Abstract
The purpose of this study was to evaluate the deliverability, tolerability, and retrievability of a novel prostatic stent (the FloStent System™) in a healthy canine model. This was a non-randomized, as-treated study. Implantations were performed using a novel fluoroscopic technique. Animals were followed up to 30 days. No stent migration occurred; the stent was well tolerated; retrieval procedures were successful. Gross pathology and histopathological findings were consistent with minimal trauma caused by the implant procedures. The FloStent prostatic implant was demonstrated adequate functionality and safety in this healthy animal model, which supports plans for future use in human studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostatic hyperplasia (BPH) is a urologic condition characterized by a progressive increase in prostate size and is a leading health problem for older men [1, 2]. It has been estimated that 50% of men aged 51–60 years and 90% of those over 80 years have evidence of prostatic enlargement [2, 3]. Though not life-threatening, BPH causes bothersome urinary symptoms, including urgency, frequency, and nocturia, which affect patient’s quality of life (QoL) [2, 4, 5]. Additionally, it has been found that healthcare costs attributed to BPH are among the top 10 most prominent and costly diseases in men older than 50 years [6].
For men with moderate-to-severe BPH symptoms who require surgery, transurethral resection of the prostate (TURP) is generally considered the gold standard; however, between 10% and 15% of these patients are deemed unfit for this procedure, and market trends suggest that a growing number of men are opting for minimally invasive treatment options [2, 4, 7, 8]. Prostatic stents were initially developed in the 1990s to be such an option and early research demonstrated that they could be easily inserted into the urethra. Initial versions of this type of device were designed to be implanted permanently and were shown to be associated with certain complications, such as recurrent infection, recurrent obstruction, urethral stricture, and stent migration [9,10,11,12,13]. Stent removal was often difficult to perform with earlier stent designs, which led to even further complications [7, 14, 15].
To overcome some of the problems associated with the permanent prostatic stents, modern implant designs now include stents that can be retrieved if necessary [2, 7, 12, 14, 16]. Retrievable nitinol stents have shown promising results in animal models and human trials across different indications [2, 7, 17,18,19,20]. The FloStent System™ (Rivermark Medical, Milwaukee, WI) is a novel nitinol stent implantable with standard flexible cystoscopes, and was developed as a potential first-line device therapy for BPH. The implant is placed into position during routine flexible cystoscopy with minimal patient recovery time and no need for a urethral catheter. The stent gently holds the prostatic urethra open to restore normal urinary function, preserve sexual function, and improve patient’s quality of life. If desired, the device can be easily retrieved or repositioned after initial implantation. The purpose of this study was to evaluate the performance and physiologic response of the FloStent System in a healthy canine model.
Methods
Study Design
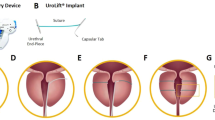
This was a non-randomized, as-treated study that enrolled six healthy, intact male canines. The weight of the animals ranged from 30.1 to 35.3 kg; age was appropriate to weight. The test article (Fig. 1) was provided steam sterilized by the device manufacturer prior to the procedures.
The study protocol was approved by the University of Wisconsin–Madison Institutional Animal Care and Use Committee (IACUC #IS00006527).
Study Procedures
Prior to enrollment, each animal was assessed by a facility veterinarian via physical exam to determine suitability for study entry. Four to five days prior to their scheduled procedure, a pre-surgical exam was performed, and the animals were weighed. Daily observations were performed beginning on the day of arrival and continuing throughout the in-life period. Animals were fasted 12–24 h prior to any anesthetized procedures. The animals were weighed again within 72 h of the procedure and within 24 h prior to termination. Anesthesia was induced and maintained according to test facility procedures, and the animals were intubated and maintained on inhalant isoflurane for continued general anesthesia. An appropriately sized intravenous catheter was placed for supportive fluid administration via constant rate infusion, and the prepuce and groin region of the animals were clipped free of hair. The animals were then transferred to the procedure room, placed in a dorsal recumbent position, secured, and attached to a patient monitor and ventilator.
The following procedures were performed for each animal enrolled in the study, unless otherwise described:
-
Spot radiograph
-
Pre-implantation retrograde urethrogram (RUG) using 11-Fr tapered dilator
-
Advancement of guidewire through 11-Fr tapered dilator; gentle dilation of distal (penile) urethra to 14-Fr over guidewire
-
Removal of guidewire
-
Backloading of implant into 8-Fr guide catheter using standard cystoscopic grasping forceps
-
Fluoroscopy-guided advancement of guide catheter, loaded with implant, through the penile urethra, prostatic urethra, and into the urinary bladder
-
Advancement of implant past tip of guide catheter, maintaining connection with grasping forceps
-
Withdrawal of guide catheter, implant, and grasping forceps, such that the implant was positioned in the prostatic urethra
-
Release of implant (opening flexible forceps)
-
Withdrawal of guide catheter and flexible forceps
-
Post-implantation RUG to confirm proper implant position and patency
-
Passage of Foley catheter through implant and into urinary bladder
-
Removal of Foley catheter through implant and into urinary bladder
-
Radiograph confirming final implant position prior to procedure end.
All successfully implanted animals were recovered according to appropriate test facility standard operating procedures and policies. They were transferred to a recovery area and monitored until they recovered from anesthesia, the endotracheal tube was removed, and the animal was stable. Once recovered, the animals were fed a portion of their regular diet ration, with regular feeding occurring the subsequent day.
Follow-Up
Cystoscopic and RUG assessments of the prostatic urethra were completed following deployment and retrieval of the implant. Imaging (both spot radiograph and live fluoroscopy, as needed) was performed immediately post-procedure and prior to termination. This methodology is similar to that used in prior prostatic stent studies in the canine model [7, 20].
Daily animal observations were performed by trained animal care staff per appropriate Biomedical Research Model Services Standard Operating Procedures and documented in the animal records. Abnormalities and adverse events (AEs) were documented, and an assessment, plan, and treatment were outlined by a testing facility veterinarian. Individual progress or regression of such instances was followed daily by a testing facility veterinarian. In-life animal activities included monitoring for evidence of urination. An AE was a description of any untoward occurrence affecting the health or well-being of an animal during the study. All reported AEs were adjudicated by the study director who was responsible for the final assessment of the seriousness of the event and its potential relationship to the procedure, device, or animal model.
Blood was collected via venipuncture at baseline and prior to euthanasia. Blood was prepared for complete blood count (CBC) with differential and serum chemistry panel.
All animals were humanely euthanized following the final follow-up imaging on their scheduled termination dates (24 ± 6 h, 7 ± 2 days, and 30 ± 3 days). After euthanasia, gross necropsies were conducted. The urethra and bladder were assessed in situ, harvested for gross examination, then fixed in formalin and sent for histologic processing. A detailed gross examination was performed of the abdominal cavity to evaluate for any damage or changes due to the treatment. Details on the assessment of gross necropsy and histopathology are provided in Appendix 1.
Statistical Analysis
The study objectives were evaluated qualitatively and, where appropriate, summarized using descriptive statistics. No formal statistical analysis was planned for this study.
Results
Stent Implantation and Removal
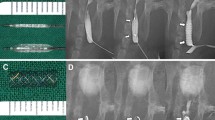
Endoscopic stent delivery was not successful in one animal due to anatomical constraints, namely the urethral caliber was too small to accommodate a standard flexible cystoscope, prompting early termination prior to test article exposure. This single animal was then exited from the study. The remaining five animals were successfully implanted using a fluoroscopic technique (Fig. 2). Pre- and post-implant RUG imaging confirmed post-implant prostatic urethral patency in all successfully implanted animals (Table 1).
All successfully implanted animals recovered normally from anesthesia and survived to their respective termination time points at 24 ± 6 h (one animal), 7 ± 2 days (two animals), or 30 ± 3 days (2 animals). Of the 30-day survival animals, implant removal was not performed in one animal in order to allow for in situ gross and histological analysis; therefore, four successful retrieval procedures were performed in this study. Pre-retrieval endoscopic evaluation of the implant was performed in these four cases, each time using a human ureteroscope.
Clinical Observations and Post-procedure Care
Overall, findings that resulted in a Subjective, Objective, Assessment, Plan were minimal in nature and not attributable to the test article. One animal presented with possible minimal urinary incontinence post-implant within the first 24 h following implantation, though, as this animal was allocated to the 24-h survival group, it was terminated before the condition had resolved; however, it was felt that the condition itself was unlikely to be pathologic. Additionally, both 30-day survival animals exhibited an increase in or maintenance of body weight at termination.
Pre-terminal Procedure
Of the five successfully implanted animals, all underwent pre-terminal imaging prior to euthanasia (Fig. 3). A summary of the pre-terminal procedure outcomes is presented in Table 2. No stent migration occurred in these animals and implant retrieval procedures were uneventful, though additional force or gentle pressure was required in two cases.
Clinical Pathology
CBC and serum chemistry were all within normal ranges at baseline and pre-termination.
Gross Necropsy
A summary of findings from the necropsy examination are found in Table 3. All implants were located in the prostatic urethra of the animal at the end of their respective survival durations, with one animal having minimal extension into the bladder.
Histology
The composite scores of the histology findings for each animal are presented in Table 4. Denudement (ulceration) of the urethral epithelium was observed in the 24-h animal, 1 of 2 (50%) 7-day animals, and 2 of 2 (100%) 30-day animals. When present, the epithelial denudement was in segmental areas. Submucosal hemorrhage was observed in variable severity in all animals, consistent with mild mucosal trauma shortly before euthanasia.
Histological changes within the prostatic parenchyma were observed in 4 of 5 (80%) animals and consisted of glandular dilation (two 7-day and two 30-day animals), glandular atrophy (one 7-day animal and two 30-day animals), mononuclear inflammatory infiltrates (one 7-day animal and two 30-day), lymphofollicular aggregates (one 7-day animal and two 30-day animals), and inflammatory casts within glandular lumina (one 7-day and two 30-day animals). The glandular atrophy was associated with the chronic mononuclear (lymphocytes, macrophages, and plasma cells) inflammation and lymphofollicular aggregates. The inflammatory casts were composed of mixed inflammatory cells (neutrophils, lymphocytes, and macrophages), as well as degenerate/necrotic cells, which could have been epithelial or inflammatory cell origin. A cross-sectional comparison of the untreated versus treated urethra is presented in Fig. 4.
Discussion
The purpose of this study was to evaluate the performance and physiologic response of the FloStent System in a healthy canine model. The study successfully met its stated objectives, which were stent delivery and stent retrieval using standard urological equipment and a channel similar in size to the working channels of common flexible cystoscopes (approximately 2.2–2.4 mm). The study also met the stated objective of assessing implant tolerability, which appeared adequate given the absence of abnormal voiding behavior in all animals.
Pre- and post-implant RUG imaging confirmed post-implant prostatic urethral patency in all implanted animals. Pre-retrieval endoscopic examination revealed that all implants were intact, remained in position where they were initially placed, and were patent without gross encrustation.
Such findings highlight the potential benefits of the FloStent device relative to earlier generation prostatic stents, in terms of reducing complication rates and the difficulty of implant removal [2, 7, 9, 13, 15, 20, 21]. The current animal study demonstrated no stent migration, which is one of the more common complications following prostatic stent placement [20, 22]. The FloStent was safely removed from all successfully implanted animals at the end of their respective survival periods, which was demonstrated up to 30 days in two animals. It has been proposed that stent-related complications generally resolve after stent removal [2, 23]. It is also notable that urethral catheter passage was successfully performed in all animals and did not compromise the integrity or position of the implant. If future trials in humans demonstrate similar results, the FloStent can be shown to be a viable option for the broader BPH population [2, 24].
Pathology and gross necropsy findings were consistent with expected transient inflammatory infiltrate seen during wound healing. The identified histologic features of mucosal denudement accompanied by submucosal fibrin, edema, hemorrhage, and congestion were consistent with mild trauma to the mucosal surface shortly before euthanasia. In total, histopathology did not reveal any unexpected findings as the prostatic tissue response to the stent seen in this investigation was consistent with what has been observed in earlier studies [2, 7, 20]. These observations do not elicit any safety concerns with FloStent implantation or removal. Additionally, the current study did not show the same histologic changes demonstrated with the long-term use of the Urolume stent, specifically, no prominent plasma cells, no polypoid hyperplasia, and no keratinizing squamous metaplasia [25].
Previously conducted studies have yielded similar findings, demonstrating the safety and feasibility of implanting a retrievable nitinol stent in a canine model [2, 7, 20]. In a study on 13 healthy canines, Yoon et al. (2006) found that the stent-induced urethral dilation and prostatic glandular atrophy persisted up to eight weeks after implant removal [7]. Crisostomo et al. conducted a study on 8 healthy beagles, which found marked enlargement of the prostatic urethral lumen and no impairment of urinary flow during a two-month follow-up. Additionally, the investigators of this study also had difficulty deploying the stent in one case as the urethral lumen of this animal was too narrow, further highlighting the difference and added difficulty of stent placement in canines versus humans [2]. In the study by Yoon et al. (2010), BPH was induced in eight beagles who were then implanted with a retrievable, barbed stent, which demonstrated increased prostatic urethra diameters immediately after and eight weeks after stent removal, in addition to extensive prostatic glandular atrophy [20]. These studies also all showed that stent retrieval can successfully be performed in the majority of cases [2, 7, 20]. Though human trials with the FloStent are required, the feasibility, safety, and efficacy of similar devices have been demonstrated in recent studies on men with BPH, with continued improvement in patient outcomes seen up to three years [16, 26,27,28,29]. Such findings show the promise and potential place in BPH therapy of these minimally invasive implants.
A limitation of the current study is that the FloStent was used in a healthy animal model, in relatively young dogs without clinical BPH; therefore, further evaluation is required to determine if its effects are consistent in men with BPH. In addition, the histological characteristics of a canine is different than those of a human, adding uncertainty as to whether or not the histologic response seen in this study will be the same in humans [20, 30]. There was also no control or comparator group, and the FloStent will eventually need to be compared to either a sham control or other BPH interventions currently used in practice. When this implant is evaluated in human trials, investigators will also need to measure patient-reported outcomes, quality of life, and other outcomes that cannot be reliably measured in animals (e.g., sexual function and foreign body sensation) [7]. Lastly, studies with greater follow-up periods are needed to ensure that the efficacy and safety of this device are sustained for long term.
Conclusion
The FloStent System performed well in a healthy canine model, demonstrating consistent deliverability, tolerability, and retrievability. A novel fluoroscopic technique was employed; results suggest that implantation via the working channel of a flexible cystoscope may be feasible in humans. No clinically significant AEs, abnormal clinical observations, or clinical pathology results attributable to the test article were encountered. Gross pathology and histopathological findings were consistent with minimal trauma caused by the implant procedures.
Data Availability
Owing to propriety nature, supporting data cannot be made available openly.
References
B. Chughtai, J.C. Forde, D.D. Thomas, L. Laor, T. Hossack, H.H. Woo et al., Benign prostatic hyperplasia. Nat. Rev. Dis. Primers 2, 16031 (2016)
V. Crisóstomo, H.Y. Song, M. Maynar, F. Sun, F. Soria, J.R. Lima et al., Evaluation of the effects of temporary covered nitinol stent placement in the prostatic urethra: short-term study in the canine model. Cardiovasc. Intervent Radiol. 30, 731–737 (2007)
S.J. Berry, D.S. Coffey, P.C. Walsh, L.L. Ewing, The development of human benign prostatic hyperplasia with age. J. Urol. 132, 474–479 (1984)
L.B. Lerner, K.T. McVary, M.J. Barry, B.R. Bixler, P. Dahm, A.K. Das et al., Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE part II-surgical evaluation and treatment. J. Urol. 206, 818–826 (2021)
L.B. Lerner, K.T. McVary, M.J. Barry, B.R. Bixler, P. Dahm, A.K. Das et al., Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE part I-initial work-up and medical management. J. Urol. 206, 806–817 (2021)
T.C. Fenter, M.J. Naslund, M.B. Shah, M.T. Eaddy, L. Black, The cost of treating the 10 most prevalent diseases in men 50 years of age or older. Am. J. Manag. Care 12, S90–S98 (2006)
C.J. Yoon, H.Y. Song, J.H. Shin, C.W. Woo, J.Y. Ro, H.K. Park et al., Covered retrievable prostatic urethral stents: feasibility study in a canine model. J. Vasc. Interv. Radiol. 17, 1813–1819 (2006)
S. Madersbacher, C.G. Roehrborn, M. Oelke, The role of novel minimally invasive treatments for lower urinary tract symptoms associated with benign prostatic hyperplasia. BJU Int. 126, 317–326 (2020)
G. Guazzoni, F. Montorsi, C. Coulange, E. Milroy, V. Pansadoro, H. Rubben et al., A modified prostatic UroLume Wallstent for healthy patients with symptomatic benign prostatic hyperplasia: a European Multicenter Study. Urology 44, 364–370 (1994)
S. Masood, H. Djaladat, C. Kouriefs, M. Keen, J.H. Palmer, The 12-year outcome analysis of an endourethral wallstent for treating benign prostatic hyperplasia. BJU Int. 94, 1271–1274 (2004)
J.E. Oesterling, S.A. Kaplan, H.B. Epstein, A.J. Defalco, P.K. Reddy, M.B. Chancellor, The North American experience with the UroLume endoprosthesis as a treatment for benign prostatic hyperplasia: long-term results. The North American UroLume Study Group. Urology 44, 353–362 (1994)
B.A. Vanderbrink, A.R. Rastinehad, G.H. Badlani, Prostatic stents for the treatment of benign prostatic hyperplasia. Curr. Opin. Urol. 17, 1–6 (2007)
G. Williams, C. Coulange, E.J. Milroy, J.P. Sarramon, H. Rubben, The urolume, a permanently implanted prostatic stent for patients at high risk for surgery. Results from 5 collaborative centres. Br. J. Urol. 72, 335–340 (1993)
C.S. Kim, H.Y. Song, I.G. Jeong, H.J. Yeo, E.Y. Kim, J.H. Park et al., Temporary placement of covered retrievable expandable nitinol stents with barbs in high-risk surgical patients with benign prostatic hyperplasia: work in progress. J. Vasc. Interv. Radiol. 22, 1420–1426 (2011)
T.S. Wilson, G.E. Lemack, R.R. Dmochowski, UroLume stents: lessons learned. J. Urol. 167, 2477–2480 (2002)
G. Kadner, M. Valerio, I. Giannakis, A. Manit, N. Lumen, B.S.H. Ho et al., Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study. World J. Urol. 38, 3235–3244 (2020)
H.Y. Song, H. Park, T.S. Suh, G.Y. Ko, T.H. Kim, E.S. Kim et al., Recurrent traumatic urethral strictures near the external sphincter: treatment with a covered, retrievable, expandable nitinol stent–initial results. Radiology 226, 433–440 (2003)
H.Y. Song, S.I. Park, H.Y. Jung, S.B. Kim, J.H. Kim, S.J. Huh et al., Benign and malignant esophageal strictures: treatment with a polyurethane-covered retrievable expandable metallic stent. Radiology 203, 747–752 (1997)
H.Y. Song, T.S. Shim, S.G. Kang, G.S. Jung, D.Y. Lee, T.H. Kim et al., Tracheobronchial strictures: treatment with a polyurethane-covered retrievable expandable nitinol stent–initial experience. Radiology 213, 905–912 (1999)
C.J. Yoon, H.Y. Song, J.H. Kim, H.G. Park, H.S. Kang, J.Y. Ro et al., Temporary placement of a covered, retrievable, barbed stent for the treatment of hormone-induced benign prostatic hyperplasia: technical feasibility and histologic changes in canine prostates. J. Vasc. Interv. Radiol. 21, 1429–1435 (2010)
D.K. Shah, R. Kapoor, G.H. Badlani, Experience with urethral stent explantation. J. Urol. 169, 1398–1400 (2003)
M.M. van Dijk, C.A. Mochtar, H. Wijkstra, M.P. Laguna, J.J. de la Rosette, The bell-shaped nitinol prostatic stent in the treatment of lower urinary tract symptoms: experience in 108 patients. Eur. Urol. 49, 353–359 (2006)
M.I. Anjum, R. Chari, A. Shetty, M. Keen, J.H. Palmer, Long-term clinical results and quality of life after insertion of a self-expanding flexible endourethral prosthesis. Br. J. Urol. 80, 885–888 (1997)
AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J. Urol. 170:530–47.
D.M. Bailey, S.J. Foley, J.P. McFarlane, G. O’Neil, M.C. Parkinson, P.J. Shah, Histological changes associated with long-term urethral stents. Br J Urol 81, 745–749 (1998)
F. Porpiglia, C. Fiori, D. Amparore, G. Kadner, A. Manit, M. Valerio et al., Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU Int. 123, 1061–1069 (2019)
F. Porpiglia, C. Fiori, R. Bertolo, D. Garrou, G. Cattaneo, D. Amparore, Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow-up. BJU Int. 116, 278–287 (2015)
F. Porpiglia, C. Fiori, R. Bertolo, A. Giordano, E. Checcucci, D. Garrou et al., 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int. 122, 106–112 (2018)
G. Yildiz, Z. Bahouth, S. Halachmi, G. Meyer, O. Nativ, B. Moskovitz, Allium™ TPS—a new prostatic stent for the treatment of patients with benign prostatic obstruction: the first report. J. Endourol. 30, 319–322 (2016)
D.P. DeKlerk, D.S. Coffey, L.L. Ewing, I.R. McDermott, W.G. Reiner, C.H. Robinson et al., Comparison of spontaneous and experimentally induced canine prostatic hyperplasia. J. Clin. Invest. 64, 842–849 (1979)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Kadlec and Doraiswamy have equity interest in Rivermark Medical, Inc. Dr. Elterman is an advisor to Rivermark Medical, Inc.
Appendix 1
Appendix 1
Gross Necropsy
A targeted gross necropsy was performed by the study director or trained test facility staff. The abdominal cavity was opened, and the bladder and urethra were assessed and photographed in situ. The bladder neck, prostatic urethra containing the treatment site, including a portion of untreated distal urethra (~ 5–10 cm, as possible) was excised and immersed in 10% neutral buffered formalin. After adequate fixation (≥ 24 h), the urethral treatment site was cut in the transverse plane in three equidistant sites and photographed on the cut surface(s). Regardless of the presence of gross lesions, a representative sample of the urethra was taken distal and proximal to the region of the treatment, where possible.
Histopathology
Histopathology was assessed by a Board-Certified veterinary pathologist. A minimum of three samples were trimmed from each treatment site. In addition, a sample immediately proximal and immediately distal to the treatment site was taken. Each sampling site was placed into an appropriately sized cassette. Representative samples of any gross lesions harvested at necropsy were placed in an appropriately sized cassette. All tissues were processed by routine methods into paraffin, and the resulting blocks were cut at approximately 5-micron thickness. All sampling sites were stained with hematoxylin and eosin (H&E). A grading scheme was used to evaluate histological findings according to the table below.
Grading Scheme for Histological Findings
Grade | Definition |
|---|---|
0 | The lesion/feature not present |
1 | The lesion/feature is present in ≤ 5% of the area of interest. The grade is equivalent to 'minimal' |
2 | The lesion/feature is present in 5–25% of the area of interest. The grade is equivalent to 'mild' |
3 | The lesion/feature is present in 25–50% of the area of interest. The grade is equivalent to 'moderate' |
4 | The lesion/feature is present in 50–75% of the area of interest. The grade is equivalent to 'moderate to severe' |
5 | The lesion/feature is present in > 75% of the area of interest. The grade is equivalent to 'marked' |
Microscopic Evaluation
All histological slides were then evaluated by light microscopy by the study pathologist according to the following:
-
Abnormal tissues—Alterations to normal architecture were described in narrative form. The severity and distribution of each lesion, as well as the likely etiology, if able to be determined, were described.
-
Urethra/bladder—The histopathology assessment documented changes to normal tissue architecture with a goal of documenting the histological features of the treatment sites, as well as the distribution of those features. Lesion characteristics assessment included, but were not limited to, necrosis, hemorrhage, edema, thrombosis/fibrin, fibrosis, and mineralization. Representative low (20 ×) and high (40 ×) magnification images stained with H&E were captured and included in the pathology report. Semiquantitative methods were employed to summarize these parameters and other common lesion features, as outlined by the study pathologist. Additional features were documented in narrative form. The proximal and distal urethral samples were assessed for the same characteristics as the lesion sites. Semiquantitative grading methods employed in the histopathology assessment were defined in the pathology report.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadlec, A., Doraiswamy, A., Bravo, M. et al. Proof-of-Concept Study Evaluating the Performance and Physiologic Response of the FloStent Prostatic Stent in a Healthy Canine Model. Biomedical Materials & Devices 2, 461–473 (2024). https://doi.org/10.1007/s44174-023-00108-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-023-00108-6