Abstract
Although antibiotics are fundamentally vital for treating human diseases, they became harmful to the ecosystem if they reach to the environment. Due to antibiotics are intensely vulnerable to oxidation, oxidation of antibiotics can be considered as a recognized tool for removal or degradation of antibiotics to save the humans and ecosystem. The existing research illuminates the kinetics of oxidative degradation of sulfafurazole antibiotic (SFZ) using chromium trioxide (CrO3) in both H2SO4 and HClO4 media. The reactions in both acidic media showed a 1: 1.33 ± 0.07 stoichiometry (SFZ: CrO3). The reliance of the rates of oxidation reactions on the reactants’ concentrations illuminated that the reactions were first order in [CrO3], whereas in [SFZ] and [H+], their orders were fractional-first and fractional-second, respectively. The rate of oxidation of SFZ in H2SO4 was discovered to be higher than that observed in HClO4. The oxidation rates were not influenced by the change in ionic strength (I) or dielectric constant (D). Addition of Cr(III) had not remarked effect on the rates. Free radical intervention tests were positive. The activation quantities were calculated then discussed. A conceivable mechanism of oxidation was anticipated. Furthermore, the rate-law expressions were also derived.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sulfonamide (sulfa) drugs are essential class of antibiotics for medication of diseases in humans as well as in animals [1, 2]. Despite, antibiotics are really required for treatment of humans, they are considered as one of the threatening pollutants for human health and ecosystem if they reach to the environment due to their involvement of complicated organic compounds [3,4,5]. The expired and unexploited sulfa drugs have several antagonistic impacts concerning humans and environment [6,7,8]. Thus, there is a great interest to ascertain operative and green treatment methodologies to remove or degrade such pollutants to safeguard human health. Traditional wastewater treatment plants were set to be ineffective for sulfa drugs degradation [9, 10]. However, antibiotics are known to impressively dispose to oxidation that can be a helpful method for antibiotics degradation [11,12,13,14,15,16,17,18]. Sulfafurazole or sulfisoxazole (SFZ) is one of sulfonamides that has antibiotic activity against Gram-negative and Gram-positive organisms. Little investigations were performed on the kinetics of oxidative degradation of sulfafurazole drug using different oxidants in various media [19,20,21].
There are many oxidants employed in oxidation reactions like permanganate, Mn(VII), [22,23,24,25,26,27,28,29,30,31,32,33,34,35], colloidal MnIVO2 [36,37,38,39,40,41,42,43,44], cerium(IV) [13, 14, 45,46,47,48,49,50,51,52], hexachloroplatinate(IV) [53,54,55,56,57,58], hexacyanoferrate(III) [59,60,61,62,63], etc. In addition, there are several investigations on the oxidation by chromic acid (H2CrVIO4) in acidic media [64,65,66,67,68,69,70,71,72]. Chromium (VI) oxide (CrVIO3) is considered as a substantial multi-electron oxidant commonly used in organic synthesis [17, 18, 73, 74]. It has a high toxicity to biological systems and carcinogenic but its reduced form, Cr(III), is approximately non-toxic [75, 76]. Thus, reductants can transform toxic Cr(VI) to Cr(III) compounds. Literature review revealed a paucity studies that interested on the oxidation kinetics by CrO3 [17, 18, 73, 74]. Therefore, our present research focuses on the description of the mechanism of CrO3 oxidation of sulfafurazole drug in both H2SO4 and HClO4 supported by a complete understanding of reactions’ kinetics. In this study, we investigated the influence of variation of the acidic medium used on the reactions’ kinetics and to understand the behavior of the reactants in these acids. Our research announce a hopeful appropriately and safe strategy with a twofold value for human and ecosystem: degradation of sulfafurazole drug and transformation of the toxic CrO3 to a relatively harmless Cr(III) compounds.
2 Results and discussion
2.1 Stoichiometry of the oxidative degradations of SFZ
The stoichiometry of oxidation of sulfafurazole (SFZ) using CrO3 in both acidic media was explored using spectrophotometry. Numerous sets of the reaction mixtures with various compositions of the main reactants (SFZ & CrO3 with a stock solution concentration of 0.1 M for each), at fixed [H+] (2.0 M) and ionic strength of 2.5 M, were reserved until realization of the reactions. Evaluation of unconsumed CrO3 showed a 1: 1.33 ± 0.07 stoichiometry (SFZ: CrO3); i.e. 3 mol of SFZ were reacted with 4 mol of CrO3. So, the reactions can be generally illustrated by Scheme (1).

The formation of Cr(III) was confirmed by the dark green precipitate, Cr(OH)3, which formed upon addition of (NH4)2S(aq) or NaOH solutions to the reaction medium.
2.2 Spectral changes
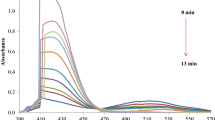
The spectral variations during sulfafurazole oxidation by CrO3 in 2.0 M of both H2SO4 and HClO4 solutions are introduced in Fig. 1(a) and (b), correspondingly. The documented spectra exhibited systematic disappearing of CrO3 band at λ = 349 nm with time. This behavior is a well-prove for oxidation of SFZ by CrO3 and reduction of the latter, Cr(VI), to Cr(III). Under similar circumstances, the decay occurred during the oxidation of SFZ in H2SO4 was discovered to be greater than that occurred in HClO4.
2.3 Effect of [CrO3]
The impact of CrO3 concentration on the oxidation rates was examined by changing its concentration in reactions’ mixtures at fixed [SFZ], [H+], I and T. The results indicated non-substantial variation in the values of the rate constants (kobs, calculated as the slopes of the first order plots) at several [CrO3] as listed in Table 1. This situation illuminated that the reactions disclosed first order reliance in [CrO3] which also confirmed by the good linearity of (ln Abs. vs. time) plots in both acidic media as appeared in Fig. 2.
2.4 Effect of [SFZ]
The rate constant kobs was determined at various [SFZ] whilst other constituents were preserved constant. Rising [SFZ] was set to enhance the rates of reactions as presented in Table 1. Linear plots of kobs vs. [SFZ] with positively kobs axes’ intercepts were obtained, Fig. 3(a). Besides, log [SFZ] vs. log kobs plots were straight with gradients of 0.76 and 0.81 in H2SO4 and HClO4, correspondingly, as shown in Fig. 3(b). So, these reactions were fractional-first orders in [SFZ] in both acids. Values of R2 and standard deviation (S.D.) are added in Table S1 in the supporting information.
2.5 Effect of [H.+]
The impact of [H+] on the rates of reactions was explored by performing the experiments at several [H+] (1.0–3.0 M) in both H2SO4 and HClO4 preserving all other variables stable. Augmenting [H+] was set to enhance the rates (Table 1). Linear plots of kobs vs. [H+]2 with positively kobs axes’ intercepts were acquired as demonstrated in Fig. 4(a) signifying fractional-second order credence in [H+]. Also, log [H+] vs. log kobs plots were straight with gradients of 1.51 and 1.64 in H2SO4 and HClO4, respectively, as presented in Fig. 4(b), confirming the fractional-second order reliance in [H+].
2.6 Effect of ionic strength (I) and dielectric constant (D)
To clarify certain information regarding to the oxidation mechanism, the influence of I on the rates of reactions was examined. So, the kinetic runs were performed at various values of I (2.5–4.0 M). In both acidic media, the gained outcomes signified that varying the values of I were set to have insignificant effects on the rates as listed in Table 1. Moreover, the impact of D was inspected by varying the water– t-butanol compositions (vol%) in the studied media. Results revealed that kobs values were appreciably unaffected with the increase in t-butanol content, reduce in D, as presented in Table S2.
2.7 Effect of [Mn(II)]
To explore the existence of Cr(IV) as one of the predicted intermediates during oxidation reactions by Cr(VI), Mn(II) was added to the reaction mixtures with its various concentrations at constant other reaction constituents. The acquired outcomes illuminated reduction in the oxidation rates with increasing [Mn(II)] confirming the existence of Cr(IV).
2.8 Effect of [Cr(III)]
The dependence of the oxidation rates on the reduction product of Cr(VI), i.e. Cr(III), was studied. For this, Cr(III) was initially added to the reactions’ mixtures with several concentrations, (4.0–12.0) × 10–4 M, at fixed other constituents. The acquired outcomes illuminated no noteworthy impact of addition of Cr(III) on the oxidation rates.
2.9 Effect of temperature
To evaluate the activation quantities, the oxidation rates were examined at different temperatures (288–328 K) at fixed other variables. The results illuminated that augmenting temperature was set to increase the oxidation rates as presented in Table S3. The activation quantities of k2 (k2 = kobs / [SFZ]), were computed (see Table 2) via Eyring and Arrhenius graphs as illustrated in Fig. 5(a) and (b), respectively.
2.10 Free radical intervention test
The feasible attending of free radicals in the present reactions was examined by supplement of acrylonitrile to the reactions’ mixtures, preserved for 4 h in an inert medium. Then, dilute the mixtures with methanol. These tests were positive where dense white precipitates were developed designating intervention of free radicals through the reactions signifying that the reactions were proceeded via generation of free radicals.
2.11 Reactions mechanism
It was stated [77] that CrO3 is hydrolyzed in water to form chromic acid as represented by Eq. (1)
The acquired positive free radical tests favored the involvement of Cr(V) species in the reactions. Also, the reduction in the oxidation rates with increasing [Mn(II)] verified the participation of Cr(IV) in the present reactions [67, 68].
On the other hand, in sulfa drug structures, two groups were identified as the protonation sites in acidic media [78, 79]. In sulfafurazole (SFZ), protonation occurs at the aromatic primary amino group and the anilinic NH2 group [80, 81]. The obtained strongly pH-dependent with fractional-second order dependence is considered as a significant prove for protonation of sulfafurazole (symbolized by S) which can be illustrated by Eq. (2),
where, SH22+ represents the protonated species of SFZ which is considered as the reactive species in the existing reactions’ kinetics. Also, the lower than unity order in [SFZ] refers to a complexation of the active species of both SFZ and the oxidant according to the following equation,
This was kinetically confirmed by the acquired positive intercepts in 1/kobs vs. 1/[S] plots [82] as presented in Fig. 6 (b). The obtained trivial effects of I and D on the oxidation rates accorded with the reactions happening amongst an ion and a neutral molecule [83, 84], i.e. between SH22+ and H2CrO4. The formed complex, [SH2 – H2CrVIO4]2+ (C), was gently decomposed in the slow stage producing free radical SFZ and Cr(V) reactive intermediates,
This stage is followed by successive rapid stages to produce the final oxidative degradation products.
The suggested mechanism guides to derive the following rate-law expressions (see Appendix S1 in the supporting information),
Equations (5) and (6) prerequisite that the graphs: 1/kobs vs. 1/[S] at fixed [H+] and 1/kobs vs. 1/[H+]2 at fixed [S] must be linear with positively 1/kobs axes’ intercepts, as were acquired in both acidic media, Fig. 6(a) and (b), respectively, proving the legality of the proposed mechanism. The rate constant k1 and the equilibrium constants K1 & K2 at 298 K were evaluated via Eqs. (5) and (6) and are inserted in Table 3.
2.12 Activation parameters
The obtained activation quantities are presented in Table 2. The high negatively ΔS# recommends construction of a rigid intermediate through the reactions [85]. Also, the positive values of ΔH≠ and ΔG≠ manifests that the intermediate construction in the rate-determining step, as proposed in the reactions mechanism, was endothermic and non-spontaneous, correspondingly [86]. The acquired higher Ea≠ illuminated that the slow stage was the decomposition of constructed complexes to yield the degradation products [17].
3 Experimental
3.1 Materials
Most employed chemicals in this research work were of Sigma-Aldrich and Fluka. Bidistilled water was used as a solvent to make all solutions. Sulfafurazole (99.6%) was utilized as supplied. Fresh solution of chromium trioxide, CrO3 (Sigma-Aldrich) was made prior to each run by dissolving the sample in bidistilled water and it was standardized spectrophotometrically [17, 18]. Solutions of H2SO4 (Fluka, 97%) and HClO4 (Sigma-Aldrich, 70%) were made by dilution with bidistilled water. Solutions of Na2SO4 and NaClO4 were made to fix the ionic strength (I) in H2SO4 and HClO4 solutions, correspondingly. t-Butanol was utilized to examine the impact of dielectric constant (D) of the reactions media.
3.2 Kinetic measurements
Kinetic measurements were conveyed out underneath pseudo-first order circumstances where [SFZ] > > [CrO3]. The values of I of the reactions’ media were attained fixed at 2.5 mol dm–3. The progresses of the reactions were monitored via spectrophotometric tool by detecting the deterioration of the absorbance of Cr (VI) with time at λ = 349 nm using a temperature– accurate double-beam Shimadzu UV-1800 spectrophotometer. The first order rate constant plots (ln Abs. vs. time) were nearly linear and the values of kobs were calculated as the slopes of these plots. The mean values of about three distinctive runs were taken and were reproducible within ± 4%.
4 Conclusions
-
1.
The kinetics of oxidative degradation of sulfafurazole (SFZ) by CrO3 in both H2SO4 and HClO4 were examined.
-
2.
The reactions in both acidic media showed a 1: 1.33 ± 0.07 stoichiometry (SFZ: CrO3).
-
3.
The rate of oxidation of SFZ in H2SO4 was discovered to be higher than that observed in HClO4. 4. The activation quantities were calculated and discussed.
-
4.
A proposed mechanism for the oxidative degradation was made.
-
5.
The rate-law expressions were also derived.
-
6.
This research announce a hopeful appropriately and safe strategy for degradation of SFZ antibiotic to safeguard the human health and ecosystem.
Data availability
All data presented in this study are available on request from the corresponding author.
References
Morrison RT, Boyd RN (2001) Organic Chemistry; 6th ed. Prentice-Hall of India: New Delhi, p 862
Chee-Sanford JC, Aminov RI, Krapac IJ, GarriguesJeanjean N, Mackie RI (2001) Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl Environ Microbiol 67:1494–1502
Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118
Santos LHMLM, Araujo AN, Fachini A, Pena A, Delerue-Matos C, Montenegro MCBSM (2010) Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater 175:45–95
Kummerer K (2009) Antibiotics in the aquatic environment—A review—Part II. Chemosphere 75:435–441
Akiyama T, Savin MC (2010) Populations of antibiotic-resistant coliform bacteria change rapidly in a wastewater effluent dominated stream. Sci Total Environ 408:6192–6201
Knapp CW, Dolfing J, Ehlert PAI, Graham DW (2010) Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ Sci Technol 44:580–587
Schmitt H, Van BP, Tolls J, Van LCL (2004) Pollution induced community tolerance of soil microbial communities caused by the antibiotic sulfachloropyridazine. Environ Sci Technol 38:1148–1153
Ingerslev F, Halling-Sorensen B (2000) Biodegradability properties of sulfonamides in activated sludge. Environ Toxicol Chem 19:2467–2473
Pérez S, Eichhorn P, Aga DS (2005) Evaluating the biodegradability of sulfamethazine, sulfamethoxazole, sulfathiazole and trimethoprim at different stages of sewage treatment. Environ Toxicol Chem 24:1361–1367
Fawzy M (2019) Removal of toxic tellurium (IV) compounds via bioreduction using flucloxacillin in aqueous acidic medium: A kinetic and mechanistic approach. J Mol Liq 292:111436
Fawzy M, Abdallah N (2020) Alqarni, Oxidative degradation of neomycin and streptomycin by cerium(IV) in sulphuric and perchloric acid solutions. J Mol Liq 312:113439
Fawzy M, Abdallah N (2020) Alqarni, Degradation of ampicillin and flucloxacillin antibiotics via oxidation by alkaline hexacyanoferrate(III): Kinetics and mechanistic aspects. Ind Eng Chem Res 59:16217–16224
Fawzy M, Abdallah N (2020) Alqarni, Oxidative degradation of some antibiotics by permanganate ion in alkaline medium: A kinetic and mechanistic approach. Trop J Pharm Res 19:1999–2007
Fawzy M, Abdallah N (2020) Alqarni, Kinetics and mechanism of oxidation of neomycin and streptomycin antibiotics by alkaline permanganate. Umm Al-Qura Univ J Appl Sci 6:1–5
Fawzy M, Abdallah N (2021) Alqarni, Mechanistic and thermodynamic aspects of oxidative removal of flucloxacillin by different oxidants in an acidic medium. J Mol Liq 325:115160
Fawzy A, Toghan A (2020) Unprecedented treatment strategy of aquatic environments. Oxidative degradation of penicillin G by chromium trioxide in acidic media and the impact of metal ion catalysts: Kinetics and mechanistic insights. ACS Omega 5:32781–32791
Fawzy A, Alqarni N, El-Gammal B, Toghan A, Hassan NA, Algarni Z (2022) Auspicious water treatment approach. Oxidative degradation of fluconazole and voriconazole antibiotics by CrO3 in different acidic environments: Kinetics, mechanistic and thermodynamic modelling. J Saudi Chem Soc 26:101396
Sharma VK, Mishra SK, Nesnas N (2006) Oxidation of sulfonamide antimicrobials by ferrate(VI) [FeVIO42-]. Environ Sci Technol 40:7222–7227
Yao J, Zeng X, Wang Z (2017) Enhanced degradation performance of sulfisoxazole using peroxymonosulfate activated by copper-cobalt oxides in aqueous solution: Kinetic study and products identification. Chem Eng J 330:345–354
Sharma VK, Kim C, Gardinali P, Varma R, Kim H (2015) Ferrate promoted oxidative cleavage of sulfonamides: Kinetics and product formation under acidic conditions. Chem Eng J 279:307–316
Hassan RM, Fawzy A, Ahmed GA, Zaafarany IA, Asghar BS, Khairou KS (2009) Acid-catalyzed oxidation of some sulfated macromolecules. Kinetics and mechanism of oxidation of kappa-carrageenan polysaccharides by permanganate ion in acid perchlorate solutions. J Mol Cat A 309:95–102
Kini K, Farokhi SA, Nandibewoor ST (2002) A comparative study of ruthenium(III) catalyzed oxidation of L-leucine and L-isoleucine by alkaline permanganate. A kinetic and mechanistic approach. Trans Met Chem 27:532–540
Hassan RM, Abdel-Kader DA, Ahmed SM, Fawzy A, Zaafarany IA, Asgar BA, Takagi HD (2009) Acid-catalyzed oxidation of carboxymethyl cellulose. Kinetics and mechanism of permanganate oxidation of carboxymethyl cellulose in acid perchlorate solutions. Cat Commun 11:184–190
Hassan RM, Dahy A, Ibrahim S, Zaafarany IA, Fawzy A (2012) Oxidation of some macromolecules. Kinetics and mechanism of oxidation of methyl cellulose polysaccharide by permanganate ion in acid perchlorate solutions. Ind Eng Chem Res 51:5424–5432
Zaafarany A, Fawzy GA, Ahmed SA, Ibrahim RM, Hassan HD (2010) Takagi, Further evidence for detection of short-lived transient hypomanganate (V) and manganate (VI) intermediates during oxidation of some sulfated polysaccharides by alkaline permanganate using conventional spectrophotometeric techniques. Carbohydr Res 345:1588–1593
Jose TP, Nandibewoor ST, Tuwar SM (2005) Mechanism of oxidation of L-histidine by heptavalent manganese in alkaline medium. E-J Chem 2:75–85
Hassan RM, Fawzy A, Alarifi A, Ahmed GA, Zaafarany IA, Takagi HD (2011) Base-catalyzed oxidation of some sulfated macromolecules: Kinetics and mechanism of formation of intermediate complexes of short-lived manganate(VI) and/or hypomanganate (V) during oxidation of iota- and lambda-carrageenan polysaccharides by alkaline permanganate. J Mol Cat A 335:38–45
Hassan RM, Fawzy A, Ahmed GA, Zaafarany IA, Asghar BH, Takagi HD, Ikeda Y (2011) Kinetics and mechanism of permanganate oxidation of iota- and lambda-carrageenan polysaccharides as sulfated carbohydrates in acid perchlorate solutions. Carbohydr Res 346:2260–2267
Asghar H, Fawzy A (2016) Kinetic, mechanistic, and spectroscopic studies of permanganate oxidation of azinylformamidines in acidic medium, with autocatalytic behavior of manganese(II). J Saudi Chem Soc 20:561–569
Fawzy SS, Ashour MA (2014) Musleh, Base-catalyzed oxidation of L-asparagine by alkaline permanganate and the effect of alkali-metal ion catalysts: Kinetics and mechanistic approach. React Kinet Mech Cat 111:443–460
Fawzy SS, Ashour MA (2014) Musleh, Kinetics and mechanism of oxidation of L-histidine by permanganate ions in sulfuric acid medium. Int J Chem Kinet 46:370–381
Halligudi LL, Desai SM, Mavalangi AK, Nandibewoor ST (2000) Free radical intervention, deamination and decarboxylation in the ruthenium(III)-catalysed oxidation of L-arginine by alkaline permanganate—a kinetic study. Trans Met Chem 26:28–35
Fawzy A, Shaaban MR (2014) Kinetics and mechanistic investigations on the oxidation of N’-heteroaryl unsymmetrical formamidines by permanganate ion in aqueous alkaline medium. Trans Met Chem 39:379–386
Fawzy NE, Guesmi II, Althagafi BH (2017) Asghar, Kinetics and mechanism of permanganate oxidations of isosorbide in different acidic media. J Sol Chem 46:613–625
Iqubal SMS, Bahafi A, Sahu M, Mishra K, Khan AA, Mohammed T (2022) Oxidation of citric acid using colloidal MnO2 in the presence of non-ionic surfactant tween-80. Asian J Pharm 16:450–452
Iqubal SMS (2009) Nanosized MnO2: preparation, characterisation and its redox activity. Int J Nanopart 1:321–328
Iqubal SMS (2022) Review on kinetic studies of α-hydroxy acids (glycolic, mandelic, citric, tartaric and malic) and some other organic compounds with water soluble nano particles of colloidal MnO2 in absence and presence of non-ionic surfactant (TX-100). J Umm Al-Qura Univ Appl Sci 8:79–84
Iqubal SMS (2022) Characterization, surface morphology and microstructure of water soluble colloidal MnO2 nanoflakes. J Umm Al-Qura Univ Appl Sci 8:33–36
Iqubal SMS (2010) Kinetics of the reduction of water soluble colloidal MnO2 by mandelic acid in the absence and presence of non-ionic surfactant triton X-100. Coll. J. 72:195–204
Kabir-ud-Din I, Iqubal SMS, Khan Z (2005) Effect of ionic and non-ionic surfactants on the reduction of water soluble colloidal MnO2 by glycolic acid. Coll Polym Sci 284:276–283
Kabir-ud-Din I, Iqubal SMS, Khan Z (2005) Oxidation of DL-tartaric acid by water soluble colloidal Mn02 in the absence and presence of surfactants. Indian J Chem 44A:2455–2461
Kabir-ud-Din I, Iqubal SMS, Khan Z (2005) Reduction of soluble colloidal MnO2 by DL-malic acid in the absence and presence of nonionic TritonX-100. Coll Polym Sci 283:504–511
Kabir-ud-Din I, Iqubal SMS (2005) Khan Z Kinetics of the reduction of colloidal MnO2 by citric acid in the absence and presence of ionic and non-ionic surfactants. Bioinorg React Mech 5:151–166
Hassan RM, Alaraifi A, Fawzy A, Zaafarany IA, Khairou KS, Ikeda Y, Takagi HD (2010) Acid-catalyzed oxidation of some sulfated polysaccharides: Kinetics and mechanism of oxidation of kappa-carrageenan by cerium(IV) in aqueous perchlorate solutions. J Mol Cat A 332:138–144
Yadav MB, Derva V, Rani A (2009) Kinetics and mechanism of uncatalyzed and silver (I) catalyzed oxidation of lysine by cerium(IV) in acid perchlorate medium. J Ind Chem Soc 86:600–604
Fawzy O (2016) of alginate and pectate biopolymers by cerium(IV) in perchloric and sulfuric acid solutions: a comparative kinetic and mechanistic study. Carbohydr Polym 138:356–364
Sumathi T, Shanmugasundaram P, Chandramohan G (2013) A kinetic and mechanistic study on the silver(I) catalyzed oxidation of l-Serine by cerium(IV) in sulfuric acid medium. J Saudi Chem Soc 17:227–235
Fawzy A (2016) Kinetic and mechanistic aspects of oxidation of aminotriazole formamidine by cerium(IV) in aqueous perchloric and sulfuric acid solutions: a comparative study. J Sol Chem 45:246–264
Thabaj KA, Chimatadar SA, Nandibewoor ST (2006) Mechanistic study of oxidation of palladium(II) by cerium(IV) in aqueous acid. Trans Met Chem 31:186–193
Fawzy RM, Hassan I, Althagafi M (2016) Morad, Cerium(IV) oxidations of sulfated polysaccharides in aqueous perchlorate solutions: A kinetic and mechanistic approach. Adv Mater Lett 7:376–382
Chimatadar SA, Madawale SV, Nandibewoor ST (2007) Mechanistic study of iodide catalyzed oxidation of L-glutamic acid by cerium(IV) in aqueous sulfuric acid medium. Trans Met Chem 32:634–641
Asghar H, Altass HM, Fawzy A (2016) Silver(I)-catalysis of oxidative deamination and decarboxylation of L-asparagine and L-histidine by platinum(IV) in perchloric acid solutions: A comparative kinetics study. J Env Chem Eng 4:617–623
Asghar HM, Altass A (2015) Fawzy, Transition metal ions-catalyzed oxidation of L-asparagine by platinum(IV) in acid medium: A kinetic and mechanistic study. Transition Met Chem 40:587–594
Fawzy BH (2015) Asghar, Kinetics and mechanism of uncatalyzed and silver(I)-catalyzed oxidation of L-histidine by hexachloroplatinate(IV) in acid medium. Transition Met Chem 40:287–295
Fawzy I (2014) of copper(II) catalyst on the oxidation of L-histidine by platinum(IV) in alkaline medium: A kinetic and mechanistic study. Transition Met Chem 39:567–576
Fawzy A (2015) Kinetics and mechanistic approach to the oxidative behavior of biological anticancer platinum(IV) complex towards L-asparagine in acid medium and the effect of copper(II) catalyst. Int J Chem Kinet 47:1–12
Fawzy IA (2015) Zaafarany, Kinetic and mechanistic investigation on the zirconium(IV)-catalyzed oxidation of L-histidine by hexachloroplatinate(IV) in acid medium. Chem Sci Rev Lett 4:608–618
Hassan RM, Ibrahim SM, Zaafarany IA, Fawzy A, Takagi HD (2011) Base-catalyzed oxidation Kinetics and mechanism of hexacyanoferrate(III) oxidation of methyl cellulose polysaccharide in alkaline solutions. J Mol Cat A 344:93–98
Fawzy A (2016) Palladium(II)-catalyzed oxidation of l-tryptophan by hexacyanoferrate(III) in perchloric acid medium: A kinetic and mechanistic approach. J Chem Sci 128:247–256
Fawzy A (2016) Kinetics and mechanism of uncatalyzed and ruthenium(III)-catalyzed oxidation of formamidine derivative by hexacyanoferrate(III) in aqueous alkaline medium. J Chem Sci 128:733–743
Durgannavar AK, Patgar MB, Chimatadar SA (2015) Oxidation of amoxicillin by hexacyanoferrate (III) in aqueous alkaline medium - a kinetic and mechanistic approach. Indian J Chem 54:1085–1091
Fawzy IA, Zaafarany J, Alfahemi I, Althagafi M (2015) Morad, Oxidation of pectate biopolymer by hexacyanoferrate(III) in aqueous alkaline medium. A kinetic and mechanistic study. Chem Sci Rev Lett 4:985–996
Hassan RM, Ahmed SM, Fawzy A, Abdel-Kader DA, Ikeda Y, Takagi HD (2010) Acid-catalyzed oxidation of carboxymethyl cellulose polysaccharide by chromic acid in aqueous perchlorate solutions. A kinetics study. Cat Commun 11:611–615
Fawzy I, Althagafi K, Khairou R, Hassan N, Yarkandi L, Almazroai T (2015) Bawazeer, Kinetics and mechanistic aspects of oxidation of iota- and lambda-carrageenans by chromium(VI) in aqueous perchlorate solutions. Chem Sci Rev Lett 4:1293–1304
Sen Gupta KK, Chakladar JK, Chatterjee AK, Chakladar JK (1973) Kinetics of the oxidation of hypophosphorous and phosphorous acids by chromium(VI). J Inorg Nucl Chem 35:901–908
Fawzy HM (2016) Altass, Ruthenium(III)-catalyzed oxidation of alginate and pectate biopolymers by chromic acid in aqueous perchlorate solutions: A comparative kinetic study. Trans Met Chem 41:115–124
Fawzy SS, Ashour MA, Musleh RM, Hassan BH (2016) Asghar, Kinetics and mechanistic approach to the chromic acid oxidation of L-tryptophan with a spectral detection of chromium(III) product. J Saudi Chem Soc 20:450–458
Khan Z (2001) Effect of manganese(II) ions on the oxidation of malic and oxaloethanoic acids by aqueous H2CrO4. Trans Met Chem 26:672–678
Fawzy RS, Jassas SA, Ahmed HM, Ali M, Abdallah MAS, Abourehab NS (2017) Abbas, Fluorenones formation via effective chromium(VI) oxidation in perchlorate solutions: Kinetic and mechanistic features. J Mater Env Sci 8:4032–4039
Fawzy N, Elguesmi II, Althagafi BH (2017) Asghar, A Study of the kinetics and mechanism of chromic acid oxidation of isosorbide, A chiral Biomass-derived substrate, in aqueous perchlorate solution. Trans Met Chem 42:229–236
Faruq UZ, Zuru AA, Odebunmi EO, Dangoggo SM (2011) Mechanism for partial oxidation of cyclohexene by chromium(VI) oxide in acetic acid. Global J Pure Appl Sci 17:117–121
Piers E, Worster PM (1977) Oxidation with chromium(VI) oxide - pyridine complex. A study of reaction parameters using cholesterol as substrate. Can J Chem 55:733–739
Fawzy O, Solo M (2021) Morad, Oxidation of barbituric and thiobarbituric acids by chromium trioxide in different acidic media: A kinetic and mechanistic aspects. J Mol Str 1229:129495
Katz SA, Salem H (1993) The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol 13:217–224
Kostecki PT (1998) Chromium in soil: perspectives in chemistry, health, and environmental regulation. J Soil Contamin 61:561–568
House HO (1972) Oxidation with chromium and manganese compounds in; modern synthetic reaction, 2nd edn. WA Benjamin, London
Uhlemann T, Berden G, Oomens J (2021) Preferred protonation site of a series of sulfa drugs in the gas phase revealed by IR spectroscopy. Eur Phys J D 75:23–35
Geiser L, Henchoz Y, Galland A, Carrupt P-A, Veuthey J-L (2005) Determination of pKa values by capillary zone electrophoresis with a dynamic coating procedure. J Sep Sci 28:2374–2380
Manzo RH, de Bertorello MM (1973) Isoxazoles I: protonation of isoxazole derivatives in aqueous sulfuric acid. J Pharm Sci 62:152–154
Prabhu M, Venkatesh G, Rajendiran N (2010) Spectral characteristics of sulfa drugs: effect of solvents, pH and cyclodextrin. J Sol Chem 39:1061–1086
Michaelis L, Menten ML (1913) The kinetics of invertase action. Bio Chem Z 49:333–369
Frost AA, Person RG (1970) Kinetics and Mechanism. Wiley Eastern, New Delhi
Amis ES (1966) Solvent Effects on Reaction Rates and Mechanism. Academic Press, New York
Freeman F, Fuselier CO, Armstead CR, Dalton CE, Davidson PA, Karchesfski EM, Krochman DE, Johnson MN, Jones NK (1981) Permanganate ion oxidations. 13. Soluble manganese (IV) species in the oxidation of 2,4(1H,3H)-pyrimidinediones (uracils). J Am Chem Soc 103:1154–1159
Hicks KW, Toppen DL, Linck RG (1972) Inner-sphere electron-transfer reactions of vanadium(II) with azidoamine complexes of cobalt(III). Inorg Chem 11:310–315
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fawzy, A., Fawzi, A. Oxidative degradation of sulfafurazole drug by chromium trioxide in different acidic media: a kinetic and mechanistic study. J.Umm Al-Qura Univ. Appll. Sci. 9, 276–284 (2023). https://doi.org/10.1007/s43994-023-00035-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00035-8