Abstract
In this article, series of semicarbazide, thiosemicarbazide, urea and thiourea derivatives have been successfully prepared via an easy synthetic strategy. The chemical structure of the prepared compounds have been confirmed based upon spectral and elemental data. The compounds showed excellent insecticidal activity against Spodoptera littoralis which causes a harmful damage to the cotton crop which represent a principal figure in economics of North Africa countries. Among all the prepared compounds, methyl-4-{[(4-chlorobenzoyl)carbamothioyl] amino}benzoate 7 showed the highest insecticidal activity against S. littoralis, with LC50 values of 9.882 for 2nd instar larvae & 102.66 for 4th instar larvae, respectively.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spodoptera littoralis is a species of moth in the family Noctuidae and it is a highly polyphagous organism that is a pest of many cultivated plants and crops [1]. Larval phase, of S. littoralis can caused huge and incredible damage to cotton crop in North Africa [2]. Larvae of this pest has minimum seven generations throughout the cotton season, moreover invading more than twenty nine other harvests and plants [3,4,5,6]. To combat the growth of this pest, scientists have improved a variety of organic pesticides [7]. The most challenge is the preparing and presenting an organic compounds which can stop or at least minimize the harmful effect of these pests and at the same time safe to human. It has been reported also that the compounds contain amide or thioamide groups were efficiently control of S. Littoralis and there are many other articles reported a significant use of pesticide materials [8,9,10,11,12,13,14,15,16]. The substitutional controller methods shows foreboding as a prospective method in fall armyworm. The administrative plans are the using of biorational control operators for instance preparing insect growth regulators and those depend on common compounds [17,18,19,20,21,22,23]. Insect growth regulator is improvement controller which is ventured to be more assure for useful living beings than regular mixes, they have been successfully employed in IPM programs in contrast to many plants and little natural materials. There is need to different pesticides which have different modes of action Juvenile hormones analogues, sesquiterpenoid moieties arranged and discharged using of the corpora allata, which important pest hormone compounds that normalize the huge variety of processes through postembryonic growth and adult reproduction in pests [24]. As well as, it improved insecticidal efficacy of a recombinant baculovirus expressing mutated JH esterase from Manduca sexta [25]. In this article, we aimed to report a novel effective S. littoralis cotton pesticides through an easy synthesis of new amides and/or thioamide derivatives and estimate their biological evaluation in contradiction of cotton leafworm.
2 Materials and methods
2.1 Measurements
Melting points of the synthesized compounds were measured on a Fisher-John mechanical tool. Elemental analysis were determined using a Vario EL C, H, N, S analyzer. On a Pye-Unicam SP3-100 spectrophotometer the IR spectra were recorded as potassium bromide disc method. 1H NMR and 13CNMR spectra were produced through Bruker 400 MHz spectrometer using tetramethylsilane (TMS) as a source of perception & concoction movements were justification as ppm. By using a Jeol JMS-400 mass spectra was accomplished. The numbers of Spodoptera littoralis pests were collected from cotton leave worm, fields of Agricultural Research Center, Sohag branch, Egypt.
2.2 Laboratory bioassay
The insecticidal effectiveness of the designed semicarbazide, thiosemicarbazide analogues were saucepan through the leaf dip bioassay methods [26,27,28,29,30,31,32]. The results of laboratory are testified here for the target compounds to find out the required concentrations which are required to kill fifty percentage (LC50) of the 2nd instar larvae & 4th instar larvae of Spodoptera littoralis bugs. In this article, 5 different concentrations of each synthesizing compounds & 0.1% tween 80 as surfactant were employed. Closely the same size ten of 2nd instar larvae & 4th instar larvae bugs where place in disks (nine centimeter diameter) of castor bean leaves which dipped in the checked concentration for 10 s, then left to dry & gave 2nd and 4th larvae, closely of the same size. The larvae were put in glass jars, and every treatment was simulated 3 times (ten larvae per each). Control disks were dipped in dist. H2O and tween 80, then moved to the untreated larvae, which were permitted to feed on castor bean for two days. Impermanence, percentage was recovered after three days for the pesticides. Mortality was redressed by Abbott’s formula [33]. The measurements of the mortality relapse line were dissected by probit analysis [34]. Harmfulness indexes were strongminded through sun equations [35].
3 Results and discussion
3.1 Chemistry
The target compounds, namely, 4-[(phenylcarbamoyl)amino]benzoic acid 2, 4-[(phenyl-carbamothioyl)amino]benzoic acid 3, 4-[(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)amino] benzoic acid 4, 4-[(benzoylcarbamothioyl)amino]benzoic acid 5, 4-{[(4-chlorobenzoyl) carbamothioyl]amino}benzoic acid 6, methyl 4-{[(4-chlorobenzoyl)carbamothioyl]amino}benzoate 7 have been successfully synthesized and their structure were established. Thus, reaction of 4-aminobenzoic acid 1 with phenyl isocyanate, phenyl isothiocyanate, benzoyl isothiocyanate and 4-chlorobenzoyl isothiocyanate in dry acetone afforded the desired compounds 2, 3, 5, 6, respectively. However, refluxing the thiourea derivative 3 with chloroacetyl chloride afforded the new 4-[(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)amino] benzoic acid 4 via cyclo-elimination manner via losing 2 mol of HCl. Also, esterification of 4-{[(4-chlorobenzoyl) carbamothioyl] amino}benzoic acid 6 with methanol in presence of conc. sulphuric acid led to the new ester compound methyl 4-{[(4-chlorobenzoyl)-carbamo-thioyl]amino}benzoate 7, (Scheme 1).
The chemical structures of the products were checked using IR, NMR spectral data, and elemental analyses. The IR spectra of molecules 2, 3, 5, 6 & 7 displayed absorption bands of NH groups around v = 3381–3201 cm−1 and characteristic stretching vibration bands due to carbonyl groups at v = 1716–1644 cm−1, respectively. Whereas, the absorption of NH groups completely disappeared in the IR spectrum of 1,3-thiazolidine derivative 4 confirming the cylization of compound 3. Also, the 1HNMR spectra of compounds 2, 3, 5, 6 and 7 revealed singlet signals at about δ = 11.04–8.02 ppm characteristic for the 2NH groups, which clearly did not recognized in the 1H NMR of 1,3-thiazolidine derivative 4, which showed instead a singlet at δ = 4.30 ppm characteristic of the CH2 group. Another evidence for formation of compound 4 comes from its 13CNMR spectrum which showed an absorption at δ = 15.60 and at δ = 188.5 ppm assigned for methylene and carbonyl carbons of 1,3-thiazolidine ring, respectively.
A plausible mechanism may suggested to rationalize the formation of 1,3-thiazolidine derivative 4 as shown in chart 1. Thus, a primary nucleophilic attack of 4-aminobenzoic acid into aryl isothiocyanate lead to the thiourea derivative 3a. Then, the free electrons of imino (NH) and thiol (SH) functions of 3a/3b attacks of chloroacetyl chloride to yield 4-[(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)amino] benzoic acid 4 via cyclization to 3c followed by elimination of 2 mol of HCl to give 1,3-thiazolidine derivative 4.
As well as, the 2-(4-chlorobenzoyl)-N-phenylhydrazinecarboxamide 9, 2-(4-chlorobenzoyl)-N-phenylhydrazine carbothioamide 10, N-phenyl-2-(pyridin-2-ylcarbonyl)hydrazinecarboxamide 11, N-phenyl-2-(pyridin-2-ylcarbonyl)hydrazinecarboxamide 12, N-phenyl-2-(pyridin-2-ylcarbonyl)-hydrazine-carbothioamide 13 were successfully synthesized and established (Scheme 2).
3.2 Experimental
3.2.1 General process for designing of products 2–7
4-[(Phenylcarbamoyl)amino]benzoic acid 2, 4-[(phenylcarbamothioyl)amino]benzoic acid 3, 4-[(benzoylcarbamothioyl)amino]benzoic acid 5, 4-{[(4-chlorobenzoyl)carbamothioyl]amino} benzoic acid (6) were synthesized in analogy to the procedure described in literature [36, 37].
Refluxing of equimolar amounts of 4-aminobenzoic acid and each of phenyl isocyanate, phenyl isothiocyanate, benzoyl isothiocyanate, 4-chlorobenzoyl isothiocyanate, respectively in dry acetone for 3 h, and the reaction was monitored by TLC affording 2, 3, 5 and 6, respectively. Also, compound 4-[(phenylcarbamothioyl)amino]benzoic acid 3 was boiled with chloroacetyl chloride for 1 h. under reflux gave 4-[(4-oxo-3-phenyl-1,3-thiazolidin-2-ylidene)amino] benzoic acid 4. Then the reaction mixture was concentrated, the precipitation compound had been collection through filtration, washed thoroughly with ethyl alcohol, dried and purification through crystallization from absolute ethyl alcohol. However, methyl-4-{[(4-chlorobenzoyl)carbamothioyl]amino}benzoate 7 was obtained via esterification of compound 4-{[(4-chlorobenzoyl) carbamothioyl]amino}benzoic acid 6 via refluxing with methanol in presence of Conc. sulfuric acid the solution for 60 min, the reaction combination allowed to transfer on icy H2O, the formed precipitation had been collection via filtration, washing with H2O, dried & purification by crystallization in absolute ethanol.
4-[(Phenylcarbamoyl)amino]benzoic acid (2): Gray powder (83% yield); melting point; 185 ℃; IR (ν−, cm−1): 3308 (OH), 3192 (NH), 3058 (CHarom), 1667 (C = O), 1644 (C = O), 1592 (C = C). 1HNMR (DMSO-d6), (δ ppm): 12.65 (OH), 9.08 (s-1H-NHexch), 8.81 (s-1H-NHexch), 7.92–7.55 (m, 9H, Harom); 13CNMR: 167.55, 154.2, 152.70, 144.49, 140.17, 139.81, 139.70, 131.72, 131.32, 131.04, 130, 129.57. Anal. for C14H12N2O3: Calcd. /found C:65.3/65.6, H: 7.2/7.4 and N: 10.91/10.93%.
4-[(Phenylcarbamothioyl)amino]benzoic acid (3): Browne crystals (85% yield), melting point; 169 ℃; IR (ν−, cm−1): 3201 (NH), 3118 (NH), 3009 (CHarom), 1685 (C = O), 1594 (C = C). 1HNMR (DMSO-d6), (δ ppm): 12.67 (OH), 10.72 (s, 1H, NHexch), 9.76 (s, 1H, NHexch), 7.97–7.39 (m, 9H, Harom).13CNMR: 189.22, 179.97, 167.43, 129.87, 128.99, 128.91 126.62, 119.23, 117.54, 113.19. Anal. for C14H12N2O2S: Calcd./found C: 61.72/61.75, H: 4.41/4.44 and N: 10.29/10.27%.
4-[(4-Oxo-3-phenyl-1,3-thiazolidin-2-ylidene)amino]benzoic acid (4): Browne crystals (80%yield), melting point; 200 ℃. IR (ν−, cm−1): 3411 (OH), 3111 (CHarom), 1718 (C = O), 1608 (C = N); 1HNMR (DMSO-d6), (δ, ppm): 11.42 (s, 1H, OH), 7.93–7.14 (m, 9H, Harom), 4.30 (s, 2H, CH2). 13CNMR: 188.05, 167.26, 165.90, 145.14, 144.61, 144.22, 131.40, 130.70, 129.58, 128.35, 15.60. Anal. for C16H12N2O3S Calcd/found: C: 61.53/61.52, H: 3.87/3.85 and N: 8.97/8.95%.
4-[(Benzoylcarbamothioyl)amino]benzoic acid (5): Yellow powder (84% yield), melting point 210 ℃. IR (ν−, cm−1): 3335 (NH), 3272 (NH), 3163 (CHarom), 1716 (C = O), 1677 (C = O), 1589(C = C); 1HNMR: 11.09 (s, 1H, OHexch), 11.04 (s, 1H, NH), 8.05–7.56 (m, 10H, Harom + NH). Anal. for C15H12N2O3S Calcd/found: C: 599.99/59.97, H: 4.03/4.01 and N: 9.33/9.30%.
4-{[(4-Chlorobenzoyl)carbamothioyl]amino}benzoic acid (6): Grey powder (80% yield), melting points; 213 ℃. IR (ν−, cm−1): 3302(NH), 3062 (CHarom), 1673 (C = O),1588 (C = C); 1HNMR (DMSO-d6), (δ ppm): 12.88 (s, 1H, OH), 10.55 (s, 1H, NH), 8.01–7.56 (m, 9H, Harom + NH); 13CNMR: 167.40, 166.94, 165.38, 143.49, 138.28, 137.28, 133.77, 131.59, 131.10, 130.67, 129.18, 128.98, 126.21, 123.99, 120.12, 113.11. Anal. for C15H11ClN2O3S Calcd/found: C: 53.82/53.79, H: 3.31/3.28 and N: 8.37/3.35%.
Methyl 4-{[(4-chlorobenzoyl)carbamothioyl]amino}benzoate (7): Grey powder (74% yield), melting point; 245 ℃. IR (ν−, cm−1): 3381 (NH), 3046(CHarom), 2922(CHalph) 1701 (C = O), 1606 (C = C). 1HNMR (DMSO d6), (δ ppm):8.02(s, 2H, NHexch), 7.99–7.04 (m, 9H, Harom), 2.61 (s, 3H, CH3). 13CNMR: 167.67, 166.99, 149.48, 138.26, 131.59, 129.20, 120.69, 115.66, 50.83(CH3). Anal. for C16H13ClN2O3S Calcd/found: C: 55.09/55.06, H: 3.76/3.74 and N: 8.03/8.01%.
3.2.2 General procedure for synthesizing of compounds 9–13
A solution of phenylisocyanate 8a or phenylisothio cyanate 8b (30 mmol) in 15 ml acetone was added to (30 mmol) of hydrazide derivatives (4-chlorobenzhydrazide, 2-naphthohydrazide and 2-hydrazinopyridine) & refluxing for three to four hours. After finishing reaction time (tested by then layer chromatography) solution moved into icy water, the subsequent products were collecting by filtration, washing thoroughly with H2O & crystallization using CH3CH2OH/ClCH2CH2Cl mixture (1:1).
2-(4-Chlorobenzoyl)-N-phenylhydrazinecarboxamide (9): Yellow powder (83% yield) melting point; 214 ℃; IR (ν−, cm−1): 3295 (NH), 3097 (CHarom), 1667 (C = O), 1633 (C = O), 1596 (C = C). 1HNMR (DMSO-d6), (δppm): 10.35 (s, 1H, NHexch), 8.83 (s, 1H, NHexch), 8.18 (s, 1H, NHexch) 7.96–6.97 (m, 9H, Harom). 13CNMR: 166.12, 156.51, 156.0, 139.95, 137.13, 131.80, 129.92, 129.12, 128.97, 122.55, 119.19. Anal. for C14H12ClN3O2 Calcd./found: C:58.04/58.01, H: 4.17/4.13 and N:14.50/14.49%.
2-(4-Chlorobenzoyl)-N-phenylhydrazinecarbothioamide (10): Brownish solid (89% yield), melting point; 140 ℃; IR (ν−, cm−1): 3296, 3184, 3147 (3 NH), 3104 (CHarom), 3005 (CHarom), 1637 (C = O), 1601 (C = C); 1HNMR (DMSO-d6), (δ ppm): 10.51 (s, 1H, NHexch), 9.80 (s, 1H, NHexch), 9.67 (s, 1H, NHexch), 7.99–7.15 (m, 10H, Harom); 13CNMR: 182.30, 166.47, 148.7, 139.84, 133.21, 132.5, 132.16, 129.84, 129.20, 128.65, 127, 126, 125, 124, 116, 114. Anal. for C14H12ClN3OS Calcd./found: C:54.99/54.96, H:3.96/3.93, and N:13.74/13.71%.
2-(Naphthalen-2-ylcarbonyl)-N-phenylhydrazinecarboxamide (11): Grey crystal (70%yield), melting point; 220 ℃. IR (ν−, cm−1): 3294 (NH), 3220 (NH), 3136 (NH), 3090 (CHarom), 1704 (C = O), 1667 (C = O), 1595 (C = C). 1HNMR (DMSO-d6), (δ ppm): 12.51 (s, 1H, NHexch), 11.23 (s, 1H, NHexch), 7.97–7.59 (m, 12H, Harom). 13CNMR: 185.4 (C = O), 179.6 (C-NH), 155.4(C–CO), other aromatic C-H carbons at 142.2, 140.2, 132.3, 130, 128.8. Anal. for C18H15N3O2 Calcd/found: C: 70.81/70.78, H: 4.95/4.92 and N: 13.76/13.74%.
N-Phenyl-2-(pyridin-2-ylcarbonyl)hydrazinecarboxamide (12): Grey powder (76%yield), melting point; 201 ℃. IR (ν−, cm−1): 3343 (OH), 3318 (NH), 3272 (NH), 3151 (NH), 3104 (CHarom), 3055 (CHarom), 1718 (C = O), 1650(C = O), 1616(C = N), 1598 (C = C). 1HNMR (DMSObd6), (δppm): 10.50(s, 1H, NHexch), 8.85 (s, 1H, NHexch), 8.05(s, 1H, NHexch), 7.46–7.26 (m, 9H, Harom). 13CNMR: 165.9, 158, 150, 149 (C–CO), 140, 139, 130, 129.8, 126, 125, 119. Anal. for C13H12N4O2 Calcd/found: C: 60.93/60.90, H: 4.72/4.70 and N: 21.86/21.83%.
N-Phenyl-2-(pyridin-2-ylcarbonyl)hydrazinecarbothioamide (13): Yellow powder (56% yield), melting point; 270 ℃. IR (ν−, cm−1): 3297 (NH), 3234 (NH), 3205 (NH), 3046 (CHarom), 1653 (C = O), 1593 (C = C); 1HNMR (DMSO-d6), (δ, ppm): 10.68 (s, 1H, NHexch), 9.71 (s, 1H, NHexch), 8.70 (s, 1H, NHexch), 8.69–7.13 (m, 9H, Harom).13CNMR: 181.12, 181, 149.74, 148.99, 139.69, 138.18, 128.52, 127.42, 125.31, 122.91. Anal. for C13H12N4OS Calcd/found: C: 57.34/57.31, H: 4.44/4.41 and N: 20.57/20.54%.
3.3 Insecticidal bio-efficacy screening
The objective designed compounds have been tested as pesticide bioefficacy for explanation as following:
3.3.1 Toxicological effectiveness checked for of 2nd instar larvae of Spodoptera littoralis after three days of treatment
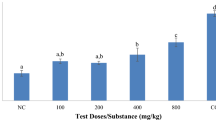
After 3 days of treatment, bioefficacy results for the designed Semicarbazide, Thiosemicarbazide, Urea and Thiourea derivatives 2–7 and 9–13 showed height to low toxicological activity against S. littoralis and the results are listed in Table 1. It has been notice that the LC50 values vary from 9.882 to 102.3, in which LC50 value of compounds 2–7 and 9–13 were 73.69, 45.10, 85.67, 59.88, 17.56, 9.88, 21.32, 54.25, 93.39, 102.3 and 96.35 ppm, respectively in which dimilin 5.946 ppm. The toxicity of chloro ester compound 7 was the most toxicological activity against 2nd instar larvae of Spodoptera littoralis than the other synthesized urea and thiourea compounds and its toxicity ratio at 1.0 with LC50 value at 102.3 ppm, followed by its source benzoic acid derivatives 6 which clearly render to the vital role played by chlorine atom attached to aromatic ring.
3.3.2 Toxicological effectiveness test for 4th instar larvae of Spodoptera littoralis after 72 h of treatment
The bioefficacy outcomes of compounds 2–7 and 9–13 against 4th instar larvae of S. littoralis are displayed in Table 1. After three days of treatment, bioefficacy outcomes showed LC50 values of compounds 2–7 and 9–13 at 164.01, 122.48, 151.08, 163.66, 111.09, 102.66, 119.36, 153.30, 166.35, 172.86 and 158.36 ppm, respectively in which dimilin 59.914 ppm. These results again confirm that the toxicity of compound 7 against 4th instar larvae of S. littoralis is the most active among the other tested compounds because LC50 value of compound 7 is 102.66 ppm and its toxicity ratio at 1.0. Again, the role played by the chlorine atom in both acid and its ester derivative is the reason for their high potency.
4 Structure-action relationship (SAR)
The results listed in Table 1 and Figs. 1, 2 showed that although the main frame structure of the designed compounds is the thiourea and its derivatives, but the existence of chlorine atom linked to the aromatic cycle may the main reason for the high activity recorded for compounds 7, 6 and 9, respectively against 2nd instar larvae and 4th instar larvae of S. littoralis. Meanwhile, the esterification of benzoic acid derivative 6 to give the ester compound 7 render it more potent and high toxic than the acid 6. Also, it is noteworthy that we expect that the presence of pyridine ring as in compound 12 will showed a good activity, unfortunately, it has not remarkable toxic effect as it notice as antibacterial and antifungal. As well as, the 1,3-thiazolidine derivative 4 showed low activity.
5 Conclusion
A series of Semicarbazide, Thiosemicarbazide derivatives have been prepared and confirmed based on spectral and elemental analyses. The toxicity of these compounds were projected against S. Littoralis (Lepidoptera: Noctuidae) & illustrated that a number of the designed compounds have respectable toxicological effectiveness as pesticides. Specifically, compound 7 which has the best insecticidal effectiveness against 2nd instar larvae and 4th instar larvae of S. littoralis than the other designed derivatives. The activity regarding compound 7 may be due to the presence of the chlorophenyl, mehtoxy groups in addition to thiourea nucleus linked to molecular structure. These results are encouraged and appreciated for additional work on the development of novel and other strong insecticides. Our work established that the urea and thiourea derivatives could effectively control against 2nd instar larvae & 4th instar larvae of S. littoralis.
Availability of data and materials
All datasets are available upon reasonable request.
Change history
19 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s43994-023-00056-3
References
Al-Shannaf HM, Desuky WM, Abd El-Halim SM (2006) Effect of some compounds on cotton leafworm, Spodoptera littoralis (Boisd.) and their predators. Egypt J Appl Sci 21:646–660
Özmen D, Kilincer N (2002) Research on the effects of diflubenzuron and hexaflumuron on Spodoptera littoralis (Boisduval) (Lepidoptera: noctuidae) larvae. Türkiye Entomoloji Dergisi 26:21–32
Nasr HM, Badawy MEI, Rabea EI (2010) Toxicity and biochemical study of two insect growth regulators, buprofezin and pyriproxyfen, on cotton leafworm Spodoptera littoralis. Pestic Biochem Physiol 98(2010):198–205
Abdelhamid AA, Elwassimy MM, Aref SA, Gad MA (2019) Chemical design and bioefficacy screening of new insect growth regulators as potential insecticidal agents against Spodoptera littoralis (Boisd.). Biotechnol Rep 24:394–401
Aiming S, Andrew P, Weiqiang Z, Murray EE, Joshua D, Li-Ting C, Jeong-Joong Y, Radchenko EV, Palyulin VA, Compans RW (2006) Nonpeptide inhibitors of measles virus entry. J Med Chem 49(17):5080–5092
Dan H, Menglei W, Siyu Z, Yisong S, Honglian Z, Cheng X, Cheng L, Yuanyan L (2008) Synthesis of novel 4, 6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (Cytotoxic) against U937 leukemia cell lines. Eur J Med Chem 43(4):846–852
Tipparaju SK, Muench SP, Mui EJ, Ruzheinikov SN, Lu JZ, Hutson SL, Kirisits MJ, Prigge ST, Roberts CW, Henriquez FL (2010) Identification and development of novel inhibitors of Toxoplasma gondii enoyl reductase. J Med Chem 53(17):6287–6300
Wei A, Yan-Ni L, Tao Y, Jian-Zhong Y, Wei-Yi P, Ying-Hong Y, You-Fu L, Yong D, Yu-Quan W (2012) Synthesis and biological evaluation of 2-(3-Fluoro-4-nitro phenoxy)-N-phenylacetamide derivatives as novel potential affordable antitubercular agents. Molecules 17(2):2248–2258
Ahmed KS, Mikhail WZA, Sobhy HM, Mostafa EM, Eldin TS, Youssef AM (2019) Effect of Lambda-Cyahalothrin as nanopesticide on cotton leafworm, Spodoptera littoralis (Boisd.). Egypt J Chem 62(7):1263–1275
Moawad SS, Sadek HE (2018) Evaluation of two ecofriendly botanical oils on cotton leaf worm, Spodoptera littoralis (Boisd) (Lepidoptera/Noctuidae). Annals of Agricultural Sciences 63(2):141–144
El-Sayed YA, El-Sayed YH (2021) Evaluation the insecticidal activity of Purpureocillium lilacinum and Cuminum cyminum and study their infection impact on some biochemical content in the haemolymph of the cotton leaf worm Spodoptera littoralis (Boisd) (Lepidoptera: Noctudiae)”. Int J Entomol Res 6(2):22–30
Soliman NN, Salam MA, Fadda AA, Abdel-Motaal M (2020) Synthesis, characterization, and biochemical impacts of some new bioactive sulfonamide thiazole derivatives as potential insecticidal agents against the cotton leafworm. Spodoptera littoralis J Agric Food Chem 68(21):5790–5805
Su NY, Scheffrahn RH (1990) Potential of insect growth regulators as termiticides: a review. Sociobiology 17(2):313–328
Steelman CD, Farlow JE, Breaud TP, schilling PE, (1975) Effects of insect growth regulators on Psorophora columbiae (Dyar and Knab) and non-target aquatic insect species in rice fields. Mosq News 35(1):67–76
Ganyard MC, Bradley JJR, Boyd FJ, Brazzel JR (1977) Field evaluation of diflubenzuron (Dimilin) for control of boll weevil reproduction. J Econ Entomol 70(3):347–350
Bowers WS, Ohta JS, Marsella PA (1979) Discovery of insect antijuvenile hormones in plants. Science 193(4253):542–547
Brooks GT (1986) Insecticide metabolism and selective toxicity. Xenobiotica 16(10):989–1002
Medina P, Smagghe G, Budia F, Tirry L, Vinuela E (2003) Toxicity and absorption of azadirachtin, diflubenzuron, pyriproxyfen, and tebufenozide after topical application in predatory larvae of Chrysoperla carnea (Neuroptera: Chrysopidae). Environ Entomol 32(1):196–203
Mitsui T, Nobusawa C, Fukami G (1984) Mode of inhibition of chitin synthesis by diflubenzuron in the cabbage armyworm, Mamestrabrassicae L. J Pestic Sci 9(1):19–26
Miura T, Takahashi RM (1974) Insect development inhibitors. Effects of candidate mosquito control agents on non-target aquatic organism. Environ Entomol 3(4):631–636
Abdel-Aziz M, Abuo-Rahma GAA, Eman AM, Taha FS (2013) New nitric oxide donating 1,2,4-triazole/oxime hybrids: synthesis, investigation of anti-inflammatory, ulceroginic liability and antiproliferative activities. Bioorg Med Chem 21:3839–3849
Alexander GM, Denis VK, Aleksey SY, Oksana VG (2017) N-substituted cyanacetohydrazides in the synthesis of 3,3-dialkyl-1,2,3,4-tetrahydroisoquinolines by Ritter reaction. Chem Heterocycl Compd 53(10):1114–1119
Magdy MH, Abd El-Mawgoude HK (2016) Use of disubstituted thiosemicarbazide in synthesis of new derivatives of 1,3,4-thiadiazole, 1,2,4-triazole and pyrazole with their antimicrobial evaluation. J Chem Res 6(40):345–350
Ishaaya I, Horowitz AR (1992) Novel phenoxy juvenile hormone analog (pyriproxyfen) suppresses embryogenesis and adult emergence of sweetpotato whitefly (Homoptera: Aleyrodidae). J Econ Entomol 85(6):2113–2117
El-Sheikh EA, Kamita SG, Vu K, Hammock BD (2011) Improved insecticidal efficacy of a recombinant baculovirus expressing mutated JH esterase from Manduca sexta. Biol Control 58:354–361
Abdelhamid AA, Elsaghier AMM, Aref SA, Gad MA, Ahmed NA, Abdel-Raheem SAA (2021) Preparation and biological activity evaluation of some benzoylthiourea and benzoylurea compounds. Curr Chem Lett 10(4):371–376
Gad MA, Aref SA, Abdelhamid AA, Elwassimy MM, Abdel-Raheem SAA (2021) Biologically active organic compounds as insect growth regulators (IGRs): introduction, mode of action, and some synthetic methods. Curr Chem Lett 10(4):393–412
Abdelhamid AA, Salama KSM, Elsayed AM, Gad MA, El-Remaily MAAA (2022) Synthesis and toxicological effect of some new pyrrole derivatives as prospective insecticidal agents against the cotton leafworm, Spodoptera littoralis (Boisduval). ACS Omega 7:3990–4000
El-Gaby MA, Ammar YA, Drar AM, Gad MA (2022) Insecticidal bioefficacy screening of some chalcone and acetophenone hydrazone derivatives on Spodopetra Frugiperda (Lepidoptera: Noctuidae). Curr Chem Lett 11(4):263–268
Jasinski JP, Akkurt M, Mohamed SK, Gad MA, Albayati MR (2015) Crystal structure of N-(propan-2-yl-carbamothioyl)benzamide. Acta Cryst 71(1):56–57
Bakhite EA, Marae IS, Gad MA, Mohamed SK, Mague JT, Abuelhassan S (2022) Pyridine derivatives as insecticides. Part 3. Synthesis, crystal structure, and toxicological evaluation of some new partially hydrogenated isoquinolines against Aphis gossypii (Glover, 1887). J Agric Food Chem 70(31):9637–9644
Ali MA, Salah H, Gad MA, Youssef MAM, Elkanzi NAA (2022) Design, synthesis, and SAR studies of some novel chalcone derivatives for potential insecticidal bioefficacy screening on Spodoptera frugiperda (Lepidoptera: Noctuidae). ACS Omega 7(44):40091–40097
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2):265–267
Finny DJ (1952) Probit analysis: a statistical treatment of the sigmoid response curve, 2nd edn. Cambridge University Press, Cambridge
Sun YP (1950) Toxicity index-an improved method of comparing the relative 378 toxicity of insecticides. J Econ Entomol 43:45–53
Brito TO, Souza AX, Mota YC, Morais VS, de Souza LT, de Souza LT, de Fátima Â, Macedo F Jr, Modolo LV (2015) Design, syntheses and evaluation of benzoylthioureas as urease inhibitors of agricultural interest. RSC Advances 5(55):44507–44515
Obradović D, Nikolić S, Milenković I, Milenković M, Jovanović P, Savić V, Rollere A, Crnogorac MĐ, Stanojković T, Grgurić-Šipka S (2020) Synthesis, characterization, antimicrobial and cytotoxic activity of novel half-sandwich Ru(II) arene complexes with benzoylthiourea derivatives. J Inorg Biochem 210:111164
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
MAG: Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review & editing. EAA: Funding acquisition, Writing-original draft, Writing-review & editing. NIA: Funding acquisition, Writing-original draft, Writing-review & editing. FMAEL: Investigation, Supervision, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review & editing. SAA: Investigation, Supervision, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review & editing. NAA: Funding acquisition, Writing-original draft, Writing-review & editing. AAA: Investigation, Supervision, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review & editing. AMME-S: Investigation, Supervision, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review & editing.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Did your research involve plants/animals?
No, did not.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to correct Nawaf I. Alsenani affiliation from 1 to 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gad, M.A., Alqurashi, E.A., Alsenani, N.I. et al. Insecticidal activity, and SAR studies of semicarbazide, thiosemicarbazide, urea and thiourea derivatives against Spodoptera littoralis (Boisd.). J.Umm Al-Qura Univ. Appll. Sci. 9, 242–251 (2023). https://doi.org/10.1007/s43994-023-00037-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00037-6