Abstract
Persimmon anthracnose, a severe disease caused by the hemibiotrophic fungus Colletotrichum horii, poses a substantial threat to China’s persimmon industry. Previous research showed that ‘Kangbing Jianshi’ cultivar exhibits strong resistance to anthracnose. Notably, ‘Kangbing Jianshi’ branches exhibit greater lignification compared with the susceptible ‘Fuping Jianshi’ cultivar. In this study, higher lignin content was observed in ‘Kangbing Jianshi’ compared with ‘Fuping Jianshi’, and this difference was associated with disease resistance. Transcriptome and metabolome analyses revealed that the majority of differentially expressed genes and differentially accumulated metabolites were primarily enriched in the phenylpropanoid biosynthesis and lignin synthesis pathways. Furthermore, significant upregulation of DkCAD1, a pivotal gene involved in lignin metabolism, was observed in the resistant cultivar when inoculated with C. horii. Transient overexpression of DkCAD1 substantially increased lignin content and improved resistance to C. horii in a susceptible cultivar. Furthermore, through yeast one-hybrid (Y1H) assays, we identified two WRKY transcription factors, DkWRKY8 and DkWRKY10, which interacts with the DkCAD1 promoter and induces its activity. Overexpression of DkWRKY8 and DkWRKY10 not only increased leaf lignin content but also enhanced persimmon tolerance to C. horii. Moreover, the expression levels of DkCAD1, DkWRKY8, and DkWRKY10 were significantly increased in response to salicylic acid and jasmonic acid in the resistant cultivar. These findings enhance our understanding of the molecular functions of DkWRKY8, DkWRKY10, and DkCAD1 in persimmons, as well as their involvement in molecular breeding processes in persimmons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persimmon (Diospyros kaki Thunb.), a member of the Diospyros genus, is a prominent commercial fruit crop of great importance. It is primarily cultivated in East Asian countries, with notable production in China, Japan, and Korea. (Luo and Wang 2008; Yamada and Sato 2016). With the expansion of persimmon cultivation, germplasm collection and analysis become increasingly important. This effort preserve genetic diversity and aids in screening and breeding for superior disease-resistant persimmon varieties (Greene and Morris 2001). The National Field Genebank for Persimmon in the Yangling District of Shaanxi Province has amassed over 1000 persimmon germplasm and varieties, exhibiting substantial genetic diversity from various regions in China and worldwide (Guan et al. 2019; Guan et al. 2020b). In a prior study, we evaluated 142 persimmon germplasms for their response to Colletotrichum horii, revealing notable variations among the examined accessions, with the most displaying high susceptibility and a few exhibiting hypersensitive reactions (Guan et al. 2022).
Persimmon anthracnose is a severe disease that affects prominent persimmon cultivation regions. It is caused by the pathogen C. horii, which was renamed to distinguish it from the broader C. gloeosporioides species complex (Weir and Johnston 2010). C. horii is highly destructive to persimmons plants, leading to leaf defoliation, fruit decay, and even plant demise (Zhang and Xu 2005). This disease has become endemic in certain Chinese regions, leading to substantial losses in both quality and production (Deng et al. 2019).
Lignin, a key component of plant cell walls, serves as the initial physical barrier against pathogen infection by inducing cell walls lignification, which protects neighboring plant cells from further damage (Khasin et al. 2021; Quiroz-Figueroa et al. 2023). Cinnamyl alcohol dehydrogenase (CAD) is a crucial enzyme in lignin biosynthesis. Research has identified key lignin synthesis enzymes (AtCAD1, AtCAD4, and AtCAD5) in Arabidopsis (Sibout et al. 2003; Kim et al. 2004; Eudes et al. 2006). In rice, the relationship between OsCAD2 and OsCAD7 is associated with the lignin content, with OsCAD2 primarily responsible for the biosynthesis of monolignols in rice lignin among the OsCAD genes (Li et al. 2009; Hirano et al. 2012). The CAD7 subfamily protein has been identified as a negative regulator, promoting host plant infection by suppressing immunity factors, including callose deposition and plant reactive oxygen species (ROS) burst (Li et al. 2019). These findings highlight the crucial role of the CAD gene in lignin synthesis and disease resistance in persimmon. However, studies on CAD protein-mediated resistance to anthracnose remain limited.
WRKY transcription factors are crucial in regulating disease resistance across various hosts, including Arabidopsis (Birkenbihl et al. 2018), rice (Li et al. 2021), cucumber (Luan et al. 2019), pepper (Hussain et al. 2019) and grape plants (Wang et al. 2020). In recent years, evidence has increasingly linked WRKY transcription factors to the signaling pathways of salicylic acid (SA) and jasmonic acid (JA) in plant defense responses. For instance, overexpression CmWRKY15-1 in chrysanthemums infected with Puccinia horiana increased endogenous SA level and an up-regulated SA synthesis pathway genes (Bi et al. 2021). Additionally, exogenous SA and JA treatments significantly induced the NtWRKY50 expression, enhancing resistance against Ralstonia solanacearum (Liu et al. 2017). Furthermore, WRKY transcription factors initiate the plant immune response by binding to cis-acting elements in specific genes promoter (Eulgem and Somssich 2007). For example, AtWRKY57 directly binds to the promoters of JAZ1 and JAZ5, suppressing the JA signal transduction pathway and negatively regulating Botrytis cinerea resistance in Arabidopsis (Jiang and Yu 2016). In pepper, CaWRKY6 enhances resistance against R. solanacearum by interacting with the CaWRKY40 promoter (Cai et al. 2015). Notably, WRKY transcription factors play crucial biological roles in interactions among economically significant crops. For instance, banana MaWRKY1 and MaWRKY2 induce resistance against C. musae by binding to pathogenesis-related genes promoters (Shan et al. 2016); In apples, overexpressing MdWRKY100 enhances resistance against to C. gloeosporioides (Zhang et al. 2019); Conversely, HbWRKY40 from Hevea brasiliensis triggers a burst of ROS in tobacco, increasing disease resistance in Arabidopsis (Yang et al. 2020); Additionally, upregulating JrWRKY21 in walnut positively regulates its resistance to C. gloeosporioides (Zhou et al. 2022). Despite these findings in various plant species, the potential role of WRKY transcription factors in persimmon disease resistance remains largely unexplored. Finally, both JA and SA are resistance hormones that activate plant defense against insect attack and necrotrophic pathogens. JA primarily regulates disease resistance against necrotrophic pathogens, whereas SA regulates broad-spectrum resistance against hemibiotrophic and biotrophic pathogens (Fu et al. 2012). These hormones typically work antagonistically, with SA and JA associated primarily with biotrophic resistance and necrotrophic resistance, respectively (Xie et al. 2022). Surprisingly, no studies have investigated the roles of SA and JA in persimmon anthracnose disease resistance.
In the present study, we conducted a comparative analysis of disease progression, lignin accumulation, and DkCAD1 gene expression (a lignin metabolism-related gene) in branches of the susceptible cultivar ‘Fuping Jianshi’ (S var.) and the resistance cultivar ‘Kangbing Jianshi’ (R var.) after C. horii inoculation. Our findings suggest that lignin may play a key role in conferring the robust disease resistance observed in R var. We preliminary verified the biological function of DkCAD1 in conferring resistance to C. horii in various persimmon varieties using a transient overexpression system in persimmon leaves. Additionally, two WRKY transcription factors, DkWRKY8 and DkWRKY10, were found to transactivate the DkCAD1 promoter upon C. horii induction. This study elucidated variations in lignin content and JA and SA levels in resistant and susceptible persimmon cultivars and demonstrated the positive contributions of DkCAD1, DkWRKY8, and DkWRKY10 to anthracnose resistance in persimmon.
Results
Morphological characteristics of persimmon anthracnose C. horii in various persimmon tissues
C. horii demonstrated the ability to infect various parts of the persimmon tree, including the trunk, branches, leaves, and fruit. Field observations revealed slight variations in disease symptoms across affected tissues. Afflicted branches exhibited the small black spots that progressively deepened into larger depressions. Over time, these sunken areas expanded, causing the bark to crack (Fig. 1A, B). On leaves, disease spots extended along the petiole and veins, leading to the formation of pink conidia (Fig. 1C, D). In fruit, affected areas exhibited depressions that evolved into soft black patches (Fig. 1E). In advanced fruit infections, substantial conidia masses became visible in the infected regions (Fig. 1F). The morphological characteristics of C. horii conidia from the diseased spots closely resembled those cultured on PDA plate (Fig. 1G, H), as previously described (Xie et al. 2010; Deng et al. 2019). Scanning electron microscopy revealed that C. horii anthracnose filtrates persimmon leaves via the elytra structure at the apex of the embryonic tube following conidia germination. Both infection structures of C. horii entered persimmon leaves through intercellular spaces and stomata, indicating multiple pathways for C. horii infections (Fig. 1I–M).
Morphological characteristics of persimmon anthracnose C. horii in various persimmon tissues. A-E Morphological characteristics of persimmon anthracnose C. horii in the new shoot (A), perennial branch (B), leaf (C), petiole (D), and fruit (E). F Stereo structure of C. horii conidia on the fruit surface of the ‘Fuping Jianzhi’ variety, as observed under the anatomical microscope. G and (H) Images from above (G) and below (H) of C. horii anthracnose cultured on the PDA plate for 10 days. I Conidia. J-M Microscopic view of C. horii invading the back of ‘Fuping Jianzhi’ leaves under scanning electron microscope. Scale bar = 10 μm
Characteristics and physiological changes in persimmon anthracnose in two persimmon cultivars
Significant differences in anthracnose symptoms were observed between the highly susceptible and highly resistant cultivars upon field inoculation with C. horii. At 3 days post inoculation (dpi), distinct black spots appeared on the branches of S var. (‘Fuping Jianshi’). As the disease progressed, these lesions rapidly expanded outward, connecting with adjacent lesions, ultimately infecting the entire branch and producing conidia by 10 dpi. In contrast, the branches of the R var. (‘Kangbing Jianshi’) showed only mild symptoms after 5 dpi, characterized by small grayish spots in the early stages of disease progression. Lesions in R var. increased minimally over time, with no conidia observed (Fig. 2A). To explore the relationship between lignin and persimmon disease resistance, we determined the lignin content in various resistant cultivars using Klason method, a reliable technique for isolating lignin fractions (Abdelrahman and Galiwango 2018). Results indicated higher lignin content in R var. compared with S var. Following C. horii inoculation, R var. displayed a noticeable increase in lignin accumulation, whereas no significant change was observed in S var., suggesting a potential role of lignin in disease resistance (Fig. 2B). Using phloroglucinol, lignin content in branches of different cultivars was assessed at various growth stages, revealing early lignin deposition in apical regions of young shoots in resistant cultivars, whereas susceptible cultivars showed minimal lignin expression at the same growth stage. The depth of lignin staining significantly increased in the semi-lignified branches of both cultivars, with a more pronounced increase observed in R var. compared with S var. (Fig. 2C).
Characteristics and physiological changes in persimmon anthracnose in two persimmon cultivars. A Characteristic of the highly resistant cultivar ‘Kangbing Jianshi’ (R var.) and the highly susceptible cultivar ‘Fuping Jianshi’ (S var.) following inoculation with C. horii. Images were taken at 0, 1-, 2-, 3-, 5-, 10-days post-inoculation (dpi). Scale bars = 1 cm. B Lignin staining on the cross sections of R var. and S var. branches. Samples were collected from the apical region of young shoots, young shoots, and semi-lignified branches. Scale bars = 10 µm. C Lignin content in branches of R var. and S var. cultivars following inoculation with C. horii. D Endogenous salicylic acid (SA) and (E) jasmonic acid (JA) contents in R var. and S var. Error bars represent standard deviation (n = 3)
SA and JA are pivotal hormones in plant disease resistance. To investigate their induction after pathogen infection, we measured SA and JA levels in R var. and S var. at various stages of C. horii disease progression. After pathogen infection, SA levels in R var. exhibited rapid elevation, peaking at 3 dpi, and gradually decreasing at 5 dpi while remaining significantly higher than preinfection levels (Fig. 2D). Similarly, JA levels followed a comparable trend in R var., whereas no distinct pattern emerged in S var. (Fig. 2E). These findings suggest that JA levels in R var. aligned with SA trends, but such alignment was not evident in S var.
Transcriptome and metabolome analysis in two persimmon cultivars infected by C. horii
To explore changes in persimmons genes associated with varying resistance levels during C. horii infection, we conducted a comprehensive analysis of the transcriptome and metabolome analysis of S var. (‘Fuping Jianshi’) and R var. (‘Kangbing Jianshi’) persimmons cultivars following inoculation with C. horii spore suspension. Branch samples were collected at different time points, including 1, 3, and 5 dpi, as well as an uninfected (CK), to identify differentially expressed genes (DEGs, Fig. 3A) and differentially accumulated metabolites (Fig. 3B). For transcriptome, we performed cluster analysis on the two groups of differential genes, and generated 12 groups of genes with different expression patterns (Fig. 3C, D). According to the clustering results, most of the genes in S var. were differentially expressed at 5 days, and more genes in R var. occurred at 1 and 3 days compared with S var., which is one of the reasons why R var. is more resistant to disease. As part of the metabolome analysis, certain metabolites associated with phenylpropanoid biosynthesis exhibited heightened expression levels during infection (Fig. 3E, F; Table S1. Matrix of normalized metabolic profile of DAMs).
Transcriptome and metabolome analysis in ‘Fuping Jianshi’ (S var.) and ‘Kangbing Jianshi’ (R var.) infected by C. horii. A, B The number of DEGs and DAMs under control and C. horii treatment. The bar chart shows the numbers of DEGs and DAMs. DEG, differentially expressed genes. DAM, differentially accumulated metabolites. 1, 3, and 5 represent the time points for collecting samples. C, D Mapman function enrichment analysis of DEGs and DAMs in S var. and R var. The histograms illustrated the –log10 of P-value of each term of enrichment. E, F Heatmaps of Metabolic profiles of DAMs, which illustrated the log2fold change Normalized Metabolic value
We performed GO, KEGG and Mapman annotation system enrichment analyses for these DEGs (Table S2. Matrix of -log10 p-value from GO annotation enrichment analysis of DEGs; Table S3. Matrix of -log10 p-value from KEGG annotation enrichment analysis of DEGs; Table S4. Matrix of -log10 p-value from Mapman annotation enrichment analysis of DEGs). Enrichment results supported by the two ontology annotation systems were concentrated in the pathways related to cell wall synthesis, lignin synthesis, jasmonic acid, and bio-stress resistance, all of which are related to the process of cells resisting the invasion of external microorganisms (Fig. 3C, D; Figure S1). In R var., the GO enrichment analysis of cluster 1 and cluster 2 in which genes were early up-regulated suggested that the salicylic acid signaling pathway and the wax biosynthetic pathway were activated, and the enrichment analysis of Mapman suggested cell wall modification, biotic stress and WRKY TF-related pathways are activated. The expression trend of cluster 1 in S var. was the same as that of cluster 2 in R var., although the enrichment results suggested the activation of salicylic acid signaling, jasmonic acid response, WRKY transcription factor and biotic stress-related pathways.
Analysis of genes and metabolites associated with SA, JA, and lignin synthesis
According to the enrichment analysis after gene clustering, salicylic acid and jasmonic acid were both involved in the activation of plant resistance. However, there is an antagonistic effect of two plant hormones when they participate in the pathway of plant resistance (Figure S2; Table S5). In the R var., the expression of salicylic acid-related genes exhibited a peculiar up-regulation at 1 day post infection, whereas the upregulation of jasmonic acid-related genes was only marginal. Among the genes upregulated by S var., the number of jasmonic acid-related genes was higher than that of salicylic acid-related genes. After 3-5 days of treatment, the number of upregulated genes associated with salicylic acid and jasmonic acid was approximately equivalent in R var., while the number of upregulated genes related to jasmonic acid was significantly more than those associated with salicylic acid in S var. after 3-5 days of treatment (Figure S2; Table S5). Therefore, we speculate that both salicylic acid and jasmonic acid-related pathways are activated in S var., but jasmonic acid is dominant, while Salicylic acid plays a role in the early reaction of R var.
In the context of lignin synthesis (Fig. 4A), we examined transcriptome and metabolome profiles across different periods and varieties in lignin synthesis pathways (Fig. 4B, C). Notably, the different expression levels of CAD1, 4CL, CCoAOMT, CCR1, COMT, F5H, and HCT related genes in resistant varieties compared with susceptible varieties (Fig. 4B; Table S6). CAD, a pivotal enzyme involved in the synthesis of three types of lignin, especially CAD1, exhibited higher expression during the early stages of infection. Moreover, the expression level of CAD1 in resistant varieties was notably elevated before 3 dpi (Table S7). According to our omics data, the upregulation of DkCAD1 may contribute to increased lignin synthesis and enhanced persimmon resistance against C. horii. We also identified certain metabolites associated with lignin biosynthesis, which exhibited decreased expression levels during infection.
The lignin synthesis pathways and the expression level of related genes. A The simplified roadmap for the lignin synthesis pathway. B Gene expression pattern in lignin synthesis pathways from RNA-seq data (Normalized gene profile with Z-score value of Log2 (TPM+1)). C Metabolic profiling in lignin synthesis pathways (Normalized metabolite value was Log2fold change between metabolite content and detection-minimum). D, E The correlation networks in lignin synthesis pathways (Orange nodes represented DEGs in R var. or S var.; Blue nodes represented DAMs in R var. or S var.; Lines illustrated correlation relationship with Pearson coefficient > 0.8)
Correlation network analysis, integrating co-expression gene and metabolite profiling, highlighted the significant involvement of the lignin synthesis pathway. By calculating correlations between gene expression profiles and metabolites, we identified relationships with a Pearson correlation coefficient greater than 0.8 (Fig. 4D, E). Strong correlations were observed among five compounds involved in the lignin synthesis pathway [sinapyl alcohol, trans-cinnamic acid, trans-P-coumaric acid, D-phenylalanine and L-(-)-phenylalanine] and multiple CAD genes, as well as WRKY8 and WRKY10. In R var., five compounds exhibited significant correlations with the expression levels of CAD7, CAD8, and CAD9. In S var., L-(-)-phenylalanine and trans-cinnamic acid content was strongly associated with the expression of lignin synthesis genes CAD6, CAD8, and CAD9. Conversely, sinapyl alcohol, trans-P-coumaric acid and D-phenylalanine content was not only linked with the expression of lignin synthesis genes CAD1, CAD7, and CAD8 but also robustly correlated with WRKY8 and WRKY10 expression. This suggests that WRKY8 and WRKY10 may play a role in regulating lignin synthesis in S var.
Enhanced resistance to C. horii in S var. through DkCAD1 overexpression promoting lignin accumulation
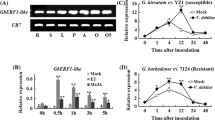
Further investigation was required to explore the relationship between lignin and disease resistance in persimmon. To do so, we isolated DkCAD1, a gene related to lignin metabolism. We observed that the expression of DkCAD1 significantly increased in the branches of R var. infected by C. horii but remained unchanged in the branches of S var. (Fig. 5A). Specifically, at 1 dpi, the expression of DkCAD1 in R var. increased by 9.5-fold compared with 0 dpi. The expression levels did not return to pre-infection levels even though they slightly declined after infection. To examine the role of DkCAD1 in the plant–pathogen interaction, we used a transient overexpression system in persimmon leaves. As expected, we observed a substantial increase in DkCAD1 transcription levels in S var. leaves after 2 days, with a >9-fold increase compared with the control, particularly in the overexpressed lines OE2, OE5, and OE6 (Fig. 5B). These results confirm the successful transformation of the DkCAD1 gene into leaves. Subsequently, we inoculated the overexpressed lines OE2, OE5, and OE6 with C. horii. After 5 dpi, we observed a significant reduction in the lesion area and disease index in OE2 and OE6 throughout the study period (Fig. 5C–E). Additionally, the lignin content of the three groups was significantly higher compared with the control (Fig. 5F). These findings demonstrate that the overexpression of DkCAD1 in persimmon effectively increases lignin content and enhances the resistance of S var. leaves against C. horii.
Enhanced resistance to C. horii in S var. through DkCAD1 overexpression promoting lignin accumulation. A Relative expression of DkCAD1 in inoculated persimmon branches. The samples were collected at 0, 1-, 2-, 3-, 5-, and 10-days post incubation (dpi). B Expression level of DkCAD1 in persimmon leaves after transient overexpression of DkCAD1 for 2 d. C Disease resistance of pMV-empty and DkCAD1 overexpressing leaves. Scale bar = 1 cm. D Quantification of the data shown in (A), images were taken at 5 dpi. E Relative disease index in DkCAD1-overexpressing leaves after inoculation for 5 d. pMV2-GFP was used as a control. F The lignin content in DkCAD1-overexpressing leaves after inoculation for 5 d. Error bars indicate the standard deviation (n=3). The letters indicate significant differences according to one-way ANOVA (Tukey’s test; p < 0.05)
Interaction between the DkCAD1 promoter and DkWRKY8 and DkWRKY10
To investigate the molecular mechanism underlying DkCAD1-mediated C. horii infection, we used the promoter of DkCAD1 as a bait to screen for two WRKY transcription factors, DkWRKY8 and DkWRKY10. Y1H yeast cells containing the DkCAD1 promoter fragment were unable to grow on SD/-Ura medium supplemented with 200 ng/ml Aureobasidin A (AbA). However, after transforming the PGADT7-DkWRKY8 and PGADT7-DkWRKY10 in Y1H yeast cells, they could grow on 200 ng/ml AbA on SD/-Leu medium, which indicated their binding to the promoter of DkCAD1 (Fig. 6B, Figure S3). Subsequent, qRT-PCR results revealed significantly elevated expression levels of DkWRKY8 and DkWRKY10 in R var. compared with S var. The relative expression of DkWRKY8 in R var. gradually increased, reaching its peak at 5 dpi, followed by a decrease at 10 dpi. Conversely, DkWRKY10 was significantly up-regulated during the early stages of infection, peaking at 2 dpi, and then declining to a lower level but remaining higher than the 0-dpi level (Fig. 6C). To assess the impact of DkWRKY8 and DkWRKY10 on the transcriptional activity of the DkCAD1 promoter, we conducted dual-luciferase assays. The results demonstrated that the LUC/REN values of DkCAD1 promoter were significantly higher when cotransferred with DkWRKY8 and DkWRKY10 than the empty vector (EV) (Fig. 6D), suggesting the positive role of DkWRKY8 and DkWRKY10 in regulating the promoter activity of DkCAD1. These findings suggest that DkWRKY8 and DkWRKY10 can interact with the promoter of DkCAD1 and positively regulate its activity.
Interaction between the DkCAD1 promoter and DkWRKY8 and DkWRKY10. A Self-activation of the DkCAD1 promoter was detected on SD/−Ura medium with AbA (200 ng/mL). B Y1H assay of DkCAD1 promoter with DkWRKY8 and DkWRKY10. The interaction was determined on the medium of SD/−Leu + AbA (200 ng/mL). C Relative expression of DkWRKY8 and DkWRKY10 in inoculated persimmon branches. The samples were collected at 0, 1-, 2-, 3-, 5-, and 10-days post incubation (dpi). D Ratios of LUC/REN on the promoter fragments of DkCAD1 to DkWRKY8 and DkWRKY10. The LUC/REN ratio of the empty vector (EV) plus promoter was set as 1. Error bars represent the standard deviation (n=5). The letters indicate significant differences according to one-way ANOVA (Tukey’s test; p < 0.05)
Enhanced resistance to C. horii in persimmon through DkWRKY8 and DkWRKY10 overexpression
To gain a deeper understanding of the roles played by DkWRKY8 and DkWRKY10 in the defense response against pathogens, we generated transgenic persimmon leaves overexpressing these genes using an Agrobacterium-mediated transient transformation system and vacuum infiltration. The overexpressed lines, namely OE1, OE2, and OE3 for both DkWRKY8 and DkWRKY10 were obtained, we then conducted a qRT-PCR analysis to assess the transcriptional abundance of DkWRKY8 and DkWRKY10, and the results indicated that these two genes exhibited a significant increase across all three overexpressed lines, with expression levels 12-fold and 14-fold higher, respectively, compared with the infiltrated control leaves (Fig. 7A, B). Subsequently, the overexpressed lines of DkWRKY8 and DkWRKY10 were inoculated with C. horii. After 5 dpi, we observed a significant reduction in the average lesion area in leaves overexpressing DkWRKY8 and DkWRKY10 compared with the control group (Fig. 7C, D). Furthermore, there was a notable decrease in the disease index of plants overexpressing DkWRKY8 and DkWRKY10 (Fig. 7E, F), accompanied by a marked enhancement in lignin accumulation in their leaves (Fig. 7G, H). Additionally, we detected the expression levels of DkCAD1 in DkWRKY8 and DkWRKY10 overexpression persimmon leaves, which suggested a positive regulation of DkWRKY8 and DkWRKY10 to the expression of DkCAD1 (Fig. 7I, J). These findings indicated that, similar to DkCAD1, DkWRKY8 and DkWRKY10 play positive regulatory roles in modulating C. horii resistance in persimmon.
Enhanced resistance to C. horii in persimmon through DkWRKY8 and DkWRKY10 overexpression. A, B The expression level of DkWRKY8 and DkWRKY10 in persimmon leaves after transient overexpression. C Disease resistance of pMV-empty, DkWRKY8, and DkWRKY10-overexpressing leaves. Bars = 1 cm. D Quantification of the data shown in (C). E, F Relative disease index in the DkWRKY8 and DkWRKY10 overexpressing leaves. ‘Fuping Jianshi’ leaves infiltrated with DkWRKY8 and DkWRKY10 and collected leaves after eight days of agroinfiltration. OE1, OE2, and OE3 represent three different lines infiltrated with pMV2-DkWRKY8 and pMV2-DkWRKY10 vectors. The pMV2-GFP was used as a control. G, H Lignin content in the DkWRKY8 and DkWRKY10 overexpressing leaves. Error bars indicate the standard deviation (n=3). I, J The expression level of DkCAD1 in transiently overexpressed lines of DkWRKY8 and DkWRKY10 in persimmon leaves. The letters indicate significant differences according to one-way ANOVA (Tukey’s test; p < 0.05)
Positive roles of DkCAD1, DkWRKY8, and DkWRKY10 in C. horii resistance modulated by SA
To investigate the impact of exogenous SA and JA on C. horii resistance, we applied these hormones to the leaves of various persimmon cultivars before inoculation. The results showed that both hormones, particularly SA, significantly reduced the severity of the disease in persimmon, especially in R var. (Figure S4). These findings suggest that SA and JA have the potential to induce resistance to anthracnose in persimmon, with a more pronounced effect in R var. To determine which hormones could influence the expression of DkCAD1, DkWRKY8 and DkWRKY10 in response to pathogen infection, we measured expression levels in the leaves of R var. and S var. inoculated with C. horii at 2 days after spraying 0.1 mM SA or JA. A substantial increase in the expression levels of DkCAD1, DkWRKY8 and DkWRKY10 was observed in the resistant cultivar following SA treatment, whereas no significant difference was observed between the susceptible cultivar and the control (Fig. 8A–C). Exogenous JA treatment also induced the expression of these three genes in two different resistant persimmon cultivars, although the differences were not as pronounced as those following SA treatment (Fig. 8D–F). In conclusion, these findings suggest that DkCAD1, DkWRKY8, and DkWRKY10 can be strongly and moderately induced by exogenous SA and JA, respectively.
Positive roles of DkCAD1, DkWRKY8, and DkWRKY10 in C. horii resistance modulated by SA. A-C Expression levels of DkCAD1, DkWRKY8 and DkWRKY10 in two persimmon cultivars inoculated with C. horii after 2 days of spraying 0.1 mM SA. D-F Expression levels of DkCAD1, DkWRKY8 and DkWRKY10 in two persimmon cultivars inoculated with C. horii after 2 days of spraying 0.1 mM JA. Samples were collected at 0, 1-, 2-, 3-, 5-, and 10-days post-inoculation (dpi). Error bars indicate the standard deviation (n=3, p < 0.05)
Discussion
Colletotrichum spp. comprises a group of fungi known for their key role as plant pathogens, capable of infecting various economically valuable crops (Dean et al. 2012). Among these fungi, C. horii is the primary causal agent responsible for persimmon anthracnose, a prevalent disease in China’s persimmon production regions. As the demand for persimmon continues to rise, this disease has emerged as a critical factor hindering industry growth. Previous research has indicated that C. horii primarily infects young shoots, leaves (particularly petioles and veins), and fruit during the spring season. Subsequently, the fungus overwinters on the diseased tissues in the form of mycelium or conidia, facilitating its spread through wind, rain, and favorable weather conditions, including high temperature and humidity (Xie et al. 2010; Hassan et al. 2018). In our previous field investigation, we observed that persimmon anthracnose primarily affected the new shoots of infected persimmon branches. Additionally, we found that a higher degree of lignification in branches was associated with a lower risk of infection. To further examine the impact of lignin on disease resistance in persimmon branches, we selected two cultivars for the present study: the susceptible cultivar ‘Fuping Jianshi’ denoted as S var., and the resistant cultivar ‘Kangbing Jianshi’ denoted as R var., based on their levels of anthracnose resistance, which were determined in our previous research (Guan et al. 2022). The symptom development in S var. following artificial inoculation was consistent with previous findings (Xie et al. 2010; Hassan et al. 2018; Deng et al. 2019). R var. exhibited minimal lesions and higher lignin content following inoculation in the field, whereas S var. displayed pronounced symptoms and lower lignin levels. Notably, the lignin deposition rate in R var. branches was significantly more rapid than that of S var., resulting in significantly greater lignin deposition during the same growth period. Based on these findings, we hypothesized that lignin plays a crucial role in conferring resistance to anthracnose in R var.
CAD genes exert marked effects on plant growth, development, and response to both abiotic and biotic stresses. In recent years, numerous studies have provided evidence for the involvement of CAD genes in a plant’s defense against pathogen infections. For instance, AtCAD5 (AtCAD_D) plays crucial roles in lignin synthesis and contribute to the resistance of Arabidopsis thaliana against Pseudomonas syringae pv. tomato infection (Sibout et al. 2003; Tronchet et al. 2010). Furthermore, the transcriptional level of TaCAD12 was found to be markedly higher in two sharp eyespot-resistant wheat lines compared with susceptible wheat lines, and it exhibited a significant increase following Rhizoctonia cerealis infection (Rong et al. 2016). The present study aimed to elucidate the divergent expression patterns of DkCAD1 in susceptible and resistant persimmon cultivars infected by C. horii. The expression of DkCAD1 in R var. significantly increased following infection, surpassing the levels observed in S var. during the same period (Fig. 5A). These findings indicate that DkCAD1 actively responds to the C. horii infection in resistant persimmon cultivars, and the high lignin content and expression level of DkCAD1 are associated with the specific persimmon response upon C. horii infection. Additionally, our investigation revealed that transiently overexpressing DkCAD1 in the leaves of S var. post inoculation resulted in a notable decrease in disease index and an increase in lignin content (Fig. 5E, F). These findings suggest that the presence of DkCAD1 potentially enhances the persimmon’s resistance to C. horii, and we hypothesized that this resistance is attributed to the facilitation of lignin accumulation.
The WRKY transcription factor exhibits the capability to coordinate with other genes, orchestrating various plant processes (Bakshi and Oelmüller 2014). For instance, JrWRKY21 enhances the transcriptional activity of JrPTI5L by binding to the W-box motif in the promoter region. This interaction indirectly stimulates the expression of the JrPR5L gene through the formation of the WRKY21–PTI5L protein complex, ultimately leading to enhanced resistance against C. gloeosporioides in walnut (Zhou et al. 2022). Additionally, PtrWRKY89 demonstrates the ability to bind to the promoters of two potential downstream target genes, PtrWRKY18 and PtrWRKY35, which can be induced by SA in Melampsora rust-infected poplar leaves (Jiang et al. 2017). In our previous study, we conducted Y1H assays to screen two DkWRKY transcription factors, namely DkWRKY3 and DkWRKY15 (Guan et al. 2020a). Overexpressing these factors resulted in the up-regulation of acetaldehyde metabolism-related gene DkPK1’s expression, as well as the positive regulation of natural deastringency in C-PCNA persimmon. Furthermore, we identified two additional WRKY transcription factors, DkWRKY8 and DkWRKY10, which bind to the promoter of DkCAD1 using the same method. We found that DkWRKY8 and DkWRKY10 expression patterns in a resistant cultivar postinoculation were similar to those of DkCAD1 (Figs. 5A and 6A). Moreover, the transient overexpression of DkWRKY8 and DkWRKY10 positively regulated the defense against C. horii infection in persimmon (Fig. 7). The results of the multiple sequence analysis demonstrated a notable similarity between DkWRKY10 and AtWRKY54 and AtWRKY70. Previous research has identified AtWRKY54 and AtWRKY70 as positive regulators that facilitate the expression of SARD1 and CBP60g during plant defense responses (Chen et al. 2021). Our investigation suggests that DkWRKY8 and DkWRKY10 may play a positive regulatory role in the defending against C. horii infection in persimmon.
Conclusions
In conclusion, our study has revealed that the resistant cultivar ‘Kangbing Jianshi’ exhibits minimal susceptibility to C. horii infection, and this resistance is attributed to its significantly higher lignin content compared with the susceptible cultivar ‘Fuping Jianshi’, which displays pronounced disease symptoms. In a resistant cultivar, the expression of DkCAD1 was observed to vary following infection. Using the DkCAD1 promoter as bait, we identified DkWRKY8 and DkWRKY10, both of which interacted with this promoter. DkWRKY8, DkWRKY10, and DkCAD1 were all positively associated with disease resistance in persimmon. Furthermore, both DkWRKY8 and DkWRKY10 were highly induced by exogenous SA but not JA in the resistant cultivar. Although further investigation is warranted, these findings represent a significant advancement in comprehending the mechanism of resistance to C. horii in diverse persimmon varieties. Therefore, this study serves as a valuable reference for the identification of additional persimmon genes associated with disease resistance and the breeding of new resistant germplasm.
Materials and methods
Plant and fungal material
Based on the previous investigation of germplasm resources regarding persimmon anthracnose resistance, the highly susceptible cultivar ‘Fuping Jianshi’ (S var.) and the highly resistance cultivar ‘Kangbing Jianshi’ (R var.) were used in this study (Guan et al. 2022). These materials were cultured at the National Field Genebank for Persimmon, located in Yangling, Shaanxi, China (34°17´42.80´´N, 108°04´8.21´´E). Leaves from the S Var. with similar healthy growth conditions were collected for further study, and C. horii strain FJ-1 was isolated from the infected S Var. branches (Fan et al. 2022), then incubated on potato dextrose agar (PDA) at 25°C in the light incubator.
Pathogen inoculation and hormone treatments
The conidial suspension for pathogen inoculation was obtained according to the previous descriptions (Hopkins and Harris 2000). The mycelia were grown on PDA plate for 7-10 days, after which they were scraped off using a scalpel and suspended in aseptic distilled water. The resulting conidial suspension was filtered through aseptic gauze and adjusted to 1×106 conidia/ml. The conidial suspension was applied to the young shoots of persimmon plants, which were subsequently bagged to maintain humidity after inoculation. Infected leaves were collected at various time points (0, 1, 3, 5, 7, and 10 days) following inoculation, frozen in liquid nitrogen, and stored at -80℃ refrigerator. Three biological repetitions were set and each consisting of 3-5 branches (Guan et al. 2022).
For SA and JA treatment, the leaves from S Var. and R Var. were sprayed with 0.1 mM SA or JA. After two days, the leaves were exposed to C. horii for a duration of two weeks in an incubator with 70% humidity and 25–28°C.
Endogenous contents of SA and JA
Endogenous SA and JA contents were determined using the previously described method (Fu et al. 2012). Briefly, 100 mg infected leaves were ground into powder and then placed in 2 ml methanol solution. The resulting homogenate was stored at -20℃ for 10 h and subsequently centrifuged at 12,000 g for 12 min at 4℃. The supernatant was dissolved in a 5% ammonia solution and purified using the Oasis MAX solid-phase extraction (SPE) column (Waters, Milford, MA). The eluted samples were then subjected to centrifuging at 12000 g for 5 min at 4℃, and the resulting supernatant was collected for the detection of SA and JA content with LC-MS (SCIEX, QTRAP5500).
Lignin content measurement and histochemical staining
The lignin content of branches was quantified using the Klason method (Fagerstedt et al. 2015). Specifically, 0.5 g (W1) samples were subjected to extraction in a Soxhlet apparatus using a mixture of ethanol and benzene (volume ratio 1:2) for 6 h. Subsequently, the extracted samples were dried to achieve a constant weight (W2). The dried powders were then treated with 72% sulfuric acid for 2 h and diluted with distilled water until the sulfuric acid concentration reached 3.0%. Following boiling and refluxing for 4 h, an insoluble residue was obtained, which was subsequently filtered and washed until it reached a pH of approximately 7. Finally, the residue was dried and weighed (W3). These measured values were utilized in the calculation of the lignin content: Lignin content (%) = (W2-W3)/W1×100.
The total lignin content was measured using the phloroglucinol-HCl staining method (Blanco-Portales et al. 2002). Specifically, an ethanol solution with 1% phloroglucinol was applied to cross- branches section to remain for 1-2 min, followed by the addition of concentrated hydrochloric acid (HCl) solution. The stained samples were observed using a stereomicroscope after the coloration process.
RNA extraction and cDNA synthesis
Total RNA was extracted from infected branches of the S var. and R var. varieties using the RNAprep Pure Plant Plus Kit (DP441, Tiangen Biotech Co., Beijing, China). Three biological replicates were included for each sample. The quality and concentration of the RNA were evaluated through gel electrophoresis analysis and spectrophotometer detection (NanoDrop 2000, Thermo Fisher Scientific, USA). For cDNA synthesis, the removal of genomic DNA and the synthesis of the first-strand cDNA were carried out using the PrimeScriptTM RT Reagent Kit with gDNA Eraser according to the manufacturer’s instructions (TaKaRa, Dalian, China).
Transcriptome analysis
The uninfected (CK), infected branch samples from S var. and R var. varieties were collected for sequencing at 1, 3, 5 dpi. The samples were then sent to Genedenovo Biotechnology Co., Ltd (Guangzhou, China) for library construction, and subsequent sequencing was conducted using Illumina HiSeqTM4000. Raw sequencing data were preprocessed with fastp and Kallisto was used for read counts qualification of each transcript and ‘Xiaoguotianshi’ was used as the reference genome (Li et al. 2023). Furthermore, basic functional annotation and difference gene analysis were performed using Gene Ontology, KEGG, and Mapman annotation (Thimm et al. 2004). DESeq2 was employed to analyze the differential expression genes between CK and 1,3,5 dpi samples. The identification of significant genes was based on a p-value threshold of less than 0.01 or a log2 fold change greater than 1. Gene expression value was represented by transcripts per million values (TPM) and normalized using a log2 (TPM +1) transformation with DESeq2. K-means clustering method was implemented for the differential genes using the hk-means function in the R package ‘factoextra’, with K set to 12.
Metabolomics analysis
Non-targeted metabolomics analysis was performed with the infected branches from S var. and R var. Each group consisted of six biological replicates. The metabolites were extracted and measured using a Liquid chromatograph-mass spectrometer (LC-MS). Agilent 1290 ultra-high-performance liquid chromatography and Thermo Fisher Scientific Q Exactive Orbitrap mass spectrometer was used in tandem for analysis. A standard and advanced analysis of the samples was conducted following detection. To identify differentially accumulated metabolites (DAMs), the screening condition was set as variable importance in the projection (VIP) ≥ 1 and T-test P < 0.05. Moreover, the relationship between genes and metabolites was examined. The average log2 value was calculated from the biological replicates, and the Pearson correlation coefficient was then calculated for the resulting data.
Quantitative real-time analysis
Quantitative real-time polymerase chain reactions (qRT-PCR) was conducted using the ABI One Step Plus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). The PCR reaction mixture consisted of a total volume of 20 µl, including 10 µl SYBR Premix Ex Taq II (TaKaRa, Dalian, China), 7.4 µl ddH2O, 1.0 µl diluted cDNA, and 0.8 µl of each primer (10 µM). The PCR conditions were as follows: an initial denaturation step at 95°C for 5 min, followed by 45 cycles of denaturation at 95 °C for 5 s, annealing at 58°C for 10 s, and extension at 72°C for 15 s. DkActin was utilized as an internal reference (Akagi et al. 2009), and data analysis was performed using the 2–ΔΔCt method (Livak and Schmittgen 2001). The primer pairs used in this study were listed in Table S8.
Dual-luciferase assay
The dual-luciferase assay was employed to investigate the transcriptional activation of DkCAD1 by DkWRKY (Hellens et al. 2005). Following the methodology described previously (Min et al. 2012), the pGreen II 002962-SK (SK) vector was used to insert the full-length sequences of DkWRKY8 and DkWRKY10 while the promoter fragments of DkCAD1 were fused into the pGreen II 0800-LUC (LUC) vectors. These constructs were then transformed into Agrobacterium tumefaciens strains GV3101 and transiently expressed in Nicotiana benthamiana leaves. After three days, the LUC and REN contents were analyzed using a dual-luciferase reporter assay system (Promega, USA). Three biological replicates were conducted, and each contained three technical replicates.
Yeast one-hybrid analysis
Yeast one-hybrid analysis was used to validate the interactions between DkWRKY8, DkWRKY10, and the promoter of DkCAD1 according to the Matchmaker™ Gold Yeast One-Hybrid Library Screening System (Clontech, USA). The DkCAD1 promoter was inserted into the pAbAi vector, and BstBI was used for digesting the recombinant plasmids. The linearized recombinant plasmids were transformed into the yeast strain Y1H and tested for Aureobasidin A (AbA) concentrations (100−500 ng/mL) on SD/-Ura medium. The coding sequence of DkWRKY8 and DkWRKY10 were cloned into the pGADT7 vectors and transferred into the competent cells with recombinant plasmids of promoter of DkCAD1. The transformants were cultured on SD/-Leu medium with supplemented with 100 and 200 ng/ml AbA, respectively. pGADT7 (AD-p53) and p53-AbAi were employed as positive controls.
Transient transformation of DkCAD1 in persimmon leaves
To ascertain the functional role of DkCAD1, DkWRKY8, and DkWRKY10 during pathogen infection, the overexpression vector pMV2-DkCAD1, pMV2-DkWRKY8, and pMV2-DkWRKY10 were constructed and transiently expressed into the S Var. leaves by vacuum infiltration (Mo et al. 2019), pMV2-GFP construct was used as control. The strain C. horii strain FJ-1 was inoculated into the overexpressed leaves for a duration of 2 days. Subsequently, images were captured at 5 days post-inoculation (dpi) to assess the relative resistance index (Guan et al. 2022), and the lignin content was determined using the Klason method (Fagerstedt et al. 2015). Six sets were conducted, each set comprising three biological replicates and each replicate consisted of a minimum of 10 leaves.
Statistical analysis
The obtained data were subjected to statistical analysis using one-way analysis of variance (AVNOA) with Duncan’s multiple range test using SPSS 22.0 software (IBM SPSS Statistics, Chicago, IL, USA). Statistical significance was considered at p < 0.05.
Availability of data and materials
All data generated or analyzed during this study are included in this published article or on its supplementary information.
Abbreviations
- C. horri :
-
Colletotrichum horii
- R var.:
-
‘Kangbing Jianshi’ cultivar
- S var.:
-
‘Fuping Jianshi’ cultivar
- Y1H:
-
Yeast one-Hybrid
- NFGP:
-
National Field Genebank for Persimmon
- CAD:
-
Cinnamyl Alcohol Dehydrogenase
- PDA:
-
Potato Dextrose Agar
- SA:
-
Salicylic Acid
- JA:
-
Jasmonic Acid
- ROS:
-
Reactive Oxygen Species
- TFs:
-
Transcription factor
- DEG:
-
Differentially Expressed Genes
- DAM:
-
Differentially Accumulated Metabolites
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- SPE:
-
Solid-Phase Extraction
- TPM:
-
Transcripts Per Million
- LC-MS:
-
Liquid Chromatography-Mass Spectrometry
- qRT-PCR:
-
Quantitative real-time polymerase chain reactions
- AbA:
-
Aureobasidin A
- dpi:
-
days post-inoculation
- C-PCNA:
-
Chinese pollination-constant non-astringent
References
Abdelrahman N, Galiwango E (2018) Klason Method: An effective method for isolation of lignin fractions from date palm biomass waste. Chem Process Eng Res 57:46–58
Akagi T, Ikegami A, Tsujimoto T, Kobayashi S, Sato A, Kono A, Yonemori K (2009) DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol 151:2028–2045. https://doi.org/10.1104/pp.109.146985
Bakshi M, Oelmüller R (2014) WRKY transcription factors: Jack of many trades in plants. Plant Signal Behav 9:e27700. https://doi.org/10.4161/psb.27700
Bi M, Li X, Yan X, Liu D, Gao G, Zhu P, Mao H (2021) Chrysanthemum WRKY15-1 promotes resistance to Puccinia horiana Henn. via the salicylic acid signaling pathway. Hortic Res 8: 6. https://doi.org/10.1038/s41438-020-00436-4
Birkenbihl RP, Kracher B, Ross A, Kramer K, Finkemeier I, Somssich IE (2018) Principles and characteristics of the Arabidopsis WRKY regulatory network during early MAMP-triggered immunity. Plant J 96:487–502. https://doi.org/10.1111/tpj.14043
Blanco-Portales R, Medina-Escobar N, López-Ráez JA, González-Reyes JA, Villalba JM, Moyano E, Caballero JL, Muñoz-Blanco J (2002) Cloning, expression and immunolocalization pattern of a cinnamyl alcohol dehydrogenase gene from strawberry (Fragaria x ananassa cv. Chandler). J Exp Bot 53:1723–1734. https://doi.org/10.1093/jxb/erf029
Cai H, Yang S, Yan Y, Xiao Z, Cheng J, Wu J, Qiu A, Lai Y, Mou S, Guan D, Huang R, He S (2015) CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J Exp Bot 66:3163–3174. https://doi.org/10.1093/jxb/erv125
Chen S, Ding Y, Tian H, Wang S, Zhang Y (2021) WRKY54 and WRKY70 positively regulate SARD1 and CBP60g expression in plant immunity. Plant Signal Behav 16:1932142. https://doi.org/10.1080/15592324.2021.1932142
Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Deng Q-e, Ding X-y, Li J-a, Cui L-k, Xu J-q (2019) Morphological characteristics and genetic diversity of Colletotrichum horii infecting persimmon tree in China. Eur J Plant Pathol 156:437–449. https://doi.org/10.1007/s10658-019-01893-x
Eudes A, Pollet B, Sibout R, Do C-T, Séguin A, Lapierre C, Jouanin L (2006) Evidence for a role of AtCAD 1 in lignification of elongating stems of Arabidopsis thaliana. Planta 225:23–39. https://doi.org/10.1007/s00425-006-0326-9
Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10:366–371. https://doi.org/10.1016/j.pbi.2007.04.020
Fagerstedt KV, Saranpää P, Tapanila T, Immanen J, Serra JA, Nieminen K (2015) Determining the composition of lignins in different tissues of silver birch. Plants (Basel) 4:183–195. https://doi.org/10.3390/plants4020183
Fan H, Li Y, Chachar S, Yang Y, Guan C (2022) Genome sequence resource of Colletotrichum horii, an important pathogenic fungus threatening persimmon production. Plant Dis 106:1052–1055. https://doi.org/10.1094/pdis-11-21-2417-a
Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong X (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486:228–232. https://doi.org/10.1038/nature11162
Greene SL, Morris JB (2001) The Case for Multiple-Use Plant Germplasm Collections and a Strategy for Implementation. Crop Sci 41:886–892. https://doi.org/10.2135/cropsci2001.413886x
Guan C, Zhang P, Hu C, Chachar S, Riaz A, Wang R, Yang Y (2019) Genetic diversity, germplasm identification and population structure of Diospyros kaki Thunb. from different geographic regions in China using SSR markers. Sci Hortic 251:233–240. https://doi.org/10.1016/j.scienta.2019.02.062
Guan C, Wang M, Zhang Y, Ruan X, Zhang Q, Luo Z, Yang Y (2020a) DkWRKY interacts with pyruvate kinase gene DkPK1 and promotes natural deastringency in C-PCNA persimmon. Plant Sci 290:110285
Guan C, Hu J, Li Y, Che Q, Yang Y (2022) Identification of new sources of resistance to anthracnose caused by Colletotrichum horii among persimmon germplasms. Horticulturae 8:180. https://doi.org/10.3390/horticulturae8020180
Guan C, Zhang Y, Zhang P, Chachar S, Wang R, Du X, Yang Y (2020b) Germplasm conservation, molecular identity and morphological characterization of persimmon (Diospyros kaki Thunb.) in the NFGP of China. Sci Hortic. 272: 109490. https://doi.org/10.1016/j.scienta.2020.109490
Hassan O, Jeon J, Chang T, Shin J, Oh N, Lee Y (2018) Molecular and morphological characterization of Colletotrichum Species in the Colletotrichum gloeosporioides complex associated with persimmon anthracnose in south korea. Plant Dis 102:1015–1024. https://doi.org/10.1094/pdis-10-17-1564-re
Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1:13. https://doi.org/10.1186/1746-4811-1-13
Hirano K, Aya K, Kondo M, Okuno A, Morinaka Y, Matsuoka M (2012) OsCAD2 is the major CAD gene responsible for monolignol biosynthesis in rice culm. Plant Cell Rep 31:91–101. https://doi.org/10.1007/s00299-011-1142-7
Hopkins DL, Harris JW (2000) A greenhouse method for screening grapevine seedlings for resistance to anthracnose. Hortscience 35:89–91. https://doi.org/10.21273/HORTSCI.35.1.89
Hussain A, Noman A, Khan MI, Zaynab M, Aqeel M, Anwar M, Ashraf MF, Liu Z, Raza A, Mahpara S, Bakhsh A, He S (2019) Molecular regulation of pepper innate immunity and stress tolerance: an overview of WRKY TFs. Microb Pathogen 135:103610. https://doi.org/10.1016/j.micpath.2019.103610
Jiang Y, Yu D (2016) The WRKY57 Transcription factor affects the expression of jasmonate zim-domain genes transcriptionally to compromise Botrytis cinerea resistance. Plant Physiol 171:2771–2782. https://doi.org/10.1104/pp.16.00747
Jiang Y, Guo L, Ma X, Zhao X, Jiao B, Li C, Luo K (2017) The WRKY transcription factors PtrWRKY18 and PtrWRKY35 promote Melampsora resistance in Populus. Tree Physiol 37:665–675. https://doi.org/10.1093/treephys/tpx008
Khasin M, Bernhardson LF, O’Neill PM, Palmer NA, Scully ED, Sattler SE, Funnell-Harris DL (2021) Pathogen and drought stress affect cell wall and phytohormone signaling to shape host responses in a sorghum COMT bmr12 mutant. BMC Plant Biol 21:391. https://doi.org/10.1186/s12870-021-03149-5
Kim S-J, Kim M-R, Bedgar DL, Moinuddin SGA, Cardenas CL, Davin LB, Kang C, Lewis NG (2004) Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. PNAS 101:1455–1460. https://doi.org/10.1073/pnas.0307987100
Li X, Yang Y, Yao J, Chen G, Li X, Zhang Q, Wu C (2009) FLEXIBLE CULM 1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice. Plant Mol Biol 69:685–697. https://doi.org/10.1007/s11103-008-9448-8
Li Y, Liao S, Mei P, Pan Y, Zhang Y, Zheng X, Xie Y, Miao Y (2021) OsWRKY93 Dually Functions Between Leaf Senescence and in Response to Biotic Stress in Rice. Front Plant Sci 12:643011. https://doi.org/10.3389/fpls.2021.643011
Li H, Sun P, Wang Y, Zhang Z, Yang J, Suo Y, Han W, Diao S, Li F, Fu J (2023) Allele-aware chromosome-level genome assembly of the autohexaploid Diospyros kaki Thunb. Sci Data 10:270. https://doi.org/10.1038/s41597-023-02175-2
Li T, Wang Q, Feng R, Li L, Ding L, Fan G, Li W, Du Y, Zhang M, Huang G, Schäfer P, Meng Y, Tyler BM, Shan W (2019) Negative regulators of plant immunity derived from cinnamyl alcohol dehydrogenases are targeted by multiple Phytophthora Avr3a-like effectors. New Phytol e 16139. https://doi.org/10.1111/nph.16139
Liu Q, Liu Y, Tang Y, Chen J, Ding W (2017) Overexpression of NtWRKY50 increases resistance to Ralstonia solanacearum and alters salicylic acid and jasmonic Acid Production in Tobacco. Front Plant Sci 8:1710. https://doi.org/10.3389/fpls.2017.01710
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Luan Q, Chen C, Liu M, Li Q, Wang L, Ren Z (2019) CsWRKY50 mediates defense responses to Pseudoperonospora cubensis infection in Cucumis sativus. Plant Sci 279:59–69. https://doi.org/10.1016/j.plantsci.2018.11.002
Luo Z, Wang R (2008) Persimmon in China: Domestication and traditional utilizations of genetic resources. Adv Hortic Sci 22:239–243. https://doi.org/10.1400/100648
Min T, Yin XR, Shi YN, Luo ZR, Yao YC, Grierson D, Ferguson IB, Chen KS (2012) Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. J Exp Bot 63:6393–6405. https://doi.org/10.1093/jxb/ers296
Mo R, Yang S, Zhang Q, Xu L, Luo Z (2019) Vacuum infiltration enhances the Agrobacterium-mediated transient transformation for gene functional analysis in persimmon (Diospyros kaki Thunb.). Sci Hortic 251:174–180. https://doi.org/10.1016/j.scienta.2019.03.002
Quiroz-Figueroa FR, Cruz-Mendívil A, Ibarra-Laclette E, García-Pérez LM, Gómez-Peraza RL, Hanako-Rosas G, Ruíz-May E, Santamaría-Miranda A, Singh RK, Campos-Rivero G, García-Ramírez E, Narváez-Zapata JA (2023) Cell wall-related genes and lignin accumulation contribute to the root resistance in different maize (Zea mays L.) genotypes to Fusarium verticillioides (Sacc.) nirenberg infection. Front Plant Sci 14: 1195794. https://doi.org/10.3389/fpls.2023.1195794
Rong W, Luo M, Shan T, Wei X, Du L, Xu H, Zhang Z (2016) A Wheat cinnamyl alcohol dehydrogenase TaCAD12 contributes to host resistance to the sharp eyespot disease. Front Plant Sci 7:1723. https://doi.org/10.3389/fpls.2016.01723
Shan W, Chen JY, Kuang JF, Lu WJ (2016) Banana fruit NAC transcription factor MaNAC5 cooperates with MaWRKYs to enhance the expression of pathogenesis-related genes against Colletotrichum musae. Mol Plant Pathol 17:330–338. https://doi.org/10.1111/mpp.12281
Sibout R, Eudes A, Pollet B, Goujon T, Mila I, Granier F, Séguin A, Lapierre C, Jouanin L (2003) Expression pattern of two paralogs encoding cinnamyl alcohol dehydrogenases in Arabidopsis. Isolation and characterization of the corresponding mutants. Plant Physiol 132:848–860. https://doi.org/10.1104/pp.103.021048
Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939. https://doi.org/10.1111/j.1365-313x.2004.02016.x
Tronchet M, Balagué C, Kroj T, Jouanin L, Roby D (2010) Cinnamyl alcohol dehydrogenase-C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Mol Plant Pathol 11:83–92. https://doi.org/10.1111/j.1364-3703.2009.00578.x
Wang D, Jiang C, Liu W, Wang Y (2020) The WRKY53 transcription factor enhances stilbene synthesis and disease resistance by interacting with MYB14 and MYB15 in Chinese wild grape. J Exp Bot 71:3211–3226. https://doi.org/10.1093/jxb/eraa097
Weir B, Johnston P (2010) Characterisation and neotypification of Gloeosporium kaki Hori as Colletotrichum horii nom. nov. Mycotaxon 111:209–219. https://doi.org/10.5248/111.209
Xie L, Zhang J-Z, Cai L, Hyde K (2010) Biology of Colletotrichum horii, the causal agent of persimmon anthracnose. Mycology 1:242–253. https://doi.org/10.1080/21501203.2010.526644
Xie Y, Sun P, Li Z, Zhang F, You C, Zhang Z (2022) FERONIA receptor kinase integrates with hormone signaling to regulate plant growth, development, and responses to environmental stimuli. Int J Mol Sci 23:3730. https://doi.org/10.3390/ijms23073730
Yamada M, Sato A (2016) Advances in table grape breeding in Japan. Breed Sci 66:34–45. https://doi.org/10.1270/jsbbs.66.34
Yang J, Wang Q, Luo H, He C, An B (2020) HbWRKY40 plays an important role in the regulation of pathogen resistance in Hevea brasiliensis. Plant Cell Rep 39:1095–1107. https://doi.org/10.1007/s00299-020-02551-x
Zhang J, Xu T (2005) Cytological characteristics of the infection in different species, varieties and organs of persimmon by Colletotrichum gloeosporioides. Mycosystema 24:116–122. https://doi.org/10.3969/j.issn.1672-6472.2005.01.020
Zhang F, Wang F, Yang S, Zhang Y, Xue H, Wang Y, Yan S, Wang Y, Zhang Z, Ma Y (2019) MdWRKY100 encodes a group I WRKY transcription factor in Malus domestica that positively regulates resistance to Colletotrichum gloeosporioides infection. Plant Sci 286:68–77. https://doi.org/10.1016/j.plantsci.2019.06.001
Zhou R, Dong Y, Liu X, Feng S, Wang C, Ma X, Liu J, Liang Q, Bao Y, Xu S, Lang X, Gai S, Yang KQ, Fang H (2022) JrWRKY21 interacts with JrPTI5L to activate the expression of JrPR5L for resistance to Colletotrichum gloeosporioides in walnut. Plant J 111:1152–1166. https://doi.org/10.1111/tpj.15883
Acknowledgements
We would like to thank State Key Laboratory of Crop Stress Biology for Arid Areas, Northwest A&F University, China for the equipment support.
One-sentence summary
DkWRKY transcription factors DkWRKY8 and DkWRKY10 enhance persimmon resistance to Colletotrichum horii by promoting lignin accumulation through DkCAD1 promotor interaction.
Funding
This research was supported by the National Natural Science Foundation of China (32272672 and 32302478) and National Key Research and Development Program of China (2022YFD2200400 and 2019YFD1000600).
Author information
Authors and Affiliations
Contributions
G.C. coordinated the project, conceived and designed the experiments. F.H., D.Y. (Yu Ding), and L.Y. collected phenotypic data and performed the overexpression experiments. S.X., D.Y. (Yuduan Ding), and L.S. analyzed all the data and wrote the manuscript. Y.Y. provided experiment materials. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All authors consent to participate.
Consent for publication
All the authors have given their consent for publication of this manuscript by Stress Biology.
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling editor: Jin-Rong Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Normalized metabolic profile of DAMs.
Additional file 2:
Table S2. Matrix of -log10 p-value from GO annotation enrichment analysis of DEGs.
Additional file 3:
Table S3. Matrix of -log10 p-value from KEGG annotation enrichment analysis of DEGs.
Additional file 4:
Table S4. Matrix of -log10 p-value from Mapman annotation enrichment analysis of DEGs.
Additional file 5:
Table S5. Normalized expression value of JA and SA related genes from RNA-seq data.
Additional file 6:
Table S6. Expression value of lignin synthesis related genes from RNA-seq data.
Additional file 7:
Table S7. Gene expression value of CAD family from RNA-seq data.
Additional file 8:
Table S8. Primers used in this study.
Additional file 9: Figure S1.
GO annotation enrichment analysis of DEGs. Figure S2. Normalized profile of SA and JA related DEGs in S var. (A) and R var. Figure S3. Promoter sequence of DkCAD1. Figure S4. Effects of exogenous SA and JA on C. horii resistance.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fan, H., Shen, X., Ding, Y. et al. DkWRKY transcription factors enhance persimmon resistance to Colletotrichum horii by promoting lignin accumulation through DkCAD1 promotor interaction. Stress Biology 4, 17 (2024). https://doi.org/10.1007/s44154-024-00154-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-024-00154-0