Abstract
The thermodynamics factors of acetaminophen and its metabolites were considered using density functional theory (DFT) at 298.15 K temperature and 1 atm. pressure. The resultant Thermodynamics factors were at that time analyzed and compared to determine the influence of temperature and pressure on the stability of the metabolite and its potential behavior in changed environments. The results display that the internal energy, enthalpy, Gibbs free energy, entropy, heat capacity at constant volume (Cv), and Cp at constant pressure (Cp) all affected by the temperature increases. The internal energy (U) of the most stable molecule increases with the increase in temperature, while the heat capacity (H) decreases with the decrease in pressure. The heat capacity and heat capacity of sulfate (APS) are stable at changed temperatures and pressures. These results will make available valued information on the Thermodynamics behavior of Acetaminophen (AP), Acetaminophen cysteine (APCys), Acetaminophen glucuronide (APGlc), and Acetaminophen sulfate (APS) metabolites which can be used to recognize their behavior in the body and how they are metabolized. Furthermore, the results of this study will be responsible for a better understanding of the thermal stability of these molecules under different conditions and guide the development of new drugs and therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acetaminophen, also known as paracetamol, is an extensively used over-the-counter pain reliever and fever reducer. It works by inhibiting the creation of prostaglandins, which are compounds that contribute to inflammation and pain. Acetaminophen is metabolized in the liver by three central pathways: glucuronidation, sulfation, and cysteine conjugation [1,2,3,4,5,6,7]. Acetaminophen cysteine is one of the metabolites created by the cysteine conjugation pathway. This metabolite is formed when acetaminophen is conjugated with the amino acid cysteine, which helps to detoxify the compound and prevent liver damage. Studies have shown that acetaminophen cysteine is a more potent antioxidant than the parent compound, acetaminophen [8,9,10]. Another major metabolite of acetaminophen is acetaminophen glucuronide, which is formed by the glucuronidation pathway. This metabolite is more water-soluble than acetaminophen and is excreted in the urine. Studies have shown that acetaminophen glucuronide is not as active as the parent compound in terms of pain relief and fever reduction [11, 12]. Finally, acetaminophen sulfate is a metabolite produced by the sulfation pathway. This metabolite is also excreted in the urine and has been shown to have similar pain-relieving and fever-reducing properties as the parent compound [12, 13].
In summary, acetaminophen is metabolized in the liver in three main pathways: glucuronidation, sulfation, and cysteine conjugation. These pathways produce acetaminophen glucuronide, acetaminophen sulfate, and acetaminophen cysteine, respectively. Every metabolite has different properties, with acetaminophen cysteine being more effective as an antioxidant, and acetaminophen glucuronide and acetaminophen sulfate being less active in terms of pain relief and fever decrease [14,15,16].
Thermodynamics factors are important tools in the investigation of chemical reactions and processes. They make available a quantitative measure of the energy changes that occur during these processes, allowing for the prediction and understanding of the Thermodynamics behavior of substances [17,18,19]. Thermodynamics factors, for instance, enthalpy, entropy, heat capacity, and free energy, can provide significant insight into the reactivity and stability of acetaminophen and its metabolites [20]. The enthalpy, or heat content, of a substance, is a measure of the energy necessary to break and form bonds during a chemical reaction [21, 22]. The entropy, or disorder, of a material, is a measure of the number of possible energy states existing in the system [23, 24]. Free energy, or Gibbs energy, and internal energy are a measure of the Thermodynamics driving force for a chemical reaction [25, 26].
In recent years, DFT has come to be a powerful tool for predicting the Thermodynamics factors of chemical systems. DFT is a quantum mechanical theory that refers to the electronic structure of a system by solving the Schrödinger equation as a function of the electron density. DFT has been far and wide used to study the Thermodynamics properties of various chemical systems, containing acetaminophen metabolites. DFT has been widely useful in the study of Thermodynamics factors, including enthalpy, entropy, and free energy [27,28,29].
There have been several studies on the use of the computational model to predict the Thermodynamics factors of acetaminophen and its metabolites. Generally, the use of DFT as a computational model has been established to be a valuable tool for predicting the Thermodynamics factors of acetaminophen and its metabolites and has contributed to a deeper understanding of their behavior in changed environments [16, 30,31,32,33].
This study aims to understand how the Thermodynamics properties of these metabolites change under different conditions. In this study, we will use DFT to calculate the Thermodynamics factors of acetaminophen, acetaminophen cysteine, acetaminophen glucuronide, and acetaminophen sulfate at 298.15 K and different temperatures and pressures. The results of this study will make available a better understanding of the stability of these acetaminophen metabolites under different conditions. This research will also contribute to the development of novel methods for predicting the Thermodynamics properties of chemical systems using DFT. The results of this study can be used to predict the stability of these metabolites under different conditions and to guide the development of new drugs and therapies.
2 Methodology and calculations
Thermodynamics is a field of physics that deals with the relationship between energy, heat, and system operation. It plays an essential role in understanding chemical reactions and material properties. In this regard, research of Thermodynamics factors such as internal energy, entropy, Gibbs-free energy, entropy, and heat capacity is essential to understand system stability under different conditions. This work deals with the Thermodynamics factors of four acetaminophen metabolites, namely acetaminophen, acetaminophen cysteine, acetaminophen glucuronide, and acetaminophen sulfate, at 298.15 K temperature and 1 atm. Pressure, and with different temperatures and pressures, using density functional theory (DFT). Internal energy (U) is the measurement of the total energy in the system and can be determined by measuring the heat absorbed or released during chemical reactions. Enthalpy (H) is the internal energy of the system and the product of pressure and volume. Gibbs free energy (G) is the energy of the system, minus the product of entropy and temperature. Entropy (S) is a measurement of system disorder and can be determined by measuring the amount of heat absorbed or released by chemical reactions. Finally, the heat capacity (C) is the amount of heat required to increase a temperature of a substance by a degree.
DFT has the advantage of predicting to predict a wide range of properties, including Thermodynamics properties, at different temperatures and pressures. The Gaussian09 [34] and GaussView05 packages [35] are widely used to perform DFT calculations and to visualize the results.
The following steps are used to investigate the stability and reactivity of acetaminophen and its metabolites using DFT:
-
1.
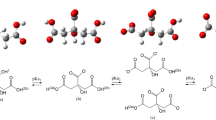
A molecule structure of acetaminophen and its metabolism is obtained., as Fig. 1. However, in Fig. 1, the color of balls are every so often used to represent dissimilar atoms where red = oxygen, gray = carbon, yellow = Sulphur, blue = nitrogen and white = hydrogen. These can be obtained from databases such as the PubChem database [36] or computational methods such as molecular modeling.
-
2.
Set the DFT calculation with the Gaussian09 package. This includes specifying the theoretical level (B3LYP), the basis set (6-31G*), and any other necessary factors.
-
3.
DFT calculations are carried out to get hold of the electronic structure and properties of the target molecule.
-
4.
In different temperatures and pressures, use the Shermo software [37] to calculate the Thermodynamics factors (e.g. internal energy, Gibbs free energy, enthalpy, entropy, and heat capacity of constant volume and pressure).
-
5.
Explore the results of the DFT calculation and compare the stability and reactivity of acetaminophen and its metabolites.
Then, the Thermodynamics factors were analyzed and compared to determine the temperature and pressure influence on the stability of acetaminophen and its metabolites.
3 Results and discussion
The Thermodynamics factors, including internal energy (U), enthalpy (H), Gibbs free energy (G), entropy (S), heat capacity at constant volume (Cv), and heat capacity at constant pressure (Cp) were considered for Acetaminophen (AP), Acetaminophen cysteine (APCys), Acetaminophen glucuronide (APGlc), and Acetaminophen sulfate (APS) metabolites using Density Functional Theory (DFT) at a temperature of 298.15 K and Pressure of 1 atm. The calculations were done using Gaussian09, gaussview05, and Shermo software. The Thermodynamics factors were analyzed to determine their influence on the stability of the four metabolites. The unit of S, Cv, and Cp is cal/mol/K, and the unit of U, H, and G is kcal/mol.
Figures 2, 3 and 4 show the entropy and heat capacity of the four metabolites of constant volume and pressure. Acetaminophen's entropy is the lowest and indicates that it is the most stable. Acetaminophen glucuronide has the highest entropy. The constant temperature capacity of Acetaminophen and its pressure is the lowest, indicating that it is the most stable, while Acetaminophen glucuronide has the highest temperature capacity. Specifically, we examine acetaminophen and acetaminophen glucuronide as we examine in detail the analytical and specific stability of the four tested compounds. The entropy of the four metabolites is lowest for acetaminophen. This means that it has lower variability or randomness compared to other metabolites. An ordered and highly complex molecular structure is indicated by a low entropy value. It also has the lowest boiling point at constant pressure and temperature. The amount of heat energy a substance can absorb before its temperature changes is known as its thermal capacity. Acetaminophen is stable when its heat capacity value is low because it requires less heat energy to raise its temperature. In contrast, acetaminophen glucuronide has the highest entropy of all metabolites. This indicates that its perturbed or random molecular structure is high compared to other metabolites. Higher entropy values are indicated by lower stiffness and higher molecular disorder.
It has the highest temperature of metabolites at constant temperature. This means that it is relatively inert because it takes more heat energy than other metabolic processes to raise its temperature.
For molecular structures, stability is the tendency of a material to maintain its structure and withstand changes when exposed to various external stimuli, including pressure and temperature.
Figure 5 shows the internal energy of Acetaminophen, Acetaminophen cysteine, Acetaminophen glucuronide, and Acetaminophen sulfate. The internal energy of Acetaminophen is the lowest among the four metabolites, indicating that it is the most stable, while Acetaminophen sulfate has the highest internal energy. The internal energy of a molecule is a measure of its total energy, including its potential energy (due to atomic arrangement) and kinetic energy (due to the motions of atoms and molecules) Four kinetics here is a brief review of the strengths of the:
-
Acetaminophen: Of the four metabolites, it has the lowest in vitro potency, indicating that it is the most stable. The lower internal energy means better ordering of the atoms and lower molecular motion, resulting in a relatively stable molecular structure.
-
Acetaminophen Cysteine: The effect of acetaminophen cysteine is not specified in the data. Without specific data, it is difficult to make conclusive statements about its stability based on internal strength alone.
-
Acetaminophen glucuronide: Acetaminophen glucuronide has a higher internal energy than acetaminophen, indicating a much less stable molecular structure Higher internal energy indicates poorer atomic structure and increased molecular mobility.
-
Acetaminophen sulfate: Acetaminophen sulfate exhibits the highest intestinal efficacy among the four metabolites. This means that its molecular structure is very unstable. Increased internal energy indicates less favorable arrangement of atoms, increased molecular mass, which can contribute to decreased instabilities.
It is important to note that internal energy alone does not provide a complete picture of the stability of a molecule. Other factors such as entropy, heat capacity, and the presence of chemical bonds or interactions play an important role in the overall stability of a compound.
Enthalpy is a thermodynamic property that represents the whole warmness content of a gadget at consistent strain. It includes the dynamics of the machine and the electricity associated with the paintings done on or appearing the gadget.
Figure 6 states that acetaminophen enthalpy is the bottom of the 4 metabolites, indicating that acetaminophen is the maximum strong A lower enthalpy value suggests that the gadget has a lower temperature and is extra thermodynamically favorable.... This shows that the molecular structure of acetaminophen is solid and easily subjected to big power adjustments.
On the other hand, some of the four metabolites, acetaminophen sulfate has the best enthalpy. This shows that it has a higher thermal balance and is less thermodynamically strong as compared to other metabolites. A better enthalpy value suggests that greater strength is related to the system, probable due to instability of molecular interactions or expanded molecular interactions This suggests that acetaminophen sulfate is incredibly volatile and liable to strength adjustments.
In Fig. 7, you can see the free energy of the four metabolites. Acetaminophen is the most stable because it has low Gibbs free energy. Acetaminophen sulfate has the most Gibbs free energy. Gibbs free energy is an important part of thermodynamics. It helps us figure out how much work can be done to keep the temperature and pressure steady. It's basically a way to show how chemical molecules are stable and work. In Fig. 7, we can see that acetaminophen has less Gibbs free energy than the other four metabolites. This means that acetaminophen is the most stable and easiest to change chemically into energy. The strong Gibbs free energy supports this expected stability. But, acetaminophen sulfate has the most Gibbs free energy. This means that it is more solid and useful against chemical changes that need energy change. The stability with little energy is figured out by the big Gibbs free energy.
The internal energy (U) and enthalpy (H) values indicate that the AP metabolite is the most stable among the four, with the lowest U and H values. The Gibbs free energy (G) values also indicate that AP is the most stable metabolite, with the lowest G value. The entropy (S) values indicate that APGlc is the least stable among the four, with the highest S values. The heat capacity at constant volume (Cv) and pressure (Cp) values indicate that AP is the most stable metabolite, with the lowest Cv and Cp values.
Also, in this study, we studied the thermodynamic factors, such as enthalpy and entropy, of the most stable acetaminophen metabolite at different temperatures and pressures. The analysis provided insight into the thermal stability of the metabolite and its potential behavior in different environments. That is why we studied the acetaminophen metabolite as a case study because it is the most stable of the metabolites under study.
From Fig. 8, as the temperature increases, the heat capacity at constant pressure also increases, indicating that more energy is necessary to maintain a constant pressure as the temperature of the acetaminophen metabolite increases.
Figure 9 shows the influence of temperature on the entropy of acetaminophen. As the temperature increases, the entropy also increases, indicating that it is a process that is favored by an increase in temperature. As the temperature increases, the entropy also increases, indicating that the acetaminophen metabolite becomes more disordered or has lower stability at higher temperatures.
From Fig. 10, as the temperature increases, the heat capacity at constant volume also increases, indicating that more energy is necessary to raise the temperature of the acetaminophen metabolite at higher temperatures.
Figure 11 illustrates the effect of temperature on the internal energy of the most stable metabolite, acetaminophen. As the temperature increases, the internal energy also increases, indicating that it is an endothermic process. The internal energy of acetaminophen increased with increasing temperatures, indicating that more energy was required to maintain the stability of the molecule at higher temperatures.
Figure 12 shows the effect of temperature on the enthalpy of acetaminophen. As the temperature increases, the enthalpy also increases, indicating that it is an endothermic process. The enthalpy of acetaminophen also increased with increasing temperature, indicating that the heat absorbed by the molecule for the duration of a reaction also increased at higher temperatures.
Figure 13 shows the effect of temperature on the Gibbs free energy of acetaminophen cysteine. As the temperature increases, the Gibbs free energy decreases, indicating that it is a process that is favored by an increase in temperature. The Gibbs free energy of acetaminophen decreased with increasing temperature, indicating that the stability of the molecule increased at higher temperatures.
The Thermodynamics stability of the most stable acetaminophen metabolite was assessed at different temperatures. The internal energy, enthalpy, Gibbs free energy, entropy, heat capacity at constant volume, and heat capacity at constant pressure were all calculated. The results illustrate that the internal energy and enthalpy of the metabolite remain relatively constant across the range of temperatures studied, with a slight decrease as temperature increases. The Gibbs free energy, however, decreases as temperature increases, indicating an increase in stability. The entropy also decreases as temperature increases, indicating a decrease in disorder. The heat capacity at constant volume and constant pressure both increase as temperature increases, indicating an increase in thermal energy. Generally, the results indicate that the acetaminophen metabolite is more stable at higher temperatures. This result could have implications for the metabolism of acetaminophen in the body, as well as for the storage and handling of the drug. Further research is needed to fully understand the implications of these results.
The Thermodynamics stability of the most stable acetaminophen metabolite was assessed at different temperatures. The results show that the internal energy, enthalpy, entropy, heat capacity at constant volume, and heat capacity at constant pressure all increase, and Gibbs free energy decrease, as the temperature increases. This suggests that the acetaminophen metabolite is more stable at lower temperatures and less stable at higher temperatures.
The Thermodynamics stability of acetaminophen was examined at different pressures using internal energy, enthalpy, Gibbs free energy, entropy, heat capacity at constant volume, and heat capacity at constant pressure.
Figure 14 displays that heat capacity at constant pressure (Cp) is a measure of the amount of heat required to raise the temperature of a system by one degree at a constant pressure. The heat capacity at the constant pressure of acetaminophen remained relatively constant with increasing pressure, indicating that the heat required to change the temperature of the molecule while maintaining constant pressure did not change considerably with pressure.
From Fig. 15 it can be seen that the entropy of acetaminophen was brought into being to be dependent on pressure, with an increase in pressure resulting in a decrease in entropy. This indicates that the system becomes more stable at higher pressures, as the increased pressure results in a decrease in the potential for spontaneous reactions to occur. This indicates that the system is becoming less Thermodynamically stable as the pressure increases.
Heat capacity at constant volume (Cv) is a measure of the amount of heat necessary to raise the temperature of a system by one degree at a constant volume as shown in Fig. 16. The heat capacity at a constant volume of acetaminophen also remained relatively constant with increasing pressure, indicating that the heat required to change the temperature of the molecule did not change significantly with pressure.
Figure 17 shows the influence of pressure on the internal energy of acetaminophen. Internal energy (U) is a measure of the total energy of a system, including both kinetic and potential energy. The internal energy of acetaminophen remained relatively constant at all pressures studied, indicating that changes in pressure do not significantly affect the internal energy of the compound.
Enthalpy (H) is a measure of the total heat energy of a system, containing both internal energy and the energy required to change the pressure and volume of the system. From Fig. 18, the enthalpy of acetaminophen also remained relatively constant at all pressures studied, indicating that changes in pressure do not significantly affect the enthalpy of the compound.
As pressure is increased, the Gibbs free energy of a substance will vary. From Fig. 19, the Gibbs free energy increases with increasing pressure, and the Acetaminophen at a constant temperature will become less stable at higher pressures.
Overall, the results put forward that acetaminophen is Thermodynamically stable at different pressures, with the stability increasing at higher pressures. This is likely due to the increased interactions between the molecule and the surrounding environment at higher pressures, leading to a decrease in entropy and an increase in Gibbs free energy. The relatively constant heat capacities at both constant volume and constant pressure, enthalpy, and internal energy suggest that the heat essential to change the temperature of the molecule does not change significantly with pressure.
4 Conclusions
The stability of acetaminophen, acetaminophen cysteine, acetaminophen glucuronide, and acetaminophen sulfate metabolites was studied using density functional theory (DFT) at 298.15 K and also with different temperatures and pressures effective. The Thermodynamics factors, including internal energy, enthalpy, Gibbs free energy, entropy, and heat capacity at constant volume and pressure, were analyzed using Gaussian09, GaussView05, and Shermo. We evaluated the internal energy, enthalpy, Gibbs free energy, entropy, heat capacity at constant volume, and heat capacity at a constant pressure of each metabolite to conclude their stability. These results will be responsible for valuable information on the Thermodynamics behavior of acetaminophen and its metabolites, which can be used to understand their behavior in the body and how they are metabolized. The Thermodynamics factors of internal energy, enthalpy, Gibbs free energy, entropy, and heat capacity of constant volume and pressure have a significant influence on the stability of Acetaminophen, Acetaminophen cysteine, Acetaminophen glucuronide, and Acetaminophen sulfate metabolites. The results of this study support the conclusion that Acetaminophen is the most stable among the four metabolites. The Thermodynamics stability of the most stable acetaminophen metabolite decreases as the temperature increases. The effect of pressure on the Thermodynamics factors of the most stable metabolite was also studied. It was detected that as the pressure increased, the Gibbs free energy, while entropy decreased, and the internal energy, enthalpy, heat capacity at constant volume, and heat capacity at constant pressure also are remaining with no change. This information is valuable for understanding the potential degradation of acetaminophen in different storage conditions, such as pressure and temperatures.
Data availability
Not applicable. Our manuscript has no associated data.
References
Vanova J, Malinak D, Andrys R, Kubat M, Mikysek T, Rousarova E, Musilek K, Rousar T, Cesla P (2022) Optimization of gradient reversed phase high performance liquid chromatography analysis of acetaminophen oxidation metabolites using linear and non-linear retention model. J Chromatogr A 1669:462956
Anderson BJ (2008) Paracetamol (acetaminophen): mechanisms of action. Pediatr Anesth 18:915–921
Mazaleuskaya LL, Sangkuhl K, Thorn CF, FitzGerald GA, Altman RB, Klein TE (2015) PharmGKB summary. Pharmacogenet Genomics 25:416–426
Hinson JA, Roberts DW, James LP, Mazaleuskaya LL, Sangkuhl K, Thorn CF, FitzGerald GA, Altman RB, Klein TE, Anderson BJ, Vanova J, Malinak D, Andrys R, Kubat M, Mikysek T, Rousarova E, Musilek K, Rousar T, Cesla P (2015) PharmGKB summary. Pharmacogenet Genomics 25:915–921
Hinson JA, Roberts DW, James LP (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196:369–405
Vale A (2003) Paracetamol (acetaminophen). Medicine (Baltimore) 31:67–68
Tan H, Stathakis P, Varghese B, Buckley NA, Chiew AL (2022) Delayed acetaminophen absorption resulting in acute liver failure. Case Rep Crit Care 2022:1–6
Karaku F, Atmaca K, Aladag B, Orhan H (2021) Cysteine conjugate of paracetamol serves as substrate for kidney cysteine S-conjugates ß-lyase: implications for drug-induced acute kidney injury. Toxicol Lett 350:S164
Stern ST, Bruno MK, Horton RA, Hill DW, Roberts JC, Cohen SD (2005) Contribution of acetaminophen-cysteine to acetaminophen nephrotoxicity II Possible involvement of the γ-glutamyl cycle. Toxicol Appl Pharmacol 202:160–171
McGill MR, Jaeschke H (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30:2174–2187
Lecoeur M, Rabenirina G, Schifano N, Odou P, Ethgen S, Lebuffe G, Foulon C (2019) Determination of acetaminophen and its main metabolites in urine by capillary electrophoresis hyphenated to mass spectrometry. Talanta 205:120108
Ali BH, Cheng Z, El-Hadrami G, Bashir AK, Mckellar QA (1996) Comparative pharmacokinetics of paracetamol (acetaminophen) and its sulphate and glucuronide metabolites in desert camels and goats. J Vet Pharmacol Ther 19:238–244
Zhao Y, Harmatz JS, Epstein CR, Nakagawa Y, Kurosaki C, Nakamura T, Kadota T, Giesing D, Court MH, Greenblatt DJ (2015) Favipiravir inhibits acetaminophen sulfate formation but minimally affects systemic pharmacokinetics of acetaminophen. Br J Clin Pharmacol 80:1076–1085
Ben-Shachar R, Chen Y, Luo S, Hartman C, Reed M, Nijhout HF (2012) The biochemistry of acetaminophen hepatotoxicity and rescue: a mathematical model. Theor Biol Med Model 9:55
Cook SF, Stockmann C, Samiee-Zafarghandy S, King AD, Deutsch N, Williams EF, Wilkins DG, Sherwin CMT, van den Anker JN (2016) Neonatal maturation of paracetamol (acetaminophen) glucuronidation, sulfation, and oxidation based on a parent-metabolite population pharmacokinetic model. Clin Pharmacokinet 55:1395–1411
Perlovich GL, Volkova TV, Bauer-Brandl A (2006) Towards an understanding of the molecular mechanism of solvation of drug molecules: a thermodynamic approach by crystal lattice energy, sublimation, and solubility exemplified by paracetamol, acetanilide, and phenacetin. J Pharm Sci 95:2158–2169
Ghiorso MS, Gualda GAR (2015) Chemical thermodynamics and the study of magmas. In: The encyclopedia of volcanoes. Academic Press, pp 143–61
Sandler SI (2003) Thermodynamics. In: Encyclopedia of physical science and technology, pp 639–657
Martha SK, Pappu S, Sarada BV, Rao TN (2022) Concept of thermodynamic studies in electrochemical storage and conversion systems. In: Encyclopedia of energy storage. Elsevier, pp 264–274
Joshi RP, McNaughton A, Thomas DG, Henry CS, Canon SR, McCue LA, Kumar N (2021) Quantum mechanical methods predict accurate thermodynamics of biochemical reactions. ACS Omega 6:9948–9959
Dorofeeva OV, Andreychev VV (2022) Benchmark thermochemistry of polycyclic aromatic hydrocarbons. J Phys Chem A 126:8315–8325
Ischia G, Cazzanelli M, Fiori L, Orlandi M, Miotello A (2022) Exothermicity of hydrothermal carbonization: determination of heat profile and enthalpy of reaction via high-pressure differential scanning calorimetry. Fuel 310:122312
Kareem RA, Gbadeyan JA (2020) Entropy generation and thermal criticality of generalized Couette hydromagnetic flow of two-step exothermic chemical reaction in a channel. Int J Thermofluids 5–6:100037
Patki P, Acharya V, Lieuwen T (2022) Entropy generation mechanisms from exothermic chemical reactions in laminar, premixed flames. Proc Combust Inst 39:1607–1614
Bhunia K, Chandra M, Kumar-Sharma S, Pradhan D, Kim SJ (2023) A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coord Chem Rev 478:214956
Arabi AA, Matta CF (2018) Adenine–thymine tautomerization under the influence of strong homogeneous electric fields. Phys Chem Chem Phys 20:12406–12412
Fu C, Liu C, Li T, Zhang X, Wang F, Yang J, Jiang Y, Cui P, Li H (2019) DFT calculations: a powerful tool for better understanding of electrocatalytic oxygen reduction reactions on Pt-based metallic catalysts. Comput Mater Sci 170:109202
Hegazy MA, Hegazy MM, Awad MK, Shawky M (2021) Chemical, electrochemical, theoretical (DFT & MEP), thermodynamics and surface morphology studies of carbon steel during gas and oil production using three novel di-cationic amphiphilies as corrosion inhibitors in acidic medium. J Mol Liq 337:116541
Sathish M, Rajasekaran L, Shanthi D, Kanagathara N, Sarala S, Muthu S (2021) Spectroscopic (FT-IR, FT-Raman, UV-Vis) molecular structure, electronic, molecular docking, and thermodynamic investigations of indole-3-carboxylic acid by DFT method. J Mol Struct 1234:130182
Horikoshi T, Kitaoka C, Fujii Y, Asano T, Xu J, Fujino T (2021) Detection of acetaminophen and its glucuronide in fingerprint by SALDI mass spectrometry using zeolite and study of time-dependent changes in detected ion amount. Analytica 2:66–75
Belal Hossen M (2019) A computational approach to investigate the biochemical properties of paracetamol and its metabolites. Biomed J Sci Tech Res 2:2. https://doi.org/10.26717/BJSTR.2019.22.003789
Uzzaman M, Shawon J, Siddique ZA (2019) Molecular docking, dynamics simulation and ADMET prediction of acetaminophen and its modified derivatives based on quantum calculations. SN Appl Sci 1:1437
Yang F, Beard DA (2006) Thermodynamically based profiling of drug metabolism and drug–drug metabolic interactions: a case study of acetaminophen and ethanol toxic interaction. Biophys Chem 120:121–134
Frisch A (2009) gaussian 09W Reference, Wallingford, USA, 25p. 470
Hratchian HP, Keith TA, Millam J (2009) Gaussian 05 user’s reference
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE (2023) PubChem 2023. Nucleic Acids Res 51:D1373–D1380
Lu T, Chen Q (2021) Shermo: a general code for calculating molecular thermochemistry properties. Comput Theor Chem 1200:113249
Funding
The author did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mekky, Ab.H. Thermodynamics analysis of acetaminophen and its metabolites using density functional theory. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00128-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00128-y