Abstract
The current century experienced many disasters affecting the human-being existence. Malaria and new corona virus (COVID-19) are two deadly infections according to the world health organization (WHO). Different types of drugs were used for their treatment, for example quinoline-based drugs. The determination of these compounds in human body or pharmaceutical tablets is crucial for assessing the quality assurance during its manufacture, also for the medication trials. This review provides the current spectrophotometric and electroanalytical methods utilized for the determination of quinoline-associated compounds, such as chloroquine, hydroxychloroquine, quinine, mefloquine, piperaquine, primaquine and amodiaquine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world experienced disastrous diseases in the recent century like malaria and Corona virus (COVID-19) which had negative impact on the life of millions of people [1]. As for malaria, it is a disease disseminates to people by the bite of Anopheles mosquitoes female mosquito. There are different types of this mosquito, among which, the deadliest is P. falciparum. The current studies by the world health organization (WHO) estimated about 250 million infected people in 2021 with about 620 thousand death cases [2]. As malaria lives on the hemoglobin in blood, the disturbance of their blood cycle vanishes them, this is achieved by the intra-parasitic cumulation of heme by the suppression of heme polymerization, which is the mechanism by which these compounds have been used for treating malaria patient [3].
When it comes to COVID-19, its transfer and effect were not similar to malaria but its spread was faster and mainly affect the respiratory system, especially at people who are elderly or have certain diseases. Efforts were made worldwide to overcome these diseases and mitigate their effect. At early stages, different medicines associated with quinoline, such as chloroquine (CQ), hydroxychloroquine (HCQ), quinine (Q), mefloquine (MQ), piperaquine (PiQ), primaquine (PrQ) and amodiaquine (AQ) were prescribed for malaria treatments [4,5,6,7,8,9,10,11,12]. It should be mentioned that, some studies illustrated the death rates of COVID-19 patients increased after using these some of these medications, therefore they are prevented from use by the world health organization (WHO). The increase in this rate may be due to counterfeit or substandard drugs [13, 14].
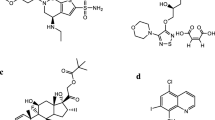
Some information regarding the structures, chemical formula and molecular weights of these compounds is shown in Fig. 1. Hydroxyl group differentiates HCQ from CQ. Both CQ and HCQ are effective against malaria, but the toxicity of CQ is higher than HCQ if used with small amounts for long time [1]. HCQ is safer but less effective compared to CQ. Many bacterial, viral, fungal infections and autoimmune diseases were mitigated by using CQ [15,16,17,18,19].
When considering Fig. 1, different quinoline-based compounds have different formulas and molecular weights, so they would have different physio-chemical properties like water solubility (Sw) and octanol-H2O partitioning coefficient (Kow). The capability of these compounds to dissolve in lipophilic lipo-philic or hydrophilic solvents is affected by Sw and Kow values. The reported values of pKa of chloroquine, piperaquine and quinine were 10.1, 5.72 and 5.1, respectively. Although piperaquine is poorly soluble in water, other drugs are soluble, like CQ (0.01 g/L), PrQ (˃ 0.03 g/L) and Q (0.5 g/L) [20, 21].
The spectrophotometric properties, such as the molar absorptivity and Sandell's sensitivity, of the studied chloroquine compounds were extracted from the literature. These properties differ based on the compound, the solvent and the analysis method. For example, CQ phosphate could form ion-pairs by reacting with either Bromocresol Green dye and Bromocresol Purple dyes, the absorbance of these colored ion-pairs can be measured spectrophotometrically at 420 nm. CQ-PO4 had Sandell's sensitivity of 17.9 × 10–3 µg/cm2 and 10.4 × 10–3 µg/cm2 when reacted with the green dye and purple dye, respectively [22]. The molar absorptivity was 17.9 × 103 L/mol.cm and 30.9 × 103 L/mol.cm with green and purple dyes, respectively [22]. CQ diphosphate could also form ion-pair complex with bromocresol purple, the absorbance measurement at 420 nm resulted in Sandell's sensitivity of 12.6 × 10–3 µg/cm2 [23].
Some of electronic properties of CQ, CQ phosphate and HCQ were evaluated by the density functional theory (DFT), shown in Table 1, which has a correlation with their properties and effect [24, 25]. These were the energy of the highest occupied molecular orbital (HOMO), energy of the lowest unoccupied molecular orbital (LUMO), energy band gap, electron affinity, ionization potential and electronegativity values. The band gap, difference between the energies of LUMO and HOMO, provides insights about the conductivity nature of the compound and its stability under different radiations as well as its ability towards electrons transfer [24, 25].
When considering the analytical methods used for the isolation and quantification of quinoline-based compounds, they were mainly chromatographic, spectrophotometric and electrochemical ones. The first method can offer wide ranges of samples to be analyzed, but these methods have some disadvantages, such as they are the most expensive, they require tedious maintenance, they are relatively slow and require specialist for running the samples and explaining the results.
On the other hand, spectrophotometric and electroanalytical methods are relatively simple, easy to use and are cost-effective. Spectrophotometric methods require the samples to be colored which is not mandatory for electrochemical methods. The analytes can be detected at very lower concentrations with wider concentration ranges by electrochemical methods contrary to the spectrophotometry. An advantage of spectrophotometric methods would be from the ability to detect more than one drug in a simultaneous determination, whereas electrochemical methods can follow the redox reaction of one analyte at a time.
Tremendous amount of work has been documented in the literature regarding the determination, quantification and pharma-kinetics of these compounds. This work aims at summarizing the outcomes of various analytical methods, especially spectrophotometric and electroanalytical ones, used for the detection of these quinoline-based compounds in various samples' types, especially in the last 10 years.
2 Reported sample preparation methods
In order to provide reliable results regarding the evaluation of the amounts of quinoline-based compounds in biological samples, the samples need to be well-prepared to remove the possible interfering molecules. Regarding this, some methods were reported in the literature, such as solid-phase extraction (SPE), liquid-phase extraction (LLE), supported liquid extraction (SLE), dispersive liquid–liquid microextraction (DLLME) and protein precipitation. All these methods are not simple, time-consuming with low selectivity. These anti-malaria compounds were detected by many analytical methods, such as spectrophotometric, electroanalytical and chromatographic ones. The last two methods were massively employed compared with the first one. Table 2 showed some of the methods used for the extraction of different quinoline-based drugs from different matrix (blood, urine, plasma, pharmaceutical tablets).
As for SPE, it is one of the simplest preparation techniques for extracting, clean-up and preconcentrating the analytes before their detection. It is suitable for water, blood, urine and food matrix. The SPE rely on using cartridges having sorbents with different affinities towards various analytes in a sample. Usually, the cartridge is washed with deionized water, conditioned with a suitable solvent for wetting the sorbent. Then, a solution containing analyte and matrix is loaded onto the device, the sorbent would retain the analyte or impurities from the sample. Finally, the analyte or impurities would be eluted from the sorbent [26, 31, 32].
As for LLE, two immiscible solvents, aqueous and organic, having different polarities are used. The analyte would partition based on its affinity towards them. Usually, the mixture, sample solution and the two different solvents, is shaken for a specific time in a separatory funnel or a test tube. The transfer of the analyte to its favorable solvent may require single or multiple extractions. Then the two phases can be eluted separately, and the phases containing the analyte can be added together. If the target analyte is eluted with the organic solvent, the medium must be passed over a drying agent to eliminate possible water droplets that may exist in the organic phase. The final solution is then undergoing direct analysis, dilution if the analyte concentration is high, or if the chromatographic method is to be used for isolation and quantification, the eluted organic solvent may be dry by evaporation, then the residue would be dissolved in a volume of the mobile phase before administrating the sample to HPLC system. Although LLE is cheaper and more efficient than SPE, its selectivity is low, it also requires further purification steps if pure product is needed and it consumes larger solvent volumes that elevate the cost and rise environmental concern [27, 32].
With regard to SLE use a cartridge or well-plates (extraction columns) with mixed principles of SPE and LLE. The solid support, fine porous particles, interacts with the analytes and impurities in an aqueous sample, upon loading them onto SLE particles surface, in an adsorption process. These species are retained by the solid support, then an organic solvent is employed for eluting the target analyte whereas interferants would still adsorb on the support [30]. It is preferable over SPE and LLE, however, the extraction columns are of single-use, hence rising environmental concern and increasing the analysis cost.
Different approaches utilized protein precipitation (PP) method whether as a single preparation step or in coupled with SPE. When used alone, the organic solvent, like CH3OH or CH3CN, were added to the sample. The internal standards can be added to the either the sample or the solvent before forming the mixture. Then, the mixture is stirred well, centrifuged and a volume of the supernatant is injected into the analytical apparatus or evaporated then reconstituted [29, 33]. About 0.3 mL methanolic solution having internal standard was used during protein precipitation of 0.05 mL of plasma sample containing PiQ [34]. Also, 0.2 mL of cold methanol was used for the protein precipitation of 0.1 mL plasma that contained Q [35]. When looking at the literature, SPE and LLE can be used for the majority of matrix whereas PP is mainly utilized for plasma and blood samples and usually associated with chromatographic methods.
2.1 Detection methods
Different analytical methods have been used for the analysis of anti-malaria compounds, particularly the ones mentioned in this work, such as spectrophotometric and electrochemical methods. A brief explanation of these methods will be presented with expressing their validity parameters, such as linearity range, recoveries, limit of detection (LOD) and limit of quantification (LOQ). As they are instrumental-based methods, calibration curves are mandatory to study the relationship between different standard concentration solutions, of the studied anti-malaria drugs, and the instrument physical property, which resulted in showing the linearity on the calibration curve. LOD and LOQ are calculated by multiplying the signal-to-noise ratio of each analytical method by 3 and 10, respectively. The validation of the reported methods was usually in accordance with the US pharmacopeia and the ICH guideline on validation of analytical procedures [36, 37].
2.2 Spectrophotometric methods
As for spectrophotometric methods, the detection of the analytes is based on their interaction with the light parts produced by the light sources in the UV–Vis spectrophotometer; ultraviolet (UV), visible (Vis) and infrared (IR) parts of the light. [38]. It was observed that this type of compounds was seen to have the maximum absorption in the UV region, and usually spectrophotometric detectors like UV or diode array detector (DAD) are linked to HPLC for the detection of these compounds after being separated by the chromatographic methods. A summary of some spectrophotometric methods presented in the literature regarding the determination of quinoline drugs is shown in Table 3.
The analysis of the quinoline-based compounds is usually performed by using a double-beam spectrophotometer for measuring their absorbance in different media. One of the requirements is choosing a suitable solvent for dissolving each compound without causing instability to the drugs during the course of analysis. Some solvents were reported to fulfil this criterion, such as distilled water, hydrochloric acid and sulfuric acid. A standard calibration curve must be established, this is by preparing different concentrations of the drug and scan their absorption at a range of wavelengths. Then extracting the highest absorbance value, at noted wavelength known as maximum wavelength (λmax), which is normally in the UV region; below 400 nm. Beer's law must be obeyed and the optimized calibration curve, which show a strong correlation between the concentration of the target analyte and their absorbance, would have a straight-line equation; y = ax ± b, in which y is the absorbance value, x denoted the analyte concentration, a is the slope whereas b is the y-intercept, can be used to determine the unknown concentration in the studied sample.
When using UV–Vis spectrophotometry, most reported analyzed samples are pharmaceutical tablets or spiked urine samples. Tablets are normally weight, crushed and homogenously powdered by using a pestle and a mortar. This was followed by weighing a certain amount of the powder, dissolving in a suitable solvent then filtrating the solution by using Whatman paper, a volume of the filtrate would be treated by SPE or LLE for extracting the analyte of interest. The final solution after the extraction may contain small volume of the extraction solvent like chloroform, hence heating the solution in the presence of HCl may be required to remove the extraction solvent [43].
In the olden days, Khalil et al. determined CQ by spectrophotometric method that based on forming ion-pair complex with Mo (V). Ascorbic acid was used for reducing Mo (IV) to Mo (V) that was then treated with ammonium thiocyanate to for a complex of red color. The presence of CQ in the sample would react with the complex which changed the color to orange-red [39].
Later on, CQ was quantified in human plasma samples by fluorescent method, in which CQ was derivatized electrochemically before being spectrophotometrically detected [40]. Samanidou et al. determined CQ and Q simultaneously by using fluorescent detector attached to RP-HPLC [31]. The analytes were extracted by SPE from biological samples and pharmaceutical preparations. They were eluted using isocratic elution using a mixture of methanol and acetonitrile as a mobile phase. The excitation wavelength (λexi) and emission wavelength (λemi) were set at 325 and 375 nm, respectively [31].
Also, CQ was quantified in pharmaceuticals using HCl as a solvent at 342 nm, the absorption maximum (λmax). High linear range was observed when applying Beer's law. The molar absorptivity was 0.0089 L.mol−1 cm−1. The method determined CQ with recoveries up to 100% [42].
To overcome the health-related problems associated with the organic solvents used in spectrophotometric measurements, Desta and Amare reported the use of distilled water instead [43]. Their approach was used when detecting CQ phosphate in tablets in which the λmax was observed at 343 nm. Beers' law was obeyed and linearity was noticed in mM concentration range [43]. Also, Singh et al. developed a spectrophotometric method for quantifying HCQ sulphate in pharmaceutical preparations. Hydrochloric acid (HCl) was employed as a solvent and 343 nm was set as the λmax. The method exhibited HCQ linear response over nM concentration range [46].
Q was quantified in pharmaceutical preparations using a complex consisted of o-sulfophenylfluorone, Q and Cu (II). Linearity was observed with acceptable validation parameters [53]. Moreover, fluorometric method was reported to be suitable for quantifying Q in pharmaceutical tablets and non-alcohol-added drinks. H2SO4 was employed for the study of Q luminescence which produced very small noise. Linearity was noticed in 0.1 × 103–1.0 × 103 nM concentration range and LOD was calculated to be 2.9 µM [54]. The Q tablets distributed in Congo republic was examined by Namegabe et al. The qualitative analysis was conducted by spectrophotometric analysis, in which tablets were dissolved in deionized water, then treated with concentrated H2SO4, left in the dark and experienced UV light at 366 nm which produced white blue florescent, this light is a fingerprint confirming Q presence [55].
As the bitter taste of soft drinks can be enhanced by the addition of Q. Q was studied in some drinks by 3rd derivative spectrophotometry at 578 nm and quantified at 320 nm [44]. Also, Q was found in tonic water and determined spectrophotometrically at 347.5 nm, with LOD around 35 mg/L [56].
The treatment of PrQ with 1,2-Naphthoquinone-4-Sulfonate yielded brown color in basic medium, pH of 10, having λmax at 485 nm. This facilitated its quantitation in tablets by spectrophotometry [52].
Both CQ and AQ were simultaneously determined by spectrophotometry. They were oxidized by KBrO3 and KIO3 and the process was studied at 342 nm and 343 nm. They were quantified in pharmaceuticals. The method showed linearity in 0.5 × 103–50 × 103 µM and 0.2 × 103–4.0 × 103 µM for CQ and AQ, respectively, with LODs of 0.06 × 103 µM and 0.4 × 103 µM for the analytes [57].
2.3 Electroanalytical methods
The most used electroanalytical methods for the analysis of quinoline-associated compounds are based on voltammetry and potentiometry. As for voltammetry, a fixed or varied potential is applied at the working electrode to cause oxidation or reduction of the analyte species. This redox process changes the analyte concentration at the working electrode. The electrochemical studies are conducted in a medium that consisted of electrolyte support. The physical properties, for examples the solubility, electrical conductivity and the electrochemical reactivity, of the studied drug influenced the used medium. The electrolyte must not react with the analyte or the product as well as it should reduce the solution resistance [58, 59].
Some electroanalytical methods like cyclic voltammetry (CV), differential pulse voltammetry (DPV), linear sweep voltammetry (LSV), square wave voltammetry (SWV), stripping voltammetry, adsorptive stripping differential pulse voltammetry (AdSDPV) and amperometry methods were reported for the detection of quinoline-associated compounds in various samples. The electroanalytical measurements were done on carbon paste electrode (CPE), glassy carbon electrode (GCE), poly vinyl chloride (PVC), boron-doped diamond electrode (BDD).
The electroanalytical methods have some merits, such as simplicity, higher selectivity, stability with high reproducibility, easily sample preparation, large linearity range and consume less amount of solvent, over the other analytical methods [60, 61]. The performance of these methods is affected by the working electrode type. As would be seen, modified electrodes have better performance, enhanced sensitivity with low LOD over unmodified ones.
The literature showed DPV as the most used electroanalytical method. It is a derivative of LSV or staircase voltammetry. During its use, a chain of voltage pulses is superimposed onto raised potential of stairsteps or linear sweep voltage. The resulted current is detected just before or after each pulse [62]. In CV, the potential of the working electrode is applied in forward and backward directions while the current is measured for each direction. Anodic current and cathodic current for oxidation and reduction of the analyte, respectively. The difference in the current between each direction is measured for SWV [63].
In relation to potentiometry, an electrochemical system having two or three electrodes is used. The analytes can be quantified by measuring the potential difference between two electrodes; working and reference electrodes. A summary of some electroanalytical methods presented in the literature regarding the determination of quinoline drugs is shown in Table 4.
Kamel and Sayour developed a potentiometric sensor for quantifying Q in soft drinks. The membrane was attached with molecular imprinted polymer (MIP). Q was determined in weak acidic medium. The improved sensor exhibited wider linearity range and LODs as low as 1.2 × 10–6 mM [64].
The electrocatalytic oxidation of Q at GCE was studied, it was found that the modification of GCE by using a gel having multiwall carbon nanotubes (MWCNTs) and 1-Butyl-3-methylimidazolium hexafluorophosphate (as ionic liquid), and conducting the work using a phosphate buffer of pH 6.8, would result in revealing the electrode mechanism to be controlled by diffusion and two electrons were involved in the oxidation process [65]. Both CV and SWV were used to study the electrochemical behavior of Q. The use of Britton–Robinson buffers of pH 10.38 with the addition of 1% CTAB was proved to enhance the signal of reduction peak current of Q. It is then postulated that the nitrogen of the Q moiety would have experienced a protonation process [68].
It was determined that, the detection of Q is improved when the working electrodes are modified. For example, Geto et al. reported improved determination of Q by modifying GCE with 4-amino-3-hydroxynaphthalene sulfonic acid. The modification enhanced the LOD as lower as 1.42 × 10–2 µM with recoveries higher than 90% [67]. Also, polymerization process would modify GCEs which resulted in lowering the LOD to 10–2 µM when detecting Q in tonic water [69]. Another method for improving the detection is by coating the working electrode by a composite. An acidic solution spiked with HAuCl4 during the electrodeposition of 3-methyl-4-nitrophenol and L-tyrosine was reported. This was to incorporate gold nanoparticles onto the created composite. The composite was used for determining Q in some biological samples [71].
Furthermore, a potentiometric sensor was fabricated for the detection of Q in human urine, in which the ion-selective electrode (ISE) was doped with melanin (as ionophore). The doping enhanced the selectivity and showed no interference from similar molecules like Q and HCQ [72].
Pristine and modified CPE were used for studying the electrochemical characteristic of CQ [73]. The systematic study over a range of buffer solutions and different pH by using CV and DPV. It showed that one irreversible oxidation peak appears when using pH 2.0–11.0 whereas another peak could be seen when using buffers with pH 5.0–7.0. The modification of the electrodes with DNA improved the sensitivity and enhanced the LOD and the recoveries [73]. Also, the modification of CPE with copper hydroxide, Cu(OH)2, nanowires improved the electrode sensitivity and lower the LOD [74].
Owing to its high surface area and higher conductivity, gold nanourchins were drop-coated onto GCEs to modify them. This was proven in the electrode performance; 0.002–1.0 µM as linear range with LOD as 1.4 nM [81]. Another modifying agents were graphene oxide and tungsten disulfide quantum dots, the modified GCE was used for the determination of CQ in human serum and medical preparations, by employing CV and DPV, better results were obtained such as wide linearity and LOD of 40 nM [75].
Oliveira et al. found that the irreversibility of the CQ anodic response could be overcome by using anodic or cathodic pretreatments of BDD before using SWV. The cathodically-pretreated BDD electrode showed a better-appeared anodic peak with higher current signal [101]. Very recently, an electrochemical electrode was fabricated by mixing graphite with SnO2 nanoparticles to modify CPE, then used it for the determination of CQ phosphate. The composite electrode showed the detection ability in the nM concentration range with LOD of 10 nM [77]. Moreover, a sensor based on using WO3/TiO2 as a photoanode and (Au nanoparticles/C/MoS2) as a photocathode was employed for the determination of CQ phosphate in river water and serum. This structure enables the sensor to be self-operated [78].
MWCNTs coated onto CPE would increase the electrode conductivity and HCQ signal when using AdSDPV method for the determination of HCQ in biological and pharmaceutical samples. This modification resulted in quantifying HCQ down to 6.0 nM [91]. Silva et al. improved the performance of CPE for the determination of HCQ in urine and tablets by treating the electrode with carbon nitride nanosheets. The electrode showed high selectivity towards HCQ with minimal noise from interfering species [93]. Matrouf et al. studied the use of direct and alternating current (DC and AC) to exfoliate graphite pencil, then applied CV for the electrodeposition of graphene oxide onto CPE. When comparing the electrodes prepared by DC and AC for the quantification of HCQ in wastewater and human urine, the latter method showed better validation parameters [95].
A modified PVC membrane with graphite rod was developed by Khalil et al. and showed good performance when determining HCQ sulphate in its forms. This composition enhanced the selectivity, sensitivity and the linear dynamic range during the potentiometric measurement [92]. BDD electrode underwent cathodic pretreatment then detected HCQ in biological samples at sub micromolar concentrations [97]. Due to the consumption of HCQ for treating COVID-19, it may be released to water, hence its monitor is important, for this purpose a composite sensor based on cork-graphite was used for its analysis in river water. The oxidation process of HCQ in the water site resulted in its removal which confirmed by BDD electrodes [99].
A fluorescent sensor based on aptamer was developed for the determination of MQ and PiQ in serum collected from malaria-infected people as well as in tablets [102].
Arguelho et al. reported a direct determination of PrQ in commercial tablets without pre-treatment. They used GCE for detecting PrQ and employed LSV and DPV and SWV for the electrochemical measurements. LODs as low as 0.18 × 104 µM were reported [82]. Modifying pencil graphite electrode (PGE) with fullerene (C60) prompted the electrode kinetic, as fullerene shuttles electrons between the recognition sites and PGE. As a result, the anodic current was enhanced by 5 folds with regard to unmodified one [83].
Thapliyal et al. showed that modifying GCEs with gold nanourchins improved its electron transfer, opposite to bare GCEs, which enhanced the electrocatalytic activity of oxidizing PrQ. The modified GCEs exhibit better validation parameters, with 10–2 µM LODs, of PrQ in urine samples when using SWV and DPV [84]. The trace amounts of PrQ in urine samples requires the use of LLE and TLC to enhance the selectivity and preconcentration of PrQ. These steps were accompanied by employing modified GCE with multi-walled carbon nanotubes. The modification of GCE was to enhanced its surface area for better electron transfer which was witnessed in lower LOD [85].
A sensor based on PVC membrane ion selective electrode (ISE) was used for detecting AQ hydrochloride [87]. Different sensors were fabricated using different plasticizers. They displayed good validation parameters; rapidity, selectivity, stability and near Nernstian response over AQ concentrations of 32 × 10–7–0.02 M in pH = 3.7–5.5 [87]. The use of hemin biosensor for electrochemical study of AQ produced a distinguished AQ oxidation peak at 0.14 V against silver/silver chloride electrode. AQ was detected in breast milk as low as µM range [88]. The modification of GCE by electrochemical polymerization of MWCNT and methyl orange was examined in detecting AQ in human urine and pharmaceutical formulations. The electrochemical oxidation of AQ was studied by CV and DPV [89]. A number of bare carbon-based electrodes were studied for AQ electrochemical characteristics. Among which, the highest oxidation current for AQ was obtained when using bare PGE. The DPV experiments on various samples resulted in 3.0 × 10–4 µM as AQ LOD with recoveries over 100% [90].
3 Conclusion and future insights
The quinoline-based compounds have been employed for treating malaria patients. This work illustrates the use of spectrophotometric and electroanalytical methods for their determination in different matrix; biological, pharmaceuticals, herbs and drinks. A limited use of spectrophotometric methods is noticed in the literature, to the contrary higher publications regarding electroanalytical methods. The use of voltametric methods exceeded the potentiometric ones. The discussion of the types of the working electrodes is presented for all modified and unmodified electrodes. When comparing the two detection methods, electrochemical methods provide faster, more reliable, cost-effective and enable detection at very lower concentration ranges compared to the spectrophotometric ones. They also do not require samples to be colored, contrary to spectrophotometric methods which usually detect analytes at higher concentrations and have poor absorbance detection for transparent samples. More studies are required for the isolation and quantification of piperaquine and primaquine due to their very limited literature work. The majority of the used working electrodes were carbon-based, additional studies need to focus on using various metal oxides, 2D inorganic compounds (like MXenes), 3D-printed sensors and metal organic frameworks for modifying the present electrodes needs consideration. Additionally, the fabrication of optoelectronic sensors for on-site determination of these drugs would be of concern.
Data availability
The data will be made available on reasonable request.
References
White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM (2014) Malaria. Lancet 383:723–735. https://doi.org/10.1016/S0140-6736(13)60024-0
World Health Organization, Malaria Key Fact Sheet, 1, 2021, https://www.who.int/en/news-room/fact-sheets/detail/malaria, accessed on 10 March 2023
Kapishnikov S, Staalsø T, Yang Y, Lee J, Perez-Berna AJ, Pereiro E, Yang Y, Werner S, Guttmann P, Leiserowitz L, Als-Nielsen J (2019) Mode of action of quinoline antimalarial drugs in red blood cells infected by Plasmodium falciparum revealed in vivo. Proc Natl Acad Sci 116:22946–22952. https://doi.org/10.1073/pnas.1910123116
Koranda FC (1981) Antimalarials. J Am Acad Dermatol 4:650–655. https://doi.org/10.1016/S0190-9622(81)70065-3
Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D’Alessandro U (2011) Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J 10:1–12. https://doi.org/10.1186/1475-2875-10-144
Palmer KJ, Holliday SM, Brogden RN (1993) Mefloquine. Drugs 45:430–475. https://doi.org/10.2165/00003495-199345030-00009
Davis TME, Hung TY, Sim IK et al (2005) Piperaquine. Drugs 65:75–87. https://doi.org/10.2165/00003495-200565010-00004
Chatterjee T, Muhkopadhyay A, Khan KA, Giri AK (1998) Comparative mutagenic and genotoxic effects of three antimalarial drugs, chloroquine, primaquine and amodiaquine. Mutagenesis 13:619–624. https://doi.org/10.1093/mutage/13.6.619
Müller O, Van Hensbroek MB, Jaffar S, Drakeley C, Okorie C, Joof D, Pinder M, Greenwood B (1996) A randomized trial of chloroquine, amodiaquine and pyrimethamine-sulphadoxine in Gambian children with uncomplicated malaria. Trop Med Int Health 1:124–132. https://doi.org/10.1046/j.1365-3156.1996.d01-13.x
Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci trends 14:72–73
Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T (2022) Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Emergencias revista de la Sociedad Espanola de Medicina de Emergencias 34:305–307. https://doi.org/10.1002/14651858.CD013587.pub2
Su-zhen LIU, Feng-ying JI, Li-mei SHI (2022) Investigation of imported malaria cases at a COVID-19 isolation point in Qingdao. Chinese J Parasitol Parasitic Diseases 40:261. https://doi.org/10.12140/j.issn.1000-7423.2022.02.021
Axfors C, Schmitt AM, Janiaud P et al (2021) Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun 12:2349. https://doi.org/10.1038/s41467-021-22446-z
Blackstone EA, Fuhr JP Jr, Pociask S (2014) The health and economic effects of counterfeit drugs. Am Health Drug Benefits 7:216–224
Lee SJ, Silverman E, Bargman JM (2011) The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol 7:718–729. https://doi.org/10.1038/nrneph.2011.150
Raoult D, Drancourt M, Vestris G (1990) Bactericidal effect of doxycycline associated with lysosomotropic agents on Coxiella burnetii in P388D1 cells. Antimicrob Agents Chemother 34:1512–1514. https://doi.org/10.1128/AAC.34.8.1512
Raoult D, Houpikian P, Tissot DH, Riss JM, Arditi-Djiane J, Brouqui P (1999) Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med 159:167–173. https://doi.org/10.1001/archinte.159.2.167
Boulos A, Rolain JM, Raoult D (2004) Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells. Antimicrob Agents Chemother 48:747–752
Rolain JM, Colson P, Raoult D (2007) Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infection in the 21st century. Int J Antimicrob Agents 30:297–308
Hung TY, Davis TM, Ilett KF (2003) Measurement of piperaquine in plasma by liquid chromatography with ultraviolet absorbance detection. J Chromatogr B 791:93–101. https://doi.org/10.1016/S1570-0232(03)00209-5
Warhurst DC, Craig JC, Adagu IS, Guy RK, MadridFivelman PBQL (2007) Activity of piperaquine and other 4-aminoquinoline antiplasmodial drugs against chloroquine-sensitive and resistant blood-stages of Plasmodium falciparum: role of β-haematin inhibition and drug concentration in vacuolar water-and lipid-phases. Biochem pharmacol 73:1910–1926. https://doi.org/10.1016/j.bcp.2007.03.011
Nagib Qarah AS, Basavaiah K, Swamy N (2017) Sensitive and selective extraction-free spectrophotometric assay of chloroquine phosphate in pharmaceuticals based on ion-pair reaction with bromocresol green and bromocresol purple. Pharm Anal Acta 8:1–9. https://doi.org/10.4172/2153-2435.1000539
Nagaraja P, Shrestha AK, Shivakumar A, Gowda AK (2010) Spectrophotometric determination of chloroquine, pyrimethamine and trimethoprim by ion pair extraction in pharmaceutical formulation and urine. J Food Drug Anal. https://doi.org/10.38212/2224-6614.2244
Noureddine O, Issaoui N, Al-Dossary O (2021) DFT and molecular docking study of chloroquine derivatives as antiviral to coronavirus COVID-19. J King Saud Uni Sci 33:101248. https://doi.org/10.1016/j.jksus.2020.101248
Ejuh GW, Fonkem C, Assatse YT, Kamsi RY, Nya T, Ndukum LP, Ndjaka JMB (2020) Study of the structural, chemical descriptors and optoelectronic properties of the drugs Hydroxychloroquine and Azithromycin. Heliyon 6:e04647. https://doi.org/10.1016/j.heliyon.2020.e04647
Tlili I, Bensghaier R, Latrous El Atrache L et al (2022) Cuttlefish bone powder as an efficient solid-phase extraction sorbent of anti-SARS-CoV-2 drugs in environmental water. Chem Pap 76:6941–6951. https://doi.org/10.1007/s11696-022-02388-6
Choemang A, Na-Bangchang K (2019) An alternative HPLC with ultraviolet detection for determination of piperaquine in plasma. J Chromatogr Sci 57:27–32. https://doi.org/10.1093/chromsci/bmy077
Jain R, Mudiam MKR, Ch R, Chauhan A, Khan HA, Murthy RC (2013) Ultrasound assisted dispersive liquid–liquid microextraction followed by injector port silylation: a novel method for rapid determination of quinine in urine by GC–MS. Bioanalysis 5:2277–2286. https://doi.org/10.4155/bio.13.188
Hanpithakpong W, Day NPJ, White NJ, Tarning J (2022) Simultaneous and enantiospecific quantification of primaquine and carboxyprimaquine in human plasma using liquid chromatography-tandem mass spectrometry. Malar J 21:169. https://doi.org/10.1186/s12936-022-04191-w
Kaewkhao K, Tarning J, Blessborn D (2021) High-throughput quantitation method for amodiaquine and desethylamodiaquine in plasma using supported liquid extraction technology. J Chromatogr B Biomed Appl 1179:122887. https://doi.org/10.1016/j.jchromb.2021.122887
Samanidou VF, Evaggelopoulou EN, Papadoyannis IN (2005) Simultaneous determination of quinine and chloroquine anti-malarial agents in pharmaceuticals and biological fluids by HPLC and fluorescence detection. J Pharm Biomed Anal 38:21–28. https://doi.org/10.1016/j.jpba.2004.12.005
Nickerson B, Colón I (2011) Liquid–liquid and solid-phase extraction techniques. In: De M (ed) Sample preparation of pharmaceutical dosage forms: challenges and strategies for sample preparation and extraction. Aapspress Springer, London
Tang C, Sojinu OS (2012) Simultaneous determination of chloroquine and its metabolite Desethyl Chloroquine in human plasma using liquid chromatography tandem mass spectrometry. Anal Lett 45:2277–2289. https://doi.org/10.1080/00032719.2012.691588
Singhal P, Gaur A, Gautam A, Varshney B, Paliwal J, Batra V (2007) Sensitive and rapid liquid chromatography/tandem mass spectrometric assay for the quantification of piperaquine in human plasma. J Chromatogr 859:24–29. https://doi.org/10.1016/j.jchromb.2007.09.021
Mirghani RA, Ericsson Ö, Cook J, Yu P, Gustafsson LL (2001) Simultaneous determination of quinine and four metabolites in plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Appl 754:57–64. https://doi.org/10.1016/S0378-4347(00)00577-6
United States Pharmacopeia (USP). (2007) The official compendia of standards, USP30-NF25
International Conference on Harmonization (ICH). Validation of analytical procedures, Methodology Q2(R2). Technical requirements for registration of pharmaceuticals for human use
Germer T, Zwinkels JC, Tsai BK (2014) Spectrophotometry: accurate measurement of optical properties of materials. Elsevier, Amsterdam
Khalil SM, Mohamed GG, Zayed MA, Elqudaby HM (2000) Spectrophotometric determination of chloroquine and pyrimethamine through ion-pair formation with molybdenum and thiocyanate. Microchem J 64:181–186. https://doi.org/10.1016/S0026-265X(99)00027-2
Amador-Hernández J, Fernández-Romero JM, Luque de Castro MD (2001) Continuous determination of chloroquine in plasma by laser-induced photochemical reaction and fluorescence. Fresenius J Anal Chem 369:438–441. https://doi.org/10.1007/s002160000652
Green MD, Nettey H, Rojas OV, Pamanivong C, Khounsaknalath L, Ortiz MG, Newton PN, Fernández FM, Vongsack L, Manolin O (2007) Use of refractometry and colorimetry as field methods to rapidly assess antimalarial drug quality. J Pharm Biomed Anal 43:105–110. https://doi.org/10.1016/j.jpba.2006.06.047
Kanakapura B, Penmatsa VK, Umakanthappa C (2016) Stability-indicating UV-spectrophotometric assay of chloroquine phosphate in pharmaceuticals. Res J Pharm Biol Chem Sci 7:251–261
Desta K, Amare M (2017) Validated UV-visible spectrometry using water as a solvent for determination of chloroquine in tablet samples. Chem Int 3:288–295. https://core.ac.uk/download/pdf/211838985.pdf
Castro e Souza MA, Reis NFA, de Souza BL, da Costa César I, Fernandes C, Pianetti GA (2020) An easy and rapid spectrophotometric method for determination of chloroquine diphosphate in tablets. Curr Pharm Anal 16:5–11. https://doi.org/10.2174/1573412914666180730123426
Ganguly A, Hwa KY (2022) Construction of zinc selenide microspheres decorated with octadecylamine-functionalized reduced graphene oxide as an effective catalyst for the dual-mode detection of chloroquine phosphate. Mater Today Chem 24:100862. https://doi.org/10.1016/j.mtchem.2022.100862
Singh A, Sharma PK, Gupta R, Mondal N, Kumar S, Kumar M (2016) Development and validation of UV-spectrophotometric method for the estimation of hydroxychloroquine sulphate. IJCT 23:237–239. http://nopr.niscpr.res.in/handle/123456789/34265
Ferraz LR, Santos FL, Ferreira PA, Maia-Junior RT, Rosa TA, Costa SP, Melo CM, Rolim LA, Rolim-Neto PJ (2014) Quality by design in the development and validation of analytical method by ultraviolet-visible spectrophotometry for quantification of hydroxychloroquine sulfate. Int J Pharm Sci Res 5:4666. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=7be4666266c1b9086053dad80b363c57abdb5d90
Sereshti H, Aliakbarzadeh G (2013) Response surface methodology optimized dispersive liquid–liquid microextraction coupled with UV-Vis spectrophotometry for determination of quinine. Anal Methods 5:5253–5259. https://doi.org/10.1039/C3AY40478A
Kalyankar TM, Kakade RB, Attar MS, Kamble AR (2013) Simultaneous spectrophotometric estimation of artesunate and mefloquine. J Chem 2013:679857. https://doi.org/10.1155/2013/679857
Deshpande SV, Patel CP, Mohite MT, Suthar JK (2016) Analytical method development of amodiaquine hydochloride in marketed formulation by UV visible spectrophotometry. Emergent Life Sci Res 2:26–29. http://emergentresearch.org/uploads/38/1801_pdf.pdf
Ragini K, Dedania Z, Dedania R (2018) Spectrophotometric method determination of artesunate and amodiaquine in combined dosage form. Int J Res Ana 5:781–786. http://ijrar.com/upload_issue/ijrar_issue_20542465.pdf
Altigani AMN, Elbashir AA (2014) Spectrophotometric method for determination of Primaquine in pharmaceutical formulations via derivatization with 1, 2-Naphthoquinone-4-sulfonate. Austin J Anal Pharm Chem 1:1019
Yamazaki C, Tominaga H, Samma M, Isono R, Kamino S, Yamaguchi T, Fujita Y (2007) Spectrophotometric determination of quinine and its related drugs with o-sulfophenylfluorone and copper (II). Bunseki Kagaku 56:879–884. https://doi.org/10.2116/bunsekikagaku.56.879
Nikolaeva AA, Korotkova EI, Lipskikh OI (2019) Determination of quinine in drugs and beverages by fluorimetric method. Becтник Кapaгaндинcкoгo yнивepcитeтa. Cepия: Xимия, 2:56–61. https://azdok.org/document/zwvwk6e1-determination-quinine-drugs-beverages-fluorimetric-method.html
Namegabe LM, Kadhesi MT, Hamuli PM, Mahano AO, Brioen P (2019) Quality control of quinine in pharmaceutical forms tablets find east of the democratic republic of Congo. Am J Anal Chem 10:415–422. https://doi.org/10.4236/ajac.2019.109029
Innocent AE, Onah I (2022) Determination of quinine in some brands of tonic water using spectrophotometric techniques. JCSN. https://doi.org/10.46602/jcsn.v47i4.793
Mohamed AA (2009) Kinetic spectrophotometric determination of amodiaquine and chloroquine. Monatsh Chem 140:9–14. https://doi.org/10.1007/s00706-008-0022-5
Manea F, Motoc S, Pop A, Remes A, Schoonman J (2012) Silver-functionalized carbon nanofiber composite electrodes for ibuprofen detection. Nanoscale Res Lett 7:331. https://doi.org/10.1186/1556-276X-7-331
Lima AB, Faria EO, Montes RH, Cunha RR, Richter EM, Munoz RA, dos Santos WT (2013) Electrochemical oxidation of ibuprofen and its voltammetric determination at a boron-doped diamond electrode. Electroanalysis 25:1585–1588. https://doi.org/10.1002/elan.201300014
Farghaly OA, Hameed RA, Abu-Nawwas AAH (2014) Analytical application using modern electrochemical techniques. Int J Electrochem Sci 9:3287–3318
Thapliyal N, Karpoormath RV, Goyal RN (2015) Electroanalysis of antitubercular drugs in pharmaceutical dosage forms and biological fluids: A review. Anal Chim Acta 853:59–76. https://doi.org/10.1016/j.aca.2014.09.054
Qian L, Durairaj S, Prins S, Chen A (2021) Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens Bioelectron 175:112836. https://doi.org/10.1016/j.bios.2020.112836
Thapliyal N, Chiwunze TE, Karpoormath R, Goyal RN, Patel H, Cherukupalli S (2016) Research progress in electroanalytical techniques for determination of antimalarial drugs in pharmaceutical and biological samples. RSC Adv 6:57580–57602. https://doi.org/10.1039/C6RA05025E
Kamel AH, Sayour HE (2009) Flow-through assay of quinine using solid contact potentiometric sensors based on molecularly imprinted polymers. Electroanalysis 21:2701–2708. https://doi.org/10.1002/elan.200904699
Zhan XM, Liu LH, Gao ZN (2011) Electrocatalytic oxidation of quinine sulfate at a multiwall carbon nanotubes-ionic liquid modified glassy carbon electrode and its electrochemical determination. J Solid State Electrochem 15:1185–1192. https://doi.org/10.1007/s10008-010-1184-8
Awasthi S, Srivastava A, Singla ML (2011) Voltammetric determination of citric acid and quinine hydrochloride using polypyrrole-pentacyanonitrosylferrate/platinum electrode. Synth Met 161:1707–1712. https://doi.org/10.1016/j.synthmet.2011.06.009
Geto A, Amare M, Tessema M, Admassie S (2012) Polymer-modified glassy carbon electrode for the electrochemical detection of quinine in human urine and pharmaceutical formulations. Anal Bioanal Chem 404:525–530. https://doi.org/10.1007/s00216-012-6171-8
Dar RA, Brahman PK, Tiwari S, Pitre KS (2012) Electrochemical studies of quinine in surfactant media using hanging mercury drop electrode: a cyclic voltammetric study. Colloids Surf B Biointerfaces 98:72–79. https://doi.org/10.1016/j.colsurfb.2012.04.035
Liu L, Tan X, Fang X, Sun Y, Lei F, Huang Z (2012) Electrochemical sensor based on molecularly imprinted polymer film prepared with functional abietic-type acids as cross-linker for the determination of quinine. Electroanalysis 24:1647–1654. https://doi.org/10.1002/elan.201200085
Buleandra M, Rabinca AA, Cheregi MC, Ciucu AA (2018) Rapid voltammetric method for quinine determination in soft drinks. Food Chem 253:1–4. https://doi.org/10.1016/j.foodchem.2018.01.130
Azadmehr F, Zarei K (2019) Fabrication of an imprinted electrochemical sensor from Ltyrosine, 3-methyl-4-nitrophenol and gold nanoparticles for quinine determination. Bioelectrochemistry 127:59–67. https://doi.org/10.1016/j.bioelechem.2019.01.001
Glasco DL, Mamaril AM, Sheelam A, Ho NH, Bell JG (2022) Selective detection of chloroquine in human urine with application at the point-of-care. J Electrochem Soc 169:077513. https://doi.org/10.1149/1945-7111/ac80d7
Radi A (2005) Accumulation and trace measurement of chloroquine drug at DNA modified carbon paste electrode. Talanta 65:271–275. https://doi.org/10.1016/j.talanta.2004.05.024
Mashhadizadeh MH, Akbarian M (2009) Voltammetric determination of some antimalarial drugs using a carbon paste electrode modified with Cu(OH)2 nano-wire. Talanta 78:1440–1445. https://doi.org/10.1016/j.talanta.2009.02.040
Srivastava M, Tiwari P, Mall VK, Srivastava SK, Prakash R (2019) Voltammetric determination of the antimalarial drug chloroquine using a glassy carbon electrode modified with reduced graphene oxide on WS2 quantum dots. Microchim Acta 186:415. https://doi.org/10.1007/s00604-019-3525-3
Silva MKL, Sousa GS, Simoes RP, Cesarino I (2022) Fabrication of paper-based analytical devices using a PLA 3D-printed stencil for electrochemical determination of chloroquine and escitalopram. J Solid State Electrochem 26:581–586. https://doi.org/10.1007/s10008-021-05075-w
AbdelHamid A, Elgamouz A, Khanfer M, Kawde AN (2023) COVID-19 chloroquine drug detection using novel, highly sensitive sno2-based electrochemical sensor. Arab J Chem 16:104674–104688. https://doi.org/10.1016/j.arabjc.2023.104674
He Y, Sun J, Yao W, Lu K, Liu D, Xie H, Huang C, Jia N (2023) A self-powered photoelectrochemical molecular imprinted sensor for chloroquine phosphate with enhanced cathodic photocurrent via stepped energy band alignment engineering. Che Eng J 451:138748. https://doi.org/10.1016/j.cej.2022.138748
Barreto FC, da Silva MKL, Cesarino I (2023) Copper nanoparticles and reduced graphene oxide as an electrode modifier for the development of an electrochemical sensing platform for chloroquine phosphate determination. Nanomater 13:1436. https://doi.org/10.3390/nano13091436
Uslu B, Doǧan B, Ozkan SA, Aboul-Enein HY (2005) Voltammetric investigation and determination of mefloquine. Electroanalysis 17:1563–1570. https://doi.org/10.1002/elan.200403255
Chiwunze TE, Thapliyal NB, Palakollu VN, Karpoormath R (2017) A. Simple, Efficient and ultrasensitive gold nanourchin based electrochemical sensor for the determination of an antimalarial drug: mefloquine. Electroanalysis 29:2138–2146. https://doi.org/10.1002/elan.201700154
Arguelho MLPM, Zanoni MVB, Stradiotto NR (2005) Electrochemical oxidation and voltammetric determination of the antimalaria drug primaquine. Anal Lett 38:1415–1425. https://doi.org/10.1081/AL-200062218
Prasad BB, Kumar A, Singh R (2016) Molecularly imprinted polymer-based electrochemical sensor using functionalized fullerene as a nanomediator for ultratrace analysis of primaquine. Carbon N Y 109:196–207. https://doi.org/10.1016/j.carbon.2016.07.044
Thapliyal NB, Chiwunze TE, Karpoormath R, Cherukupalli S (2017) Fabrication of highly sensitive gold nanourchins based electrochemical sensor for nanomolar determination of primaquine. Mater Sci Eng C 74:27–35. https://doi.org/10.1016/j.msec.2016.12.126
Pedrozo-Penafiel MJ, Almeida JMS, Toloza CAT, Larrude DG, Pacheco WF, Aucelio RQ (2019) Square-wave voltammetric determination of primaquine in urine using a multi-walled carbon nanotube modified electrode. Microchem J 150:104201. https://doi.org/10.1016/j.microc.2019.104201
Nate Z, Gill AA, Chauhan R, Karpoormath R (2021) Polyaniline-cobalt oxide nanofibers for simultaneous electrochemical determination of antimalarial drugs: Primaquine and proguanil. Microchem J 160:105709. https://doi.org/10.1016/j.microc.2020.105709
Malongo TK, Blankert B, Kambu O, Amighi K, Nsangu J, Kauffmann JM (2006) Amodiaquine polymeric membrane electrode. J Pharm Biomed Anal 41:70–76. https://doi.org/10.1016/j.jpba.2005.10.014
Valente CO, Garcia CAB, Alves JPH, Zanoni MVB, Stradiotto NR, Arguelho MLP (2012) Electrochemical determination of antimalarial drug amodiaquine in maternal milk using a hemin-based electrode. ECS Trans 43:297–304. https://doi.org/10.1149/1.4704970
Chiwunze TE, Palakollu VN, Gill AAS, Kayamba F, Thapliyal NB, Karpoormath R (2019) A highly dispersed multi-walled carbon nanotubes and poly (methyl orange) based electrochemical sensor for the determination of an antimalarial drug: Amodiaquine. Mater Sci Eng C Mater Biol Appl 97:285–292. https://doi.org/10.1016/j.msec.2018.12.018
Karakaya S, Kartal B, Dilgin Y (2020) Ultrasensitive voltammetric detection of an antimalarial drug (amodiaquine) at a disposable and low cost electrode. Monatshefte Fur Chemie 151:1019–1026. https://doi.org/10.1007/s00706-020-02637-y
Ghoreishi SM, Attaran AM, Amin AM, Khoobi A (2015) Multiwall carbon nanotube-modified electrode as a nanosensor for electrochemical studies and stripping voltammetric determination of an antimalarial drug. RSC Adv 5:14407–14415. https://doi.org/10.1039/C4RA16357E
Khalil MM, Mostafa SM, Masoud AA, Correa AA (2016) A Novel Coated Graphite Sensor for Potentiometric Determination of Hydroxychloroquine Sulfate. Sch Rep 1:1–10. https://d1wqtxts1xzle7.cloudfront.net/52679618/ScholReps-V1-I2-000004-libre.pdf?1492571470=&response-content-disposition=inline%3B+filename%3DA_novel_coated_graphite_sensor_for_poten.pdf&Expires=1679862622&Signature=gjnUcIgKuRxWrFr2pWMMv0yW~0AgKvUxWgV9szrUi5UMWBfUy6gwJQ0CzSx3GSCdHoTgmA2EgTK4z3WcbObmIZMmMl-O-6NtpIa4oIterd-fnegXLks29AN~TYnZ2k0hDdaAf47si4l3ppvIeM1QFOiaNxxyF3Vzhbmq5H3w39geilhYbXVQCEeZnVDLWtVx6O8wBSn~HLg4zAq6rQme91GxSQxqHKhOt6aZtgNRkc7aoEncppT1IAKMUHImuiZFQwMgBtdqKLeEx0WqOsHZYYK5uWXPV~7pKeBu-Zm8-YuvG5pA~ZVjRv2QNDmkh5Sh~GK4kDZ5KJMFFEBBbCJkgQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA
de Oliveira S, Silva J, Sant’Anna MVS, Gevaerd A, Lima JBS, Monteiro MDS, Carvalho SWMM, Sussuchi EM (2021) A novel carbon nitride nanosheets-based electrochemical sensor for determination of hydroxychloroquine in pharmaceutical formulation and synthetic urine samples. Electroanalysis 33:2152–2160. https://doi.org/10.1002/elan.202100170
George JM, Mathew B (2021) Cyclodextrin-mediated gold nanoparticles as multisensing probe for the selective detection of hydroxychloroquine drug. Korean J Chem Eng 38:624–634. https://doi.org/10.1007/s11814-020-0719-7
Matrouf M, Loudiki A, Ouatmane FZ, Chhaibi B, Alaoui OT, Laghrib F, Farahi A, Bakasse M, Lahrich S, Mhammedi ME (2022) Effect of graphite exfoliation way on the efficiency of exfoliated graphene for the determination of hydroxychloroquine in urine and waste water. J Electrochem Soc 169:097505. https://doi.org/10.1149/1945-7111/ac915d
Khoobi A, Ghoreishi SM, Behpour M, Shaterian M, Salavati-Niasari M (2014) Design and evaluation of a highly sensitive nanostructure-based surface modification of glassy carbon electrode for electrochemical studies of hydroxychloroquine in the presence of acetaminophen. Colloids Surf B Biointerfaces 123:648–656. https://doi.org/10.1016/j.colsurfb.2014.10.002
Deroco PB, Vicentini FC, Oliveira GG, Rocha-Filho RC, Fatibello-Filho O (2014) Square-wave voltammetric determination of hydroxychloroquine in pharmaceutical and synthetic urine samples using a cathodically pretreated boron-doped diamond electrode. J Electroanal Chem 719:19–23. https://doi.org/10.1016/j.jelechem.2014.01.037
de Araújo DM, Paiva SDSM, Henrique JMM, Martínez-Huitle CA, Dos Santos EV (2021) Green composite sensor for monitoring hydroxychloroquine in different water matrix. Materials 14:4990. https://doi.org/10.3390/ma14174990
Medeiros de Araújo D, Dos Santos EV, Martínez-Huitle CA, De Battisti A (2022) Achieving electrochemical-sustainable-based solutions for monitoring and treating hydroxychloroquine in real water matrix. Appl Sci 12:699. https://doi.org/10.3390/app12020699
Carvalho MS, Rocha RG, de Faria LV, Richter EM, Dantas LM, da Silva IS, Muñoz RA (2022) Additively manufactured electrodes for the electrochemical detection of hydroxychloroquine. Talanta 250:123727. https://doi.org/10.1016/j.talanta.2022.123727
Oliveira GG, Azzi DC, Silva TA, de Oliveira PR, Fatibello-Filho O, Janegitz BC (2020) Sensitive voltammetric detection of chloroquine drug by applying a boron-doped diamond electrode. C 6:75. https://doi.org/10.3390/c6040075
Coonahan ES, Yang KA, Pecic S, De Vos M, Wellems TE, Fay MP, Andersen JF, Tarning J, Long CA (2021) Structure-switching aptamer sensors for the specific detection of piperaquine and mefloquine. Sci Transl Med 13:eabe1535. https://doi.org/10.1126/scitranslmed.abe1535
Funding
No fund was received regarding this manuscript.
Author information
Authors and Affiliations
Contributions
HA conducted the whole work.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alessa, H. Recent spectrophotometric and electroanalytical methods used for the determination of quinoline-based compounds. J.Umm Al-Qura Univ. Appll. Sci. 9, 378–390 (2023). https://doi.org/10.1007/s43994-023-00051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00051-8