Abstract
Alcohol abuse is dangerous to one’s health and contributes to disorders such as neurotoxicity and hepatotoxicity worldwide. Twenty-five male albino rats, each weighing 200 ± 10 g, were divided into five groups. For 4 weeks, rats in the control group were given only a regular chow diet and ad libitum. Rats in the alcohol group received an oral gavage of alcohol at a dose of 40 mg/kg body weight each day. Rats in the alcohol and avocado extract group received oral gavage doses of 250 mg/kg b. wt./day of avocado extract and 40 mg/kg b. wt. of alcohol. Rats in the alcohol and mustard seed extract group received oral gavage doses of 250 mg/kg/day of mustard seed extract and 40 mg/kg/day of alcohol, respectively. Rats were given alcohol and a mixture of avocado and mustard seed extract at a dose of 250 mg/kg body weight each day by oral gavage for 4 weeks. Our results showed increased levels of hydrogen peroxide (H2O2) and lipid peroxidation in the liver and brain tissues, decreased glutathione content, glutathione peroxidase, superoxide dismutase, catalase, and glutathione-S-transferase. In rats consumed excessive alcohol, there was an increase in the activity of the tumor marker α-l-fucosidase in sera. Additionally, the liver and brain tissues of the alcoholic group showed decreased cytochrome c oxidase activity. Furthermore, changes in the expression levels of the genes for brain α-secretase and liver alcohol dehydrogenase (ADH) were observed. The administration of extracts from avocado and mustard seeds improved the state of oxidative stress and restored antioxidant enzyme activity. The expression levels of brain α-secretase and liver ADH genes were almost fully recovered at the molecular level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The most abused legal substance is alcohol. The third leading cause of death worldwide is alcoholism, which presents a serious public health concern. Drinking excessive amounts of alcohol results in acute alcohol poisoning with acidosis, heart failure, and respiratory depression brought on by dysfunction of the autonomic nervous system and the brain [1]. Alcoholism in many forms, including alcoholic hepatitis, liver hypertrophy, and polyneuropathy, is caused by heavy alcohol use over time [2]. Alcohol intake, alcohol abuse problems, and upper gastrointestinal cancer are all directly linked to liver cirrhosis [3]. Several metabolic processes help the body get rid of alcohol. Alcohol dehydrogenase (ADH), the cytochrome P450 (CYP2E1) system, also known as the microsomal ethanol oxidizing system (MEOS), mostly CYP2E1, and catalase (CAT) are the predominant enzyme systems involved in this process, which is carried out by the liver [4]. ADH mediates 80% of ethanol metabolism, followed by MEOS for the majority of the remaining 40%, and CAT for a very tiny portion. However, when the free fatty acid supply for peroxisomal -oxidation is increased, the percentage contribution of CAT can rise [5]. Chronic alcoholism is known to be affected by free radicals and to have oxidative damage, which is a change in the ratio of antioxidants to oxidants [6]. Acute and chronic ethanol therapy has been shown to enhance the formation of reactive oxygen species (ROS), decrease cellular antioxidant levels, and cause oxidative stress in a variety of organs, most notably the liver and brain tissues. Rats exposed to hepatotoxicity were reported to have higher LPO levels and lower SOD and GSH activities.
Alcohol targets a variety of channel proteins, receptor molecules, and signalling pathways in the brain, according to the evidence [7]. Alzheimer's disease (AD), the most prevalent type of dementia, is characterized by a progressive deterioration in cognitive function, which usually begins with memory loss. Alcohol consumption is a frequent but preventable exposure, and understanding how alcohol affects AD may help show that there is morbidity [8]. Oxidative stress might be a significant factor in the development of AD. Antioxidants included in diet are thought to lower the incidence of AD [9]. Alzheimer's patients' brains are less toxic when antioxidants are present. Alcohol use has been linked to an increased risk of dementia and has been proposed as a probable menace factor for AD or impaired cognitive impairment [10]. The creation of potent medications to treat liver issues can benefit greatly from the discovery of novel chemicals from medicinal plants. Additionally, herbal products have the benefit of being more affordable and popular, better suited to the human body, having less adverse effects, and being simpler to keep [11]. Plant products' antioxidant capabilities are extremely important in defending the liver. Through a multi-goal, multi-track strategy at the cellular and molecular levels as well as general body change at the levels of the organ system, herbal medicines could treat Alzheimer's disease. Research into AD using Chinese medicine has advanced significantly [12, 13]. In recent years, research has focused on different parts of avocado plants. It has been established that the fruit has therapeutic potentials in alleviation of the deleterious impacts of several diseases. Up to 33% of the oil in avocados comes from monounsaturated fatty acids, which are thought to alter the composition of fatty acids in heart and kidney membranes and increase the absorption of lutein and beta-carotene [14]. In white mice, the avocado fruit has anti-fat and antioxidant properties. Vitamins A, C, and K, as well as the carotenoids and flavonoids carotenes, zeaxanthin, and lutein, are antioxidants found in mustard seeds [15]. However, the hepatoprotective activity of avocado fruits [16] and mustard seeds [17] might be because of their antioxidant and free radical scavenging qualities.
2 Materials and methods
Adult male albino rats (Sprague Dawley) weighing 200–210 g obtained from the animal house centre, King Khalid University, Saudi Arabia, were used in the present study. The local ethical committee approved the design of the experiment, and the protocol conforms to the guidelines of the National Institutes of Health (NIH). All measures were taken to minimize the number of rats used and their suffering. Animals were kept in standard polyethylene cages under good ventilation in a room with a 12:12 h light/dark cycle, control temperature (22 ± 1 °C), relative humidity (40–60%) and received a balanced laboratory chow diet and water was added ad-libitum throughout the experimental period.
2.1 Animal grouping
Five groups of five rats each were randomly assigned to experimental animals. The groupings were as follows:
Control group rats were used as a standard, healthy control group. They were fed a regular chow diet and had unlimited access to water for 4 weeks.
Rats in the alcohol group were given alcohol orally for 4 weeks at a dose of 40 mg/kg body weight every day.
Drinking alcohol and avocado extract: For 4 weeks, rats were given 40 mg/kg of body weight of alcohol and 250 mg/kg of watery avocado fruit extract.
Alcohol with mustard seed extract: for 4 weeks, rats were given 40 mg of alcohol per kilogram of body weight and 250 mg of a watery mustard seed extract per kg of body weight.
Alcohol and mixture of mustard and avocado seeds: for 4 weeks, rats were given an oral dose of 40 mg/kg body weight of alcohol and 250 mg/kg body weight of a watery extract of mustard and avocado fruit.
2.2 Tissue sampling and extraction
Rats who had fasted overnight were slaughtered at the conclusion of the treatment session. Blood was taken and centrifuged for 15 min at 3000 rpm in a clean centrifuge tube. Separated, labelled, and stored at 20 °C for biochemical analysis are sera that have not been haemolyzed. The animals were dissected, and samples of their liver tissues were taken and weighed for each animal. For biochemical and molecular examination, liver tissue samples were homogenized in 10% phosphate buffered saline (PBS), pH 7.4. Tissue samples under investigation are frozen in dry ice and kept at 80 °C for upcoming molecular analysis.
2.3 Biochemical study
Thiobarbituric acid reactive compounds (TBARS), which produce lipid peroxidation, were estimated according [18], and hydrogen peroxide (H2O2) concentration is estimated according to the procedure of Fossati [19]. Glutathione peroxidase (GPx) activity is determined by the process of Paglia [20]. Reduced glutathione (GSH) content is described by the scheme of Beutler [21]. Glutathione-S-transferase (GST) endeavour is revealed by the procedure of Habig [22]. Superoxide dismutase (SOD) activity was undertaken along with Nishikimi [23]. The method described by Aebi was used to measure the activity of the catalase enzyme (CAT) [24]. The amount of cytochrome c oxidase present in the liver and brain was calculated using double-antibody sandwich ELISAs. Cytochrome c oxidase (CCO) subunit 1 (MT-C01) monoclonal antibody should be added to the enzyme, which has already been coated.
2.3.1 1-Gene expression
Using the RNeasy mini kit (Qiagen), total RNA was extracted from the liver and brain tissues in accordance with the manufacturer's instructions. Following the manufacturer's instructions, single-stranded cDNA was produced using the cDNA Synthesis Kit (Invitrogen, USA). PCR was used to intensifying up the genes for ADH and α-secretase utilizing a specific PCR technique. ALD, F: GCTAAGAAGTTTTCACTGGA-TGC, R: ACCTCTTTCCAGAG CGAAGC, and beta-secretase, F: GCATGATCATTGG-TGGTATC, R: CCATCTTGAGATCTTG-ACCA, specific primers were used in combination with Hot Star Taq DNA polymerase (Promega, USA) to amplify the ADH and -secretase genes. A BioRad Thermocycler was used to carry out each PCR amplification. The amplicon band intensity of expressed genes in different groups were compared with control group.
2.3.2 2- Histological study
The brain and liver of each rat were extracted as soon as possible and kept in 10% neutral buffered formalin. The fixed liver specimens were processed for dehydration using grades of ethanol (70, 80, 90, 95, and 100%), cleaned in two changes of xylene, and impregnated with two changes of molten paraffin wax using an automatic tissue processor (Sakura, Japan). A microtome (Model RM2245, Leica, Germany) was used to cut serial sections of sections (4–5 m) thick after the specimens had been blocked out and embedded using an embedding station (Sakura, Japan). We used a Leica, Germany auto cleaner (Model 5020) to stain the sections with hematoxylin and eosin. The mounted specimens were examined using an optical microscope (Olympus Microscope BP53 with Digital Camera, Japan) to look for alterations in the hepatic tissues of each rat that was the subject of the study.
2.4 Statistical analysis
All the grouped data were subjected to statistical analysis using the Prism (GraphPad Prism, 6.01) tool. Group differences were evaluated using Duncan’s multiple range tests and one-way analysis of variance (ANOVA). The mean ± standard deviation of five animals per group was utilized for each value, and a P value of less than 0.05 was deemed significant.
3 Results
3.1 Biochemical alterations
In Figs. 1 and 2, LPO and H2O2 increased in the ALC group and decreased in ALC + AVC, ALC + SEED, and ALC + MIX groups as compared with the ALC group.
In Fig. 3 a, b reduced GSH and their relative investigated enzymes decreased in the ALC group but augmented in the treated groups related to the ALC group in different tissues.
Liver (Fig. 4a) and brain (Fig. 4b) SOD and CAT activity decreased in the ALC group when compared with the control one, and avocado and mustard seeds increased their activity in all treated groups when compared with the ALC group.
In Fig. 5 liver and brain cytochrome c oxidase activity decreased in ALC as equated with the control but their activity increased in the other groups that related to the alcohol group. In Fig. 6 the activity of serum α- l-fucosidase increased in ALC and its activity decreased in ALC + AVC, ALC + SEED, and ALC + MIX groups as compared with the ALC group.
3.2 Molecular alterations
The ALDH gene displayed in Fig. 7a revealed that an amplicon measuring around 440 bp was found in every sample. In the alcohol group, the band intensity rose (lane 2). In contrast to the control group (lane 1), the expression of the ADH gene progressively rose in the alcohol + avocado (lane 3), alcohol + master seeds (lane 4), and alcohol + mix (lane 5) groups.
The brain β-secretase gene, in Fig. 7b, has approximately 400 bp observed with all the examined samples. Band intensity increased in the alcohol group (lane 2). While the expression of the β-secretase gene gradually decreased in the alcohol + avocado group (lane 3), alcohol + mustard seeds group (lane 4), and alcohol + mix (lane 5) when compared with the control group (lane 1) or alcohol group (lane 2).
3.3 Histopathological alterations
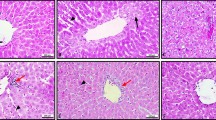
Within the control group, all rats' liver architecture showed intact and well-preserved normal histological components of the portal regions and hepatic lobules (Fig. 8). The liver of the control rat displayed normal morphology and no lesion area. Hepatocyte parallel cords are divided by sinusoidal gaps (Fig. 8A, B). Sections of the liver from the alcohol-treated animal group showed signs of severe alcohol-related steatosis, pyknotic nuclei, and necrosis (arrowheads and arrows, respectively) (Fig. 8C, D). The liver architecture of the avocado + alcohol-treated group, the liver portion of the avocado + alcohol-treated group, and the mustard + alcohol-treated group all improved and did not exhibit any histological changes (Fig. 8E, F, and G, respectively). the mice in the avocado/mustard + alcohol-treated group, the liver section of those animals displayed improved liver architecture (Fig. 8H, I).
Exemplary light micrographs illustrating the histopathological modifications resulting from the application of plant extract therapy on the morphology of liver tissue. A, B Hepatocyte parallel cords (H) and sinusoidal gaps (arrows) divide the normal morphology and lesion region of the control rat liver; significant necrosis (arrowheads), pyknotic nuclei (arrows), and alcohol-related steatosis (star) are present in the alcohol group (C, D). Small- and large-droplet fat are the usual components of steatosis in fatty liver disease. The E group treated with avocado extract + alcohol and the F group treated with mustard + alcohol showed improved liver architecture with normal hepatic structure, but some hepatocytes (arrows) still had a small number of steatoses (G). The group that consumed avocado, mustard, and alcohol H, I demonstrated improved liver architecture with normal hepatic structure (H & E; 400 × magnification from the original)
The architecture of the brains of all the control group’s rats, as shown in Fig. 9, showed that normal neuronal pyramidal and granulocyte cells, as well as normal blood vessels, had been conserved and remained intact in the cerebral cortex (Fig. 9A, B). Alcohol-treated group, the sections of the brain in the alcohol-treated animal group exhibited the Alcohol group showed a reduction in the level of organized cytoplasm surrounding the nuclei, differences in the cytoplasm surrounding the pyknotic nuclei of cells from alcoholic sections have been marked with arrows associated with dilated blood vessels (BV) (Figs. 9C, D). Avocado + alcohol-treated group, the brain section of the Avocado + alcohol-treated group, and the mustard + alcohol-treated group showed improvement in brain architecture and absence of histological alterations (Fig. 9E) and (Figs. 9F, G) respectively. In the avocado/mustard + alcohol-treated group, the brain section of the animals of the group treated with avocado/mustard + alcohol showed improvement in brain architecture (Figs. 9H, I).
Representative light micrographs showing the histopathological alterations brought about by the application of plant extract therapy to the shape of brain tissue. A, B By using light microscopy, the control rat brain cortex's normal pyramidal cells (P), granulocytes (G), and blood vessels (BV) could be seen. A, D The cytoplasm around the pyknotic nuclei of cells from alcoholic sections differed, with arrows indicating dilated blood vessels (BV); the alcohol group displayed a decrease in the amount of structured cytoplasm surrounding the nuclei E The group administered alcohol along with avocado extract, and F the group administered alcohol along with mustard: demonstrating improved brain architecture with normal brain structure; G The group that received avocado/mustard + alcohol treatment H, I had improved brain architecture with normal brain structure (H & E; 400 × magnification from the original)
4 Discussion
Numerous metabolic and non-metabolic disorders are brought on by oxidative stress, which is aggravated by pro-oxidants like alcohol. The reactive oxygen species (ROS) are emanated in the liver because of the significant metabolic abnormalities caused by alcohol misuse and/or alcoholism [25]. Insufficient ROS removal accelerates cell aging by damaging proteins, lipids, and enzymes in the membrane [26]. Alcohol misuse most likely causes oxidative stress through both an increase in prooxidant production and a decrease in antioxidant defenses. Chronic drinking is thought to be the primary cause of cellular membrane degradation due to the rise in LPO and H2O2 levels as well as the imbalance in the oxidant-antioxidant profile [27]. GSH participates in the removal of H2O2 and lipid peroxides. Since excessive alcohol use depletes antioxidants in cells like GSH, GPx, and GST activity in rats, it's possible that the increased production of ROS by drinking alcohol is the cause of the antioxidants' decline in the liver and brain [27, 28]. Oxygen, on the other hand, is thought to be crucial in alcohol-related liver and brain damage. Alcohol exposure has been proven to produce antioxidant and protective effects by SOD and CAT. However, when the free fatty acid supply for peroxisomal-oxidation is increased, the percentage contribution of CAT can rise [29]. A peroxidase-like process involving catalase is responsible for the oxidation of alcohols to the corresponding aldehydes. Alcohol misuse, on the other hand, is linked to fatty acid accumulation in the liver, and this accumulation is dose- and time-dependent with alcohol consumption [30]. The impact of alcohol abuse causes hepatopathy [31] and tumorgenicity. Alpha-L-fucosidase enzyme found in the lysosomal compartment and it involved in the catabolism of fucose-containing glycoproteins in several tissues including the liver [32]. Its rise in the alcoholic administered rats related to the control rats could demonstrate the tumorgenicity of alcohol misuse and hepatic cancer. α-L-fucosidase is employed as a liver function and tumor marker [33]. Additionally, cytochrome c oxidase (CCO), it catalyzes the electron transport from cytochrome c to oxygen in the mitochondrial cristae and is dependent on oxidative metabolism for energy production. Additionally, cytochrome P450 (P450) is crucial for the metabolism of xenobiotics, and drinking alcohol raises P450 activity [34]. Also, CCO dysfunction has a correlated effect on cancer progression [35]. Alcohol metabolism is the process by which ethanol is converted to acetaldehyde in the liver to be metabolized. While alcohol dehydrogenase (ADH) is the primary enzyme system engaged in this process, other systems, primarily cytochrome P450 2E1, CAT, and SOD, may also be implicate [34, 36]. They also have a major impact on the processes by which alcohol-derived electrons are transferred from ADH to the reducing equivalent NAD + , from ethanol to molecular oxygen through the cytochrome system, and from H2O2 to water through CAT [37]. It is commonly known that 80% of ethanol metabolism is regulated by ADH, with the cytochrome system controlling the majority of the remaining 20% and CAT controlling the other 5%. Additionally, CCO is crucial for neurons and hepatocytes, and areas of the brain that are frequently used for associative memory processes have increased vulnerability to CCO inhibition. On the other hand, the primary oxidative pathway for early ethanol metabolism is made up of human liver ADH [38]. Oxidation of alcohol to acetaldehyde is catalyzed by ADH and primarily takes place in the liver, with a smaller amount occurring in the gastric mucosa. Alcohol consumption is increased in chronic alcoholics by inducing the P450-group member microsomal ethanol oxidizing system [38]. The ADH gene’s importance in reducing the effects of alcohol intoxication on liver tissue is investigated by looking at how the toxicity of alcohol misuse affects the expression of the gene in alcoholic-treated rats [39].
One of the possible risk factors for Alzheimer’s disease (AD) is alcohol drinking. Numerous cognitive issues, including alcoholism, are linked to drinking. Alcohol usage is reasonable to increase the chance of developing AD because to the effects it has on cognition, brain diseases, and brain chemistry [40]. According to the prevalent idea, the “amyloid hypothesis,” accumulation of the Aβ peptide in protein aggregates causes pathophysiological changes that eventually result in cognitive failure in the aged brain due to failing defence and compensating mechanisms [41]. By breaking off the amyloid precursor protein, α- and β-secretases produce beta-amyloid. The discovery of potential therapeutic targets for the treatment or prevention of AD because of the amyloid hypothesis includes the secretases-proteases that are involved in the production of Aβ [42, 43]. Elevated secretase gene expression in the alcoholic community may underscore heavy drinkers’ long-term consequences on AD. However, drinking alcohol has been linked to an increased risk of dementia and Alzheimer's disease [10]. Enhanced vulnerability to CCO inhibition is found in brain regions most often engaged in associative memory functions [44]. Mitochondria contain both the Aβ-regulating secretases and the precursor protein amyloid [45]. Microglia and astrocytes have been demonstrated to produce Aβ, a primary constituent of senile plaques, in both animal models and human brains. It protects against CCO release and mitochondrial enlargement caused by different ROS and is attached to the outer mitochondrial membrane [35].
The therapeutic administration of aqueous extracts from mustard and avocado seeds shows promise in preventing the onset and adverse effects of alcohol intoxication. The effects of avocado and mustard seed extracts proved beneficial in alleviating various risk factors. These include a noticeable improvement in the antioxidant system and the prevention of elevated oxidative stress resulting from alcohol misuse. Moreover, the administration of aqueous mustard and avocado seed extracts led to an increased expression of the ADH gene in the liver and the β-secretase gene in brain tissues.
The findings of this study imply that mustard and avocado seeds may be able to regulate the antioxidant/oxidant system as well as molecular alterations in addition to efficiently treating alcohol dependence. This study also proposes the potential use of avocado and mustard seed extracts as complementary therapeutic agents to mitigate the hepatotoxicity and neurotoxicity associated with alcohol consumption. Avocado fruit phytochemicals mitigate the proliferation of precancerous and malignant cell lines and trigger apoptosis [46]. Different avocado pieces are frequently utilized in African traditional medicines to treat epilepsy and juvenile convulsions [47]. Aquatic avocado extract has anticonvulsant action [47]. When compared to the alcoholic group, the serum of the alcoholic + avocado group shows a decrease in the activities of α-l-fucosidase due to avocado's ability to scavenge free radicals [46] stated that avocado fruit’s polyphenols may provide a prestigious dietary approach to cancer prevention. Avocado fruit has a lot of phytochemicals that are crucial in preventing the growth of cancer cells [48].
Avocado fruit has antioxidant activities due to its polyphenolic compounds [49]. High increase in lipid peroxidative indices and H2O2 levels in both liver and brain tissues attenuated with avocado fruit when compared with the alcoholic group and this agrees with [50]. The antioxidant properties of avocado maintain the action of GPx, GST, CAT, SOD, and GSH level in the liver and brain. So, avocados could improve the gene expression of liver ALD due to their antioxidant properties. Also, the avocado extract can restore β-secretase gene expression in rats’ brains, the explanation of avocado’s role may be due to their antioxidant action. Avocados decrease the upregulation of the β-secretase gene in alcoholic groups nearly to the control group indicating their role in AD treatment. Avocados may directly affect the genetic expression of the β-secretase gene in brain tissue, which would explain their protective effects. Considering this, the study suggests using avocado to shield the human brain from AD, especially if you’ve been consuming alcohol for a while. Therefore, exposure to prolonged alcohol consumption may produce oxidative stress and may cause the production of CCO in brain tissues, that substantially triggers the apoptotic pathways and be the cause of the disturbance of cognitive abilities in rats.
The vital vitamin B-complex found in Brassica juncea seeds is a fantastic source of support for the neurological system, metabolism, and enzyme synthesis [51]. Indian mustard seeds exhibit antioxidant properties both in vitro and in vivo [52] so, Indian mustard seeds' antioxidant and free radical-scavenging abilities account for their hepato- and neuroprotective effects. Additionally, investigations conducted in vivo on mice showed that mustard seeds reduce plasma LPO and increase SOD, CAT, and GPx activity [53]. AD is treated or prevented by a variety of conventional herbal remedies, some of which have been created as nutraceuticals or functional foods [54]. Herbal extracts seem to have more positive effects on cognitive functioning than negative ones in AD animal models and cell models. The hypothesised causal roles of antioxidant activities and GSH depletion in apoptosis are supported by data showing that the release of mature cytochrome c from mitochondria is a biological response to GSH depletion on apoptosis or survival [55]. Cytochrome c, after it has been liberated from mitochondria, activates downstream caspases, which are the molecules responsible for carrying out the apoptotic signaling cascade [56]. Mustard seeds inhibit tumor cell proliferation and have anticancer action [53]. These results are consistent with our own, which indicated that the alcohol + mustard seed group had lower levels of α-l-fucosidase than the control group. Therefore, mustard seeds may be regarded as a food that has anti-tumor properties. Mustard seeds' possible antioxidant properties [53] may be the reason for the improvement of ALD gene expression but the improvement was more noticed in the mixture group. On the other hand, the protective role of mustard seeds on neurotoxicity was more obvious in the alcohol + mustard seeds group as it inhibits the expression of brain β-secretase as correlated with the control. This finding supports mustard seeds' significant contribution to the prevention of AD. The mixture of mustard seeds and avocado has more effect in the alleviation of secretase gene expression (Fig. 7b). This protective action of mustard seeds may be due to their potential antioxidants [57].
5 Conclusion
The abuse of alcohol disrupts the delicate balance of oxidants and antioxidants in both the liver and brain tissues. However, alcohol intoxication contributes to the liver’s defence mechanism against this toxicity by upregulating the expression of genes linked to alcohol dehydrogenase activity and decreasing cytochrome c oxidase activity. Furthermore, alcohol consumption elevates the gene expression of secretase, which can lead to neuropathies such as AD. The therapeutic administration of aqueous extracts from mustard and avocado seeds shows promise in preventing the onset and adverse effects of alcohol intoxication. The effects of avocado and mustard seed extracts proved beneficial in alleviating various risk factors. These include a noticeable improvement in the antioxidant system and the prevention of elevated oxidative stress resulting from alcohol misuse. Moreover, the administration of aqueous mustard and avocado seed extracts led to an increased expression of the ADH gene in the liver and the β-secretase gene in brain tissues. The findings of this study imply that mustard and avocado seeds may be able to regulate the antioxidant/oxidant system as well as molecular alterations in addition to efficiently treating alcohol dependence. This study also proposes the potential use of avocado and mustard seed extracts as complementary therapeutic agents to mitigate the hepatotoxicity and neurotoxicity associated with alcohol consumption.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Pagliaro LA, Pagliaro AM (2022) Drug and Substance Abuse Among Older Adults: Identification, Analysis, and Synthesis, 1st edn. Routledge, London. Doi: https://doi.org/10.4324/9781003010333
Stickel F, Datz C, Hampe J, Bataller R (2017) Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver 11:173
Yi S-W, Hong J-S, Yi J-J, Ohrr H (2016) Impact of alcohol consumption and body mass index on mortality from nonneoplastic liver diseases, upper aerodigestive tract cancers, and alcohol use disorders in Korean older middle-aged men: prospective cohort study. Medicine 95:39
Teschke R (2018) Alcoholic liver disease: alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 6:106
Hermoso DAM, Shimada LBC, Gilglioni EH, Constantin J, Mito MS, Hermoso APM, Salgueiro-Pagadigorria CL, Iwamoto ELI (2016) Melatonin protects female rats against steatosis and liver oxidative stress induced by oestrogen deficiency. Life Sci 157:178–186
Maithreyi R, Janani AV, Krishna R, Shweta A, Edwin RR, Mohan SK (2010) Erythrocyte lipid peroxidation and antioxidants in chronic alcoholics with alcoholic liver disease. Asian J Pharm Clin Res 3(3):183–185
Rahman S, Engleman EA, Bell RL (2016) Recent advances in nicotinic receptor signalling in alcohol abuse and alcoholism. Prog Mol Biol Transl Sci 137:183–201
Shield KD, Parry C, Rehm J (2013) Chronic diseases and conditions related to alcohol use. Alcohol Res 35:155
Casetta I, Govoni V, Granieri E (2005) Oxidative stress, antioxidants and neurodegenerative diseases. Curr Pharm Des 11:2033–2052
Laitinen MH, Ngandu T, Rovio S, Helkala E-L, Uusitalo U, Viitanen M, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M (2006) Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement Geriatr Cogn Disord 22:99–107
Al-Asmari AK, Al-Elaiwi AM, Athar MT, Tariq M, Al Eid A, Al-Asmary SM (2014) A review of hepatoprotective plants used in saudi traditional medicine. Evid Based Complement Alternat Med 2014:890842
Wu J, Wang Y, Zhang Z, Yu B (2015) Herbal medicine in the treatment of Alzheimer’s disease. Chin J Integr Med 21:102–107
Chang Z, Wang Y-C, Tian D, Hu W-Y, Wang Z-Y, Liu G-L, Ma H-P, Hu Y-L, Wu B, Han Z-Y (2022) Medication rules in herbal medicine for mild cognitive impairment: a network pharmacology and data mining study. Evid Based Complement Alternat Med 2022:2478940
Majeed T, Bhat NA (2022) Health benefits of plant extracts. In: Shabir AM, Annamalai MK, Manzoor AS (eds) Plant extracts: applications in the food industry. Academic Press, London, pp 269–294
Verma A, Sharma A, Rai PK (2019) Impact of soxhlet extraction method on oil yield and antioxidant potential of Brassica juncea. J Pharmacogn Phytochem 8(4):1134–1137
Jimenez P, Garcia P, Quitral V, Vasquez K, Parra-Ruiz C, Reyes-Farias M, Garcia-Diaz DF, Robert P, Encina C, Soto-Covasich J (2021) Pulp, leaf, peel and seed of avocado fruit: a review of bioactive compounds and healthy benefits. Food Rev Intl 37:619–655
Parikh H, Pandita N, Khanna A (2015) Phytoextract of Indian mustard seeds acts by suppressing the generation of ROS against acetaminophen-induced hepatotoxicity in HepG2 cells. Pharm Biol 53:975–984
Buege JA, Aust SD (1978) [30] Microsomal lipid peroxidation. In: Methods in enzymology. Elsevier, Amsterdam, pp 302–310
Fossati P, Prencipe L, Berti G (1980) Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26:227–231
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Beutler E (1963) Improved method for the determination of blood glutathione. J lab clin Med 61:882–888
Habig W, Pabst M, Jakoby W (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Masalkar PD, Abhang SA (2005) Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin Chim Acta 355:61–65
Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB (2012) Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal 16:1295–1322
Shati AA, Elsaid FG (2009) Effects of water extracts of thyme (Thymus vulgaris) and ginger (Zingiber officinale Roscoe) on alcohol abuse. Food Chem Toxicol 47:1945–1949
Chauhan NM, Mohan Karuppayil S (2021) Dual identities for various alcohols in two different yeasts. Mycology 12:25–38
Melis M, Carta G, Pistis M, Banni S (2013) Physiological role of peroxisome proliferator-activated receptors type α on dopamine systems. CNS Neurol Disord Drug Targets 12(1):70–77. https://doi.org/10.2174/1871527311312010012. (PMID: 23394525)
Cannady R, Nimitvilai-Roberts S, Jennings SD, Woodward JJ, Mulholland PJ (2020) Distinct region-and time-dependent functional cortical adaptations in C57BL/6J mice after short and prolonged alcohol drinking. eNeuro. Doi: https://doi.org/10.1523/ENEURO.0077-20.2020
Rehm J, Gmel GE Sr, Gmel G, Hasan OSM, Imtiaz S, Popova S, Probst C, Roerecke M, Room R, Samokhvalov A (2017) The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction 112:968–1001
Hutchinson WL, Du M, Johnson PJ, Williams R (1991) Fucosyltransferases: differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology 13:683–688
AlSalloom AAM (2016) An update of biochemical markers of hepatocellular carcinoma. Int J Health Sci (Qassim) 10:121
Gonzalez FJ (2005) Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res 569(1–2):101–110. https://doi.org/10.1016/j.mrfmmm.2004.04.021. (PMID: 15603755)
Srinivasan S, Guha M, Dong DW, Whelan KA, Ruthel G, Uchikado Y, Natsugoe S, Nakagawa H, Avadhani NG (2016) Disruption of cytochrome c oxidase function induces the Warburg effect and metabolic reprogramming. Oncogene 35:1585–1595
Bak MJ, Truong V-L, Ko S-Y, Nguyen XNG, Ingkasupart P, Jun M, Shin JY, Jeong W-S (2016) Antioxidant and hepatoprotective effects of procyanidins from wild grape (Vitis amurensis) seeds in ethanol-induced cells and rats. Int J Mol Sci 17:758
Silva-Adaya D, Garza-Lombó C, Gonsebatt ME (2021) Xenobiotic transport and metabolism in the human brain. Neurotoxicology 86:125–138
Agarwal DP, Goedde HW (2012) Alcohol metabolism, alcohol intolerance and alcoholism. Springer, Berlin
Eriksson CJP (2001) The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin Exp Res 25:15S-32S
Bagnardi V, Blangiardo M, La Vecchia C, Corrao G (2001) Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health 25(4):263–270
Hou Y-C, Huang C-L, Lu C-L, Zheng C-M, Lin Y-F, Lu K-C, Chung Y-L, Chen R-M (2021) The role of plasma neurofilament light protein for assessing cognitive impairment in patients with end-stage renal disease. Front Aging Neurosci 13:657794. https://doi.org/10.3389/fnagi.2021.657794.PMID:34122041;PMCID:PMC8192845
de Strooper B, Vassar R, Golde T (2010) The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol 6:99–107
Sasaoka N, Sakamoto M, Kanemori S, Kan M, Tsukano C, Takemoto Y, Kakizuka A (2014) Long-term oral administration of hop flower extracts mitigates Alzheimer phenotypes in mice. PLoS ONE 9:e87185
Gonzalez-Lima F, Cada A (1998) Quantitative histochemistry of cytochrome oxidase activity. In: Gonzalez-Lima F (ed) cytochrome oxidase in neuronal metabolism and Alzheimer’s disease. Springer, Boston
Anandatheerthavarada HK, Biswas G, Robin M-A, Avadhani NG (2003) Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161:41–54
Ding H, Han C, Guo D, Chin Y-W, Ding Y, Kinghorn AD, D’Ambrosio SM (2009) Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr Cancer 61:348–356
Ojewole JAO, Amabeoku GJ (2006) Anticonvulsant effect of Persea americana Mill (Lauraceae) (Avocado) leaf aqueous extract in mice. Phytother Res 8:696–700. https://doi.org/10.1002/ptr.1940
Larijani LV, Ghasemi M, AbedianKenari S, Naghshvar F (2014) Evaluating the effect of four extracts of avocado fruit on esophageal squamous carcinoma and colon adenocarcinoma cell lines in comparison with peripheral blood mononuclear cells. Acta Med Iran 52(3):201–205
Pahua-Ramos ME, Ortiz-Moreno A, Chamorro-Cevallos G, Hernández-Navarro MD, Garduño-Siciliano L, Necoechea-Mondragón H, Hernández-Ortega M (2012) Hypolipidemic effect of avocado (Persea americana Mill) seed in a hypercholesterolemic mouse model. Plant Foods Hum Nutr 67:10–16
Al-Dosari MS (2011) Hypolipidemic and antioxidant activities of avocado fruit pulp on high cholesterol fed diet in rats. Afr J Pharm Pharmacol 5(12):1475–1483
Sarwar MF, Sarwar MH, Sarwar M, Qadri NA, Moghal S (2013) The role of oilseeds nutrition in human health: a critical. J Cereals oilseeds 4(8):97–100
Singh S, Bhatia A, Tomer R, Kumar V, Singh B, Singh SD (2013) Synergistic action of tropospheric ozone and carbon dioxide on yield and nutritional quality of Indian mustard (Brassica juncea (L.) Czern.). Environ Monit Assess 185:6517–6529
Yuan H, Zhu M, Guo W, Jin L, Chen W, Brunk UT, Zhao M (2011) Mustard seeds (Sinapis Alba Linn) attenuate azoxymethane-induced colon carcinogenesis. Redox Rep 16:38–44
Geun Kim H, Sook OhM (2012) Herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr Pharm Des 18(1):57–75
Ghibelli L, Coppola S, Fanelli C, Rotilio G, Civitareale P, Scovassi AI, Ciriolo MR (1999) Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J 13:2031–2036
Sharma DR, Sunkaria A, Bal A, Bhutia YD, Vijayaraghavan R, Flora SJS, Gill KD (2009) Neurobehavioral impairments, generation of oxidative stress and release of pro-apoptotic factors after chronic exposure to sulphur mustard in mouse brain. Toxicol Appl Pharmacol 240:208–218
Dua A, Chander S, Agrawal S, Mahajan R (2014) Antioxidants from defatted Indian Mustard (Brassica Juncea) protect biomolecules against in vitro oxidation. Physiol Mol Biol Plants 20:539–543
Acknowledgements
This work was supported by King Abdulaziz City for Science and Technology (KACST) student research number AT-37-15.
Funding
This study was funded by KACST, AT-37-15., Aishah Abdullah AL Qahtani.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AAAQ. The first draft of the manuscript was written by all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AL-Qahtani, A.A., Shati, A.A., Al-Doaiss, A.A. et al. Mitigating alcohol-induced neurohepatotoxicity in male albino rats with avocado and mustard. J.Umm Al-Qura Univ. Appll. Sci. (2024). https://doi.org/10.1007/s43994-024-00124-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43994-024-00124-2