Abstract
Numerous diseases have been linked to oxidative stress, emphasizing its role in pathogenicity. This study employed an ultrasound-assisted extraction process to obtain extracts from the bark of Murraya koenigii (L) Spreng. A thorough analysis was conducted on these dry extracts to determine their total phenolic and flavonoid content across four solvent fractions: n-hexane, ethyl acetate, n-butanol, and aqueous. Among the solvent fractions, ethyl acetate and n-butanol showed higher levels of total flavonoid content (TFC) and total phenolic content (TPC) compared to the others. The ethyl acetate fraction displayed the highest TPC (58.27 ± 4.20 mg GAE/g) and TFC (878.67 ± 40.38 mg QE/g) at 250 µg/mL concentration. Furthermore, the in-vitro antioxidant capacity of the ethyl acetate fraction was assessed for its ability to scavenge DPPH and hydrogen peroxide, revealing a concentration-dependent effect with IC50 values of 154.6 ± 6.2 and 55.12 ± 4.28 μg/mL, respectively. In a controlled experiment using streptozocin-induced diabetic rats, administering UMBE-EA at doses of 100 and 200 mg/kg notably improved blood glucose levels and body weight. Furthermore, several biochemical indicators, such as total protein levels, serum insulin, and the activities of enzymes like SGOT, SGPT, and ALP, as well as liver glycogen, showed significant enhancements. The antioxidant enzyme activities, including SOD, CAT, and GSH, returned to more favorable levels, and TABAR levels were normalized. In conclusion, the findings from this study strongly suggest that UMBE-EA possesses substantial anti-diabetic efficacy in Wistar rats, primarily attributed to its remarkable antioxidant properties and capacity to scavenge free radicals. These results provide valuable insights into the potential therapeutic benefits of UMBE-EA in diabetes management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hyperglycemia and aberrant glucose metabolism are hallmark symptoms of diabetes mellitus [1], a condition intricately linked with insufficient insulin secretion, whether absolute or relative, often accompanied by peripheral resistance to insulin. Diabetes presents a significant public health challenge, affecting nations across the globe [2]. Notably, approximately 90% of all diabetes cases globally are attributed to type 2 diabetes mellitus (T2DM) [3]. The prevalence of obesity has surged nearly threefold since 1975, contributing to a twofold increase in global diabetes diagnoses over the past two decades [4]. Obesity arises from a chronic energy imbalance marked by excessive caloric intake, often from processed foods, reduced physical activity, and increased sedentary behaviors [5]. In 2021, projections indicated that diabetes would impact approximately 11% of individuals aged 20 to 79 worldwide, with expectations of this figure rising to 12% by 2045 [3, 4]. Notably, the most substantial increases in prevalence between 2021 and 2045 are anticipated in low- and middle-income countries, with the global epidemiology of T2DM transitioning from primarily affecting middle-aged and older individuals to becoming increasingly prevalent among younger populations, particularly young adults, adolescents, and children [6, 7].
Diabetes-related chronic kidney disease (CKD) stands as a prevalent and severe long-term consequence of diabetes, with T2DM patients accounting for 20–50% of all CKD cases. CKD represents 50% of end-stage renal disease occurrences and is the most common cause of chronic kidney disease globally [8, 9]. It not only impairs the quality of life but also escalates cardiovascular morbidity and mortality [9]. Reactive oxygen species (ROS) are generated at higher rates in both types of diabetes, and there is compelling experimental and clinical evidence linking oxidative stress to diabetes development. Elevated ROS and/or reactive nitrogen (RNS) species contribute to oxidative stress [10]. For individuals at high risk of diabetes, drug therapy may be necessary when lifestyle interventions prove ineffective or unsustainable. The primary objectives of pharmacological intervention are glycemic control and management of cardiovascular risk factors [11]. While insulin replacement therapy and synthetic oral hypoglycemics are essential for glycemic control, they are not without limitations, often accompanied by undesirable side effects. Therefore, addressing diabetes complications in clinical practice while minimizing side effects remains a formidable challenge. The utilization of medicinal plants shows great promise in diabetes treatment due to their cost-effectiveness and minimal adverse effects.
Numerous herbal remedies have undergone extensive animal studies and human clinical trials, with some yielding significant improvements in diabetes management without discernible adverse effects. Murraya koenigii (L) Spreng leaves very commonly known as curry leaves, belongs to the Rutaceae family, and is distributed widely across tropical and subtropical regions [12, 13]14]. Although the use of M. koenigii in conventional medications and treatments has gained attention in recent years, few investigations have been conducted to determine its pharmacological and medicinal effectiveness in promoting health benefits and treating disease.
Currently, there is a lack of research examining the preventative advantages of M. koenigii bark extracts against streptozocin-induced diabetes in rats. In the present study, methanolic extracts of M. koenigii bark were evaluated for in vitro antioxidant activity and for their protective effects against streptozocin-induced diabetes in Wistar rats.
2 Methodology
2.1 Extract preparation
Fresh bark from M. koenigii was acquired from a local spice market in Delhi, India, and underwent authentication by Dr. Md. Irfan Khan, an expert from Integral University in India. The Department of Pharmacognosy and Phytochemistry has received a voucher specimen (#21,413-MK/IU). The name of the plant is M. koenigii (Linn.) Spreng. with the original publication information: Syst. Veg. 2: 315 1817 has been confirmed by the website "World Flora Online" at http://www.theplantlist.org. To eliminate the dust and dirt, barks were properly washed with tap water, then shade-dried and crushed.
2.2 Ultrasound-assisted extraction
Four hundred grams (400 g) of M. koenigii coarse powder were soaked in methanol–water (1:3 v/v) and subjected to ultrasound-assisted extraction using an ultrasonic bath system with a 60 kHz/200 W ultrasound frequency/electric power. Centrifugation at 3100 g (4000 rpm) for 10 min separated the solid–liquid combination from the aqueous phase containing the polyphenols. The aqueous solvent was removed via freeze-drying, resulting in dried crystals known as ultrasonic-assisted M. koenigii bark extract (UMBE) [15]. The extract yield was estimated using the formula:
2.3 Solvent–Solvent Partitioning of the Extract
In 500 mL of 25% methanol in water, 10 g of UMBE was dissolved, and the resultant mixture was partitioned four times each against n-hexane, ethyl acetate, and n-butanol and concentrated by vacuum dryer [16].
2.4 Quantification of phenolics and flavonoids
Total phenolic content (TPC) of UMBE was determined using the Folin–Ciocalteau method with gallic acid equivalents (GAEs) as the unit of measurement [17]. Gallic acid equivalent was employed as a standard reference (y = 0.0059x + 0.0855, R2 = 0.996), was used to express phenolic content.
Flavonoid concentrations were determined colorimetric method [16]. A reaction mixture was prepared by adding 1.5 mL of methanol with 100 µL of 10% AlCl3 (aluminium chloride), and 100 µL of 1 M potassium acetate with 500 µL of the extract. The mixture was left to remain at room temperature for 30 min. The absorbance at 425 nm was determined by UV/Visible spectrophotometer. Quercetin solutions of serial dilution ranging from 10 to 100 mg/mL in methanol were used to prepare the standard curve (y = 0.0061x + 0.0121, R2 = 0.997) is used to calculate the total flavonoid concentration, which is expressed as mg quercetin equivalent/g of extract (QE).
2.5 Antioxidant activity in-vitro
The antioxidant potential of the ethyl acetate fraction derived from UMBE (UMBE-EA) was evaluated by assessing its capacity to neutralize 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•) and hydrogen peroxide.
An extract and standard sample are present in the absorbance curve, with A1 denoting the absorbance with the extract present.
2.5.1 DPPH radical-scavenging assay
UMBE-EA concentrations were added to a DPPH methanolic solution, and the absorbance was measured at 517 nm after incubation. IC50 values were calculated [18].
2.5.2 Hydrogen peroxide assay
UMBE-EA was tested for its ability to neutralize hydrogen peroxide using a phosphate buffer solution. Absorbance was measured at 230 nm against a blank solution. Quercetin was employed as the standard compound for comparison [18].
2.6 Animals
In the present study Wistar rats of weight range 175 ± 25 g were used. Under conventional laboratory circumstances light/dark cycle (12:12 h). Polypropylene cages were used for housing the animals with six animals per cage at temperature ranging 23 ± 2 ºC and humidity 50 ± 5%.
2.6.1 Acute toxicity study
The acute toxicity study meticulously adhered to the guidelines outlined by the Organization for Economic Co-operation and Development (OECD). Its purpose was to evaluate the potential harmful effects of UMBE. Rats that had not eaten all night were split into six groups. They received higher doses (500, 1000, 1500, 2000, and 2500 mg/kg) of the medication. A control group was designated and provided with a vehicle exclusively for their use. To detect any signs of toxicity and behavioral changes, animals were continuously monitored for an hour. of toxicity and mortality, if any, and signs of lethargy, restlessness, writhing, convulsions, and piloerection. Acute toxicity symptoms were then monitored for the next six hours at regular intervals, and then again after 24 h [19].
2.7 Experimental design and Induction of diabetes
Rats that had fasted overnight were induced into experimental diabetes by administering a single dose of streptozocin solution (40 mg/kg bw/ 0.1 M citrate buffer, pH 4.5) intraperitoneally. Following seven days of STZ treatment, plasma glucose levels were measured. The study was conducted on diabetic mice (200 mg/dL plasma glucose levels) that had been identified by the elevated plasma glucose levels. A complete treatment is mentioned in table (Table 1). Upon completion of this study, the animals were euthanized through cervical dislocation, and their livers were harvested for further analysis (Table 1).
2.8 Biochemical parameters
Blood glucose levels were evaluated utilizing EasyMax® test strips, following the guidelines provided in the user manual. Insulin levels were quantified through a rat insulin (INS) ELISA Kit (Cat # MBS2024348) obtained from MyBioSource. Tissue markers were examined using conventional methods for assessing liver glycogen [20], SGOT, SGPT [21], and ALP [22]. Lowry et al., 1951 method was used for protein estimation [23]. A liver homogenate was prepared by blending an ice-cold solution of 10% potassium chloride. Subsequently, the mixture underwent centrifugation at 4 °C for 30 min to separate the post-mitochondrial supernatant (PMS). Subsequently, the activities of glutathione (GSH) were evaluated using this PMS fraction [24], superoxide dismutase (SOD), catalase (CAT) [25], malondialdehyde (MDA), the resultant product of lipid peroxidation, reacts with TBA to generate a colored substance. MDA measurement by TBA reactivity is the method most commonly used to measure lipid peroxidation. A liver homogenate sample was tested for thiobarbituric acid-reactive substances (TBARS) [26].
2.9 Statistical analysis
Data were analyzed using one-way ANOVA followed by Tukey post-tests, with p < 0.05 considered statistically significant. Values are expressed as mean ± SD.
3 Results
3.1 Extract mass and fraction yield
To determine the percentage yield of extracts, dried plant material was used, while drying plant material was used to determine the percentage yield of fractions (Table 2).
3.2 Phenolic and flavonoids quantification
3.2.1 Total phenol content (TPC)
Total phenol content, determined by the Folin–Ciocalteau method, is quantified in Gallic acid equivalents using the standard curve as a reference. UMBE ethyl acetate fraction had the highest amount of phenolics among all samples, demonstrating that TPC increased with increasing extract and fraction concentrations (Fig. 1 A-D).
3.2.2 Total flavonoid content (TFC)
The findings of the present investigation showed that TFC increased with UMBE and extract and fraction concentrations. The fraction of ethyl acetate that contains the most flavonoids is compared to other fractions (Fig. 2 A-D).
3.3 In-vitro antioxidant assay
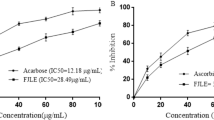
3.3.1 DPPH radical-scavenging activity
An increase in radical-scavenging activities was noted with higher concentrations of UMBE-EA. This extract, distinguished by elevated phenolic and flavonoid contents, exhibited notable efficacy in scavenging free radicals, displaying an IC50 value of 154.6 ± 6.2 μg/mL (refer to Table 3).
3.3.2 Hydrogen Peroxide Scavenging
Concentration-dependent scavenging of hydrogen peroxide by the extract was observed. The IC50 of UMBE-EA and quercetin for scavenging H2O2 were 55.12 ± 4.28 and 21 ± 0.8 μg/mL, respectively (Table 3).
3.4 Acute toxicity study
M. koenigii ultrasound-assisted extract ethyl acetate fraction named (UMBE-EA) contained the highest level of phenolics and flavonoids selected for the animal study. During an acute toxicity assessment, the administration of UMBE-EA in escalating doses, reaching up to 1 g/kg, did not induce any adverse effects. Additionally, all rats survived throughout the experimental period. In comparison to rats treated with the vehicle, those given the substance maintained normal body weight and food intake levels. Subsequent studies were conducted using concentrations set at 100 and 200 mg/kg.
3.5 Impact of UMBE-EA treatment on body weight and blood sugar
The plasma glucose levels of every experimental rat were measured on days 0, 14, and 21 of the treatment. When compared to normal rats, the blood glucose levels of diabetic rats treated with STZ were significantly higher. On days 15 and 21 of therapy, blood glucose levels were considerably (p ≤ 0.005) lower after treatment with UMBE-EA at 200 mg/kg compared to diabetic rats. Furthermore, the blood glucose levels of the rats given glibenclamide were significantly (p < 0.005) lower (Fig. 3A). At first, body weight was also tracked. Finally, a substantial shift in body weight was seen in the treated groups, despite the nonsignificant change initially observed (Fig. 3 B-C).
Effect of treatment A. Blood glucose level (mg/dL) at 0, 14, and day 21 and B. Initial change in body weight (g) C. Final change in body weight of different groups of rats. The data is shown as mean ± SD (n = 6). *p ≤ 0.05, ** p ≤ 0.005 in comparison to the diabetic control group and ##p ≤ 0.05 in comparison to the normal control group
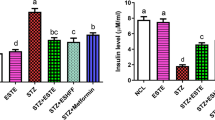
3.6 Effect of UMBE-EA on insulin, SGOT, SGPT, ALP, and liver glycogen
In diabetic rats, SGOT, SGPT, and ALP activities significantly increased in comparison to the normal control group, whereas insulin and glycogen levels significantly decreased. Significant increases in body weight, insulin level, glycogen content, and SGOT, SGPT, and ALP activities were seen over a 21-day course of therapy with UMBE-EA at both 100 and 200 mg/kg, as compared to controls (p < 0.005) (Table 4).
3.7 Effect of UMBE-EA on CAT, SOD, GSH and TBARS
Streptozocin administration actively damages various macromolecules in rats resulting in the induction of oxidative stress. In diabetic rats, oxidative stress caused a significant (p ≤ 0.05) decrease in the activities of CAT, SOD, and GSH while significantly increasing the lipid peroxidation product malonaldehyde (p ≤ 0.05) in contrast with normal control rats. Administration of UMBE-EA at a dose of 100 and 200 mg/kg and glibenclamide the values were returned to normal levels (Table 5).
4 Discussion
Murraya koenigii (L) Spreng, referred to as "curry leaves," belongs to the Rutaceae family. High blood glucose disorder has traditionally been treated with M. koenigii. Various herbal treatments have been the subject of several animal research and human clinical trials. Some of these studies found considerable improvements in the control of diabetes without discernible negative effects. Using an ultrasonic-assisted methanol extract from M. koenigii (UMBE) bark, male Wistar rats with diabetes induced by streptozocin (STZ) were evaluated. In the present study, ultrasonic-assisted extraction of M. koenigii bark was performed to isolate plants' antioxidant compounds. Extract quantification for phenolics and flavonoids showed ethyl acetate fractions were rich in phenols and flavonoids. Flavonoids and phenolic compounds, found abundantly in various natural sources, exhibit remarkable antioxidant properties. Owing to their abundance in a variety of natural sources, flavonoids and phenolic compounds are exceptional antioxidants [16]. The inclusion of phenolics and flavonoids in extracts or fractions could potentially augment the antioxidant efficacy of plant bioactive components. The DPPH assay is a frequently used method to evaluate the free radical scavenging capacity of various substances [18]. Usually, a higher concentration of phenolic compounds and flavonoids performs better DPPH-scavenging activity. The phenolics in extracts may also be responsible for scavenging H2O2 since they can provide electrons, neutralising it into water [17]. While hydrogen peroxide (H2O2) is not inherently highly reactive, it has been demonstrated to sometimes induce cytotoxicity by producing hydroxyl radicals within cells. Hence, eliminating H2O2 throughout the food system is crucial [27]. The antioxidant properties of phenolic compounds have been linked to their ability to neutralize free radicals and mitigate the oxidative stress associated with diabetes [28]. These strong antioxidant and anti-diabetic properties could be attributed to the phenolics, and flavonoids found in this plant. In order to cause diabetes mellitus in experimental rat models, streptozocin (STZ) is commonly used. When 40 mg/kg of STZ is injected intraperitoneally, the pancreatic β-cells that secrete insulin are moderately destroyed. This process involves DNA strand breakage, ultimately leading to a decrease in endogenous insulin production [29]. A sudden drop in body weight that results from increased structural protein breakdown or muscle disruption in STZ-induced diabetes is indicative of the condition [30]. When UMBE-EA 200 mg/kg was administered to diabetic rats caused by streptozocin (STZ), there was a notable reduction in plasma glucose levels and a corresponding rise in insulin levels. The enhancement of insulin secretion from remaining or revived pancreatic β-cells may be the likely mechanism by which UMBE-EA lowers plasma glucose levels in diabetic mice [30]. A significant increase in body weight was observed following UMBE-EA feeding, in contrast to the glibenclamide-treated and diabetic control groups. This suggests that UMBE-EA has a protective effect against gluconeogenesis and muscle wasting.
Furthermore, the capacity to lower high plasma glucose levels appears to be the cause of the capacity to protect body weight reduction. Diabetes-induced decreases in plasma total protein were observed in rats. Exaggerated proteolysis reduced protein synthesis, and a negative nitrogen balance are all signs of apparent metabolic renal changes in experimental diabetes [31]. UMBE-EA treated diabetic rats the plasma protein level was improved. Insulin is essential for controlling the amount of glycogen in different organs, as glycogen is the primary source of glucose in cells. Insulin stimulates the production of glycogen by blocking glycogen phosphorylase and activating glycogen synthase. However, the amount of hepatic glycogen was significantly reduced in diabetic rats with low insulin levels [28]. Due to increased insulin secretion, liver glycogen levels in diabetic rats receiving UMBE-EA treatment show a tendency toward normalization. Alkaline phosphatase (ALP), serum glutamate pyruvate transaminase (SGPT), and serum glutamate oxaloacetate transaminase (SGOT) are all reliable indicators of liver function. Rats given streptozotocin (STZ) to produce diabetes showed signs of liver necrosis, which resulted in increased plasma levels of SGOT, SGPT, and ALP, presumably because the liver cytosol released these enzymes into the bloodstream [3]. GSH is a potent antioxidant that has been demonstrated to guard against damage from free radicals [32] Almost all mammalian tissue contains it, which is composed of the three amino acids glutamic acid, cysteine, and glycine [32]. In the context of diabetes, elevated glucose levels can lead to the deactivation of essential antioxidant enzymes such as SOD, CAT, and GSH through a process called protein glycation. This phenomenon induces oxidative stress, ultimately resulting in an increase in malondialdehyde levels [30]. Catalase, SOD, and GSH levels were dramatically raised after taking UMBE-EA. GSH functions as a detoxifying molecule and an antioxidant, scavenging free radicals [33]. The flavonoid content of M. koenigii may contribute to its ability to raise GSH levels and display antioxidant properties [34]. The activities of glutathione, superoxide dismutase, and catalase were normalized after the administration of UMBE-EA. This signifies the effectiveness of UMBE-EA in alleviating oxidative stress in the diabetic liver.
5 Conclusion
As a result of ultrasonic-assisted extraction, the Murraya koenigii bark contains high levels of total phenolics and flavonoids. The antioxidant properties of the extract were efficient in scavenging DPPH and hydrogen peroxide. The extract may have raised insulin levels, which improved glucose uptake and metabolism and preserved glycemic homeostasis. Increased levels of the antioxidant enzymes CAT, SOD, and GSH showed an antioxidant impact in vivo. All things considered; these results show that M. koenigii bark extract has a potent therapeutic impact that aids in the management of diabetes. The use of M. koenigii bark extract as a nutraceutical supplement for diabetes has to be supported by additional research and clinical studies that explore the additional mechanisms needed to show its therapeutic efficacy with a strong ethical and medical foundation. The traditional usage of M. koenigii is validated by its anti-diabetic and antioxidant qualities to cure diabetes.
Data availability statement
All data generated or analysed during this study are included in this published article.
References
Banday MZ, Sameer AS, Nissar S (2020) Pathophysiology of diabetes: an overview. Avicenna J Med 10(4):174–188
de Boer IH et al (2020) Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int 98(4):839–848
Sun H et al (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
Koye DN et al (2018) The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 25(2):121–132
Hall KD et al (2022) The energy balance model of obesity: beyond calories in, calories out. Am J Clin Nutr 115(5):1243–1254
Chen L, Magliano DJ, Zimmet PZ (2012) The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 8(4):228–236
Magliano DJ et al (2019) Trends in incidence of total or type 2 diabetes: systematic review. BMJ 366:l5003
Kianmehr H et al (2022) Potential gains in life expectancy associated with achieving treatment goals in us adults with type 2 diabetes. JAMA Netw Open 5(4):e227705–e227705
Hoogeveen EK (2022) The epidemiology of diabetic kidney disease. Kidney and Dialysis 2:433–442. https://doi.org/10.3390/kidneydial2030038
Halim M, Halim A (2019) The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr 13(2):1165–1172
Lingvay I et al (2022) Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet 399(10322):394–405
Balakrishnan R et al (2020) Medicinal profile, phytochemistry, and pharmacological activities of murraya koenigii and its primary bioactive compounds. Antioxidants. https://doi.org/10.3390/antiox9020101
Nalli Y et al (2018) Correction: four new carbazole alkaloids from murraya koenigii that display anti-inflammatory and anti-microbial activities. Org Biomol Chem 16(11):1994
Adebajo AC et al (2006) Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine 13(4):246–254
Rehman K, Akash MSH (2016) Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci 23(1):87
Agbo MO et al (2015) Antioxidant, total phenolic and flavonoid content of selected nigerian medicinal plants. Dhaka Univ J Pharm Sci 14(1):35–41
Kujala TS et al (2000) Phenolics and betacyanins in red beetroot (beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem 48(11):5338–5342
Agbo, M.O., et al., Antioxidative polyphenols from Nigerian mistletoe Loranthus micranthus (Linn.) parasitizing on Hevea brasiliensis. Fitoterapia, 2013. 86: p. 78–83.
Ekanayake, C.P., et al., Acute and Subacute Toxicity Studies of the Ethyl Acetate Soluble Proanthocyanidins of the Immature Inflorescence of Cocos nucifera L. in Female Wistar Rats. Biomed Res Int, 2019. 2019: p. 8428304.
Carroll N, Longley R, Roe J (1956) The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem 220:583–593
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Kind PR, King EJ (1954) Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol 7(4):322–326
Lowry OH et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Lawrence RA et al (1974) Glutathione peroxidase activity in rat lens and other tissues in relation to dietary selenium intake. Exp Eye Res 18(6):563–569
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
Wu M-L et al (1996) Mechanism of Hydrogen Peroxide and Hydroxyl Free Radical-Induced Intracellular Acidification in Cultured Rat Cardiac Myoblasts. Circ Res 78(4):564–572
Vázquez-Ruiz Z et al (2023) Effect of dietary phenolic compounds on incidence of type 2 diabetes in the “Seguimiento Universidad de Navarra” (SUN) cohort. Antioxidants. https://doi.org/10.3390/antiox12020507
Matough FA et al (2012) The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J 12(1):5–18
Noor A, Gunasekaran S, Vijayalakshmi MA (2017) Improvement of insulin secretion and pancreatic β-cell function in streptozotocin-induced diabetic rats treated with aloe vera extract. Pharmacognosy Res 9(Suppl 1):S99-s104
Figueira, M.F., et al., Diabetic rats present higher urinary loss of proteins and lower renal expression of megalin, cubilin, ClC-5, and CFTR. Physiol Rep, 2017. 5(13).
Lobo, V., et al., Free radicals, antioxidants and functional foods: Impact on human health. 2010. 4(8): 118.
Dasari S, Ganjayi MS, Meriga B (2018) Glutathione S-transferase is a good biomarker in acrylamide induced neurotoxicity and genotoxicity. Interdiscip Toxicol 11(2):115–121
Erkan, N., et al., Antibacterial Activities of Essential Oils Extracted from Leaves of Murraya koenigii by Solvent-Free Microwave Extraction and Hydro-Distillation. Natural Product Communications, 2012. 7(1): 1934578X1200700139.
Funding
The project has not received any financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author report no declaration of interest. The author alone are responsible for the content and writing of the paper.
Ethical approval
The Biomedical Ethics Committee of Umm Al-Qura University reviewed and accepted the use of animals [HAPO-02-K-012–2023-02–1452].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahzad, N. Glycemic homeostasis of ultrasound-assisted Murraya koenigii (Linn.) Spreng. bark extract in streptozocin-induced diabetes in rats. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00143-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00143-z