Abstract
Rare diseases (RDs) are a group of lifetime incapacitating or fatal diseases affecting nearly 3.5–5.9% of the global population, reaching 263–446 million individuals. RDs possess a genotypic-phenotypic pleiotropic nature so that the same disease can manifest with different outcomes. This renders definitive diagnosis challenging and thus hinders providing appropriate treatment, if available. Since 80% of rare diseases have a genetic origin, evolution in genetic diagnosis owing to the NGS has widely contributed to proper diagnosis and hence facilitating the future implementation of precision medicine. Currently, treatments covering less than 3% of rare diseases are US Food and Drug Administration (FDA) approved. Besides, RDs have a very high economic burden. This review sheds the light on Egyptian achievements and efforts in the field of rare diseases to prioritize the rare genomic diseases to be studied in Egypt. This will grab the attention towards conducting further studies that target Egyptians, to include the under-recognized populations potentially affected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Population architecture and genetic homogeneity in Egypt
Egypt is the 30th largest country in the world (1,001,450 km2) and the 4th largest country in Africa by population after Nigeria, Ethiopia, and Congo (107 million people in 2023) [1, 2]. Egypt has different ethnic groups: 95% are Arabs and 5% are minorities: Copts, Nubians, Bedouins, and Berbers [3]. The major two ethnic groups (Arabs and Copts) were proven to be genetically homogenous and to originate from the same ancestor [4]. The overall reported prevalence of consanguinity in Egypt is 35%, which is higher than the worldwide consanguinity level (25%) [5]. This plays a vital role in the development of rare diseases as most rare diseases are autosomal recessive requiring 2 mutated copies to be manifested. Since consanguinity increases the chances of receiving 2 mutated copies, hence it increases the chance of rare disease development.
Rare disease introduction
Rare diseases (RDs) are a group of lifetime incapacitating or fatal diseases that occur in 3–6% of the population [6]. Since the definition of rare diseases depends on the population size, it varies across different countries. Around 263–446 million persons worldwide are affected by 7000 rare diseases [6, 7]. RDs can be classified into 33 groups according to the organ affected [8].
In Egypt, there is no specific definition of “Rare Diseases” in the procedures and standards of the Egyptian Ministry of Health and Population (MoHP) [9]. Egypt is following the European Union’s definition of rare diseases which is “a rare disease is one that affects no more than 1 person in 2,000” [10].
At least 80% of rare diseases are due to genetic factors, while the remaining 20% are due to infection and exposure to environmental and teratogenic factors [11]. Since RDs are genetically and phenotypically heterogeneous, so the same disease can manifest with different outcomes. This makes the standard diagnosis inconclusive. This is one of the major problems facing the management of RDs, along with the absence of a standard diagnostic protocol, the scarcity of well-trained physicians in the rare disease field, the lack of coordination between the healthcare systems, and the limited public knowledge about rare diseases [12]. Also, the complexity of the disease and the limited number of patients represents a great challenge to the process of rare diseases drug development. This is why the worldwide expenditure on rare diseases drug development still represents 3–9% of the drug market, and less than 3% of these drugs are Food and Drug Administration (FDA) approved [13, 14]. Moreover, RDs have a very high economic burden due to the cost of diagnoses, medical resources, productivity loss, special equipment, daily care, and family spending on drugs or non-traditional treatments [13].
Status of rare diseases in Egypt
In Egypt, there is no registry for rare disease patients, which hinders the precise prevalence estimation of these patients. Also, the lack of appropriate physician training, molecular diagnosis, standard treatment protocols, and drugs hinders the reduction of the rare disease burden in Egypt. Moreover, consanguinity is among the most prevailing social phenomena predisposing to rare genetic diseases, especially in Middle Eastern countries.
On the other hand, public awareness about rare diseases is noticeable in Egypt. This was prominent through the celebration of Rare Disease Day and through the conferences and media articles organized by some associations such as the National Association for Rare Diseases (NARD), the Arab-German Young Academy of Sciences and Humanities (AGYA), Egyptian Group for Orphan Renal Diseases (EGORD), and the Egyptian Scientific Foundation of Rare Diseases in Children [15,16,17].
Status of rare diseases diagnosis and treatment in Egypt
Rare disease diagnosis begins with history taking, clinical examination, clinical investigation, routine laboratory tests, and biochemical tests. If these results are inconclusive, genetic screening is thus required. Genetic testing begins with the targeted screening of known disease-associated candidate genes if the disease is genetically characterized. This includes single gene sequencing, microarray, and copy number variation analysis. If these were inconclusive, whole-exome sequencing (WES) would be used to discover new variants in the genes related to the disease [18]. The variants are analyzed to identify whether they are correlated with the disease. If WES was inconclusive, whole-genome sequencing (WGS) would be used to identify new pathogenic genes or gene modifiers [19, 20]. The link between the gene and the disease is confirmed by the segregation analysis of the affected persons in the patient’s family. Then, the identified variant is modeled in an in vivo animal model to reveal its functional effect. Indeed, whole genome sequencing greatly improved the diagnosis of rare diseases [21].
Notably, at most, 50% of patients will be diagnosed, and the remaining 50% will be missed due to the limited sharing of genomic data between healthcare providers worldwide and the inability of current methods to assess the different pathological genetic mechanisms causing rare diseases [22]. For example, microarrays miss some copy number variations due to their low resolution, the non-Mendelian inheritance challenges NGS and the high GC content impedes the unequal coverage [23].
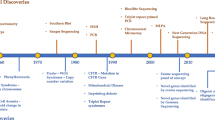
In Egypt, since 2000, MoHP had established hypothyroidism screening for neonates, and in 2015, phenylketonuria screening was added [24, 25]. Since 2021, MOHP has expanded neonatal screening tests to include 17 other congenital genetic disorders. Fifteen of these tests are for worldwide rare diseases such as propionic acidemia, glutaric aciduria type 1, ornithine transcarbamylase deficiency, citrullinemia type 1, argininemia, classic galactosemia, isovaleric acidemia, tetrahydrobiopterin deficiency (BH4), homocystinuria, congenital adrenal hyperplasia, methylmalonic acidemia, maple syrup urine disease, glucose-6-phosphate dehydrogenase deficiency (G6PD), tyrosinemia type 1, and cystic fibrosis [26]. If left undiagnosed and untreated, most of these diseases can result in mental retardation, serious health issues, or even death [27].
In 2021, the presidency launched an initiative to early diagnose and treat spinal muscular atrophy bearing its high cost. This paves the way for governmental and private genetic laboratories to develop and provide different genetic tests for these diseases [28].
Also, there are some non-governmental organizations that aim to care for, improve lifestyle, and provide treatment for the rare disease patients such as Forsat Hayat [15].
Status of orphan drugs in Egypt
Orphan drugs in Egypt have no specific regulations and are regulated by Ministerial Decree No. 2015/415 for drug authorization [9]. Also, the Egyptian Ministry of Health and Population (MoHP) admits the authorization of some supervising bodies such as the European Medicines Agency, the USA Food and Drug Administration, the Japanese Ministry of Health, and the Australian Therapeutic Goods Administration, Labour, and Welfare.
The pricing of those drugs is regulated by Egyptian law (Ministerial Decrees No. 1991/314, 2009/373, and 2012/499). Egypt applies the least customer price available in the reference countries with a 10% discount and a profitable margin for distributors and pharmacists. The reference countries are Australia, Austria, Belgium, Canada, Denmark, Germany, Finland, France, Iceland, Ireland, Netherlands, Japan, Luxemburg, the USA, New Zealand, Norway, Sweden, Switzerland, the UK, Italy, Portugal, and Spain [9]. During the registration phase of the drugs, a pricing committee from the Central Administration of Pharmaceutical Affairs (CAPA) reviews the pricing applications and documents, and MoHP approves them.
The access of Egyptian rare disease patients to orphan drugs increased following the development of a tool for multi-criteria decision analysis (MCDA). This tool is concerned with the reimbursement of orphan drugs based on a differential threshold since orphan drugs exceed the conventional cost-effectiveness thresholds (CETs) which hamper their reimbursement [29].
Egyptian rare diseases research and clinical trials
Egypt has given great attention to scientific research. This was prominent by the increase of the research budget from 0.27% in 2011 to 0.96% in 2020 from the gross domestic product (GDP) [30]. Currently, Egypt is the 35th in research and development spending worldwide, the 2nd in the Arab countries, and the 1st in Africa [31]. The increase in research and development also affected rare disease studies (Fig. 1). This sheds the light on the necessity of the alignment of the efforts of scientists, physicians, policymakers, and healthcare providers to tackle the challenges facing rare disease care and treatment.
The number of rare diseases Egyptian studies over decades. Above each column is the number of studies performed in this decade. The search methodology was mentioned in the Appendix
Sixty-three percent of the expenditure on research and development is by the governmental sector, 37% is by the private sector, and 1% is by international non-profit organizations. This is also in accordance with the difference in the number of publications in the rare disease field between the public and the non-public sectors. The highest contribution to rare disease Egyptian publications is from the National Research Center, Ain Shams University, and Cairo University as governmental institutions and the American University in Cairo and New Giza University as private institutions (Fig. 2). The majority of the rare diseases’ studies were held at Egypt’s capital (Cairo) with 67% contribution. Seventeen out of 27 governates (Dakahlia, Asyut, Alexandria, Gharbia, Sohag, Monufia, Sharqia, New Valley, Suez, Beni Suef, Minya, Faiyum, Qalyubia, Aswan, Port Said, Beheira, and Luxor) were also involved with 33% contribution.
Egyptian institutions performing rare disease research. Some are public institutions and some are non-public either private or non-profit organizations. Next to each column is the percentage of the studies by which the institution contributed to the Egyptian rare disease studies. The search methodology was mentioned in the Appendix
Since genetic mutations vary in different countries, it is important to perform research on the Egyptian population to know the frequency of the different mutations in the Egyptian population not only the worldwide known mutations. The aim of the research in the field of rare diseases is the early diagnosis of these cases to improve their lifestyle, prevent more deterioration, and alleviate the disabling symptoms [22]. Also, this will pave the way for family planning [32].
The majority of the rare disease studies in Egypt focus on rare developmental anomalies, rare inborn errors of metabolism, and rare neurological diseases (Fig. 3).
Rare diseases’ classification of the Egyptian rare disease studies. Next to each column is the percentage of the studies on this category of rare diseases in the Egyptian literature. The search methodology was mentioned in the Appendix
In 2021, Egypt launched the Egyptian Genome Project (EGP) to unravel the Egyptian genetic landscape, establish an Egyptian reference genome, and link the genotypic and phenotypic data for creating a diagnostic target panel tailored for the Egyptian population. As a subsidiary of EGP, rare disease studies are launched and will be held in two phases. The first is a pilot study to reach a genetic diagnosis for undiagnosed patients and to explore the RDs with the highest socio-economic burdens in Egypt. Based on this, it is possible to prioritize the most prevalent RDs in Egypt. The second phase aims to establish a genotypic/phenotypic correlation in specific rare diseases [33]. This will pave the way for personalized medicine neglecting the “One size fits all” approach. The personalized medicine approach categorizes patients by their genomic mutations in order to provide the maximum drug effect with the lowest drug toxicity.
According to the recommendations of the international rare diseases and orphan drugs regulations, Egypt is collaborating with international organizations in many rare diseases clinical research and is developing new tools in the healthcare system in order to offer fair access of rare disease patients to orphan drugs. However, Egypt still has to develop an epidemiological database for rare diseases and encourage research on rare diseases in the pharmaceutical companies to develop orphan drugs.
Conclusion
Rare diseases represent a great economic and social burden worldwide. A major contributor to rare diseases is consanguinity. In Egypt, there is no registry for rare disease patients, which hinders the precise prevalence estimation of these patients. Also, the lack of appropriate molecular diagnosis hinders the reduction of the rare disease burden in Egypt. Genomics can reduce the burden of rare diseases by providing quicker and more precise diagnostic tools. This will offer rare disease patients the chance for personalized medicine alleviating the family’s suffering and Egypt’s economic burden. This sheds the light on the importance of the national project “Egyptian Genome Project—Rare diseases project” to decrease the rare disease burden.
Availability of data and materials
All data in this study are included in this manuscript and in the Appendix.
References
Population - The World Factbook, https://www.cia.gov/the-world-factbook/field/population/country-comparison
Egypt: country data and statistics, https://www.worlddata.info/africa/egypt/index.php
Major Ethnic Groups in Egypt - WorldAtlas, https://www.worldatlas.com/articles/what-is-the-ethnic-composition-of-egypt.html
Taha, T., Elzalabany, S., Fawzi, S., Hisham, A., Amer, K., Shaker, O.: Allele frequency comparative study between the two main Egyptian ethnic groups. Forensic Sci Int. 313, 110348 (2020). https://doi.org/10.1016/j.forsciint.2020.110348
Shawky RM, Elsayed SM, Zaki ME, El-Din SMN, Kamal FM. Consanguinity and its relevance to clinical genetics. Egypt J Medical Hum Genetics. 2013;14:157–64. https://doi.org/10.1016/j.ejmhg.2013.01.002.
Wakap SN, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneau V, Murphy D, Cam YL, Rath A. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28:165–73. https://doi.org/10.1038/s41431-019-0508-0.
Rare diseases - IFPMA, https://www.ifpma.org/subtopics/rare-diseases/#:~:text=There%20are%20between%205%2C000%20to,to%20400%20million%20people%20worldwide.
Orphadata rare diseases - Classifications of rare diseases, http://www.orphadata.org/cgi-bin/rare_free.html#classifmodal
PharmaBoardroom - Orphan Drugs & Rare Diseases: Egypt, https://pharmaboardroom.com/legal-articles/orphan-drugs-rare-diseases-egypt/
European commission - Research and innovation - Rare diseases, https://research-and-innovation.ec.europa.eu/research-area/health/rare-diseases_en#:~:text=In%20the%20European%20Union%2C%20a,million%20people%20in%20the%20EU.
Bartek, R.J., Basile, E.M., Burns, K.& S.J., Frohnmayer, G.C.D., Gallin, F.A.R.F.E., Griggs, D.D.C.F.R.C., Kelley, U. of R.S. of M.S., Khosla, C., Knowles, S.U.M., Lewis, U. of N.C. at C.H.R.J., Linehan, L.A.J., Milliner, S.U.D.S., Mimura, M.C.C., Parrish, B.J.A., Pyeritz, M.G.H.R.E., Sokolovsky, U. of P.J., Thoene, M.P.A.C.J.G.: Rare Diseases and Orphan Products: Accelerating research and development. In: Profile of Rare Diseases (2010)
Walkowiak, D., Bokayeva, K., Miraleyeva, A., Domaradzki, J.: The awareness of rare diseases among medical students and practicing physicians in the Republic of Kazakhstan. An Exploratory Study. Frontiers Public Heal. 10, 872648 (2022). https://doi.org/10.3389/fpubh.2022.872648
Rare Diseases at FDA | FDA, https://www.fda.gov/patients/rare-diseases-fda
Gombocz M, Vogler S. Public spending on orphan medicines: a review of the literature. J Pharm Policy Pract. 2020;13:66. https://doi.org/10.1186/s40545-020-00260-0.
Misr Measurement and Control “MMC” sponsors the Forsa Foundation to support people with rare diseases, https://aleqaria.com.eg/post/details/82606/%D9%85%D8%B5%D8%B1-%D9%84%D9%84%D9%82%D9%8A%D8%A7%D8%B3-%D9%88%D8%A7%D9%84%D8%AA%D8%AD%D9%83%D9%85-mmc
The Annual Congress of The Egyptian Scientific Foundation of Rare Diseases in Children | Leaders, https://leaders-org.com/events/esfrd/
National Association for Rare Diseases - NARD | Facebook, https://www.facebook.com/onlineNARD/
Vinkšel M, Writzl K, Maver A, Peterlin B. Improving diagnostics of rare genetic diseases with NGS approaches. J Community Genetics. 2021;12:247–56. https://doi.org/10.1007/s12687-020-00500-5.
Bergant G, Maver A, Peterlin B. Whole-genome sequencing in diagnostics of selected Slovenian undiagnosed patients with rare disorders. Life. 2021;11:205. https://doi.org/10.3390/life11030205.
Zastrow DB, Kohler JN, Bonner D, Reuter CM, Fernandez L, Grove ME, Fisk DG, Network UD, Yang Y, Eng CM, Ward PA, Bick D, Worthey EA, Fisher PG, Ashley EA, Bernstein JA, Wheeler MT. A toolkit for genetics providers in follow-up of patients with non-diagnostic exome sequencing. J Genet Couns. 2019;28:213–28. https://doi.org/10.1002/jgc4.1119.
Investigators, T. 100, 000 Genomes Project Pilot, Smedley, D., Smith, K.R., Martin, A., Thomas, E.A., McDonagh, E.M., Cipriani, V., Ellingford, J.M., Arno, G., Tucci, A., Vandrovcova, J., Chan, G., Williams, H.J., Ratnaike, T., Wei, W., Stirrups, K., Ibanez, K., Moutsianas, L., Wielscher, M., Need, A., Barnes, M.R., Vestito, L., Buchanan, J., Wordsworth, S., Ashford, S., Rehmström, K., Li, E., Fuller, G., Twiss, P., Spasic-Boskovic, O., Halsall, S., Floto, R.A., Poole, K., Wagner, A., Mehta, S.G., Gurnell, M., Burrows, N., James, R., Penkett, C., Dewhurst, E., Gräf, S., Mapeta, R., Kasanicki, M., Haworth, A., Savage, H., Babcock, M., Reese, M.G., Bale, M., Baple, E., Boustred, C., Brittain, H., Burca, A. de, Bleda, M., Devereau, A., Halai, D., Haraldsdottir, E., Hyder, Z., Kasperaviciute, D., Patch, C., Polychronopoulos, D., Matchan, A., Sultana, R., Ryten, M., Tavares, A.L.T., Tregidgo, C., Turnbull, C., Welland, M., Wood, S., Snow, C., Williams, E., Leigh, S., Foulger, R.E., Daugherty, L.C., Niblock, O., Leong, I.U.S., Wright, C.F., Davies, J., Crichton, C., Welch, J., Woods, K., Abulhoul, L., Aurora, P., Bockenhauer, D., Broomfield, A., Cleary, M.A., Lam, T., Dattani, M., Footitt, E., Ganesan, V., Grunewald, S., Compeyrot-Lacassagne, S., Muntoni, F., Pilkington, C., Quinlivan, R., Thapar, N., Wallis, C., Wedderburn, L.R., Worth, A., Bueser, T., Compton, C., Deshpande, C., Fassihi, H., Haque, E., Izatt, L., Josifova, D., Mohammed, S., Robert, L., Rose, S., Ruddy, D., Sarkany, R., Say, G., Shaw, A.C., Wolejko, A., Habib, B., Burns, G., Hunter, S., Grocock, R.J., Humphray, S.J., Robinson, P.N., Haendel, M., Simpson, M.A., Banka, S., Clayton-Smith, J., Douzgou, S., Hall, G., Thomas, H.B., O’Keefe, R.T., Michaelides, M., Moore, A.T., Malka, S., Pontikos, N., Browning, A.C., Straub, V., Gorman, G.S., Horvath, R., Quinton, R., Schaefer, A.M., Yu-Wai-Man, P., Turnbull, D.M., McFarland, R., Taylor, R.W., O’Connor, E., Yip, J., Newland, K., Morris, H.R., Polke, J., Wood, N.W., Campbell, C., Camps, C., Gibson, K., Koelling, N., Lester, T., Németh, A.H., Palles, C., Patel, S., Roy, N.B.A., Sen, A., Taylor, J., Cacheiro, P., Jacobsen, J.O., Seaby, E.G., Davison, V., Chitty, L., Douglas, A., Naresh, K., McMullan, D., Ellard, S., Temple, I.K., Mumford, A.D., Wilson, G., Beales, P., Bitner-Glindzicz, M., Black, G., Bradley, J.R., Brennan, P., Burn, J., Chinnery, P.F., Elliott, P., Flinter, F., Houlden, H., Irving, M., Newman, W., Rahman, S., Sayer, J.A., Taylor, J.C., Webster, A.R., Wilkie, A.O.M., Ouwehand, W.H., Raymond, F.L., Chisholm, J., Hill, S., Bentley, D., Scott, R.H., Fowler, T., Rendon, A., Caulfield, M.: 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care — Preliminary Report. New Engl J Med. 385, 1868–1880 (2021). https://doi.org/10.1056/nejmoa2035790
Hartley T, Lemire G, Kernohan KD, Howley HE, Adams DR, Boycott KM. New diagnostic approaches for undiagnosed rare genetic diseases. Annu Rev Genom Hum G. 2020;21:1–22. https://doi.org/10.1146/annurev-genom-083118-015345.
Boycott KM, Hartley T, Biesecker LG, Gibbs RA, Innes AM, Riess O, Belmont J, Dunwoodie SL, Jojic N, Lassmann T, Mackay D, Temple IK, Visel A, Baynam G. A diagnosis for all rare genetic diseases: the horizon and the next frontiers. Cell. 2019;177:32–7. https://doi.org/10.1016/j.cell.2019.02.040.
Egypt launches 2nd phase of screening newborns for genetic disorders - EgyptToday, https://www.egypttoday.com/Article/1/108942/Egypt-launches-2nd-phase-of-screening-newborns-for-genetic-disorders
Egyptian Neonatal screening program.
SIS, S.I.C.: Egypt launches initiative for early detection of 19 genetic diseases in newborns, begins free treatment of spinal muscular atrophy-SIS, https://www.sis.gov.eg/Story/158530/Egypt-launches-initiative-for-early-detection-of-19-genetic-diseases-in-newborns%2C-begins-free-treatment-of-spinal-muscular-atrophy?lang=en-us
Shawky RM, Elsayed NS, Ibrahim DS, Seifeldin NS. Profile of genetic disorders prevalent in northeast region of Cairo. Egypt Egypt J Medical Hum Genetics. 2012;13:45–62. https://doi.org/10.1016/j.ejmhg.2011.10.002.
Egypt Center For Research and Regenerative Medicine, http://www.ecrrm.ac.eg/
Fasseeh A, Elezbawy B, Korra N, Roushdy M, Seyam A, Hayek N, Rahman NA, Abdelhamid S, Fasseeh N, Saad A, Elagamy A, Mahmoud A, Sedrak A, Elshazly K, Eldebeiky M, Talaat M, Maher N, Abdelaziz R, Refaat R, Akeel S, Adel R, Khalil S, Abaza S, Kalo Z. HPR180 eligibility of orphan drugs for preferential reimbursement in Egypt. Value Health. 2022;25:S265. https://doi.org/10.1016/j.jval.2022.09.1309.
Expenditure on research and development (% of GDP), https://data.albankaldawli.org/indicator/GB.XPD.RSDV.GD.ZS
Ministry of Higher education: Egypt is the 35th globally and the first in Africa in spending on scientific research, https://www.youm7.com/story/2022/12/27/%D8%A7%D9%84%D8%AA%D8%B9%D9%84%D9%8A%D9%85-%D8%A7%D9%84%D8%B9%D8%A7%D9%84%D9%89-%D9%85%D8%B5%D8%B1-%D8%A7%D9%84%D9%8035-%D8%B9%D8%A7%D9%84%D9%85%D9%8A%D8%A7-%D9%88%D8%A7%D9%84%D8%A3%D9%88%D9%84%D9%89-%D8%A3%D9%81%D8%B1%D9%8A%D9%82%D9%8A%D8%A7-%D9%81%D9%89-%D8%A7%D9%84%D8%A5%D9%86%D9%81%D8%A7%D9%82-%D8%B9%D9%84%D9%89/6024318#:~:text=%D9%88%D8%A3%D8%B4%D8%A7%D8%B1%20%D8%A7%D9%84%D9%88%D8%B2%D9%8A%D8%B1%20%D8%A5%D9%84%D9%89%20%D8%A3%D9%86%20%D9%85%D8%B5%D8%B1,%D8%AF%D9%88%D9%84%D8%A9%20%D8%A7%D9%84%D8%A5%D9%85%D8%A7%D8%B1%D8%A7%D8%AA%20%D8%A7%D9%84%D8%B9%D8%B1%D8%A8%D9%8A%D8%A9%20%D8%A7%D9%84%D9%85%D8%AA%D8%AD%D8%AF%D8%A9%D8%8C%20%D9%88%D8%AC%D8%A7%D8%A1%D8%AA
Esquivel-Sada D, Nguyen MT. Diagnosis of rare diseases under focus: impacts for Canadian patients. J Community Genetics. 2018;9:37–50. https://doi.org/10.1007/s12687-017-0320-x.
Neurology TL. Rare diseases: maintaining momentum. Lancet Neurol. 2022;21:203. https://doi.org/10.1016/s1474-4422(22)00046-1.
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the article. T.T.: Revision of the article. D.A., Z.E., G.A, S.E.: Acquisition, analysis, interpretation of the data. Y.G.: Acquisition. T.E.: Conception, design of the work. K.A.: Revision of the article. All authors agreed both to be personally accountable for the author’s own contributions and ensure the accuracy and integrity of every part of the work. All authors approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable because this article does not contain any studies with human or animal subjects performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
Dr. Khaled Amer is a co-author of this study and an Editorial Board member of the journal. He was not involved in handling this manuscript during the submission and review processes. The rest of the authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Methodology
Literature searches for relevant articles were performed using the following keywords (rare diseases Egypt), (rare diseases “Egypt”, rare genetic diseases “Egypt”) in Google scholar, PubMed, Egyptian knowledge bank (EKB), Scopus, and The Catalogue for Transmission Genetics in Arabs (CTGA) databases. The references of the primary studies were further manually analyzed for additional relevant studies. The inclusion criteria were original articles, case reports, editorials, dissertations, thesis, opinion studies, or experience reports indexed in the selected databases, Egyptian patients, one or more authors affiliated to Egypt, English language, and no selection criteria on the date. The exclusion criteria were review articles, guidelines, book chapters, and studies not performed by Egyptians. Readcube was used as a reference manager to store and organize papers and exclude duplicates. All the studies were imported into the Rayyan application to decide on the study eligibility. Three independent reviewers decided on the inclusion and exclusion of the articles. The following data were extracted from each study: article title, country of authors, year of publication, and study type.
Results
The systematic searches identified a total of 1886 references in the five selected databases. Of these, duplicates were excluded by Readcube. Only 479 met the inclusion criteria. 1407 were excluded by the title and abstract.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taha, T., Ahmed, D., El-Gammal, Z. et al. A brief insight into the rare diseases in Egypt. J Rare Dis 2, 6 (2023). https://doi.org/10.1007/s44162-023-00010-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44162-023-00010-1