perfusion Mode Platforms
A perfusion flow HTS platform exhibits the dynamics of a laminar fluid and consists of several components, i.e., inlets and outlets for reagent/media, desired microfluidic branching and networks, and a mechanism to mix reagents. The perfusion flow platform controls the environmental condition in the microfluidic chip; this is regulated by controlling the medium/reagent flow, dissolved gas, temperature, pH, and shear stress [87]. Polydimethylsiloxane (PDMS) is commonly used to fabricate microfluidic devices because of its ability for oxygen and gas permeability, optical transparency, ease of manufacturability, and low cost [11, 88, 89]. However, the application of PDMS is limited because it cannot mimic the complex architecture of tissue and organ. As the geometrical complexity increases, sequential integration of the PDMS layer is required, making the process labor-intensive and expensive [90]. Investigation of alternative and new material to overcome the disadvantage of PDMS is appealing to researchers these days. For example, hydrogel-based material is used to mimic the function of the nephron in the vascular network [91], demonstrating the potential of using hydrogel as a possible alternative.
Among several techniques that evolved to fabric perfusion flow, HTS platforms, laminates, molding, and additive manufacturing (AM) are the most commonly used. The layers are cut and bonded in laminate to form a microfluidic channel. Thus, the microfluidic channel may be used in preclinical testing, biochemical analysis, and detection [92]. Molding is another technique widely used to fabricate the perfusion-mode microfluidic platform where a liquid or malleable material is shaped using a mold. There are three processes: replica molding, injection molding, and hot embossing. Replica molding is commonly used in biomedical applications since it is easy and cost-effective. For example, the hydrogel is cast in a mold and cured [93], and PDMS is shaped similarly. The setting/crosslinking is done enzymatically, thermally, or by photocrosslinking. Additive Manufacturing (AM), a sequential layer-by-layer manufacturing technique, is intriguing and desirable. Among existing AM techniques, extrusion-based and stereolithography printing are commonly used. Extrusion-based 3D bioprinting is widely used because of its versatility and affordability for biomedical applications [94]. In extrusion-based printing, the material is deposited layer-by-layer by a heating filament or pressure/gravity deposition to form the model. In contrast, stereolithography applies layer-to-layer polymerization of photosensitive monomer resin to create a model [95].
Perfusion Flow Fluid Mixers
The perfusion flow HTS platform is laminar, characterized by a low Reynolds number, i.e., the ratio of fluid momentum to viscous force. The value of the Reynolds number in an internal laminar flow falls below the critical value of approximately 2300. Due to the laminar flow inside the microfluidic platform, fluid mixing in the channel is required. The fluid mixing in the perfusion platform occurs through convection and molecular diffusion [100]. Péclet number represents the ratio of mass transport due to convection to diffusion, and it determines whether diffusion or convection dominates the means of mass transport. In the microfluidic channel, turbulent mixing cannot inherently slow mixing. The channel mixing may be intensified by additional energy input or by exploiting fluidic design elements [100]. The process is commonly termed active and passive mixing, respectively.
Passive microfluidic mixing applies geometry modulation in microfluidic channels (Fig. 5). The mixing occurs because of chaotic advection that manipulates the laminar flow or molecular diffusion by increasing the surface contact and time between different fluid flows [101]. The mixer includes lamination, intersecting channels, grooved staggered herringbone, and surface chemistry technology [102]. The flowing fluid is initially divided into multiple channels in the lamination mixer to increase the contact area between fluids and is gathered downstream (Fig. 5A) [103]. The mixing efficiency in this approach depends on the number of channels. In intersecting channel, the fluid is divided, rearranged, and combined in the stream (Fig. 5B) [104, 105]. The channels are modified to get intersecting channels by varying the channel length and creating bimodal width distributions [104]. This approach might lead to a mixing index of 0.95 [106].
Architectures of passive microfluidic mixers: A the design of a Lamination micromixer employing a split and a recombine pattern for mixing [109]; B illustration of microfabricated multi-intersecting channels of varying lengths, as a testament to intricate mixing capabilities [104]; C demonstration of a flat zig-zag (top) [110] and a square-zigzag micromixer (bottom) [111], as innovative designs for efficient microfluidic mixing; D Presenting a Staggered herringbone mixer [112] as a sophisticated solution for achieving a precise and controlled symphonic mixing in passive microfluidic systems
The zigzag mixer is one of the most commonly used passive mixing geometry with mixing efficiency of up to 96% [107]. It does not require other additional control units and can be easily integrated into the microfluidic device (Fig. 5C). Another geometric alteration involves using a barrier in the microfluidic channel, such as an embedded barrier. The barriers are applied in different formations such as single periodic, double periodic, alternating, single and double, and aperiodic [108]. The slanted wells and grooved staggered herringbone pattern mixer involve grooving those shapes in the wall directly on the microfluidic channel (Fig. 5D).
Active microfluidic mixing components are complex to fabricate, including an external valve control unit that cannot be fully integrated into a microfluidic platform [113]. A Pressure-driven active mixer uses the generation of perturbation in the fluid stream by pulsating velocity. It utilizes simple channels such as T-channel [114] setting and multiple side/cross channels [115, 116]. Applying an electric field in a microfluidic platform to generate electrohydrodynamic instability can also help in mixing fluids [117]. When the electric field is used, the instabilities are formed in the vortices [118]. An electrode array-based electric mixer was demonstrated to create vortices to enhance the mixing of fluids [119]. The sound field mixing involves an acoustic/ultrasonic method to create an acoustic stream to improve the mixing. For example, a piezoelectric transducer operating at 450MHz was used to mix and stir the fluid perpendicular to the flow direction [120]. Magnetic field mixing is done by the application of either magnetic microparticles, incorporation of ferrofluids, or magnetic microactuators [121]. The magnetic field manipulation also allows fluid mixing in the microfluidic platform; 96% mixing efficiency has been observed with this approach [122]. To induce a blending effect, actuators such as stirrer bars are rotated inside a microfluidic platform by applying a benchtop stirrer plate [123]. The diffusion in the microfluidic channel increases with the increase in temperature; thermal field mixing uses the same approach for mixing fluid. The accelerated mixing behavior was observed in a T-junction of two miscible fluids. However, the flow rate was critical in the thermal mixing [124].
Perfusion Flow Mode Platforms in High Throughput Applications
Perfusion flow mode platforms offer great advantages for manipulating cells and enabling the formation of spheroids. Strategies use microfluidics for transporting stimuli-responsive cell-laden hydrogels into pre-defined device regions confined by specific structures, such as pillars or phase-guides [125,126,127]. An example of a commercially available technology is the OrganoPlate™ culture platform (Mimetas BV, the Netherlands). It is marketed as a two-lane or three-lane hydrogel chip containing cells, enabling varying cell–cell interactions [82, 128,129,130]. Several studies have also used valves to isolate or fluidically connect multiple culture chambers. Visone et al. presented a chip containing multiple cell culture chambers and a valve layer separated by thick polydimethylsiloxane (PDMS) membrane [131]. Gao et al. used a microfluidic channel combined with vacuum-actuated chambers for one-step cell seeding and anticancer drug testing [132].
Pneumatic pumping is another way to guide different mediums to different cell types [133]. Wang et al. reported a multilayer pneumatic pump-based microfluidic array platform for cell cytotoxicity screening of mammalian cell lines. The microfluidic channels were isolated by pneumatically actuated elastomeric valves into parallel rows for cell seeding and, subsequently, into parallel columns for toxin exposure [134]. Kim et al. further developed a similar device with 64 cell culture chambers, in which on-chip generation of drug concentrations is possible, enabling drug combination screening applications with pair-wise combinations of drug concentrations and cells [29].
On-chip drug screening is based on the controlled diffusive mixing of solutions in continuous laminar flow inside a network of microfluidic channels, at low Reynolds number, which has been proven as a simple and versatile approach to generate (drug) concentration gradients [135]. Ye et al. presented a high-content multiparametric screening method (plasma membrane permeability, nuclear size, mitochondrial transmembrane potential and intracellular redox states) for human liver carcinoma (HepG2) responding to multiple anti-cancer drugs with different concentrations. This device provided a possible solution for cancer treatment screening by rapidly extracting tumor cell response to several drugs with little sample consumption [136, 137]. Ostrovidov et al. investigated cell viability through the spatially controlled release of drug from a hydrogel covering on a microfluidic gradient generator, which also had the potential to be used for drug discovery [138]. Chung et al. developed a microfluidic HTS platform for imaging thousands of single cells in a continuous flow channel [139]. Such platforms have also been used for performing on-chip serial-dilution-based cell viability assays Field [140] and generating nonlinear concentration gradients for cell assays using an asymmetrical network in microfluidic concentration generators [141]. A high throughput study of cell apoptosis by drug combinatorial concentrations has also been performed by using a microfluidic concentration generator with repeated splitting and mixing of the source solutions in a radial channel network [142].
The electrical properties of cells have been used as well for cell analysis in microfluidics-based HTS platforms. Cell-based impedance spectroscopy is a label-free and non-destructive method to measure the dielectric properties of biological samples. By investigating impedance changes during cell apoptosis, Meissner et al. developed a microelectronics-based microfluidic system for the real-time investigation of cell changes during drug-induced cytotoxicity [143]. Hsiung et al. reported a dielectrophoresis (DEP)-based microfluidic chip for cell perfusion culture used to study anticancer drug-induced cell apoptosis [144]. Microfluidic chips with integrated microelectrodes can also be used to monitor neurotransmitter release from neural cells and to study drug effects, which is important to discover drugs for diseases related to brain functions [145].
Future Directions
A notable trend involves the integration of tissue organoids into HTS platforms. Organoids are multiple-cell-laden ECM models that can mimic the biophysical and biological functions of the target tissue. They can be derived from embryonic stem cells, induced pluripotent stem cells (iPSCs) as synthetic counterparts, and organ-restricted adult stem cells. The iPSCs differentiate and tend to self-categorize into constructs resembling essential features of the tissues intended to represent [146]. These processes are under investigation, and the application of organoids within precision oncology is in the early stages. Further optimizations and standardizations are required to achieve some goals, such as genetic profiling of tumor and non-tumor organoids for identifying new targeted therapy screening for antitumor drugs, encompassing efficacy and pharmacological toxicity tests [147].
There is a trend for bioprinting organoids based on bioprinting techniques are scaffold-based and scaffold-free approaches. The scaffold-based process provides excellent structural support for cells, incorporating tissue-specific mechanical properties, bioactive cues, and reservoirs of growth factors, adhesion, and responses [148]. The architecture of the scaffolds can be appropriately controlled to enhance the biological significance of the engineered tissue construct. Recent advances in additive manufacturing methods, including extrusion, stereolithography, drop-on-demand, and laser-induced forward transfer, have enabled researchers to create intricate structures with controlled properties and architectures. These methods yield scaffolds with high porosity and controllable pore size [149,150,151,152,153].
Applying bioprinting to generate 3D tumor models results in a well-organized spatial distribution of tumor cells. This approach facilitates cell–cell and cell-extracellular matrix (ECM) interactions, addressing the limitation of vascularization in other models. Bioprinting allows for creating an in vitro 3D cancer model with enhanced biomimicry, more accurately representing intrinsic biology and drug response. A recent study employed bioprinting to create a model for cervical cancer using bioinks consisting of HeLa cells encapsulated in gelatin, alginate, and fibrinogen hydrogel. HeLa cells were printed with materials in the ECM, among others, and were subsequently compared with 2D studies. The comparison revealed differences in cell proliferation, protein expression of ECM components, and increased chemoresistance to the drug Paclitaxel in the 3D model [153]. The vascular function is vital for nutrient delivery and metabolite removal. Naive incorporation of endothelial cells in 3D bioprinted models leads to the formation of vessels without efficient perfusion. Attempts to mimic vessels using molds like microacupuncture needles don't accurately replicate vessel morphology [154]. To address this, integrating 3D bioprinting with microfluidic technology is now trending to facilitate the creation of organized vessels with efficient perfusion and enhance culture efficiency [155].
Unlike previous HTS systems, which were limited to combining two drugs and introducing a linear concentration gradient, µHST offers enhanced capabilities in sample handling and assay throughput [29]. Due to the nonlinearity of cellular responses to drug concentrations, most drug screening techniques require testing doses over multiple orders of magnitude to determine the 50% inhibitory concentration (i.e., IC50) [156]. A potential direction includes increasing the use of mixing agents in a single process to test combinatory drugs. Another promising direction involves the introduction of multi-organ chips, an extension of the organ-on-a-chip concept, which interconnects diverse organ microarchitectures to facilitate the study of drug metabolism and disease progression [157,158,159]. These chips are designed to resemble vessels, enhance our understanding of complex organ interactions, and shed light on human pathology and drug characteristics. The human-on-a-chip approach, involving interconnected human organ cultures on a single chip, can yield valuable drug metabolism data to improve clinical experiment efficiency [160, 161]. As chip data approaches the realism of human experiments, it offers a secure platform to explore disease characteristics across genders and age groups, expanding the scope of clinical research to include rare diseases and individual differences.
Lastly, the efficacy of HTS platforms and their applications in large-scale analyses heavily relies on automation. While much of the existing HTS research centers on the 2D culture of mammalian cells on microfluidic channel surfaces, there is a growing acknowledgment that replicating in vivo conditions is more faithfully achieved through cells thriving in a 3D culture environment. The standard procedure unfolds as a seamless sequence of automated operations, from a liquid handling system to the swift screening plate reader. This automated orchestration streamlines processes and promises to unlock new dimensions in high-throughput screening.
Data Availability
No data supporting documents as the review article process.
References
G. Du, Q. Fang, J.M.J. den Toonder, Microfluidics for cell-based high throughput screening platforms—a review. Anal. Chim. Acta 903, 36–50 (2016)
G. Wu, S.K. Doberstein, HTS technologies in biopharmaceutical discovery. Drug Discov. Today 11(15–16), 718–724 (2006)
J. Hong, J.R. Lukes, Microfluidic systems for high-throughput screening, in Encyclopedia of Microfluidics and Nanofluidics. ed. by D. Li (Springer, Boston, 2008)
E. Brouzes et al., Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. 106(34), 14195–14200 (2009)
M. Sesen, T. Alan, A. Neild, Droplet control technologies for microfluidic high throughput screening (μHTS). Lab Chip 17(14), 2372–2394 (2017)
P. Neužil et al., Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discov. 11(8), 620–632 (2012)
M.-H. Wu, S.-B. Huang, G.-B. Lee, Microfluidic cell culture systems for drug research. Lab Chip 10(8), 939–956 (2010)
W. Zheng et al., Fabrication of biomaterials and biostructures based on microfluidic manipulation. Small 18(16), e2105867 (2022)
E.M. Payne et al., High-throughput screening by droplet microfluidics: perspective into key challenges and future prospects. Lab Chip 20(13), 2247–2262 (2020)
Y. Zhang, N.T. Nguyen, Magnetic digital microfluidics—a review. Lab Chip 17(6), 994–1008 (2017)
S. Halldorsson et al., Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 63, 218–231 (2015)
J. Melin, S.R. Quake, Microfluidic large-scale integration: the evolution of design rules for biological automation. Annu. Rev. Biophys. Biomol. Struct. 36, 213–231 (2007)
J. Hong, J.B. Edel, A.J. Demello, Micro-and nanofluidic systems for high-throughput biological screening. Drug Discov. Today 14(3–4), 134–146 (2009)
Z. Wang et al., High-density microfluidic arrays for cell cytotoxicity analysis. Lab Chip 7(6), 740–745 (2007)
O.J. Dressler et al., Droplet-based microfluidics: enabling impact on drug discovery. J. Biomol. Screen. 19(4), 483–496 (2014)
S.M. Paul et al., How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 9(3), 203–214 (2010)
F. Pammolli, L. Magazzini, M. Riccaboni, The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 10(6), 428–438 (2011)
Y. Fan et al., Engineering a brain cancer chip for high-throughput drug screening. Sci. Rep. 6, 25062 (2016)
B. Schuster et al., Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 11(1), 1–12 (2020)
M. Najah et al., Droplet-based microfluidics platform for ultra-high-throughput bioprospecting of cellulolytic microorganisms. Chem. Biol. 21(12), 1722–1732 (2014)
K. Woodruff, S.J. Maerkl, A high-throughput microfluidic platform for mammalian cell transfection and culturing. Sci. Rep. 6, 23937 (2016)
N. Ye, J. Qin, W. Shi, X. Liu, B. Lin, Cell-based high content screening using an integrated microfluidic device. Lab Chip 7(12), 1696–1704 (2007)
T. Thorsen, S.J. Maerkl, S.R. Quake, Microfluidic large-scale integration. Science 298(5593), 580–584 (2002)
D. Di Carlo, L.Y. Wu, L.P. Lee, Dynamic single cell culture array. Lab Chip 6(11), 1445–1449 (2006)
P.A. Romero, T.M. Tran, A.R. Abate, Dissecting enzyme function with microfluidic-based deep mutational scanning. Proc. Natl. Acad. Sci. U.S.A. 112(23), 7159–7164 (2015)
S.L. Sjostrom et al., High-throughput screening for industrial enzyme production hosts by droplet microfluidics. Lab Chip 14(4), 806–813 (2014)
Z. Wang et al., High-throughput isolation of fetal nucleated red blood cells by multifunctional microsphere-assisted inertial microfluidics. Biomed. Microdevices 22(4), 75 (2020)
X. Chen, H. Chen, D. Wu, Q. Chen, Z. Zhou, R. Zhang, 3D printed microfluidic chip for multiple anticancer drug combinations. Sens. Actuators B 276, 507–516 (2018)
J. Kim et al., A programmable microfluidic cell array for combinatorial drug screening. Lab Chip 12(10), 1813–1822 (2012)
J. Zhong et al., When robotics met fluidics. Lab Chip 20(4), 709–716 (2020)
C. Jiu-Sheng, J.-H. Jiang, Droplet microfluidic technology: Mirodroplets formation and manipulation. Chin. J. Anal. Chem. 40(8), 1293–1300 (2012)
X. An, P. Zuo, B.-C. Ye, A single cell droplet microfluidic system for quantitative determination of food-borne pathogens. Talanta 209, 120571 (2020)
F. Fu et al., Cells cultured on core–shell photonic crystal barcodes for drug screening. ACS Appl. Mater. Interfaces 8(22), 13840–13848 (2016)
I.U. Khan et al., Continuous-flow encapsulation of ketoprofen in copolymer microbeads via co-axial microfluidic device: Influence of operating and material parameters on drug carrier properties. Int. J. Pharm. 441(1–2), 809–817 (2013)
W. Li et al., Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 47(15), 5646–5683 (2018)
K. Wang et al., Droplet generation in micro-sieve dispersion device. Microfluid. Nanofluid. 10(5), 1087–1095 (2011)
S.-Y. Teh et al., Droplet microfluidics. Lab Chip 8(2), 198–220 (2008)
P. Cui, S. Wang, Application of microfluidic chip technology in pharmaceutical analysis: a review. J. Pharm. Anal. 9(4), 238–247 (2019)
K. Doufène et al., Microfluidic systems for droplet generation in aqueous continuous phases: a focus review. Langmuir 35(39), 12597–12612 (2019)
H. Wennerström, J. Balogh, U. Olsson, Interfacial tensions in microemulsions. Colloids Surf. A 291(1–3), 69–77 (2006)
Y. Zhao, S.K. Cho, Micro air bubble manipulation by electrowetting on dielectric (EWOD): transporting, splitting, merging and eliminating of bubbles. Lab Chip 7(2), 273–280 (2007)
P. Zhu, L. Wang, Passive and active droplet generation with microfluidics: a review. Lab Chip 17(1), 34–75 (2017)
S. Sharma et al., Droplet-based microfluidics, in Microfluidic Diagnostics. (Springer, Berlin, 2013), pp.207–230
G. Nurumbetov, N. Ballard, S.A. Bon, A simple microfluidic device for fabrication of double emulsion droplets and polymer microcapsules. Polym. Chem. 3(4), 1043–1047 (2012)
T. Trantidou et al., Droplet microfluidics for the construction of compartmentalised model membranes. Lab Chip 18(17), 2488–2509 (2018)
L. Shui et al., Geometry-controlled droplet generation in head-on microfluidic devices. Appl. Phys. Lett. 93(15), 153113 (2008)
M. Rhee et al., Versatile on-demand droplet generation for controlled encapsulation. Biomicrofluidics 8(3), 034112 (2014)
Y. Ding, X.C. i Solvas, A.J.A. DeMello, “V-junction”: a novel structure for high-speed generation of bespoke droplet flows. Analyst 140(2), 414–421 (2015)
H.S. Kim et al., Raman spectroscopy compatible PDMS droplet microfluidic culture and analysis platform towards on-chip lipidomics. Analyst 142(7), 1054–1060 (2017)
J. Yao et al., The effect of oil viscosity on droplet generation rate and droplet size in a T-junction microfluidic droplet generator. Micromachines 10(12), 808 (2019)
M.Y.A. Jamalabadi et al., Effect of injection angle, density ratio, and viscosity on droplet formation in a microfluidic T-junction. Theor. Appl. Mech. Lett. 7(4), 243–251 (2017)
F. Ushikubo et al., Y-and T-junction microfluidic devices: effect of fluids and interface properties and operating conditions. Microfluidics Nanofluidics 17(4), 711–720 (2014)
P. Umbanhowar, V. Prasad, D.A. Weitz, Monodisperse emulsion generation via drop break off in a coflowing stream. Langmuir 16(2), 347–351 (2000)
M.L. Cordero, F. Gallaire, C.N.J.P.O.F. Baroud, Quantitative analysis of the dripping and jetting regimes in co-flowing capillary jets. Phys. Fluids 23(9), 094111 (2011)
L. Li et al., Electro-hydrodynamics of droplet generation in a co-flowing microfluidic device under electric control. Colloids Surf A Physicochem Eng Asp 586, 124258 (2020)
T. Cubaud, T.G.J.P.O.F. Mason, Capillary threads and viscous droplets in square microchannels. Phys. Fluids 20(5), 053302 (2008)
A. Lashkaripour et al., Performance tuning of microfluidic flow-focusing droplet generators. Lab Chip 19(6), 1041–1053 (2019)
M. Michelon et al., High-throughput continuous production of liposomes using hydrodynamic flow-focusing microfluidic devices. Colloids Surf. B Biointerfaces 156, 349–357 (2017)
S. Okushima et al., Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir 20(23), 9905–9908 (2004)
J. Zhang et al., One-step fabrication of supramolecular microcapsules from microfluidic droplets. Science 335(6069), 690–694 (2012)
C.-X. Zhao, Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv. Drug Deliv. Rev. 65(11–12), 1420–1446 (2013)
C.-H. Yang, K.-S. Huang, J.-Y. Chang, Manufacturing monodisperse chitosan microparticles containing ampicillin using a microchannel chip. Biomed. Microdevice 9(2), 253–259 (2007)
S. Yang et al., Microfluidic synthesis of multifunctional Janus particles for biomedical applications. Lab Chip 12(12), 2097–2102 (2012)
Z. Nie et al., Janus and ternary particles generated by microfluidic synthesis: design, synthesis, and self-assembly. J. Am. Chem. Soc. 128(29), 9408–9412 (2006)
Y.-T. Yang et al., A side-by-side capillaries-based microfluidic system for synthesizing size-and morphology-controlled magnetic anisotropy janus beads. Adv. Powder Technol. 26(1), 156–162 (2015)
Z. Zhu, C.J. Yang, Hydrogel droplet microfluidics for high-throughput single molecule/cell analysis. Acc. Chem. Res. 50(1), 22–31 (2017)
L. Li et al., Nanoliter microfluidic hybrid method for simultaneous screening and optimization validated with crystallization of membrane proteins. Proc. Natl. Acad. Sci. 103(51), 19243–19248 (2006)
M.T. Guo et al., Droplet microfluidics for high-throughput biological assays. Lab Chip 12(12), 2146–2155 (2012)
Y. Wang et al., Advances of droplet-based microfluidics in drug discovery. Expert Opin. Drug Discov. 15, 969–979 (2020)
L. Li et al., High-throughput and ultra-sensitive single-cell profiling of multiple microRNAs and identification of human cancer. Chem. Commun. 55(70), 10404–10407 (2019)
H. Zec, D.J. Shin, T.-H. Wang, Novel droplet platforms for the detection of disease biomarkers. Expert Rev. Mol. Diagn. 7159(February), 1–15 (2014)
D. Pekin et al., Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 11(13), 2156–2166 (2011)
D. Dressman, H. Yan, G. Traverso, K.W. Kinzler, B. Vogelstein, Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc. Natl. Acad. Sci. U.S.A. 100(15), 8817–8822 (2003)
F. Diehl et al., BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat. Methods 3(7), 551–559 (2006)
L. De Mattos-Arruda et al., Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 6, 8839 (2015)
M.M. Kiss, L. Ortoleva-Donnelly, N.R. Beer, J. Warner, C.G. Bailey, B.W. Colston, J.M. Rothberg, D.R. Link, J.H. Leamon, High-throughput quantitative polymerase chain reaction in picoliter droplets. Anal. Chem. 80(23), 8975–8981 (2008)
P.J. Tighe et al., ELISA in the multiplex era: potentials and pitfalls. Proteomics Clin. Appl. 9(3–4), 406–422 (2015)
S.X. Leng, J.E. McElhaney, J.D. Walston, D. Xie, N.S. Fedarko, G.A. Kuchel, ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 63(8), 879–884 (2008)
J.U. Shim, R.T. Ranasinghe, C.A. Smith, S.M. Ibrahim, F. Hollfelder, W.T.S. Huck et al., Ultrarapid generation of femtoliter microfluidic droplets for single-molecule-counting immunoassays. ACS Nano 7(7), 5955–5964 (2013)
J. Lim et al., Ultra-high throughput detection of single cell beta-galactosidase activity in droplets using micro-optical lens array. Appl. Phys. Lett. 103(20), 203704 (2013)
S. Gu et al., A droplet-based microfluidic electrochemical sensor using platinum-black microelectrode and its application in high sensitive glucose sensing. Biosens. Bioelectron. 55, 106–112 (2014)
W. Feng, E. Ueda, P.A. Levkin, Droplet microarrays: from surface patterning to high-throughput applications. Adv. Mater. 30(20), e1706111 (2018)
H. Li et al., Splitting a droplet for femtoliter liquid patterns and single cell isolation. ACS Appl. Mater. Interfaces 7(17), 9060–9065 (2015)
S. Pennathur, Flow control in microfluidics: are the workhorse flows adequate? Lab Chip 8(3), 383 (2008)
H.A. Stone, S. Kim, Microfluidics: basic issues, applications, and challenges. AIChE J. 47(6), 1250–1254 (2001)
J. Gaudioso, H. Craighead, Characterizing electroosmotic flow in microfluidic devices. J. Chromatogr. A 971(1–2), 249–253 (2002)
P. Occhetta, R. Visone, M. Rasponi, High-throughput microfluidic platform for 3D cultures of mesenchymal stem cells, in 3D Cell Culture. (Springer, Berlin, 2017), pp.303–323
S. Damiati et al., Microfluidic devices for drug delivery systems and drug screening. Genes 9(2), 103 (2018)
S. Torino et al., PDMS-based microfluidic devices for cell culture. Inventions 3(3), 65 (2018)
A. Miri et al., Bioprinters for organs-on-chips. Biofabrication 11, 042002 (2019)
X. Mu et al., Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip 13(8), 1612–1618 (2013)
F. An et al., A laminated microfluidic device for comprehensive preclinical testing in the drug ADME process. Sci. Rep. 6(1), 1–8 (2016)
S. Lv et al., Micro/nanofabrication of brittle hydrogels using 3D printed soft ultrafine fiber molds for damage-free demolding. Biofabrication 12(2), 025015 (2020)
Z. Gu et al., Development of 3D bioprinting: from printing methods to biomedical applications. Asian J. Pharm. Sci. 15, 529–557 (2019)
G. Gaal et al., Simplified fabrication of integrated microfluidic devices using fused deposition modeling 3D printing. Sens. Actuators B Chem. 242, 35–40 (2017)
D. Gao et al., Evaluation of the absorption of methotrexate on cells and its cytotoxicity assay by using an integrated microfluidic device coupled to a mass spectrometer. Anal. Chem. 84(21), 9230–9237 (2012)
Q. Chen et al., Qualitative and quantitative analysis of tumor cell metabolism via stable isotope labeling assisted microfluidic chip electrospray ionization mass spectrometry. Anal. Chem. 84(3), 1695–1701 (2012)
D. An, K. Kim, J. Kim, Microfluidic system based high throughput drug screening system for curcumin/TRAIL combinational chemotherapy in human prostate cancer PC3 cells. Biomol. Ther. 22(4), 355 (2014)
K. Woodruff, S.J. Maerkl, A high-throughput microfluidic platform for mammalian cell transfection and culturing. Sci. Rep. 6(1), 1–12 (2016)
T.H. Schulte, R.L. Bardell, B.H. Weigl, Microfluidic technologies in clinical diagnostics. Clin. Chim. Acta 321(1–2), 1–10 (2002)
D. Li, Encyclopedia of Microfluidics and Nanofluidics (Springer, Berlin, 2008)
C.-Y. Lee et al., Passive mixers in microfluidic systems: a review. Chem. Eng. J. 288, 146–160 (2016)
J. Cha et al., A highly efficient 3D micromixer using soft PDMS bonding. J. Micromech. Microeng. 16(9), 1778 (2006)
B. He et al., A picoliter-volume mixer for microfluidic analytical systems. Anal. Chem. 73(9), 1942–1947 (2001)
A. Bertsch et al., Static micromixers based on large-scale industrial mixer geometry. Lab Chip 1(1), 56–60 (2001)
T. Tofteberg et al., A novel passive micromixer: lamination in a planar channel system. Microfluid. Nanofluid. 8(2), 209–215 (2010)
C.Y. Lee et al., Experimental and numerical investigation into mixing efficiency of micromixers with different geometric barriers, in Materials Science Forum. Trans Tech Publ., Zurich (2006)
D.S. Kim et al., A barrier embedded chaotic micromixer. J. Micromech. Microeng. 14(6), 798 (2004)
W. Raza, K.-Y. Kim, Unbalanced split and recombine micromixer with three-dimensional steps. Ind. Eng. Chem. Res. 59(9), 3744–3756 (2019)
C.-H.D. Tsai, X.-Y. Lin, Experimental study on microfluidic mixing with different zigzag angles. Micromachines 10(9), 583 (2019)
H. Wang et al., 3D printed microfluidic lab-on-a-chip device for fiber-based dual beam optical manipulation. Sci. Rep. 11(1), 1–12 (2021)
V.J. Shenoy et al., Design and characterization of a 3D-printed staggered herringbone mixer. Biotechniques 70(5), 285–289 (2021)
X. Niu et al., Active microfluidic mixer chip. Appl. Phys. Lett. 88(15), 153508 (2006)
I. Glasgow, N. Aubry, Enhancement of microfluidic mixing using time pulsing. Lab Chip 3(2), 114–120 (2003)
X. Niu, Y.-K. Lee, Efficient spatial-temporal chaotic mixing in microchannels. J. Micromech. Microeng. 13(3), 454 (2003)
P. Tabeling et al., Chaotic mixing in cross-channel micromixers. Philos. Trans. R. Soc. Lond. Ser. A: Math. Phys. Eng. Sci. 362, 987–1000 (2004)
P. Eribol, A. Uguz, Experimental investigation of electrohydrodynamic instabilities in micro channels. Eur. Phys. J. Spec. Top. 224(2), 425–434 (2015)
S. Dong et al., Mixing enhancement of electroosmotic flow in microchannels under DC and AC electric field. J. Appl. Fluid Mech. 13(1), 79–88 (2020)
J. Huang et al., An electro-thermal micro mixer, in 2011 6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems. IEEE (2011)
G.G. Yaralioglu et al., Ultrasonic mixing in microfluidic channels using integrated transducers. Anal. Chem. 76(13), 3694–3698 (2004)
E.-S. Shanko et al., Microfluidic magnetic mixing at low reynolds numbers and in stagnant fluids. Micromachines 10(11), 731 (2019)
S.H. Lee et al., Effective mixing in a microfluidic chip using magnetic particles. Lab Chip 9(3), 479–482 (2009)
K.S. Ryu et al., Micro magnetic stir-bar mixer integrated with parylene microfluidic channels. Lab Chip 4(6), 608–613 (2004)
B. Xu et al., Thermal mixing of two miscible fluids in a T-shaped microchannel. Biomicrofluidics 4(4), 044102 (2010)
Y. Wang et al., Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 8(3), 1677–1685 (2018)
P. Cui, S. Wang, Application of microfluidic chip technology in pharmaceutical analysis: a review. J Pharm Anal 9(4), 238–247 (2019)
P. De Stefano, E. Bianchi, G. Dubini, The impact of microfluidics in high-throughput drug-screening applications. Biomicrofluidics 16(3), 031501 (2022)
K.I.W. Kane et al., Automated microfluidic cell culture of stem cell derived dopaminergic neurons. Sci. Rep. 9(1), 1796 (2019)
A. Petrosyan et al., A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun. 10(1), 3656 (2019)
N.R. Wevers et al., A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 15(1), 23 (2018)
R. Visone et al., A simple vacuum-based microfluidic technique to establish high-throughput organs-on-chip and 3D cell cultures at the microscale. Adv. Mater. Technol. 4(1), 1800319 (2018)
Y. Gao, P. Li, D. Pappas, A microfluidic localized, multiple cell culture array using vacuum actuated cell seeding: integrated anticancer drug testing. Biomed. Microdevices 15(6), 907–915 (2013)
M.A. Unger et al., Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116 (2000)
E.S. Park, A.C. Brown, M.A. DiFeo, T.H. Barker, H. Lu, Continuously perfused, non-cross-contaminating microfluidic chamber array for studying cellular responses to orthogonal combinations of matrix and soluble signals. Lab Chip 10, 571–580 (2010)
N.L. Jeon, S.K.W. Dertinger, D.T. Chiu, I.S. Choi, A.D. Stroock, G.M. Whitesides, Generation of solution and surface gradients using microfluidic systems. Langmuir 16(22), 8311–8316 (2000). https://doi.org/10.1021/la000600b
J. Qin et al., Microfluidic devices for the analysis of apoptosis. Electrophoresis 26(19), 3780–3788 (2005)
N. Ye et al., Cell-based high content screening using an integrated microfluidic device. Lab Chip 7(12), 1696–1704 (2007)
S. Ostrovidov et al., Controlled release of drugs from gradient hydrogels for high-throughput analysis of cell-drug interactions. Anal. Chem. 84(3), 1302–1309 (2012)
K. Chung et al., Imaging single-cell signaling dynamics with a deterministic high-density single-cell trap array. Anal. Chem. 83(18), 7044–7052 (2011)
S. Sugiura, K. Hattori, T. Kanamori, Microfluidic serial dilution cell-based assay for analyzing drug dose response over a wide concentration range. Anal. Chem. 82(19), 8278–8282 (2010). https://doi.org/10.1021/ac1017666
Š. Selimović, W.Y. Sim, S.B. Kim, Y.H. Jang, W.G. Lee, M. Khabiry, H. Bae, S. Jambovane, J.W. Hong, A. Khademhosseini, Exponential concentration gradients in microfluidic devices for cell studies, in Proceedings of the ASME 2011 Summer Bioengineering Conference. ASME 2011 Summer Bioengineering Conference, Parts A and B. Farmington, Pennsylvania, USA. June 22–25, 2011. pp. 99–100. ASME
C.G. Yang et al., A radial microfluidic concentration gradient generator with high-density channels for cell apoptosis assay. Lab Chip 11(19), 3305–3312 (2011)
R. Meissner et al., Distinguishing drug-induced minor morphological changes from major cellular damage via label-free impedimetric toxicity screening. Lab Chip 11(14), 2352–2361 (2011)
L.C. Hsiung et al., Dielectrophoresis-based cellular microarray chip for anticancer drug screening in perfusion microenvironments. Lab Chip 11(14), 2333–2342 (2011)
Y. Chen, C. Guo, L. Lim, S. Cheong, Q. Zhang, K. Tang, J. Reboud, Compact microelectrode array system: tool for in situ monitoring of drug effects on neurotransmitter release from neural cells. Anal. Chem. 80, 1133–1140 (2008)
H. Xu et al., Organoid technology and applications in cancer research. J. Hematol. Oncol. 11(1), 116 (2018)
C. Wang et al., Three-dimensional in vitro cancer models: a short review. Biofabrication 6(2), 022001 (2014)
B. Chan, K. Leong, Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J. 17(4), 467–479 (2008)
N.S. Bhise et al., Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 190, 82–93 (2014)
P.-A. Vidi et al., Disease-on-a-chip: mimicry of tumor growth in mammary ducts. Lab Chip 14(1), 172–177 (2014)
E.W. Esch, A. Bahinski, D. Huh, Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 14(4), 248–260 (2015)
Y.S. Zhang, A. Khademhosseini, Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine 10(5), 685–688 (2015)
Y. Zhao et al., Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6(3), 035001 (2014)
C.J. Mandrycky et al., Organ-on-a-chip systems for vascular biology. J. Mol. Cell. Cardiol. 159, 1–13 (2021)
D.B. Kolesky et al., 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26(19), 3124–3130 (2014)
J.Y. Yun et al., Log-scale dose response of inhibitors on a chip. Anal. Chem. 83(16), 6148–6153 (2011)
H. Fan, U. Demirci, P. Chen, Emerging organoid models: leaping forward in cancer research. J. Hematol. Oncol. 12(1), 142 (2019)
S.H. Lee, J.H. Sung, Organ-on-a-chip technology for reproducing multiorgan physiology. Adv. Healthc. Mater. 7(2), 1700419 (2018)
K. Shinha et al., A pharmacokinetic-pharmacodynamic model based on multi-organ-on-a-chip for drug-drug interaction studies. Biomicrofluidics 14(4), 044108 (2020)
D.E. Ingber, Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 23(8), 467–491 (2022)
Z.A. Li, R.S. Tuan, Towards establishing human body-on-a-chip systems. Stem Cell Res. Ther. 13(1), 431 (2022)
Acknowledgements
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We thank Dr. Ali Mousavi (Orthopedic Research Center, Mashhad University of Medical Sciences, Mashhad, Iran) for his comments and contributions to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhusal, A., Yogeshwaran, S., Goodarzi Hosseinabadi, H. et al. Microfluidics for High Throughput Screening of Biological Agents and Therapeutics. Biomedical Materials & Devices (2024). https://doi.org/10.1007/s44174-024-00169-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44174-024-00169-1