Abstract
Ferulic acid (FA), also known as hydroxycinnamic acid, is a sweet-smelling and natural antioxidant present in the cell walls of plants and bran of cereals, making it a common dietary component. FA possesses diverse medicinal properties, including anti-inflammatory, anti-arrhythmic, antioxidant, anti-bacterial, anti-thrombotic, anti-cancer, anti-diabetic, anti-cardiovascular, neuroprotective, anti-apoptotic, antifibrotic, anti-platelet, anti-aging, anti-melanogenesis, angiogenesis promoting, and skin damage reducing effects. Judging by these outstanding credentials of FA, this study investigated the hepatoprotective, spermato-protective, and hemato-protective effects of FA against 2MEETH-induced spermatotoxicity, hematotoxicity, and hepatotoxicity in rats. After oral administration of 2MEETH for 30 days, the number of abnormal and dead spermatozoa, as well as serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and activity of alcohol dehydrogenase (ADH), were significantly increased. In contrast, the number of normal and live spermatozoa, sperm motility and concentration, serum total cholesterol level, red blood cell (RBC), packed cell volume (PCV), and white blood cell (WBC) counts were significantly decreased compared to the control group. FA treatments did not have any effect on all the sperm and hematological parameters checked, but significantly lowered the serum levels of AST, ALT, and ADH activity compared to rats administered with only 2MEETH. Based on the results, it is concluded that FA may not possess spermato- and hemato-protective effects against 2MEETH-induced spermatotoxicity and hematotoxicity but may possess a hepatoprotective effect in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cellular oxidative stress ensues due to the imbalance between the endogenous antioxidant defence setup and the generation of free radicals, which favours the latter. In the testes, the excessive free radicals generated attack the cellular macromolecular components, leading to oxidative tissue damage [1,2,3]. These cellular alterations are caused by the negative consequences of free radicals such as the hydroxyl radical (HO), hydrogen peroxide (H2O2), and superoxide anion radical (O2) [4]. Sperm and germ cells are prone to oxidative stress due to the availability of high amounts of very long-chain and long-chain unsaturated fatty acids, making them susceptible to free radical-induced lipid peroxidation (LPO) and cellular damage [4, 5]. LPO is extremely harmful to the function of sperm and the membrane structure of germ cells, causing several cytopathological and morphological alterations [6,7,8], as well as cell death [9, 10]. Also, LPO affects sperm motility, thereby compromising the ability of sperm to fertilize an egg [11, 12]. In addition to motility, both concentration and morphology are other sperm indices affected by excessive free radical production [13]. In the testes, there is a tendency for altered tissue physiology or DNA damage due to elevated free radical generation, which is potentially risky to reproduction in males [14, 15]. Hence, guarding sperm synthesis and function from oxidative stress is very important.
2-Methoxyethanol (2MEETH) has a wide range of applications in industries, including those that produce and use textile dyes, varnishes, printing inks, and anti-icing additives [16]. Organs deleteriously affected by 2MEETH include the thymus, bone marrow, foetus, and testes [17]. Exposure to 2MEETH has been documented to diminish white blood cell count, cause developmental problems, and lead to destruction of the testes and thymocytes [18]. 2MEETH-induced teratogenicity and embryotoxicity occur following its metabolism to 2-methoxyacetic acid [19] by alcohol dehydrogenase and 2-methoxyacetaldehyde dehydrogenase [20]. The product of this metabolism, 2-methoxyacetic acid, is readily detectable in body fluids of exposed individuals and is eliminated in urine [21]. As a teratogen, 2MEETH can cause hematopoietic and reproductive system toxicities in humans and other species of animals [22]. 2MEETH targets and damages the testes, which is a hallmark of its acute toxicity [23, 24]. Administration of 2MEETH for at least 2 days (at 0.5 g/kg) significantly reduces the weight of the testes, while degenerated pachytene spermatocytes are observed within a day when 0.1 g/kg/day of 2MEETH is administered [25]. Repeated administration of 2MEETH causes continuous spermatocyte deterioration [25] due to cellular activation of apoptosis in the testes [26]. 2MEETH exposures (at 2.6 mg/m3) in painters working in a shipyard have been reported to cause severe alterations in spermatogenesis, leading to oligospermia and azoospermia, as well as a lowered spermatozoa count [27].
Ferulic acid (FA; C10H10O4; 194.18 g/mol) is a phenolic acid that was first extracted from Ferula foetida [28]. FA is a highly abundant and ubiquitous phytocompound derived from cinnamic acid that naturally exists in the cell walls of plants, where it is covalently attached as side chains [28]. As a by-product of phenylalanine and tyrosine metabolism, FA is present in every plant and is relatively non-toxic. It possesses diverse medicinal properties, including anti-inflammatory, anti-arrhythmic, antioxidant, anti-bacterial, anti-thrombotic, anti-cancer, anti-diabetic, anti-cardiovascular, and neuroprotective effects [29]. The presence of three different structural or functional group attachments to the benzene ring in the FA structure could be responsible for its ability to scavenge free radicals [30]. Some examples of foods, vegetables, and fruits rich in FA include sweet corn, oranges, carrots, tomatoes, rice bran, whole grains, grapes, parsley, spinach, rhubarb, and cereals such as barley, rye, oats, and wheat. Unlike other phenolic acids, FA is swiftly absorbed and remains in the blood for a longer time.
Cis-FA is a yellow oil that exhibits its highest UV absorption at 316 nm, while trans-FA has two absorption peaks at 284 and 307 nm. Previous studies have shown that FA interacts with UV filters, leading to positive effects on the functional properties of sunscreen [31]. Through in vitro assessments of sun protection factor (SPF), critical wavelength, and UV transmittance, we established these interactions. Additionally, ferulic acid significantly influenced the in vitro antioxidant activity, resulting in a remarkable 90% increase in antioxidant potential [31]. The experimental design analysis conclusively demonstrated the synergistic relationship between UV filters and FA [32]. In terms of in vivo application, researchers have provided evidence of FA's effectiveness in mitigating the harmful effects of UV radiation, such as erythema, photoaging, and skin cancer [31]. Three separate in vivo studies utilized a solar simulator to induce inflammatory reactions and incorporated FA into topical solutions containing vitamins [31]. This incorporation not only enhanced the chemical stability of the vitamins but also augmented the photoprotective effects by reducing levels of erythema and apoptosis of corneocytes [33,34,35].
The present study aimed to investigate the effect of FA on 2MEETH-induced hematotoxicity, spermatotoxicity, and tissue damage in rats, based on the previously mentioned medicinal properties of FA.
2 Materials and methods
2.1 Substances administered, chemicals and kits
The administered toxicant, 2MEETH (C3H8O2), was produced by BDH Laboratory Supplies in Poole, England. The phenolic acid used for treatment, FA (C10H10O4; CAS: 1135–26-6), was produced by AK Scientific in Union City, CA, USA. All other chemicals used were of analytical grade. Diagnostic kits for the estimation of total cholesterol, AST, and ALT were produced by Randox Laboratories Ltd. in the United Kingdom.
2.2 Experimental animal and the study design
Male experimental rats weighing 200 g (n = 20) were used in this study. They were housed in the animal facility of our department and provided with constant access to rat feed and potable drinking water. After acclimatization for four weeks, the rats were divided into four groups, each consisting of five animals, and were administered the test substances as shown in Table 1 below.
2.3 Sacrifice, blood sample collection and processing
On the 31st day, the rats were euthanized in accordance with documented guidelines for the handling and utilization of research animals [38]. All handling of the animals was carried out humanely. Blood samples were collected from each rat via the retroorbital sinus and divided into 10 mL plain tubes and 5 mL EDTA bottles. The samples in the former were centrifuged at 3000 rpm for 10 min, and the resulting supernatant (serum) was used to determine the levels of various biochemical parameters. The samples in the EDTA bottles were used for haematological analyses. In addition, the spermatic cord of each rat was excised, and sperm cells were collected to determine various sperm characteristics and morphologies.
2.4 Sperm analyses
2.4.1 Estimation of sperm concentration, motility, viability (live and dead), normal and abnormal cells
The sperm concentration of the rats was determined using an improved Neubauer hemocytometer (LABART, Germany), based on the protocol of Zemjanis [39]. Sperm motility was assessed according to the method of Cheesbrough [40]. The viability of sperm cells (dead and live) was determined using the Eosin-Nigrosin stain as described by Zemjanis [39]. Sperm morphological abnormalities (abnormal and normal sperm cells) were evaluated using the method described by Wells and Awa [41].
2.5 Hematological assays (PCV, WBC and RBC)
The percentage packed cell volume (PCV) as well as the white blood cell (WBC) and red blood cell (RBC) counts of each rat were estimated using the protocol described by Dacie and Lewis [42].
2.6 Estimations of serum levels of total cholesterol, AST, ALT and activity of serum ADH
The levels of AST and ALT in the serum of rats were estimated following the protocols provided by the manufacturer for each diagnostic kit. The activity of ADH in the serum was assessed using the method described by Walker [43].
2.7 Statistical analyses
For each biochemical assay, the data obtained were analysed using ANOVA. The level of significance among the different groups (n = 5) was determined using Tukey's test in version 8 of GraphPad Prism. The results are presented as mean ± standard deviation in bar charts. Only p values < 0.05 were considered statistically significant.
3 Results
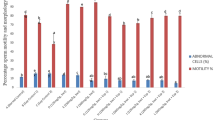
3.1 Effect of FA treatments on abnormal sperm cell count in 2MEETH-administered rats
The percentage of abnormal sperm cells was significantly increased (p < 0.05) following administration of 2MEETH compared to the percentage recorded in control rats (Fig. 1). Treatment with FA (2MEETH + FA) did not result in a significant effect (p > 0.05) compared to rats exposed to 2MEETH only.
Effect of FA treatments on number of abnormal sperm cells in 2MEETH administered rats. Each of the bars stands for mean ± standard error of mean. *Significantly different compared with control (p < 0.05); #significantly different compared with 2MEETH only (p < 0.05). 2MEETH 2 methoxyethanol, FA ferulic acid
3.2 Effect of FA treatments on normal sperm cell count in 2MEETH-administered rats
The percentage of normal sperm cells was significantly decreased (p < 0.05) following administration of 2MEETH compared to the percentage recorded in control rats (Fig. 2). Treatment with FA (2MEETH + FA) did not result in a significant effect (p > 0.05) compared to rats exposed to 2MEETH only.
Effect of FA treatments on number of normal sperm cells in 2MEETH administered rats. Each of the bars stands for mean ± standard error of mean. *Significantly different compared with control (p < 0.05); #significantly different compared with 2MEETH only (p < 0.05). 2MEETH 2 methoxyethanol, FA ferulic acid
3.3 Effect of FA treatments on dead sperm cell count in 2MEETH-administered rats
The percentage of dead sperm cells was significantly increased (p < 0.05) following administration of 2MEETH compared to the percentage recorded in control rats (Fig. 3). Treatment with FA (2MEETH + FA) did not result in a significant effect (p > 0.05) compared to rats exposed to 2MEETH only.
Effect of FA treatments on number of dead sperm cells in 2MEETH administered rats. Each of the bars stands for mean ± standard error of mean. *Significantly different compared with control (p < 0.05); #significantly different compared with 2MEETH only (p < 0.05). 2MEETH 2 methoxyethanol, FA ferulic acid
3.4 Effect of FA treatments on live sperm cell count in 2MEETH-administered rats
The percentage of live sperm cells was significantly decreased (p < 0.05) following administration of 2MEETH compared to the percentage recorded in control rats (Fig. 4). Treatment with FA (2MEETH + FA) did not result in a significant effect (p > 0.05) compared to rats exposed to 2MEETH only.
Effect of FA treatments on number of live sperm cells in 2MEETH administered rats. Each of the bars stands for mean ± standard error of mean. *Significantly different compared with control (p < 0.05); #significantly different compared with 2MEETH only (p < 0.05). 2MEETH 2 methoxyethanol, FA ferulic acid
3.5 Effect of FA treatments on motility of sperm cells in 2MEETH-administered rats
The percentage of motile sperm cells was significantly decreased (p < 0.05) following administration of 2MEETH compared to the percentage recorded in control rats (Fig. 5). Treatment with FA (2MEETH + FA) did not result in a significant effect (p > 0.05) compared to rats exposed to 2MEETH only.
3.6 Effect of FA treatments on sperm concentration in 2MEETH-administered rats
The concentration of sperm was significantly reduced (p < 0.05) following 2MEETH administration compared to the sperm concentration in control rats (Fig. 6). Moreover, treatments with FA (2MEETH + FA) did not exhibit any significant effect (p > 0.05) compared to rats exposed to 2MEETH alone.
Effect of FA treatments on sperm cell concentration in 2MEETH administered rats. Each of the bars stands for mean ± standard error of mean. *Significantly different compared with control (p < 0.05); #significantly different compared with 2MEETH only (p < 0.05). 2MEETH 2 methoxyethanol, FA ferulic acid
3.7 Effect of FA treatments on RBC, WBC and PCV in 2MEETH-administered rats
The RBC (Fig. 7), WBC (Fig. 8), and PCV (Fig. 9) of rats were significantly decreased (p < 0.05) following 2MEETH administration compared to control rats. Furthermore, treatment with FA (2MEETH + FA) resulted in a significant (p < 0.05) additional reduction in RBC, WBC, and PCV levels in rats compared to those exposed to 2MEETH alone.
3.8 Effect of FA treatments on serum total cholesterol concentration in 2MEETH administered rats
The concentration of total cholesterol in the serum was significantly decreased (p < 0.05) in rats administered 2MEETH compared to the concentration recorded in control rats (Fig. 10). Treatment with FA (2MEETH + FA) further significantly reduced (p < 0.05) the serum total cholesterol of rats compared to those administered 2MEETH only.
Effect of FA treatments on serum total cholesterol in 2MEETH administered rats. Each of the bars stands for mean ± standard error of mean. *Significantly different compared with control (p < 0.05); #significantly different compared with 2MEETH only (p < 0.05). 2MEETH 2 methoxyethanol, FA ferulic acid
3.9 Effect of FA treatments on serum levels of ALT and AST in 2MEETH administered rats
The levels of serum ALT (Fig. 11) and AST (Fig. 12) were significantly elevated (p < 0.05) by 2MEETH administrations in rats compared to the control group. However, co-administration of FA and 2MEETH (2MEETH + FA) significantly reduced (p < 0.05) the serum levels of these markers of tissue damage compared to rats administered with 2MEETH only.
3.10 Effect of FA treatments on serum level of ADH in 2MEETH administered rats
The activity of serum ADH (Fig. 13) was significantly elevated (p < 0.05) in rats administered with 2MEETH compared to the control group. However, co-administration of FA and 2MEETH (2MEETH + FA) significantly reduced (p < 0.05) the serum activity of this enzyme involved in metabolizing 2MEETH, compared to rats administered 2MEETH only.
4 Discussion
The presence of free radicals can significantly impact the quality of sperm, affecting parameters such as count, motility, and morphology, leading to reduced fertility [44]. Free radicals cause oxidative stress, which can contribute to abnormal sperm production, decreased sperm count, and fragmented sperm DNA, all leading to infertility. High oxygen pressure can also reduce the rate and motility of sperm, but adding catalase to the culture medium can prevent this effect [11]. Furthermore, high oxygen pressure can increase the production of hydrogen peroxide by spermatozoa, decreasing sperm motility [45]. Reactive oxygen species (ROS) produced by leukocytes or spermatozoa can also have negative effects on sperm function in infertile men. To prevent oxidative stress in sperm, ROS concentrations must be continuously kept low by inactivating them. Antioxidants found mainly in seminal plasma play a crucial role in maintaining sperm health and fertility. If antioxidants are removed from semen, for example during washing, sperm can become vulnerable to oxidative damage. The antioxidant properties of semen allow sperm to combat oxidative stress conditions [46]. Seminal antioxidants can inactivate ROS generated within the semen under normal conditions. An imbalance between ROS production and inactivation of these molecules by seminal antioxidants is believed to be one of the causes of oxidative stress conditions. In this study, 2MEETH-induced alterations in sperm morphology and parameters were recorded. These changes may be attributed to the destruction of testicular cells by 2MEETH, a potent testicular toxicant that produces free radicals, as previously reported by Somade et al. [47] and Somade et al. [48]. Our findings on sperm parameters are consistent with the study of Somade et al. [36], which reported a significant elevation in the number of abnormal and dead sperm cells, as well as a significant reduction in sperm count, and in the number of normal, live, and motile sperm cells in rats. FA treatment did not show any significant therapeutic effect against 2MEETH-induced spermatotoxicity, which we believe may be due to the simultaneous administration of both substances throughout the study duration. Once again, the findings from this study are in line with the previous study reported by Somade et al. [36], which reported the ineffectiveness of the simultaneous administration of 2MEETH and syringic acid for 30 days, leading to a significant elevation in the number of abnormal and dead sperm cells, as well as a significant reduction in sperm count, and in the number of normal, live, and motile sperm cells in rats. However, in contrast to our findings, Hasanein et al. [49] reported the preventive effect of FA against lead-induced suppressed spermatogenesis in rats. We believe that the differences in the potency of toxicity and site of primary attack of the two toxicants investigated in both studies may account for the contrasting results.
Looking at other studies, Yousef et al. [50] observed the presence of various bacteria in fresh bull semen. They discovered that the newly synthesized silver nanoparticles (AgNPs) with carbon content (referred to as AgC NPs) had a minimal inhibitory concentration (MIC), ensuring that they did not negatively impact the measured sperm parameters. Additionally, the application of AgC NPs did not harm the ultrastructure of bull sperm and exhibited limited interaction with the internalization process of sperm. Consequently, it can be concluded that AgC NPs have the potential to serve as an alternative antibiotic agent for bull semen extenders, offering a viable option for cold storage with no significant cytotoxic effects on the sperm. Also, in a study conducted by Carvalho et al. [51], prolonged exposure (34 days) to CBD at doses of 15 and 30 mg/kg negatively affects the quality of sperm, leading to DNA damage and lipid peroxidation in sperm cells. However, there were no observed genotoxic effects in leukocyte cells. The disruption of spermatogenesis, specifically from the meiotic to spermiogenesis stages, due to dysregulation of the endocannabinoid system, along with the induction of oxidative damage in the nuclei of male gametes, suggests that these detrimental effects may contribute to the decline in sperm quality in CBD-treated mice. Additionally, CBD's impact on sperm motility can be partially attributed to its inhibition of mitochondrial activity, resulting in a deprivation of energy for the exposed spermatozoa, while Sun et al. [52] reported that the presence of per- and polyfluoroalkyl substances (PFASs) is linked to negative effects on sperm quality, and their toxic mechanisms may contribute to harm in the testicles and epididymis. These mechanisms involve disorders in testosterone synthesis, oxidative stress, changes in membrane lipid composition, and the entry of calcium ions into sperm cells.
Haematopoiesis is a crucial physiological process in mammals that is responsible for the formation of all the cellular components of blood, including platelets, white blood cells (WBCs), and red blood cells (RBCs). These cells play important roles in sustaining life by facilitating tissue oxygenation (RBCs), controlling infections/infestations (WBCs), and maintaining haemostasis (platelets). The PCV, also known as haematocrit, is a measure of the volume of red blood cells in the bloodstream. It is a valuable diagnostic tool for identifying conditions such as polycythaemia (abnormally high red blood cell count) and anaemia (abnormally low red blood cell count) [53]. Haematopoiesis can be influenced by various factors such as chronic and acute diseases [54, 55], malignancies [56], environmental pollutants [57], plant extracts [58], and exposure to conventional drugs [59]. In this study, alterations in the haematological indices (PCV, WBC, and RBC) induced by 2MEETH were observed, marked by a significant decrease in RBC, WBC, and PCV. These effects on the PCV and RBC are indications of 2MEETH's potential to cause anaemia in rats, which has been previously documented by Starek et al. [60] and Adeyemo-Salami and Farombi [61]. White blood cells (WBC) and their differentials, including monocytes, eosinophils, neutrophils, basophils, and lymphocytes, are involved in the body's defence mechanism and are also associated with inflammation [62]. Therefore, the decrease in WBC in this study could be an indication of 2MEETH-induced inflammatory reaction, which may have called for the recruitment of white blood cells to the affected sites in a bid to protect against the 2MEETH-induced injury, which may be responsible for the reduced WBC count in rats. Furthermore, our findings in this study are corroborated by the study of Hamdi et al. [63], which also reported a significant reduction in PCV, WBC, haemoglobin, and RBC counts following exposure of rats to 2MEETH. The inability of FA to protect against 2MEETH-induced hematotoxicity in rats may suggest that the phytocompound lacks hemato-protective capability against the toxicant or that the ineffectiveness of FA could be due to the simultaneous administration with the toxicant throughout the study, as previously reported by Somade et al. [36] in a study that used syringic acid for the treatment of 2MEETH-induced hepatotoxicity in rats.
Cholesterol, a type of lipid, plays various roles, including serving as a precursor in the biosynthesis of bile acids and steroid hormones. It is obtained from foods that contain fat, where it exists as cholesteryl esters, and can also be synthesized mainly in the liver, adrenal cortex, intestine, and reproductive tissues [64]. In most species studied, cholesterol is the primary sterol found in ejaculated sperm, along with other sterols such as cholesterol esters, cholesterol sulphate, cholesta-7,24-dien-3β-ol, desmosterol sulphate, and desmosterol (the immediate precursor of cholesterol) [65,66,67,68,69]. Some of these molecules are believed to be important regulators of sperm function [70]. Our study showed that the administration of 2MEETH significantly reduced serum total cholesterol levels. This may be due to altered cholesterogenesis caused by 2MEETH-induced toxicity, where the rate-limiting enzyme (HMG CoA reductase) could have been inhibited, or the substrate for the enzyme (mevalonic acid) may be unavailable for cholesterol biosynthesis, as reported by Somade et al. [36]. FA’s intervention was not able to restore the serum level of total cholesterol, suggesting that the phytocompound may not have the ability to protect against the 2MEETH-induced alteration in cholesterogenesis following 30 days of simultaneous administration to rats.
Hepatotoxicity refers to damage to liver cells that occurs when they are exposed to substances that are harmful to the liver. Examples of such substances include alcohol, such as ethanol [71], drugs, such as acetaminophen [72], environmental toxins, like dimethyl nitrosamine [73], and certain food additives. This injury impairs liver function and destroys the hepatic cell membrane, leading to the release and increased levels of markers of hepatic function, including AST, alkaline phosphatase, GGT, and ALT into the bloodstream [73]. In this study, the elevation of serum AST and ALT levels induced by 2MEETH was recorded, suggesting the induction of hepatotoxicity in rats following exposure, as previously reported by Somade et al. [36]. FA's intervention demonstrated a hepatoprotective effect against 2MEETH-induced liver damage by lowering the elevated serum levels of liver function enzymes. This hepatoprotective effect may be attributed to the antioxidant and free radical scavenging potentials of FA, previously documented by Esmat et al. [74] against liver injury induced by cisplatin and by Roghani et al. [75] against the hepatotoxicity induced by methotrexate in mice.
ADH is an enzyme that contains zinc and is present in large quantities in the liver. Its function is to facilitate the oxidation of alcohol into aldehyde. Similarly, 2MEETH is metabolized by ADH into 2-methoxyacetaldehyde (2MAD), which is then further oxidized to 2-methoxyacetic acid by aldehyde dehydrogenase [76]. Although the parent compound 2MEETH is not toxic on its own, its metabolite 2-methoxyacetic acid is highly toxic. Therefore, ADH plays an active role in promoting 2MEETH-induced toxicity. Repeated exposure to 2MEETH increases tissue toxicity due to the induction of ADH activity, which forms 2-methoxyacetic acid from 2MEETH [76]. In this study, the elevated serum activity of ADH is a response to 2MEETH exposure since the former is induced for the metabolism of the latter. This exposure may have resulted in liver damage, causing the passage of the enzyme from the liver into the blood of the rats, as previously reported by Somade et al. [36]. Again, FA's intervention demonstrated a hepatoprotective effect against 2MEETH-induced liver damage by lowering the elevated serum level of ADH. This effect may be attributed to the antioxidant and free radical scavenging potentials of FA against the 2MEETH-induced toxicity, which may have protected hepatic cells against the damaging effect of the reactive intermediate (2-methoxyacetic acid), as previously reported for syringic acid against 2MEETH-induced toxicity in rats [36]. The levels of ethanol and acetaldehyde are influenced by the functioning of ADH and ALDH enzymes during the detoxification process. Therefore, FA treatment prevented against the production of free radicals that could arise through the metabolism of alcohol into acetaldehyde and acetate, which has the potential to disrupt the oxidative equilibrium of cells and heighten the susceptibility to oxidative stress [77, 78].
In conclusion, this study recorded 2MEETH-induced hematotoxicity, spermatotoxicity, and hepatotoxicity in rats. However, FA was unable to protect against the 2MEETH-induced hematotoxicity and spermatotoxicity. Nevertheless, it demonstrated a hepatoprotective effect in the rats.
Data availability
All data are included in the manuscript.
References
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Priya PH, Reddy PS (2012) Effect of restraint stress on lead-induced male reproductive toxicity in rats. J Exp Zool A Ecol Genet Physiol 317:455–465
Nirupama M, Devaki M, Nirupama R, Yajurvedi HN (2013) Chronic intermittent stress-induced alterations in the spermatogenesis and antioxidant status of the testis are irreversible in albino rat. J Physiol Biochem 69:59–68
Sikka SC (2001) Relative impact of oxidative stress on male reproductive function. Curr Med Chem 8:851–862
Parodi J (2014) Motility, viability, and calcium in the sperm cells. Syst Biol Reprod Med 60:65–71
Leong CT, D’Souza UJ, Iqbal M, Mustapha ZA (2013) Lipid peroxidation and decline in antioxidant status as one of the toxicity measures of diazinon in the testis. Redox Rep 18:155–164
Farombi EO, Abarikwu SO, Adesiyan AC, Oyejola TO (2013) Quercetin exacerbates the effects of subacute treatment of atrazine on reproductive tissue antioxidant defence system, lipid peroxidation and sperm quality in rats. Andrologia 45:256–265
Collodel G, Federico MG, Geminiani M, Martini S, Bonechi C, Rossi C, Figura N, Moretti E (2011) Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod Toxicol 31:239–246
Mupfiga C, Fisher D, Kruger T, Henkel R (2013) The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst Biol Reprod Med 59:304–311
Maheshwari A, Misro MM, Aggarwal A, Sharma RK, Nandan D (2009) Pathways involved in testicular germ cell apoptosis induced by H2O2 in vitro. FEBS J 276:870–881
Aitken RJ, Clarkson JS (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 81:459–469
Aitken RJ (1999) The Amoroso Lecture. The human spermatozoon—a cell in crisis? J Reprod Fer 115:1–7
Agarwal A, Mulgund A, Sharma R, Sabanegh E (2014) Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med 60:206–216
Sepaniak S, Forges T, Fontaine B, Gerard H, Foliguet B, Guillet-May F, Zaccabri A, Monnier-Barbarino P (2004) Negative impact of cigarette smoking on male fertility: from spermatozoa to the offspring. J Gynecol Obstet Biol Reprod (Paris) 33:384–390
Doreswamy K, Muralidhara, (2005) Genotoxic consequences associated with oxidative damage in testis of mice subjected to iron intoxication. Toxicol 206:169–178
Somade OT, Ajayi BO, Olushola MO, Omoseebi EO (2020) Methyl cellosolve-induced renal oxidative stress and time-dependent upregulation of pro-inflammatory cytokines, apoptotic, and oncogenic markers in rats. Toxicol Rep 7:779–787
Beattie PJ, Brabec MJ (1986) Methoxyacetic acid and ethoxyacetic acid inhibit mitochondrial function in vitro. J Biochem Toxicol 1:61–70
Boatman RJ (2005) International industry initiatives to improve the glycol ether health effects knowledge base. Toxicol Lett 156:39–50
Sleet RB, Greene JA, Welsch F (1988) The relationship of embryotoxicity to disposition of 2-methoxyethanol in mice. Toxicol Appl Pharmacol 93:195–207
Welsch F (2005) The mechanism of ethylene glycol ether reproductive and developmental toxicity and evidence for adverse effects in humans. Toxicol Lett 156:13–28
Groeseneken D, Veulemans H, Masschelein R, Van Vlem E (1989) Experimental human exposure to ethylene glycol monomethyl ether. Int Arch Occup Environ Health 61:243–247
Chapin RE, Lamb JC (1984) Effects of ethylene glycol monomethyl ether on various parameters of testicular function in the F344 rat. Environ Health Perspect 57:219–224
Holladay SD, Comment CE, Kwon J, Luster MI (1994) Fetal hematopoietic alterations after maternal exposure to ethylene glycol monomethyl ether: prolymphoid cell targeting. Toxicol Appl Pharmacol 129:53–60
Li LH, Wine RN, Chapin RE (1996) 2-Methoxyacetic acid (MAA)-induced spermatocyte apoptosis in human and rat testes: an in vitro comparison. J Androl 17:538–549
Foster PM, Creasy DM, Foster JR, Gray TJ (1984) Testicular toxicity produced by ethylene glycol monomethyl and monoethyl ethers in the rat. Environ Health Perspect 57:207–217
Ku WW, Wine RN, Chae BY, Ghanayem BI, Chapin RE (1995) Spermatocyte toxicity of 2-methoxyethanol (ME) in rats and guinea pigs: evidence for the induction of apoptosis. Toxicol Appl Pharmacol 134:100–110
Welch LS, Schrader SM, Turner TW, Cullen MR (1988) Effects of exposure to ethylene glycol ethers on shipyard painters: II. Male reproduction. Am J Ind Med 14:509–526
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep 4:86–93
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40:92–100
Itagaki S, Kurokawa T, Nakata C, Saito Y, Oikawa S, Kobayashi M, Hirano T, Iseki K (2009) In vitro and in vivo antioxidant properties of ferulic acid: a comparative study with other natural oxidation inhibitors. Food Chem 114:466–471
Peres DD, Sarruf FD, de Oliveira CA, Velasco MVR, Baby AR (2018) Ferulic acid photoprotective properties in association with UV filters: multifunctional sunscreen with improved sunscreen with improved SPF and UVA-PF. J Photochem Photobiol B Biol 185:46–49
Peres DD, Ariede MB, Candido TM, de Almeida TS, Lourenço FR, Consiglieri VO, Kaneko TM, Velasco MVR (2017) Quality by design (QbD), process analytical technology (PAT), and design of experiment applied to the development of multifunctional sunscreens. Drug Dev Ind Pharm 43:246–256
Lin FH, Lin JY, Gupta RD, Tournas JA, Burch JA, Selim MA, Monteiro-Riviere NA, Grichnik JM, Zielinski J, Pinnell SR (2005) Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol 125:826–832
Oresajo C, Stephens T, Hino PD, Law RM, Yatskayer M, Foltis P, Sreekumar P, Pinnell SR (2008) Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J Cosmet Dermatol 7:290–297
Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, Paolino D, Bonina F (2000) In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm 199:39–47
Somade OT, Oyinloye BE, Ajiboye BO, Osukoya OA, Adeyi OE (2022) Effect of syringic acid on steroid and gonadotropic hormones, hematological indices, sperm characteristics and morphologies, and markers of tissue damage in methyl cellosolve-administered rats. Biochem Biophys Rep 32:101360
Chowdhury S, Ghosh S, Das AK, Sil PC (2019) Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front Pharmacol 10:27
NRC (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington
Zemjanis R (1970) Collection and evaluation of semen. In: Zemjanis R (ed) Diagnostic and therapeutic techniques in animal reproduction, 2nd edn. Williams and Wilkins Co, Baltimore, pp 139–156
Cheesbrough M (2000) Laboratory Practice in Tropical Countries, Cambridge university low price edition, pp. 130–132.
Wells ME, Awa OA (1970) New technique for assessing acrosomal characteristics of spermatozoa. J Diary Sci 53:227
Dacie JV, Lewis SM (1991) Practical Haematology, 7th edn. ECBS, London, p 37
Walker JRL (1992) Spectrophotometric determination of enzyme activity: alcohol dehydrogenase (ADH). Biochem Educ 20:42–43
Saleh RA, Agarwal A (2002) Oxidative stress and male infertility: from research bench to clinical practice. J Androl 23:737–752
Baker MA, Aitken RJ (2004) The importance of redox reguy lated pathways in sperm cell biology. Mol Cell Endocrinol 216:47–54
Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ (2014) Antioxidants for male subfertility. Cochrane Database Syst Rev 1:CD007411
Somade OT, Ajayi BO, Adeyi OE, Adeshina AA, James AS, Ayodele PF (2020) Ethylene glycol monomethyl ether-induced testicular oxidative stress and time-dependent up-regulation of apoptotic, pro-inflammatory, and oncogenic markers in rats. Metab Open 7:100051
Somade OT, Ajiboye BO, Osukoya OA, Jarikre TA, Oyinloye BE (2023) Syringic acid ameliorates testicular oxidative stress via the conservation of endogenous antioxidant markers and inhibition of the activated Nrf2-Keap1-NQO1-HO1 signaling in methyl cellosolve-administered rats. Pharmacol Res Modern Chin Med 6:100207
Hasanein P, Fazeli F, Parviz M, Roghani M (2018) Ferulic acid prevents lead-induced testicular oxidative stress and suppressed spermatogenesis in rats. Andrologia 50:e12798
Yousef MS, Abdelhamid HN, Hidalgo RF, Gomez-Gascon L, Dorado J (2021) Antimicrobial activity of silver-carbon nanoparticles on the bacterial flora of bull semen. Theriogenol 161:219–227
Carvalho RK, Rocha TL, Fernandes FH, Goncalves BB, Souza MR, Araujo AA, Barbosa CC, Silva DM, Campos HM, Tomazett MV, Ghedini PC, Guimaraes FS, Andersen ML, Santos FCA, Mazaro-Costa R (2022) Decreasing sperm quality in mice subjected to chronic cannabidiol exposure: new insights of cannabidiol-mediated male reproductive toxicity. Chem-Biol Interact 351:109743
Sun Z, Wen Y, Wang B, Deng S, Zhang F, Fu Z, Yuan Y, Zhang D (2023) Toxic effects of per- and polyfluoroalkyl substances on sperm: epidemiological and experimental evidence. Front Endocrinol (Lausanne) 14:1114463
Mondal H, Budh DP (2021) Hematocrit. Stat Pearls, (2021) (Internet), https://www.ncbi.nlm.nih.gov/books/NBK542276/
Joly BS, Coppo P, Veyradier A (2017) Thrombotic thrombocytopenic purpura. Blood 129:2836–2846
Mansi K, Lahham J (2008) Effects of Artemisia sieberi Besser (a. herba-alba) on heart rate and some hematological values in normal and alloxan-induced diabetic rats. J Basic Appl Sci 4:57–62
Zhang P, Zong Y, Liu M, Tai Y, Cao Y, Hu C (2016) Prediction of outcome in breast cancer patients using test parameters from complete blood count. Mol Clin Oncol 4:918–924
Ibrahim M, Noor S, Lashari Y, Rizwan S, Rehman A, Shahzad F (2018) Effects of environmental pollution on changes in blood biochemical parameters. Pak-Euro J Med Life Sci 1:18–21
Ajagbonna OP, Onifade KI, Suleiman U (1999) Hematological and biochemical changes in rats given extract of Calotropsis procera. Sokoto J Veter Sci 1:36–42
Lubran MM (1989) Hematologic side effects of drugs. Ann Clin Lab Sci 19:114–121
Starek A, Szymczak W, Zapor L (2008) Hematological effects of four ethylene glycol monoalkyl ethers in short-term repeated exposure in rats. Arch Toxicol 82:125–136
Adeyemo-Salami OA, Farombi EO (2021) The effect of ethylene glycol monomethyl ether on hematological parameters in Wistar rats. Arch Bas App Med 9:121–124
Adeyemo-Salami OA, Ewuola EO (2015) Hematological effects of repeated graded doses of the methanol extract of Paullinia pinnata (Linn.) leaves in Wistar albino rats. Pharmacog Res 7:S34–S38
Hamdi L, Djemli S, Arkoub FZ, Boukarine R, Khelili K, Abdennour C, Tahraoui A (2021) The protective effect of wild mustard (Sinapis arvensis L.) pollen seeds against the toxicity of a solvent (EGME) in Wistar rats. J Animal Behav Biometeorol 9:2130
Al-Otaibi SZ, Bajaber A (2021) Effect of green coffee bean extract on steroid hormones synthesis, blood lipids and body weight in rats. Int J Food Sci Nutr Diet 10:499–507
Legault Y, Bouthillier M, Bleau G, Chapdelaine A, Roberts KD (1979) The sterol and sterol sulfate content of the male hamster reproductive tract. Biol Reprod 20:1213–1219
Awano M, Kawaguchi A, Morisaki M, Mohri H (1989) Identification of cholesta-7,24-dien-3β-ol and desmosterol in hamster cauda epididymal spermatozoa. Lipids 24:662–664
Nikolopoulou M, Soucek DA, Vary JC (1985) Changes in the lipid content of boar sperm plasma membranes during epididymal maturation. Biochim Biophys Acta 815:486–498
Agrawal P, Magargee SF, Hammerstedt RH (1988) Isolation and characterization of the plasma membrane of rat cauda epididymal spermatozoa. J Androl 9:178–189
Lin DS, Connor WE, Wolf DP, Neuringer M, Hachey DL (1993) Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. J Lipid Res 34:491–499
Langlais J, Roberts KD (1985) A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res 12:183–224
Shankari SG, Karthikesan K, Jalaludeen AM, Ashokkumar N (2010) Hepatoprotective effect of morin on ethanol-induced hepatotoxicity in rats. J Basic Clin Physiol Pharmacol 21:277–294
Kamel GAM, Harahsheh E, Hussein S (2022) Diacerein ameliorates acetaminophen hepatotoxicity in rats via inhibiting HMGB1/TLR4/NF-κB and upregulating PPAR-γ signal. Mol Biol Rep 49:5863–5874
Somade OT, Akinloye OA, Ugbaja RN, Idowu MA (2020) Cnidoscolus aconitifolius leaf extract exhibits comparable ameliorative potentials with ascorbate in dimethylnitrosamine-induced bone marrow clastogenicity and hepatotoxicity. Clin Nutr Exp 29:36–48
Esmat MA, Osman A, Hassan RE, Hagag SA, El-maghraby TK (2022) Hepatoprotective effect of ferulic acid and/or low doses of γ-irradiation against cisplatin-induced liver injury in rats. Hum Exp Toxicol. https://doi.org/10.1177/09603271221136205
Roghani M, Kalantari H, Khodayar MJ, Khorsandi L, Kalantar M, Goudarzi M, Kalantar H (2020) Alleviation of liver dysfunction, oxidative stress and inflammation underlies the protective effect of ferulic acid in methotrexate-induced hepatotoxicity. Drug Des Devel Ther 14:1933–1941
Kawamoto T, Matsuno K, Kayama F, Hirai M, Arashidani K, Yoshikawa M, Kodama Y (1990) Effect of ethylene glycol monomethyl ether and diethylene glycol monomethyl ether on hepatic metabolizing enzymes. Toxicol 62:265–274
Peana AT, Sánchez-Catalán MJ, Hipólito L, Rosas M, Porru S, Bennardini F, Romualdi P, Caputi FF, Candeletti S, Polache A, Granero L, Acquas, (2017) Mystic acetaldehyde: the never-ending story on alcoholism. Front Behav Neurosci 11:81
El-Mas MM, Abdel-Rahman AA (2019) Role of alcohol oxidative metabolism in its cardiovascular and autonomic effects. Adv Exp Med Biol 1193:1–33
Funding
We did not receive any.
Author information
Authors and Affiliations
Contributions
OEA and OTS designed and supervised the study, did the investigation, provided the resources, and were involved in the project administration. OTS provided the methodology and validated it, also did the formal analysis, wrote the manuscript original draft, reviewed, and edited the manuscript for submission. SAR provided the methodology and participated in the analysis. BTS, AEO, IMA, OOO, GMO, and ORS provided the resources, were involved in the project administration, and participated in the analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any competing interest to declare.
Ethical approval
Approval to proceed with this research was granted by the local Institutional Animal Care and Use Committee (IACUC) of the Department of Biochemistry at the Federal University of Agriculture, Abeokuta (FUNAAB), with approval number FUNAABBCHREC 998460—IDRD2022/073.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adeyi, O.E., Somade, O.T., Rahman, S.A. et al. The effect of ferulic acid on 2-methoxyethanol-induced spermatotoxicity, hematotoxicity and hepatotoxicity in rats. J.Umm Al-Qura Univ. Appll. Sci. 10, 1–11 (2024). https://doi.org/10.1007/s43994-023-00069-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00069-y