Abstract
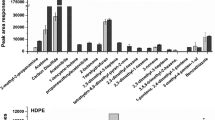
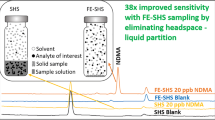
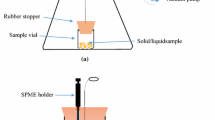
This study addresses the critical issue of detecting volatile extractables released during the clinical use of medical devices which can pose a serious threat to patient health. ISO 10993-18:2020 introduces the concept of the Analytical Evaluation Threshold (AET) to determine the necessary analytical sensitivity for detecting these extractables. Compounds at or above the AET must be reported for toxicological risk assessment. Currently, Static Headspace (SHS) analysis serves as a supplementary technique for volatile analysis, but its variable signal responses and limited sensitivity have hindered the establishment of a defined AET. In response to this challenge, this study explores the potential of Dynamic Headspace (DHS) gas chromatography-mass spectrometry (GC–MS) analysis to achieve the required sensitivity levels for robust toxicological risk assessment of volatile compounds from medical devices. The development of the DHS method involves optimizing several parameters, including incubation temperature, trapping volume/time, adsorbent type, and drying time. The study rigorously evaluates DHS extraction performance and compares it to traditional SHS and Stir Bar Sorptive Extraction (SBSE) GC–MS methods. By applying these newly developed methods to saline extracts from various medical devices and materials, the study demonstrates that DHS achieves over a tenfold increase in sensitivity compared to conventional volatile organic compound (VOC) analysis methods. This enhanced sensitivity enables the quantification of a significantly greater number of VOCs, thereby improving safety assessments for volatiles present in medical devices. With optimized conditions and robust data, DHS methods emerge as a valuable tool for VOC analysis to support comprehensive toxicological risk assessments in the context of medical device safety evaluation.
Graphical Abstract










Similar content being viewed by others
Data Availability
Data will be made available on request.
References
U.S. Pharmacopea, USP<1664> Assessment of Drug Product Leachables Associated with Pharmaceutical Packaging/Delivery Systems, 2017.
U.S. Pahamacopia, USP<661>Plastic Packaging Systems and Their Materials of Construction, 2017.
ICH M7(R2) Guideline on assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk, 2023.
C. B’Hymer, Residual solvent testing: a review of gas-chromatographic and alternative techniques. Pharm. Res. 20(3), 337–344 (2003). https://doi.org/10.1023/a:1022693516409
D.R. Jenke, J.M. Jene, M. Poss, J. Story, T. Tsilipetros, A. Odufu, W. Terbush, Accumulation of extractables in buffer solutions from a polyolefin plastic container. Int. J. Pharm. 297(1–2), 120–133 (2005). https://doi.org/10.1016/j.ijpharm.2005.03.010
K.A. Favela, M.J. Hartnett, J.A. Janssen, D.W. Vickers, A.J. Schaub, H.A. Spidle, K.S. Pickens, Nontargeted analysis of face masks: comparison of manual curation to automated GC × GC processing tools. J. Am. Soc. Mass Spectrom. 32(4), 860–871 (2021). https://doi.org/10.1021/jasms.0c00318
F.C. Su, M.C. Friesen, A.B. Stefaniak, P.K. Henneberger, R.F. LeBouf, M.L. Stanton, X. Liang, M. Humann, M.A. Virji, Exposures to volatile organic Compounds among healthcare workers: modeling the effects of cleaning tasks and product use. Ann. Work Expo. Health 62(7), 852–870 (2018). https://doi.org/10.1093/annweh/wxy055
A. Steinemann, N. Nematollahi, B. Rismanchi, N. Goodman, S.D. Kolev, Pandemic products and volatile chemical emissions. Air Qual. Atmos. Health 14(1), 47–53 (2021). https://doi.org/10.1007/s11869-020-00912-9
N. Lin, N. Ding, E. Meza-Wilson, A. Manuradha Devasurendra, C. Godwin, S. Kyun Park, S. Batterman, Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ. Int. 144, 105740 (2020). https://doi.org/10.1016/j.envint.2020.105740
A. Arbin, S. Jacobsson, K. Hänninen, A. Hagman, J. Östelius, Studies on contamination of intravenous solutions from PVC-bags with dynamic headspace GC-MS and LC-diode array techniques. Int. J. Pharm. 28(2), 211–218 (1986). https://doi.org/10.1016/0378-5173(86)90247-4
O. Ezquerro, B. Pons, M.T. Tena, Development of a headspace solid-phase microextraction-gas chromatography-mass spectrometry method for the identification of odour-causing volatile compounds in packaging materials. J. Chromatogr. A 963(1–2), 381–392 (2002). https://doi.org/10.1016/s0021-9673(02)00211-x
ISO 10993–18:2020/Amd 1:2022, Biological evaluation of medical devices — Part 18: Chemical characterization of medical device materials within a risk management process — Amendment 1: Determination of the uncertainty factor, 2022.
A. Kremser, M.A. Jochmann, T.C. Schmidt, Systematic comparison of static and dynamic headspace sampling techniques for gas chromatography. Anal. Bioanal. Chem. 408(24), 6567–6579 (2016). https://doi.org/10.1007/s00216-016-9843-y
L.F. Gyorgy Vas, K. Comstock, J. Cole, Extractable and leachable testing for pharmaceutical packaging finished pharmaceutical products, and medical devices: an analytical perspective. LCGC Suppl. 18(3), 5–14 (2020)
E. Haddadi, E. Mehravar, F. Abbasi, K. Jalili, Expandable styrene/methyl methacrylate copolymer: Synthesis and determination of VOCs by combined thermogravimetry/differential thermal analysis-gas chromatography/mass spectrometry. J. Appl. Polym. Sci. 124(6), 4711–4720 (2012). https://doi.org/10.1002/app.35513
R.C. Silva, P.M.S. Aguiar, F. Augusto, Coupling of dynamic headspace sampling and solid phase microextraction. Chromatographia 60(11), 687–691 (2004). https://doi.org/10.1365/s10337-004-0446-y
W. Wojnowski, T. Majchrzak, T. Dymerski, J. Gębicki, J. Namieśnik, Dynamic headspace sampling as an initial step for sample preparation in chromatographic analysis. J. AOAC Int. 100(6), 1599–1606 (2019). https://doi.org/10.5740/jaoacint.17-0206
A. Marquez, M.P. Serratosa, J. Merida, L. Zea, L. Moyano, Optimization and validation of an automated DHS–TD–GC–MS method for the determination of aromatic esters in sweet wines. Talanta 123, 32–38 (2014). https://doi.org/10.1016/j.talanta.2014.01.052
L. Moyano, M.P. Serratosa, A. Marquez, L. Zea, Optimization and validation of a DHS-TD-GC-MS method to wineomics studies. Talanta 192, 301–307 (2019). https://doi.org/10.1016/j.talanta.2018.09.032
I. Guiffard, T. Geny, B. Veyrand, P. Marchand, A. Pellouin-Grouhel, B.L. Bizec, E. Bichon, Quantification of light polycyclic aromatic hydrocarbons in seafood samples using on-line dynamic headspace extraction, thermodesorption, gas chromatography tandem mass spectrometry, based on an isotope dilution approach. J. Chromatogr. A 1619, 460906 (2020). https://doi.org/10.1016/j.chroma.2020.460906
A.K. Katyal, R.D. Morrison, Chapter 11 – Forensic Applications of Contaminant Transport Models in the Subsurface, 2007.
D.M. Butterfield, R.P. Lipscombe, T.D. Gardiner, Safe sampling volume determinations of 12 volatile organic compounds on Carboxen 1003, Carbopack-X & Tenax-TA. J. Chromatogr. A 1626, 461369 (2020). https://doi.org/10.1016/j.chroma.2020.461369
J.H. Lee, S.A. Batterman, C. Jia, S. Chernyak, Ozone artifacts and carbonyl measurements using tenax GR, tenax TA, carbopack B, and carbopack X adsorbents. J. Air Waste Manag. Assoc. 56(11), 1503–1517 (2006). https://doi.org/10.1080/10473289.2006.10464560
O. Vyviurska, M. Hanobiková, A.A. Gomes, I. Špánik, Multivariate optimization of dual-sorbent dynamic headspace extraction of volatiles in wine analysis. Food Chem. 365, 130449 (2021). https://doi.org/10.1016/j.foodchem.2021.130449
Y. Liu, Z. Wang, W. Wang, J. Xing, Q. Zhang, Q. Ma, Q. Lv, Non-targeted analysis of unknown volatile chemicals in medical masks. Environ. Int. 161, 107122 (2022). https://doi.org/10.1016/j.envint.2022.107122
U. Colareta Ugarte, P. Prazad, B.L. Puppala, L. Schweig, R. Donovan, D.R. Cortes, A. Gulati, Emission of volatile organic compounds from medical equipment inside neonatal incubators. J. Perinatol. 34(8), 624–628 (2014). https://doi.org/10.1038/jp.2014.65
F.A. Franchina, G. Purcaro, Chapter Six - Sample preparation strategies for comprehensive volatile fingerprinting, in Comprehensive Analytical Chemistry. ed. by C.E.I. Cordero (Elsevier, Amsterdam, 2022), pp.155–184. https://doi.org/10.1016/bs.coac.2022.02.001
V. Ahmadi-Moshiran, A.A. Sajedian, A. Soltanzadeh, F. Seifi, R. Koobasi, N. Nikbakht, M. Sadeghi-Yarandi, Carcinogenic and health risk assessment of respiratory exposure to acrylonitrile, 1,3-butadiene and styrene in the petrochemical industry using the US environmental protection agency method. Int. J. Occup. Saf. Ergon. 28(4), i–ix (2022). https://doi.org/10.1080/10803548.2022.2059171
M. Wijeweera Patabandige, K. Nahan, J.A. Young, B. Oktem, E.M. Sussman, B.H. Yun, S. Wickramasekara, Thermal extraction: an alternative headspace GC–MS method for volatile extractables from medical device materials. Biomed. Mater. Dev. (2023). https://doi.org/10.1007/s44174-023-00103-x
ISO, 10993–12:2021, Biological evaluation of medical devices — Part 12: Sample preparation and reference materials, 2021.
P.A. Lamprea Pineda, K. Demeestere, M. Sabbe, J. Bruneel, H. Van Langenhove, C. Walgraeve, Effect of (bio)surfactant type and concentration on the gas-liquid equilibrium partitioning of hydrophobic volatile organic compounds. J. Hazard. Mater. 443, 130320 (2023). https://doi.org/10.1016/j.jhazmat.2022.130320
Y. Cheng, H. He, C. Yang, G. Zeng, X. Li, H. Chen, G. Yu, Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 34(6), 1091–1102 (2016). https://doi.org/10.1016/j.biotechadv.2016.06.007
M.A. Anderson, Influence of surfactants on vapor-liquid partitioning. Environ. Sci. Technol. 26(11), 2186–2191 (1992)
T. Jumepaeng, D.L. Luthria, S. Chanthai, The effect of surfactant on headspace single drop microextraction for the determination of some volatile aroma compounds in citronella grass and lemongrass leaves by gas chromatography. Anal. Methods 4(2), 421–428 (2012). https://doi.org/10.1039/C2AY05646A
N. Ochiai, K. Sasamoto, F. David, P. Sandra, Recent Developments of Stir Bar Sorptive Extraction for Food Applications: Extension to Polar Solutes. J. Agric. Food Chem. 66(28), 7249–7255 (2018). https://doi.org/10.1021/acs.jafc.8b02182
O. Zuloaga, N. Etxebarria, B. González-Gaya, M. Olivares, A. Prieto, A. Usobiaga, 18 - Stir-bar sorptive extraction, in Solid-Phase Extraction. ed. by C.F. Poole (Elsevier, Amsterdam, 2020), pp.493–530. https://doi.org/10.1016/B978-0-12-816906-3.00018-2
F. David, P. Sandra, Stir bar sorptive extraction for trace analysis. J. Chromatogr. A 1152(1–2), 54–69 (2007). https://doi.org/10.1016/j.chroma.2007.01.032
B.L. Armstrong, A.F. Senyurt, V. Narayan, X. Wang, L. Alquier, G. Vas, Stir bar sorptive extraction combined with GC-MS/MS for determination of low level leachable components from implantable medical devices. J. Pharm. Biomed. Anal. 74, 162–170 (2013). https://doi.org/10.1016/j.jpba.2012.10.019
A. Prieto, O. Basauri, R. Rodil, A. Usobiaga, L.A. Fernández, N. Etxebarria, O. Zuloaga, Stir-bar sorptive extraction: a view on method optimisation, novel applications, limitations and potential solutions. J. Chromatogr. A 1217(16), 2642–2666 (2010). https://doi.org/10.1016/j.chroma.2009.12.051
M. Kawaguchi, R. Ito, K. Saito, H. Nakazawa, Novel stir bar sorptive extraction methods for environmental and biomedical analysis. J. Pharm. Biomed. Anal. 40(3), 500–508 (2006). https://doi.org/10.1016/j.jpba.2005.08.029
J.M.F. Nogueira, Novel sorption-based methodologies for static microextraction analysis: A review on SBSE and related techniques. Anal. Chim. Acta 757, 1–10 (2012). https://doi.org/10.1016/j.aca.2012.10.033
Acknowledgements
This research was funded in part by CDRH Critical Path initiative and an appointment to the Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and the U.S. FDA. Special thanks go to Drs. Keaton Nahan, Robert Elder and Adrijo Chakraborti for their help in project initiation and data processing. The authors would like to acknowledge Drs. Michael Eppihimer and Victoria Nawaby for their guidance and support throughout the project.
Disclaimer
Findings and conclusions in this paper have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy. The mention of commercial products, their sources, or their use in this study is not to be construed as either an actual or implied endorsement of such products by the US Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wijeweera Patabandige, M., Hill, J., Herath, A. et al. Dynamic Headspace GC–MS Method to Detect Volatile Extractables from Medical Device Materials. Biomedical Materials & Devices (2024). https://doi.org/10.1007/s44174-023-00145-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44174-023-00145-1