Abstract
In this research, a novel L-tryptophan-vanillin Schiff base and three complexes were synthesised, characterization, and their biological activities were also determined. The physicochemical parameters of the compounds investigated revealed that, Schiff base ligand (SL) melting point = 84–85 °C, Schiff base ligand–Fe2+ complex = 245–246 °C, Schiff base ligand–Co2+complex = 271–272 °C, and Schiff base ligand–Ni2+complex > 350 °C. Molar conductivities was found to be 10,300, 5000, 17,300, and 52,900 Sm2 mol−1 for the Schiff base and the complexes, respectively. It indicates that the complexes are non-electrolytic in nature. The magnetic susceptibilities of the complexes were found to be higher than the spin-only values due to unpaired electrons (Fe2+ complex = 5.73 BM, Co2+ complex = 4.52 BM, and Ni2+ complex = 3.46 BM), suggesting octahedral geometries. Electronic absorption (max) for Fe2+ = 480, Co2+ = 520 nm, while Ni2+ shows two bands at 480 and 570 nm, which signifies the n → π* and π → π* transitions, respectively. Their crystallinity index was also assessed using the PXRD and it revealed that complexes are purely crystalline. Antimicrobial test results revealed that Co2+ and Fe2+ complexes have excellent activities against gram-negative bacteria (E. coli, F. Shigella, and S. Typhi). They are all effective against MRSA, K. Pneumoniae., and S. Pneumoniae, as well as A. niger and C. albicans. Therefore, based on the findings from the study, it was concluded that the Schiff base is a bidentate ligand, and octahedral geometries and paramagnetic were suggested for the complexes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Schiff bases derives from amino acids and flavones such as vanillin and quercetin, as well as their metal complexes, exhibit a wide range of biological activities [1]. High affinity for chelation by Schiff bases towards transition metals is utilized for preparing their complexes, and due to its polyhydroxylated chemical structure, vanillin easily forms complexes with species with free orbitals [2, 3].
The potentials of vanillin-Schiff base ligands in inorganic chemistry can be very well understood from the biological activities properties exhibited by their complexes [4]. Vanillin Schiff’s bases and its derivatives has been found to have different therapeutic activities such as antibacterial, analgesic, antiviral, anti-inflammatory, antitubercular, etc. It have been found to be potent drugs in the pharmaceutical industry and possess a wide spectrum of pharmacological activities like antifungal, antibacterial, antiviral, anti-inflammatory etc. [5].
Vanillin Schiff bases are found to be active against gram-positive gram-negative bacterial strains and some fungal species [6, 7]. L-Tryptophan (Trp) is one of the essential amino acid required for the biosynthesis of proteins in the body, it is significant for nitrogen balance, maintenance of muscle mass, and body weight in humans [8].
Vanillin-primary amine condensation products have biological activity as well as good complexation abilities with metal ions [9]. This study focused on the synthesis, characterization, and testing for antimicrobial activities of a Schiff base ligand derived from the condensation of o-vanillin with L-tryptophan and complexes of their ligands with Fe2+, Co2+, and Ni2+ ions. Transition metal complexes with bidentate Schiff base ligands that contain nitrogen, oxygen, or sulphur donor atoms contribute immensely to biological systems [10]. Numerous studies carried out on transition metal complexes derived from Schiff bases have pointed out their significance due to their properties and wider biological applications [11,12,13]. Fugu et al. [14] opined that, Schiff bases derived from 4-hydroxysalicylaldehyde and amines have good anticancer activity [15].
Transition metal ions are part of the significant species needed to keep human body healthy and active because several biological functions in the body depend upon their presence while their absence may lead to deficiency diseases [16]. Hossain et al. [17] explained that, most notable metals that exist in human body are in the form of ions such as: Fe2+, Co2+, Ni2+, Ca2+, Cu2+, Zn2+, and Cr2+. Transition metal complexes are observed to be generally more biologically active than the uncomplexed ligands, which are formed due to the presence of metal moieties [18, 19].
Therefore this study aimed to synthesized, characterize, and evaluate the /biological activities of Fe2+, Co2+ and Ni2+ complexes derived from vanillin–tryptophan Schiff base ligand.
2 Methodology
2.1 Materials and methods
Materials and method used to synthesized and characterized the compounds were listed in supplementary files. All the chemicals used for this research are of analytical grade (AR) obtained from Sigma Aldrich and other reliable chemical vendors. The chemicals were used as purchased.
2.1.1 Syntheses of Schiff-base ligand (SL)
The method used to synthesise the ligand was adopted from that of [20] with minor modifications. In 15 ml of ethanolic solution of vanillin (0.30 g, 2 mmol) was mixed with a solution of l-tryptophan (0.4 g, 2 mmol) and 1 ml of dilute sodium hydroxide solution (0.056 g, 1 mmol) served as a catalyst which will help to abstract the hydrogens from the primary amines which reacted with oxygen of the flavone to formed water molecule as the bye product of the reaction. The reaction mixture was stirred for about 10 min and refluxed over a water bath for 8 h at 60 °C. The coloured precipitate that formed was filtered, washed with 50% cold ethanol, and rinsed with diethyl ether. It was allowed to dry in a vacuum desiccator to a constant weight, which gave a yield of 72.0% for the Schiff base ligand (SL). The yellowish product was recrystallized in methanol for further purification.
2.1.2 Syntheses of the complexes
The complexes were prepared using standard methods of metal: ligand ratio 2:1 adopted from [21–23] with some minor modifications. An ethanolic solution of Schiff base (20 mmol) was prepared and added drop wise into the solutions of metal (II) chloride (10.0 mmol), FeCl2, CoCl2 and NiCl2 which were stirred for about 20 min. The solution of the Schiff base was added to the solution of the metal ions because it served as electron(s) donor to the metal ions in solution. Therefore the same concentrations were prepared and refluxed in a water bath at 60 °C for 6 h. The pH of the mixture was adjusted to about 7.5 with a few drops of potassium hydroxide solution, and the coloured solids that formed were filtered, washed with 50% cold ethanol, and finally dried in a vacuum desiccator to a constant weight. The synthetic routes for the Schiff base and the metal complexes are summarised in Eqs. (1) and (2) respectively:


2.2 Physical measurements
The solubilities of the Schiff base and metal complexes were checked in water, 50% ethanol solution, and 50% dilute DMSO solution respectively. The melting points of the complexes were also determined to establish their purity using an electrically heated melting point apparatus (SMP10, Fascia, Stuart; Bibby Scientific), and molar conductivity was assessed to test the electrolytic nature of the complexes (with a Hann Scientific electrical conductivity meter). The wavelength of maximum absorption (max) of the Schiff base and the complexes was measured using an aqueous solution of the compounds in a UV/visible spectrophotometer (Jenway, 7315) in a 1 cm cuvette at 200–780 nm in an FT-IR spectrometer (Nicolet iS5, Thermo Scientific). Elemental analyses was performed using the phase crystal analysis software and the report obtained from the PXRD data. Magnetic susceptibility followed by an x-ray diffraction technique were used to determine the geometries, degree of crystallinity, and morphological properties of the complexes.
2.3 Assessment of antimicrobial activities: (i) minimum inhibitory concentration (MIC)
The MIC values were determined in accordance with the standard method proposed by the Clinical and Laboratory Standards Institute [24] using broth dilution techniques. McFarland's turbidity standard was developed and used in the study. 1 ml of sterilised MH medium was added to each of the clean, dried, and sterilised test tubes, followed by 1 ml of 0.5 MCF bacterial suspension, which contained about 1–2 108 CFU per mL of bacterial load. 1 mL of a 2000 g/mL solution of the test compounds was transferred into each test tube and serially diluted to give concentrations of 1000, 500, 250, 125, 62.5, 31.25, and 15.625 g/mL.
The solution was added to the mixture, followed by 0.1 mL of the bacterial suspension in a normal saline solution. The test tubes were incubated at 37 °C for 24 h to enhance bacterial growth. The test tubes with the lowest and highest turbidity were examined and compared with the standard, and the results were carefully recorded.
2.3.1 Minimum bactericidal concentration (MBC)
The MBC was assessed using the method developed by [25, 26] to ascertain whether the test pathogens were completely killed or just inhibited in their growth. Mueller–Hinton agar was sterilised and poured into Petri dishes, and the media was allowed to cool and solidify. An aliquots samples that showed sensitivity to the MIC test were carefully selected and subjected to MBC evaluation. They were subculture and incubated at 28 °C for 7–14 days in some cases. To determine CFU, the plates of the media were taken for microscopy and observed for bacterial growth.
2.3.2 Minimum fungicidal concentration (MFC)
Methods were adopted to carry out this test using micro broth dilution technique with little modifications [27,28,29]. Two fungal spp (Aspergillus Niger and Candida albicans) isolates were used, grown for 7 days at 28 °C incubation period in potato dextrose agar. The stock was diluted with normal saline solution to corresponding concentration of 1 × 105 cfu /mL and viability of the fungi spp was confirmed using the sabouraud dextrose agar plates and counting the number of cfu/mL.
Two fold serially diluted solution of the test compounds were prepared to obtained 200 µg/ml, 100 µg/ml, 50 µg/ml, 25 µg/ml and 12.5 µg/ml respectively. A micro pipette was used to transfer 0.1 ml of the test fungal strain solution into the test-tubes containing the mixture with varied concentrations of the compounds. The mixture was incubated at 38 °C for 72 h after which the turbidity was observed and compared with standard for the growth of the pathogens.
The turbidity (growth) between the test-tube which contained lowest and the highest concentration of the compound were examined and compared with standard. The results were carefully recorded in accordance with clinical and laboratory standard institute [30]. Samples that showed sensitive to the MIC test were carefully selected and subjected to MBC evaluation. They were sub-cultured and incubated at 28 °C for 7–14 days in some cases. To determine CFU, the plates of the media were taken for microscopy and observed for bacterial growth.
3 Results and discussion
The results in Table 1 indicate the colour of the Schiff base and the metal complexes, percentage yields, and solubilities in some solvents (Fig. 1). It revealed that the Schiff base ligand is sparingly soluble in water but readily dissolved in DMSO and 50% ethanol solutions, while the complexes are soluble in water and DMSO, respectively. It also indicated that the Schiff base has a lower melting point (84 °C) than the complexes, but the Ni2+ complex has the highest melting temperature of > 350 °C, followed by the Cobalt II complex (271 °C) and the Iron II complex (245 °C). Molar conductivity measurement shows that both the ligand and the complexes were non-electrolytic in nature, and magnetic susceptibility measurement obtained at room temperature revealed that the complexes are paramagnetic with observed magnetic moments as: Fe2+ = 5.73 B.M., Co2+ = 4.52 B.M., and Ni2+ = 3.46 B.M., respectively, which are slightly higher than the spin only values due to unpaired electrons, suggesting an octahedral geometry for all the complexes. This is similar to the work of [31, 32].
Results in Table 2 show electronic transitions within the Schiff base and the complexes scanned from 300 to 750 nm in ethanolic solutions (Figs. 2, 3, 4), which indicate some bands as n- and -type transitions from non-bonding to anti-bonding molecular orbitals and delocalization of pi-electrons within the benzene rings. The absorption bands assigned and the proposed geometries of the complexes are as follows: Fe2+ absorbs at 460–480 nm, Co2+ absorbs at 500–520 nm, and Ni2+ absorbs at 369–480 and 550–570 nm, respectively. This result is in agreement with that of [33].
The results of the elemental analysis of the Schiff base and the complexes revealed the actual compositions of the newly synthesised compounds. The experimental values obtained correspond to the calculated compositions of the constituent atoms present in each proposed compound, as shown in Table 3 above
Results in Table 4 above show FT-IR spectra bands of the ligand and the complexes at 1628 v (HCN, str), 382.47 v (O–H, str), and 362.12 cm–1, respectively. The FT-IR analysis revealed that the bonding of the Schiff base to the metal centres has occurred as expected. In the Schiff base ligand, a strong, sharp band observed at 1663 cm1 can be assigned to the V (HCN) azomethine stretching vibration. When the Schiff base ligand was reacted to form complexes with the metal ions, the band shifted to lower frequencies in the 401.64–419.78 cm–1 range for v(MN) and 385.2–535.30 cm–1 range for v(MO), indicating that new bonds had formed and azomethine had been coordinated to the metal ions via Oxygen and Nitrogen donor atoms.
The symmetric carbonyl stretching v(COO−) is also shifted to a higher frequency from 1362 cm–1 to 535.30, 588.73, and 410.36 cm–1 regions for Fe2+, Co2+, and Ni2+ complexes, respectively. Similarly, the v (C–O) phenolic band in the complexes shows a significant shift to lower frequencies of 1282 cm–1 in the ligand and 1026.32, 1025.14, and 1017.85 cm–1 for the metal ions, respectively. The spectra of the complexes show broad bands between 3208.51, 3211.86, and 3362.07 cm–1, which are attributed to O–H stretching vibrations from phenolic groups (Figs. 5, 6, and 7). Information obtained from the FT-IR results of some of the major functional groups in the Schiff base and the complexes revealed that the Schiff base is a bidentate ligand and that it is bound to the metal ions through the phenolic oxygen and azomethine nitrogen atoms to form octahedral complexes.
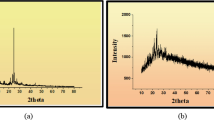
Table 5 shows the results of the PXRD pattern of the complexes at laboratory temperature (303 K) with an XRD machine (RIGAKU) (Figs. 8, 9, 10). The complexes' degree of crystallinity, crystallite sizes, and average crystallite size were measured between 10 and 120 o at the wavelength of CuK1 radiation = 1.5418 nm. The XRD results are further supported by the interplanar distance and the crystallite sizes (d) of 19.41, 21.32, and 22.16, which correspond to Fe2+–SL, Co2+–SL, and Ni2+–SL, respectively.
Table 6 shows the sensitivity test results of the products against some selected pathogens used for the study. The Schiff base ligand is mildly sensitive to one-gram + ve and two-gram – ve bacteria and fungal spp, the Fe2+ complex is very sensitive to Klebsiella P., Shigella, and a fungi spp C. albican., and the Co2+ complex is effective against Streptococcus P., Klebsiella P., E. coli, and A. niger. While the Ni2+ complex proved to be very effective and positive in sensitivity tests for Streptococcus, Klebsiella, E. coli, and the fungal species Aspergillus niger.
The data in Table 7 above shows results for the zone of inhibition tests of the products against some of those pathogens that showed positive sensitivity (MIC) tests (Plate 1). It also indicates the regions (in mm) where those pathogens growth are inhibited or killed by the compounds, with the Schiff base having the smallest inhibition zone (5, 7, 10, 8, and 7 mm), followed by the Fe2+ complex (12, 22 and 25 mm), Ni2+ (13, 16, 21, and 23 mm), and the Co2+ complex having the largest inhibition zone (10, 18, 20, 23, and 26 mm, respectively). The negative test result for the control agar plate is less than 0.01 mm. This indicates that all the microbes grow freely in the absence of the compounds.
Table 8 shows the MBC and MFC results of the test compounds, based on the preliminary in vitro antimicrobial activities of the ligand and the complexes. They were screened for their minimum inhibitory concentrations and in vitro antimicrobial actions against the selected microbes (Table 8). The minimum active concentrations were measured in g/mL, and two standards were used (Augmentin and Terbinafine for Fugal spp.). The results revealed that the complexes were more significantly active for killing the pathogens than the ligand, and the overall activities of all three complexes were found to be very effective, even at very low concentrations of less than 250 g/mL, which is being considered to be the minimum concentrations of the Schiff base and complexes required to kill (99.99%) of the pathogens completely.
3.1 Conclusion
In this study, a novel Schiff base ligand (SL) and metal (II) complexes were synthesized, characterised and their antimicrobial activities were assessed. The results confirmed the proposed molecular formula of the Schiff base and the complexes (C19H18N2O4 and MC38 H36N4O8), the spectroscopic studies revealed that the Schiff base is bidentate ligand while complexes are mononuclear with 2 mol of the ligand bonded to 1 mol of the metal ion with the molecular formula [M-(SL)2. H2O], where SL = Schiff base ligand and M = Co2+ and Ni2+, respectively.
The geometries of the complexes were obtained from electronic spectral and magnetic moments as Fe2+ = 5.73 BM, Co2+ = 4.52 BM, and Ni2+ = 3.46 BM, respectively, which are higher than the spin-only values due to unpaired electrons, suggesting that, all the complexes are octahedral. Molar conductivity values of the complex in 10–3 M DMSO solution confirmed that they are non-electrolytic. The result is in line with the findings of [34] and [35].
The FT-IR spectra of the Schiff base and the complex shows some vibration bands at 1628 v (HCN, str), 382.47 v (O–H, str), and 362.12 cm–1, revealed the binding nature of the Schiff base to the metal centres (Eq. 1 & 2). The complexes UV/visible electronic scan gives supportive evidence as Fe2+ 460–480 nm, Co2+ shows only one band at 500–520 nm, and Ni2+ shows two absorption bands at 369–480 and 550–570 nm, respectively. The crystals morphology and size of the complexes, a PXRD pattern was scanned in the range of 2 values from 10 to 120° at wavelength CuK1 radiation = 1.5418. The results in (Table 5) revealed that the complexes are crystalline with high crystallinity indices of 72, 80, and 84%, respectively [36].
3.1.1 Assessment of the biological activities of the compounds
The antimicrobial activities studies of the compounds were carried out in vitro a suggested by [31] and [36] using some clinical strains of microbes: MRSA, ESBL K. Pneumoniae, S. pneumonia, ESBL E. coli, Shigella spp., and S. typi and two fungi species: A. nigger and C. albicans.), The test compounds demonstrated better activities than the conventional antibiotics (Augmentin and Terbinafine) used for the study.
The results revealed that the SL–Ni2+ complex shows the highest activity, followed by SL–Co2+, SL–Fe2+and the Schiff base ligand respectively.
Data availability
Data will be available on special request.
References
Abu Bakar SN, Bahron H, Kassim K (2010) Synthesis and characterization of a novel Schiff base derived from 2,4,6-trimethyl-m-phenylenediamine with o-vanillin and its metal complexes. In: CSSR 2010 - 2010 International Conference on Science and Social Research, pp 463–466. https://doi.org/10.1109/CSSR.2010.5773821
Adam MSS, Makhlouf MM, Alharbi A, El-Metwaly NM (2022) Novel isatin-based complexes of Mn (II) and Cu (II) ions: characterization, homogeneous catalysts for sulphides oxidation, bioactivity screening and theoretical implementations via DFT and pharmacokinetic studies. J Mol Liq 351:118620
Adam MSS, El-Hady OM, Makhlouf MM, Bayazeed A, El-Metwaly NM, Mohamad ADM (2022) Effect of oxy-vanadium (IV) and oxy-zirconium (IV) ions in O, N-bidentate arylhydrazone complexes on their catalytic and biological potentials that supported via computerized usages. J Taiwan Inst Chem Eng 132:104168
Agbese S, Shallangwa G, Idris S (2018) Synthesis, Characterization and antimicrobial evaluation of Co(II), Ni(II) and Cu(II) Schiff base complexes of (Z)-4-(1-pyridin-4- ylimino)propyl)phenol. Trop J Nat Prod Res 2(9):429–432. https://doi.org/10.26538/tjnpr/v2i9.4
Akhter S, Zaman HU, Mir S, Dar AM, Shrivastava S (2017) Synthesis of Schiff base metal complexes: a concise review. Eur Chem Bull 6(10):475. https://doi.org/10.17628/ecb.2017.6.475-481
Alfi AA, Alharbi A, Qurban J, Abualnaja MM, Abumelha HM, Saad FA, El-Metwaly NM (2022) Molecular modelling and docking studies of new antioxidant pyrazole-thiazole hybrids. J Mol Struct 1267:133582
Al-jeboori FHA, Al-shimiesawi TAM (2021) Synthesis of Cr (III)-aspartate and Cu(II)-aspartate complexes as antidiabetic compound. Indones J Pharm. https://doi.org/10.22146/ijp.2484
Gupta AK, Orthaber A (2018) Alkynyl coinage metal clusters and complexes –syntheses, structures, and strategies. Chem A Eur J 24(30):7536–7559. https://doi.org/10.1002/chem.201704667
Asgharpour Z, Farzaneh F, Ghiasi M, Azarkish M (2017) Synthesis, characterization, density functional theory studies and antibacterial activity of a new Schiff base dioxomolybdenum(VI) complex with tryptophan as epoxidation catalyst. Appl Organomet Chem 31(11):1–10. https://doi.org/10.1002/aoc.3782
Barbosa VT, De Menezes JB, Carinhanha J, Santos C, Derk ML (2019) Characterization and stability of the antimony-quercetin complex. Tabriz Univ Med Sci 7(3):113–117. https://doi.org/10.15171/jcvtr.2015.24
Boduszek-Urowska B (2011) Synthesis, spectroscopy and magnetic properties of transition-metal complexes with aminophosphonate derivatives of pyridine. Mater Sci-Pol 29(2):105–111. https://doi.org/10.2478/s13536-011-0026-4
Clinical and Laboratory Stanadards Institute (2019) Performance Standard antimicrobial susceptibility testing 29th, edi Supplement M100 Wayne CLSI (2019). 54(58):104–162
Dhanaraj CJ, Johnson J (2014) Spectrochimica acta part A: Molecular and Biomolecular Spectroscopy Synthesis, characterization, electrochemical and biological studies on some metal (II) Schiff base complexes containing quinoxaline moiety. Spectrochim Acta Part A: Mol Biomol Spectrosc 118:624–631. https://doi.org/10.1016/j.saa.2013.09.007
Fugu MB, Ndahi NP, Paul BB, Mustapha AN (2013) Synthesis, characterization and antimicrobial studies of some vanillin schiff base metal (II) complexes. J Chem Pharm Res 5(4):22–28
Khaidir HBSS, Tajuddin AM, Bohari KR, Yamin M (2019) Synthesis, characterization and anticancer activity of mono- and dinuclear Ni(II) and Co(II) complexes of a Schiff base derived from o-vanillin. Polyhedron, Elsevier J 16:84–92
Haddad HH (2016) A new Schiff base derivatives designed to bind metal ion (Cu, Co): thermodynamics and biological activity studies. Am J Anal Chem 07(05):446–451. https://doi.org/10.4236/ajac.2016.75041
Hossain M, Zakaria C, Zahan MK-E (2018) Metal complexes as potential antimicrobial agent: a review. Am J Heterocycl Chem 4(1):1–21. https://doi.org/10.11648/j.ajhc.20180401.11
Kaczmarek MT, Jastrzab R, Hołderna-Kedzia E, Radecka-Paryzek W (2009) Self-Assembled synthesis, characterization and antimicrobial activity of zinc(II) Salicylaldimine complexes. Inorg Chim Acta 362(9):3127–3133. https://doi.org/10.1016/j.ica.2009.02.012
Maalik A, Khan FA, Mumtaz A, Mehmood A, Azhar S, Atif M, Karim S, Altaf Y, Tariq I (2014) Within a pharmacological applications of quercetin and its derivatives: a short review. Trop J Pharm Res 13(9):1561–1566. https://doi.org/10.4314/tjpr.v13i9.26
Malik A, Goyat G, Vikas K, Verma KK, Garg S (2018) Coordination of tellurium (IV) with Schiff base derived from o-vanillin and 3-aminopyridine. Int J Chem Sci 16(2):1–10
Gabera M, El-Ghamry HA, Fathallaa SK, Mansou MA (2018) Synthesis, spectroscopic, thermal and molecular modelling studies of Zn2+, Cd2+ and UO2 2+ complexes of Schiff bases containing triazole moiety. Antimicrob, Anticancer, Antioxid DNA Binding Stud. https://doi.org/10.1016/j.msec.2017.11.004
Mustafa SK, Alsharif MA (2018) Copper (Cu ) an essential redox-active transition metal in living system. Am J Anal Chem Rev Artic. https://doi.org/10.4236/ajac.2018.91002
Mohamed GG, Omar MM (2006) Metal complexes of schiff bases : preparation, characterization and biological activity. Turk J Chem 30:361–382
Yarkandi NH, El-Ghamry HA, Gaber M (2017) Synthesis, spectroscopic and DNA binding ability of CoII, NiII, CuII and ZnII complexes of Schiff base ligand (E)-1-(((1Hbenzo[d]imidazol-2-yl)methylimino) methyl) naphthalen-2-ol Xray crystal structure determination of cobalt (II) complex. Mater Sci Eng. https://doi.org/10.1016/j.msec.2017.02.171
Nagesh GY, Mruthyunjayaswamy BHM (2015) Synthesis, characterization and Biological relevance of some metal (II) complexes with oxygen, nitrogen and oxygen (ONO) donor Schiff base ligand derived from thiazole and 2-hydroxy-1-naphthaldehyde. J Mol Struct 1085:198–206. https://doi.org/10.1016/j.molstruc.2014.12.058
Nathally Claudiane de Souza Santos, Regiane Bertin de Lima Scodro, Eloı´sa Gibin Sampiron, Andressa Lorena Ieque,2 Hayalla Correˆa de Carvalho, Thais da Silva Santos, Luciana Dias Ghiraldi Lopes, Paula Aline Zanetti Campanerut-Sa´, Vera Lucia Dias Siqueira, Katiany Rizzieri Caleffi-Ferracioli, Jorge Juarez Vieira Teixeira and Rosilene Fressatti Cardoso (2020) Minimum bactericidal concentration techniques in Mycobacterium tuberculosis: a systematic review
Neacşu VA, Maxim C, Mădălan AM, González-arellano MC, Soriano S, Andruh M (2018) New complexes of Ni(II) and Co(III) with a Schiff-base ligand derived from o –vanillin. Crystal structure, magnetic and catalytic properties of a dissymmetric binuclear nickel(II) complex. Polyhedron. https://doi.org/10.1016/j.poly.2018.05.007
Amer S, El-Wakiel N, El-Ghamry H (2013) Synthesis, spectral, antitumor and antimicrobial studies on Cu(II) complexes of purine and triazole Schiff base derivatives. J Mol Struct. https://doi.org/10.1016/j.molstruc.2013.06.059
Sani S, Hussain SY (2017) A convenient method to synthesis and characterization of Ni(II) and Zn(II) Schiff base complexes. Int J Innov Res Dev 6(12):8–13. https://doi.org/10.24940/ijird/2017/v6/i12/DEC17084
Saranraj P, Devi D (2018) Effect of potassium sorbate on the inhibition of growth of fungi isolated from spoiled bakery products. Life Sci Arch (LSA) Rev. https://doi.org/10.22192/lsa.2017.3.2.6
Shaimaa K, Fathalla A, El-Ghamry HA, Gaber M (2021) Ru(III) complexes of Triazole based Schiff base and Azo dye ligands: an insight into the molecular structure and catalytic role in oxidative dimerization of 2-aminophenol. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2021.108616
Sheyin F, Ndukwe GI, Iyun ORA, Anyam JV, Habila JD (2018) Phytochemical and anti-microbial screening of crude extracts of natal fig (FicusNatalensisKraus). J Appl Sci Environ Manag 22(9):1457. https://doi.org/10.4314/jasem.v22i9.16
Suresh MS, Prakash V (2010) Preparation and characterization of Cr(III), Mn(II), Co(III), Ni(II), Cu(II), Zn(II) and Cd(II) chelates of schiffs base derived from vanillin and 4-Amino antipyrine. Int J Phys Sci 5(14):2203–2211
Yan Y, Zhang GX, Gran B, Fallarino F et al (2010) Upregulates regulatory Tcells via tryptophan catabolized and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol 185(10):5953–5961
Zhang Y, Wang X, Ding L (2010) Interaction between tryptophan-vanillin Schiff base and herring sperm DNA. J Serbian Chem Soc 75(9):1191–1201. https://doi.org/10.2298/JSC100128107Z
Zubair M, Sirajuddin M, Ullah K, Haider A, Perveen F, Hussain I, Ali S, Nawaz M (2020) Synthesis, structural peculiarities, theoretical study and biological evaluation of newly designed O-Vanillin based azomethines. J Mol Struct 1205:127574. https://doi.org/10.1016/j.molstruc.2019.127574
Acknowledgements
The authors gratefully acknowledged the technical effort of Dr. A. Abdulelil, Dr. I. U. Nkole and all Laboratory technologists in the department of chemistry Ahmadu Bello University, Zaria.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed on the Syntheses of the ligand and metal complexes. The main laboratory work was carried out by Salisu Abubakar. The first draft of the manuscript was written by Salisu Abubakar and all the authors commented on the first version of the manuscript. Prof. G. A. Shallangwa majorly supervised the entire research work and also makes some corrections on the manuscript. Dr. I. Abdulkadir, Analyzed and interpreted the spectroscopic data as well as antimicrobial screening results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abubakar, S., Shallangwa, G.A. & Ibrahim, A. Syntheses and determination of activities of some metal(II) complexes with derivatives of a novel vanillin–tryptophan Schiff base ligand. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00129-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00129-x