Abstract
Antifungal azole drugs like fluconazole, itaconazole and ketoconazole are widely used for treatment of fungal related diseases including aspergillosis and mucormycosis. This study aimed at biosynthesis of fluconazole, itaconazole and ketoconazole containing oil/water (O/W) nanoemulsions (NE) using Eucalyptus essential oil and its effect against aspergillosis and mucormycosis. Nanoemulsions were synthesized having eucalyptus essential oil, surfactants: tween 80, and co-surfactant: ethanol. Zeta potential, pH, conductivity and droplet size of nano-formulations were studied by using Zeta sizer. Nanoemulsions were analyzed by UV–VIS, FT-IR and fluorescent techniques. Stability studies were conducted by storing the nanoemulsions at different conditions for 60 days. Anti-aspergillosis, anti-mucormycosis and drug release pharmokinetics were evaluated. Average size of nanoemulsions ranged from 245 to 415 nm along with zeta potential from − 9.20 to − 25.4 mV. Encapsulation efficiency of drug loaded nanoemulsions was ranged from 40 to 50%. Nano-droplets displayed stability after 60 days of storage. Considerable anti-aspergillosis and anti-mucormycosis activities were detected. Among all formulations, F1NEs depicted high antifungal activity against Aspergillus strains MTCC 277, MTCC 343 as observed by zone of inhibition (ZOI) values. Against Mucor spp strain MTCC 3373, visual pictures clearly showed substantial inhibition in fungal growth. Pharmokinetic study shown that all nanoformulations showed Korsmeyer–Peppas model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
After COVID 19 pandemic, researchers have recognized COVID-19–linked severe co-infections in COVID-19 patients such as: mucormycosis, aspergillosis, coccidioidomycosis, and saccharomycosis [1, 2]. Aspergillosis and mucormycosis are deadly diseases having mortality rate of 68% [3].Invasive aspergillosis and mucormycosis is predominantly instigated by Aspergillus and Mucor spp common indoors and outdoors fungal strains. Post COVID-19, these fungal infections stances a serious health risk for sternly immune-compromised persons due to unregulated use of steroids to cure COVID patients [4].The symptoms associated with aspergillosis include: headache, stiffness, running nose, cough, chest pain, fever, blood in cough, and reduced smelling power [5]. Symptoms associated with mucormycosis include: around the nose blackish discolouration, stuffy and bleeding nose, black crusts oozing out from nose, teeth and jaw loosening, numbness and one-sided facial pain, swelling of eyes; blurred vision and problems in respiratory symptoms [6].
Azole based drugs like fluconazole, itaconazole and ketoconazole are widely used an effective anti-fungal drugs [1]. Owing to having high mol weight, high lipophilicity index, low BBB penetration and low hydrophilicity and consequently low bioavailability, their use as antifungal agents is limited [7]. Fungal resistance after prolong use of azole derivatives is another problem [8]. In this regard, Nazzaro et al. [7] cited that the problem of multiple fungal drug resistance can be resolved by using combination of essential oils (EO) with antifungal drugs. Authors also noticed synergistic antifungal activity of azole based drugs with some essential oils (EOs) [9]. Based on above background we hypothesize that by encapsulating azole based drugs in eucalyptus essential oil, infection of fungus causing aspergillosis and mucormycosis can be mitigated effectively.
In recent years, to deliver therapeutic drugs to target sites, nanoemulsions technology is proposed is an efficient delivery system. Jaiswal et al. [10] proposed that nanoemulsions (NE):water-in-oil (W/O) and oil-in-water (O/W) types are an emulsion system with particle size distribution from 50 to 1000 nm. Drugs placed in nanoemulsions droplet is free from light, air, and any kind of hard stressful environment, therefore, as delivery system nanoemulsions not only improve bioavailability but also protects drugs from oxidation and hydrolysis [11,12,13]. NEs have potent potential to by-pass drugs across P-glycoprotein (P-gp) in BBB and cell membranes in CNS patients [14, 15] thus great attention has been paid to NE technology recently [16].
Plant based bioactives compounds as drug delivery carriers are tremendously used to cure various diseases other medical problems [17]. It was proposed that bioactives based NEs have better biocompatibility than synthetic delivery forms [18]. Studies have documented that drugs co-used with natural compounds is an alternative strategy to resolve multi drug resistance (MDR) in fungi [15]. However, still research in this area is infancy. Among plant based bioactives, essential oils (EO) have used immensely as drug carries systems in ethno-medicine. Being volatile, aromatic and low MW, EOs are a complex mixture of bioactive compounds [19]. Owing to this, diverse roles of EOs have been cited [20]. Since natural products are less expensive and considered safer, they could be explored for their synergistic interactions with drugs of choice for treatment of fungal infections which might result in more cost effective and safer formulations [21, 22]. Eucalyptus globules, native to Tasmanian and South-East Australia, member of Myrtaceae family is one of most widely spread genera. The leaves of this plant are used to extract Oleum Eucalypti (eucalyptus oil) worldwide and has long history to be used as traditional medicine in ancient times. Due to enriched presence of 1,8-cineole in its essential oil, leaf extracts from this plant is widely used as a raw material in pulp, cosmetic, pharmaceutical, food beverage, aromatherapy and phototherapy [19,20,21,22]. Some in vitro studies have reported the combination of oils with fluconazole and amphotericin B against candidal infections [23]. Vörös-Horváth et al. [24] reported formulation of tioconazole in tea tree oil (Melaleuca alternifolia Essential oil) against Onychomycosis. Some earlier studies pointed out the use of plant based essential oils from Citrus limonum Risso, Salvia officinalis L, Origanumm ajorana L as drug carrier system for Ifosfamide, Epirubicin and observed a dramatic increase in the biological activities of synthetic drugs [15]. However, novelty of this study lies in the fact that the role of eucalyptus essential oil as azole based drug delivery system in relationship with aspergillosis is still not well documented. Hence the aim of this study was to formulate azole drug-loaded nanoemulsions with eucalyptus essential oil and evaluation of its anti-aspergillosis and anti-mucormycosis potential.
2 Material and methods
2.1 Extraction of EO and GC-FID analysis
EO was extracted from fresh leaves of Eucalyptus globules by hydro-distillation method and stored in dark bottles at 4 °C for further studies as described previously [14]. EO was extracted from fresh leaves of Eucalyptus globules growing at vicinity of campus. The plants were authenticated by Botany Dept and voucher with number BT105 was deposited in Dept of Biotechnology. Essential oil was extracted by hydro-distillation method and stored in dark bottles at 4 °C for further studies as described previously [26]. To identify bioactive compounds in EO, GC-FID study was carried out (GC-FID, 2045 Chemtron Pvt Ltd, India). The specifications of column were: 2 m long, stainless steel having 10% OV-17 on 80–100% mesh Chromosorb W (HP). Nitrogen was used as carrier gas at flow rate of 35 ml/min. 0.2 µl EO sample was used. The temperatures for detector and injector were: 220 °C and 270 °C. Oven ramping conditions were: 100 °C (firstly maintained) ramped to 200 °C at 3 °C/min. Bioactive constituents in EO were identified by comparing relative retention times (RT) of GC-FID spectra of EO with authentic standards [(eucalyptol, citral, eugenol and geraniol, Sigma-Aldrich (St. Louis, MO, USA)] and literature data.
2.2 Preparation of drug loaded nanoemulsions
Eucalyptus globules EO based NEs having drugs Flucaconazole, Itraconazole, and Ketoconazole were manufactured following [25]. Drugs were purchased from local market. Briefly, three nanoemulsion formulations designated as F1-F3 (F1: Flucaconazole, Fungiset 200 mg, Leford pvt Ltd, F2: Itraconazole, Itrasys 200 mg, Leford pvt ltd, and F3:Ketoconazole Ketofly 200 mg, Leford pvt Ltd) were prepared. Mass I: Methanol was added to the respective drug and stirred for 1 h at 37 °C, followed by Eucalyptus oil was added (5% v/v) drop by drop and stirred until transparent solution is obtained. Mass II: Tween 80 (2 v/v%), distilled water and SDS (0.30 w/w%) were mixed in water and heated at 50 °C until transparent mass was obtained. Mass I was added to Mass II drop wise with constant shaking at 1500 rpm for 40 min. The mixture was exposed to ultrasonication (Citizen, CDT, UC-2000) at 30 kHz for 30 min at 35 °C.
2.3 Physiochemical studies
All nanoemulsions were subjected to microscopy analysis using optical microscope and analyzed by in-built MicroView tool (Debro DM RL) as described in [26]. Nanoemulsions were also assessed by taking turbidity at OD at 600 nm and % transmittance by UV–VIS spectrophotometer (LT 291 Labtronics pvt ltd, Ambala, India). pH and conductivity of formulated nanoemulsions were also studied by using pH meter (LT 10 Labtronics pvt ltd, Ambala, India) and conductometer (LT 26 Labtronics Pvt Ltd, Ambala, India) at room temperature. Dilution test was made by taking 1.5 mL of nanoemulsions in 9.5 mL of H2O observed for phase inversion. Dye solubility (o/w test) test was achieved by taking diluted methylene blue dye which was added to 3.0 mL of nanoemulsions, mixed and noticed for phase inversion with the help of microscope.
2.4 Determination of particle size distribution
To observed particle size of F1-F3 nanoemulsions, particle size distribution was performed. It was executed using a Particle Size Analyzer (Malverrn Zetasizer Nano ZS90, Germany). 100 µl of nanoemulsions were diluted with water (1:400) before using the instrument. The analysis was performed in triplicate at 25 °C.
2.5 Determination of zeta potential
Zeta potential of F1-F3 nanoemulsions were also evaluated by using Zetasizers Nano ZS, (Malverrn Zetasizer Nano ZS90, Germany). 100 µl of nanoemulsions were diluted with distilled water in ratio1:600 and put into the cuvette and measured the zeta potential. Analysis was performed in triplicate manner (n = 3).
2.6 Stability of nanoemulsions
Stability of F1-F3 NE formulations having drug were evaluated. For this 5 ml of each NE having drug were taken into glass vials and sealed. These vials were kept at RT (37 ± 1 °C), 20 °C, 4 °C, for 60 days. NE samples were analyzed visually for clarity, phase separation and drug precipitation, pH and droplet size. The F1-F3 NE formulations were also subjected to centrifugal stability studies. The NE samples were centrifuged at 6000 rpm for 30 min then observed for clarity, phase separation, precipitation of drug. Analysis was performed in triplicate manner (n = 3). Thermodynamic stability studies were performed for observing any physical stability by subjecting the F1-F3 NE formulations to stress conditions of freeze thaw cycling (12 h at 4 °C and 12 h at 37 °C for period of 7 days).
2.7 Fingerprint analysis
Fomulated nanoemulsions (F1-F3) were characterized by FT-IR, UV- and fluorescence spectroscopy as described in Agnish et al. [26].
2.8 Antifungal activity
To analyze the antifungal effect of drug loaded NEs on fungal strains, Aspergillus niger (MTCC 277) and Aspergillus oryzae (MTCC 343), and Mucor spp. (MTCC 3473) were procured from IMTECH, Chandigarh (India). Briefly, from 7 day old actively growing pure fungal culture plates, fungal mycelia agar discs (8 mm) were taken and suspended in 10 mL of autoclaved H2O. As inoculum, 150 µl of fungal suspension was spread on to PDA having plates. About 200 µl of drug loaded NE formulations were impregnated onto sterilized paper discs (15 mm in diameter) and located in middle of agar plates followed by incubation at 37 °C for 7 days. Pure drugs fluconazole (500 µg), itraconazole (500 µg), and ketoconazole (500 µg), were taken as positive control.
2.9 Drug entrapment efficiency (DEE)
DEE was calculated by dispersing 2.00 mL of NEs (F1-F3) in 6 mL of methanol, stirred for 30 min at 150 rpm, at 35 °C and examined at 210 nm using UV spectrometer (LT 291 Labtronics Pvt Ltd, Ambala, India). DEE % = [TDC- FDC/TDC] × 100; TDC: total drug concentration, FDC: free drug concentration, TDC: theoretical drug concentration.
2.10 Drug kinetics
Pharmokinetic profile of F1-F3 NE formulations was calculated as defined previously [26]. In brief, Drug loaded NEs 3 mL (having 100 mg of drug) were placed in a dialysis bag (LA411-135, MW cut off 12 kDa-14 kDa, Himedia Pvt Ltd, India). Bags were firmly tagged and suspended in 100 ml of PBS and stirred at 120 rpm at 37 °C. 3 mL of aliquot was taken at defined intervals and analysed at 215 nm by UV–VIS spectrophotometer (LT 291 Labtronics pvt ltd, Ambala, India). Higuchi model, Korsmeyer–Peppas, zero order and 1st order kinetic models were studied [26].
2.11 Statistical analysis
One way ANOVA including Post Hoc Tukey HSD (beta) was subjected to data having antifungal activity and physiochemical properties (https://www.socscistatistics.com/tests/anova/default2.aspx). For antifungal, different nanoformulations (F1-F3), designated as group A, were compared with respective controls (pure drug), designated as group B, using stat tool. For Statistical analysis of physiochemical properites of nanoformulations, F1 formulation was designated as T1, F2 formulation was designated as T2, F3 formulation was designated as T3 and subjected to Post Hoc Tukey HSD (beta) for pairwise comparisons.
3 Results
3.1 Compositional profiling
Compositional profile identified by GC-FID analyses is demonstrated beside with their abundances in Fig. 1A. GC-FID examination of Eucalyptus essential oil revealed the incidence of 39 peaks. GC-FID profile confined major peaks along with many small peaks. The major constituents were eucalyptol (1,8 cineole) (21%), α-pinene (10%), Trans-Geraniol (7.4%), Beta-myrcene (4.5%) and citral (2.9%).
3.2 Synthesizes of EO based NE
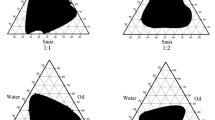
In this study, 3 different drug loaded nanoemulsions designated as F1 (Flucaconazole-loaded NE), F2 (Itraconazole-loaded NE) and F3 (Ketoconazole-loaded NE) were developed and main physiochemical parameters studied. To formulate three nanoemulsions, co-surfactant ethanol and tween 80 non-ionic surfactant were used. All NEs were uniformly distributed, globules were smooth and circular in shape, and discrete and non-aggregated (Fig. 1B, Table1). Particle size of F1-F3 nanoemulsions were in the range of 245- 415 nm (Table 1). F2 nanoemulsions were smaller (mean diameter 245 nm) followed by F3 and F2 nanoemulsions. Dye solubility testy indicated that O/W NEs were stable as no phase inversion was detected.
PDI (polydispersibility index) was in the range of 0.285–0.359 for F1 to F3 nanoemulsions (Table 1). Zeta potential (ZP) of nanoformulations was: F1: − 9.20 mV, F2: − 20.2 mV and F3: − 25.4 mV, respectively (Table 1). pH of drug loaded nanoformulations were in range of 5.0–8.0 for F1 to F3 nanoemulsions (Table 1). F1, F2 and F3 nanoemulsions displayed conductivity values 0.544, 0.589 and 0.485 mS/cm, respectively. Turbidity of drug loaded NEs are shown in Fig. 1. According to Abs600, turbidity of F1 NE was 0.184, F2: 0.117 and F3: 0.154. As illustrated in the photos of NEs, F1 NE was more turbid than F2 NE which may be due to more particle size of droplets.
3.3 Fingerprint analysis of NEs
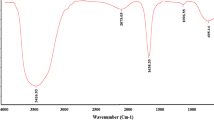
Under UV a sharp peak at about 360 nm was detected (Fig. 2). Notably, when drugs were encapsulated, all nanoemulsions depicted decreased absorbance when compared to pure drug UV-spectra. In fluorescent studies, pure drug displayed one major peak at 500 nm and minor peak at 450 nm (Fig. 3). FT-IR peak spectra of pure drugs are illustrated in Fig. 4. FT-IR spectrum of pure drugs showed broad peak at 3400 cm−1due to Imidazole ring structure (C–N stretching). Sharp peak at 2960 cm−1 was due to stretching vibrations of aliphatic CH2 group. Two prominent peaks were observed at 2700 cm−1 only in F3 and F2NEs can be ascribed to O–H stretching of alcohols. Additionally, peak in the range of 1000 cm−1 corresponds to C–C stretching vibrations of alkenes. Peak at 750 cm−1 attributed to C-H bending were due to aliphatic ether.
3.4 Storage stability
The F1-F3 nanoemulsions were assessed in centrifugation analysis. Centrifugal studies results indicated that, no precipitation of drug, phase inversion, cracking or creaming. Thermodynamic studies revealed that formulated NEs were physically stable under freeze thaw stress like conditions (Fig. 5) as no sign of cracking, flocculation and phase separation was observed. To determine long-term steadiness and shelf-life and of F1–F3 NEs, stability of NEs were studied at three different temperatures for 60 days (Fig. 6). In F1, F2 and F3NEssize of droplet hardly changed at -20 °C and 4 °C, but, Ostwald Repining (OR) was noticed at 37 °C.
3.5 Anti-fungal activity
Antifungal potential of formulated NEs is illustrated in Table 2 and supplementary Figures 1–3. Formulated F1 NEs depicted high antifungal activity against Aspergillus strains MTCC 277, MTCC 343 as observed by zone of inhibition (ZOI) values which were: 2.0 cm and 2.8 cm, respectively. F2 and F3 NEs also depicted sufficient fungal inhibition against MTCC 277. Notably, against fungal strain MTCC 343, total inhibition in growth was observed. Positive control also depicted fungal inhibition against all strains as cited in Table 2. Against Mucor spp strain MTCC 3373, although no clear ZOI was observed, however, visual pictures clearly showed substantial inhibition in fungal growth was observed when compared to negative control (supplementary Figure 3). It was further observed that F2 NE showed synergetic effect in antifungal activity against A. niger and Mucor spp strains.
3.6 Drug release kinetics
Drug entrapment efficiency (DEE) of F1-F3 NEs is illustrated in Table 1. DEE improved considerably coupled with reducing the size of nano-droplets. DEE of F2NE was higher (50%) compared to others. For instance: compared with DEE of F1 NE (40%), DEE of F2 was 50% and DEE of F3 was 45%. Drug release (DR) of F1-F3 NEs and pure drug was studied. As shown in Fig. 7, it was observed that due to low water solubility and lipophilic nature, pure drug showed In vitro drug release of 30–45% at 72 h, while F1-F3NEs DR was 62%-92% in 72 h. To get understanding about the mechanism of drug release in pharmokinetic studies, four kinetic models like: 0 order,1st order, Korsmeyer–Peppas and Higuchi models were studied. Release of drug from F-F3 NEs was examined with these models. To depict release pattern of drug from NEs, correlation coefficient (R2) value was studied (Figs. 8, 9, 10). For F1–F3 NEs R2 value was 0.92 (F1), 0.86 (F2) and 0.88 (F3). The exponent release diffusion values were: n = 0.64 (F1), and 0.69 (F2) and 0.68 (F3).
4 Discussion
GC-FID profile indicated the presence of major constituents such as: eucalyptol (1,8 cineole) (21%), α-pinene (10%), Trans-Geraniol (7.4%), Beta-myrcene (4.5%) and citral (2.9%). As cited earlier, Eucalyptus essential oil was rich in eucalyptol (1,8 cineole) [19, 20]. Due to these bioactives molecules, EO oil has marvelous applications in health- and medical-related research [20,21,22].
Three different azole drug loaded nanoemulsions designated as F1 (Flucaconazole-loaded NE), F2 (Itraconazole-loaded NE) and F3 (Ketoconazole-loaded NE) were developed and main physiochemical parameters studied. To formulate three nanoemulsions, co-surfactant ethanol and tween 80 non-ionic surfactant were used. Usually, co-surfactants are used for lessening interfacial bonding stress aid flexibility to NE to assume various curvatures [25]. No gravitational separation of formulated F1–F3 NEs were observed, indicating kinetically stability of formulated NEs. Particle size and distribution (PDI) are key factors in order to ascertain physical stability of nanoemulsions [25]. F1–F3 nanoemulsions were in the range of 245–415 nm (Table 1). Various factors like charge, permeability, thickness, adsorption rate could be the reason for different size of droplets [27, 28]. Dye solubility testy indicated that O/W NEs were stable as no phase inversion was detected. PDI (polydispersibility index) was in the range of 0.285–0.359 for F1 to F3 nanoemulsions (Table 1). PDI is pivotal factor that determines stability of formulations. The PDI can be range from 0–1, where 0 denotes monodisperse system and 1 indicates polydisperse system [29]. Low PDI values usually indicate uniformity in droplet size of nanoemulsions thus in this regard nanoemulsions tend to be stable during long time storage [10]). Small particle size formulations usually accompanied by low PDI value. For instance: F2 nanoemulsions have particle size of 245 nm along with 0.285 PDI value than F1 nanoemulsions which poses particle size of 415 nm and 0.359 PDI value. Less particle size along with PDI values also assist to increase stability of nanoemulsions against the creaming and sedimentation because diffusion rate and brownian movement of globules is higher than sedimentation and creaming rate induced by gravity force [30]. Zeta potential (ZP) of nanoformulations was: F1: − 9.20 mV, F2: − 20.2 mV and F3: − 25.4 mV, respectively (Table 1). Zeta potential indicates the charge on the droplets that indicates degree of repulsions between the “like charge particles” surrounding the nearby particles [25]. Earlier studies revealed that ZP feature to the stability of nanoemulsions by measuring interactive forces between the nanoparticles at macroscopic scale [31]. In order to attain kinetically stable nanoformulations, ZP should be in the range of ± 30 mV. In our study, ZP values obtained were in the range of − 9.20 to − 25.4 mV, which were revealing of electrostatically stabilized NEs. The negative charge of all nanoemulsions is probably due to the anionic groups present in surfactants and –co-surfactants, hence there are less chances of aggregation of nanoemulsions in the biological environment and during storage shelf life [25]. pH of drug loaded nanoformulations were in range of 5.0–8.0 for F1 to F3 nanoemulsions (Table 1) which were in the acceptable range as adequate and non-irritant for skin use [32, 33]. Conductivity of F1–F3 nanoemulsions were: 0.544, 0.589 and 0.485 mS/cm, respectively. Conductivity depicts ability of emulsions to pass electric current [34]. Similar observations were noticed by Fraooq et al. [35] while formulating miconazole nanoemulsions for treatment of Candidiasis albicans. As illustrated in the photos of NEs, F1 NE was more turbid than F2 NE which may be due to more particle size of droplets. Various factors like: shape, size and concentration have been attributed to optical properties of NEs [36, 37].
Notably, when drugs were encapsulated, all nanoemulsions depicted decreased absorbance when compared to pure drug UV-spectra, indicating that drugs were effectively encapsulated during preparation of NEs. It was coverage of mixed surfactant and co-surfactant that reduced the absorbance of pure drugs when encapsulated in nanoemulsions [11]. Similar observation has been noticed by Laxmi et al. [25] upon encapsulating artemether O/W nanoemulsions. In fluorescent studies, pure drug displayed one major peak at 500 nm and minor peak at 450 nm (Fig. 3). Upon drug encapsulation, a sharp increase fluorescent emission was noticed. Boni et al. [38] also reported exorbitant increase in fluorescent signals in micro-emulsions. Authors claimed nano-droplets act as optical signal amplifier thus enhanced the scattering of light was observed. Functional group analysis of pure drugs and NE encapsulated drugs was studied byFourier Transform Infrared Spectrophotometer (FT-IR) [39].This powerful tool depicts various kinds of chemical bonds in samples by generating an IR spectrum. FT-IR gives energy levels that proportionate to intermolecular interaction, molecular structure, and the type of chemical bonding. FT-IR was additional, used to assess encapsulation of drug with in nanoemulsions [25].When compared to pure drug, NEs showed a dissimilar FT-IR spectrum. It was also evident that there was peak, which was formed when drug was encapsulated (Fig. 4). For e.g.: in all NEs, one prominent band at 1600 cm−1 was observed which was absent in pure drugs. Some major and minor peaks which were present in pure drug IR spectrum were disappeared upon encapsulation. Emergence of new FT-IR bands indicated that azole drugs were efficiently encapsulated in nano-emulsions droplets. Similar observations have been noticed earlier by researchers using essential oils while encapsulating synthetic drugs [11, 36].
All formulated F1–F3 NEs were evaluated for stability analysis. In drug delivery system, stability of NEs is pivotal factor to practice as anti-microbial agents [25, 36]. The F1-F3 nanoemulsions were assessed in centrifugation analysis. Centrifugal studies results indicated that, no precipitation of drug, phase inversion, cracking or creaming. pH values also showed almost similar results before and after storage at different conditions (data not shown), indicating that encapsulation prevented degradation of bioactives upon storage. pH values are very important in determining stability of nanoemulsions. Change in the pH value indicates probable chemical reaction occurrence upon storage that can affect final product quality [40].To determine long-term steadiness and shelf -life and of F1–F3 NEs, stability of NEs were studied at three different temperatures for 60 days. In F1, F2 and F3NEssize of droplet hardly changed at − 20 °C and 4 °C, but, Ostwald Repining (OR) was noticed at 37 °C. OR is a process in which smaller droplet merged to bigger ones owing to diffusion of nano-droplets via prevailing aqueous phase [41].These results were in consonance of earlier studies [42] citing that NEs are often prone to creaming, and due to OA. Hence, cold environments were obligatory to avert, Ostwald flocculation, ripening and coalescence. It indicated that F1–F3 NEs were stable over 60 days at 4 °C and could be best candidates for industrial applications.
Antifungal potential of formulated NEs revealed that F1NEs depicted high antifungal activity against Aspergillus strains MTCC 277, MTCC 343 as observed by zone of inhibition (ZOI). F2 and F3 NEs also displayed high fungal inhibition against MTCC 277. Notably, against fungal strain MTCC 343, total inhibition in growth was observed. Against Mucor spp strain MTCC 3373, although no clear ZOI was observed, however, visual pictures clearly showed substantial inhibition in fungal growth was observed when compared to negative control (supplementary Figure 3). Notably, it was further observed that F2 NE displayed synergetic effect in antifungal activity against A. niger and Mucor spp strains. From ZOI values when compared with pure drug (+ control), it was apparent that was antifungal activity of pure drug increased upon encapsulation. Similar kind of observations on dramatic increase in the antimicrobial activities have been reported in synthetic drugs Ifosfamide, Epirubicin when encapsulated essential oils from Citrus limonum Risso, Salvia officinalis L, Origanumm ajorana [15, 43, 44]. Fluconazole and itraconazole are triazole derivative based antifungals whereas ketoconazole is imidazole-derivative antifungal drug. The possible anti-fungal mechanism is that these entire drug loaded NEs exert its antifungal activity by inhibiting the synthesis of ergosterol, the principal sterol in the fungal cell membrane. It is thereby alter fungal membrane functions, integrity and permeability thus leakage of essential elements (e.g., amino acids, potassium), and impaired uptake of precursor molecules e.g., purine and pyrimidine precursors to DNA) [45, 46].
DEE improved considerably coupled with reducing the size of nano-droplets. Previously, it was reported that that nano-emulsion drop size has a pivotal effect on the DEE [47].NEs having less size can have more DEE in emulsion systems which may be ascribed to condensed mean droplet diameter [48]. It was observed that due to low water solubility and lipophilic nature, pure drug showed In vitro drug release of 30–45% at 72 h, while F1-F3NEsDR was 62%-92% in 72 h. This could be accredited due to small size of nano-droplets and eventually larger surface area of small droplets of nanoemulsions which provided nanoemulsions improved release profile than plan pure drug. To depict release pattern of drug from NEs, correlation coefficient (R2) value was studied. For F1-F3NEs R2 value was 0.92 (F1), 0.86 (F2) and 0.88 (F3), indicating that formulations followed Korsmeyer–Peppas model. The exponent release diffusion values were: n = 0.64 (F1), and 0.69 (F2) and 0.68 (F3), that entitles that formulations followed Fickian diffusion. Our results were in consonance with earlier study reporting artemethernano-emulsions [25]. In present study we were unable to ascertain role F1-F3 nanoformulations using some in vivo models. Hence further studies may be required using in vivo models, which was limitation of current study, to ascertain the role of drug loaded NEs.
5 Conclusion
Formulations were kinetically stable and morphologically separate, circular and good. F2 nanoemulsions size and turbidity was less than all other formulations. Storage stability results indicated that F1-F3 NEs were stable for 60 days. Globule size of nanoemulsions ranged from 245 to 415 nm along with zeta potential from − 9.20 to − 25.4 mV. Encapsulation efficiency of drug loaded nanoemulsions was ranged from 40 to 50%. Considerable anti-aspergillosis and anti-mucormycosis activities were detected. Among all formulations, F1NEs depicted high antifungal activity against Aspergillus strains MTCC 277, MTCC 343 as observed by zone of inhibition (ZOI) values. Against Mucor spp strain MTCC 3373, visual pictures clearly showed substantial inhibition in fungal growth. NEs displayed decent antiaspergillosis and antimucormycosis activities. Overall, it was suggested that formulated drug loaded eucalyptus essential oil based NEs in pharmaceutical based industries to conceptualize antifungal drugs in future. Further studies may be required using in vivo models to ascertain the role of drug loaded NEs.
Availability of data and materials (data transparency)
Not applicable.
References
Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B (2020) Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med 8:e48–e49
Chang CC, Senining R, Kim J, Goyal R (2020) An acute pulmonary Coccidioido mycosisco infection in a patient presenting with multifocal pneumonia with COVID-19. J Investig Med High Impact Case Rep 8:2324709620972244
Ventoulis I, Sarmourli T, Amoiridou P, Mantzana P, Exindari M, Gioula G (2020) Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of Saccharomyces in the ICU. J Fungi (Basel) 6:98
John TM, Jacob CN, Kontoyiannis DP (2021) When Uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel) 15(7):298
Schweer KE, Bangard C, Hekmat K, Cornely OA (2014) Chronic pulmonary aspergillosis external icon. Mycoses 57:257–270
Reid G, Lynch JP 3rd, Fishbein MC, Clark NM (2020) Mucormycosis. Semin Respir Crit Care Med 41:99–114
Nazzaro F, Fratianni F, Coppola R, De Feo V (2017) Essential oils and antifungal activity. Pharmaceuticals 10:86
Canuto MM, Gutiérrez F (2002) Antifungal drug resistance to azoles and polyenes. Lancet Infect Dis 2:550–563
Rosato A, Vitali C, Piarulli M, Mazzotta E, Argentieri MP, Mallamaci R (2009) In vitro synergic efficacy of the combination of Nystatin with the essential oils of Origanum vulgare and Pelargonium graveolens against some Candida species. Phytomedicine 16:972–975
Jaiswal M, Dudhe R, Sharma PK (2015) Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 5:123–127
Yan H, Bao CC, Yu C, Kong D, Shi J, Lin Q (2019) Preparation of biodiesel oil-in-water nanoemulsions by mixed surfactants for bifenthrin formulation. RSC Adv 9:11649–11658
Nagajyothi M, Pramod K, Bijin EN, Jomon N, Baby J (2015) Nanoemulsified system of a poorly water soluble drug. Res J Pharm Dosage Form Technol 7:169–174
Ines GRM, Juliana MC, Araceli OM, Laura ST, Olga MB (2015) Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll 52:438–446
Bonferoni MC, Rossi S, Sandri G et al (2019) Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics 11:84
Sharma M, Grewal K, Jandrotia R, Batish DR, Singh HP, Kohli RK (2022) Essential oils as anticancer agents: potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed Pharmacother 146:112514
Samimi MS, Mahboobian MM, Mohammadi M (2021) Ocular toxicity assessment of nanoemulsion in-situ gel formulation of fluconazole. Hum ExpToxicol 40(12):2039–2047
Saw PE, Soyoung L, Sangyong J (2019) Naturally occurring bioactive compound-derived nanoparticles for biomedical applications. Danced Therap 2:1800146
Attallah OA, Shetta A, Elshishiny F, Mamdouh W (2020) Essential oil loaded pectin/chitosan nanoparticles preparation and optimization via Box–Behnken design against MCF-7 breast cancer cell lines. RSC Adv 10:8703–8708
Ishfaq A, Shukla S, Beraiya S, Tripathi SK (2018) Biochemical and pharmacological applications of essential oils in human health especially in cancer prevention. Anti-Cancer Agents Med Chem 18:1815–1827
Figueiredo AC (2017) Biological properties of essential oils and volatiles: sources of variability. Nat Volatiles Essent Oils 4:1–13
Hermana A, Herman AP (2014) Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: a review. J Pharm Pharmacol 67:473–485
Marena GD, dos Santos Ramos MA, Carvalho GC, Junior JAP, Resende FA, Corrêa I, Ono GYB, Araujo VHS, de Camargo BAF, Bauab TM, Chorilli M (2022) Natural product-based nanomedicine applied to fungal infection treatment: a review of the last 4 years. Phyother Res 36:2710–2745
Mishra L, Gupta S (2021) Fluconazole and curcumin loaded nanoemulsion against multiple drug resistance dermatophytes. Biomed Pharmacol J 14(4):2085–2094
Vörös-Horváth B, Das S, Salem A, Nagy S, Böszörményi A, Kőszegi T, Pál S, Széchenyi A (2020) Formulation of tioconazole and Melaleuca alternifolia essential oil pickering emulsions for onychomycosis topical treatment. Molecules 25:5544
Laxmi M, Bhardwaj A, Mehta S, Mehta A (2015) Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif Cells Nanomed Biotechnol 43(5):334–344
Agnish S, Sharma AD, Kaur I (2022) Nanoemulsions (O/W) containing Cymbopogon pendulus essential oil: development, characterization, stability study, and evaluation of in vitro anti-bacterial, anti-inflammatory, anti-diabetic activities. BioNanoSci 12:540–554
Qian C, Decker EA, Xiao H, McClements DJ (2012) Nanoemulsion delivery systems: influence of carrier oil on β-carotene bioaccessibility. Food Chem 135:1440–1447
Silva HD, Cerqueira MA, Vicente AA (2015) Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J Food Eng 167:89–98
Baboota S, Shakeel F, Ahuja A, Ali J, Shafiq S (2007) Design development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm 57:315–332
Rajalaksmi R, Mahesh K, Ashok Kumar CK (2011) A critical review on nano emulsions. Int J Innov Drug Discov 1(1):1–8
Mahajan HS, Dinger SB (2011) Design and In vitro evaluation of nanoemulsion for nasal delivery of artemether. IJNDD 3:272–277
Hashem FM, Shaker DS, Ghorab MK et al (2011) Formulation, characterization, and clinical evaluation of microemulsion containing clotrimazole for topical delivery. AAPS PharmSciTech 12:879–886
Morsi NM, Mohamed MI, Refai H, El Sorogy HM (2014) Nanoemulsion as a novel ophthalmic delivery system for acetazolamide. Int J Pharm PharmSci 6:227–236
Talegaonkar S, Tariq M, Alabood RM (2015) Design and development of O/W nanoemulsion for the transdermal delivery of ondansetron. Bull Pharm Res 1:18–30
Farooq U, Akhtar R, Muhammad Z, Ghulam A, Maria R, Farman A, Shabbir A, Zeeshan J, Zoya A, Humayun R, Rana K, Mahmood A, Shayan M, Naseem A, Kanwal A (2021) Nanoemulsions as novel nanocarrieres for drug delivery across the skin: in-vitro, in-vivo evaluation of miconazole nanoemulsions for treatment of Candidiasis albicans. Des Monomers Polym 24(1):240–258
Kumar A, Kanwar R, Mehta SK (2021) Eucalyptus oil-based nanoemulsion: a potent green nanowagon for controlled delivery of emamectin benzoate. ACS Agric Sci Technol 2:76–88
Tomaszewsk E, Soliwod K, Kadziol K, Tkacz-Szczesn B, Celichowski G, Cichomski M, Szmaj W, Grobelny J (2013) Detection limits of DLS and UV–Vis spectroscopy in characterization of polydisperse nanoparticles colloids. Nanomaterials 2013:1–10
Boni M, Nastasa V, Andrei IR, Staicu A, Pascu M (2015) Enhanced fluorescence emitted by microdroplets containing organic dye emulsions”. Biomicrofluidics 24:014126
Okpo S, Otaraku IJ (2020) GC-FID and FT-IR characterization of lemongrass oil extracted with SOXHLET extraction apparatus using ethanol as solvent. IOSR J Eng 10:33–38
Bernardi DS, Daniela Pereira TA, Maciel NR, Bortoloto J, Viera GS, Oliveira GC, Rocha-Filho PA (2011) Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J Nanobiotechnology 9:44
Ziani KH, Chang Y, McLandsborough L, McClements DJI (2011) Influence of surfactant charge on antimicrobial efficacy of surfactant-stabilized thyme oil nanoemulsions. J Agric Food Chem 59:6247–6255
Kim J, Gao Y, Hebebrand C, Peirtsegaele P, Helgeson ME (2013) Polymer–surfactant complexation as a generic route to responsive viscoelastic nanoemulsions. Soft Matter 9:6897
Dangkong D, Limpanasithikul W (2015) Effect of citral on the cytotoxicity of doxorubicin in human B-lymphoma cells. Pharm Biol 53:262–268
Erdogan A, Ozkan A (2013) A comparative study of cytotoxic, membrane and DNA damaging effects of Origanum majorana’s essential oil and its oxygenated monoterpene component linalool on parental and epirubicin-resistant H1299 cells. Biologia 68:754–761
Allen D, Wilson D, Drew R, Perfect J (2015) Azole antifungals: 35 years of invasive fungal infection management. Expert Rev Anti Infect Ther 13:787–798
Gao T, Zhou H, Zhou W, Hu L, Chen J, Shi Z (2016) The fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules 21(6):770
Wen-Chien Lu, Huang D-W, Chiun-C R, Wang C-H, Tsai J-C, Huang Y-T, Li P-H (2018) Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal 26:82–89
Jena S, Das H (2006) Modeling of particle size distribution of sonicated coconut milk emulsion: effect of emulsifiers and sonication time. Food Res Int 39:606–611
Acknowledgements
Not applicable.
Funding
Dept of Science and Technology, Govt. of India, DST/SEED/SCSP/STI/2019/253.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate (include appropriate approvals or waivers)
Not applicable.
Consent for publication (include appropriate statements)
Yes.
Competing interests (include appropriate disclosures)
Nil.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, A.D., Kaur, I. & Chauhan, A. Anti-aspergillosis and anti-mucormycosis potential of eucalyptus essential oil based O/W nanoemulsions containing azole based drugs from Eucalyptus globulus. J.Umm Al-Qura Univ. Appll. Sci. 10, 313–329 (2024). https://doi.org/10.1007/s43994-023-00108-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00108-8