Abstract
Bovine lactoferrin (bLf), a glycoprotein with iron, Several of Lf's functions intervene through various cell receptors. Its antibacterial, antiparasitic, anti-inflammatory, and antioxidant effects Additionally, it has been found that Lf has immunomodulating. We looked into the protective effects of Lf on various brain tissue indicators and histopathological examination of streptozotocin (STZ) induced diabetic rats. This research aimed to check the potential protective effect of lactoferrin verses brain lesion in STZ induced diabetic rat. The rats were split into five groups: Control (C), Diabetic rat (T2D), T2D + Insulin, T2D + Lactoferrin, and T2D + Insulin + Lactoferrin. Diabetes was created in rat groups by administration STZ (60 mg/kg body weight), then rat was sacrificed. The tissues of the Brain and serum had been chosen to analyze biochemical brain tissue indicators, Serum Neuron-Specific Enolase (SNE), Serum Brain-Derived Neurotrophic Factor (BDNF), and Tissue Necrosis Factor-Alpha (TNF-Alpha). Also, H&E staining was utilized to measure the histological changes of the brain and Comet assay of brain tissue samples for detection of DNA damage. In T2D rat, Lactoferrin corrected aberrant serum levels of SNE, BDNF and TNF-Alpha, in addition, Lactoferrin improves brain pathological changes might be through hypoglycemic, and/or anti-inflammatory actions and reduced DNA damage as compared to diabetic untreated rats. It could be concluded that oral administration of lactoferrin to diabetic rats alone or in combination with insulin treatment for six weeks was found to attenuate the harmful effects of diabetes on brain biomarkers as well as the pathological lesions that occur in the brain tissues of diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
On mental evaluations of data handling speed and executive capability, diabetes is connected to subpar performance. Markers of both neurodegeneration and cerebrovascular sickness play a significant job in interceding the association [1]. Diabetes and every indication of brain disease were highly correlated. There was areas of strength between every one of these markers and worse memory, handling rate, and leader capability scores. Formal intervention concentrates on uncovered that the relationships of diabetes with handling velocity and chief capability were altogether mediated by marks of cerebrovascular and degenerative illness [2]. Strong evidence from prior studies has connected T2DM to dementia, Alzheimer’s disease, and late-life cognitive impairment [1]. However, it is unclear what structural brain damage underlies the connection. White matter lesions (WMLs), cerebral microbleeds (CMBs), and stroke are only a few examples of the macro- and microvascular conditions that diabetes causes in the brain [2].
Focuses on the use of population-based neuroimaging have also uncovered an association between diabetes and neurodegenerative markers such as global and mean fleeting curve decline in middle age or later. Despite the fact that there are conflicting clinicopathological data linking diabetes to the prevalence of neurodegenerative diseases (amyloid plaques and neurofibrillary tangles) [3], atherosclerosis, cerebrovascular disease and neurodegeneration have all been linked to diabetic pathophysiological pathways from oxidative stress implicated, hyperglycemia, inflammation and insulin resistance [2]. For instance, according to Qiu et al. [4], CMBs in addition to disruption of white matter integrity have been linked to slowed processing speed and subpar executive function. Additionally, follow-up researches had been demonstrated a link between diabetes and an accelerated rate of brain atrophy, along with a reduction in processing power and executive function.
DNA damage could be related to T2DM and its complications mainly due to oxidative pressure. Little is known that DNA fixation disorders may contribute to the overall level of DNA damage in T2DM, which may thus be associated with genomic precarity leading to malignant growth [5].
Neuron-specific enolase (NSE) is a neuronal glycolytic enzyme that is abundant in the neurons of brain gray matter and its production is upregulated during ischemia and axon injuries. Brain-derived neurotrophic factor (BDNF) serves as a neurotransmitter modulator, and participates in neuronal plasticity, which is essential for learning and memory. Tumor necrosis factor alpha (TNF-Alpha,) is a proinflammatory cytokine produced by microglia during neuroinflammation and exerts both homeostatic and pathophysiological roles in CNS [1, 2].
Bovine lactoferrin (bLf), a glycoprotein with iron, is mostly found in bodily fluids and milk [6]. Several of Lf's functions intervene through various cell receptors [7]. Its antibacterial, anti-inflammatory, and antioxidant effects have all been demonstrated [8,9,10]. Additionally, it has been found that Lf posses immunomodulating [11], anticarcinogenic [12], and improved apoptosis characteristics [13]. we looked into the protective effects of Lf against the diabetes-associated brain tissue damage and DNA damage including measurement of brain tissue biomarkers, NSE, BDNF and TNF- Alpha.Brain histopathological analysis was also done in diabetic rats.
2 Materials and methods
2.1 Medications
Radiance Organic Process Company, a New Zealand company, provided the lactoferrin. A. Insulin (Mixtard, Novo Nordisk).
2.2 Animals
The study used (60) healthy male Sprague–Dawley rats, 150 g on average. Their fasting blood sugar levels were in the range of 100–150 mg/dL. Ten animals were used as control, healthy rats. To induce diabetes in 50 rats, intraperitoneal infusion (IP) of 60 mg/kg body weight [14] of streptozotocin was used. A heavy fatty meal was offered to the rats 3 days before streptozotocin injection [15]. Blood tests were performed 48 h after the injection to check for hyperglycemia (> 300 mg/dL). Forty rats were classified as diabetes once their blood glucose levels exceeded 300 mg/dL. After being kept untreated for 6 weeks, all rats (40 diabetics and 10 normal) were divided into the corresponding exploratory groups. The Ethics Committees approval number for allowing animal experiments was (RZ10094, Faculty of Science, Mansoura University, Egypt).
2.3 Experimental groups
-
1.
Group I (Control): Ten rats, received 0.1 mL of saline solution subcutaneously every day for 6 weeks along with saline P.O via rat stomach tube.
-
2.
Group 2, diabetic-untreated rats: 10 rats, received saline P.O and were daily injected (SC) with 0.1 mL of saline solution for 6 weeks. mL
-
3.
Group 3, diabetic rats-treated with insulin: 10 rats, received 1 mL of saline administered orally each day and 2U of insulin according to manufacturer instruction (Mixtard, Novo Nordisk) given SC every day for 6 weeks.
-
4.
Group 4, diabetic rats treated with lactoferrin. Ten rats, received daily dose of Lf (200 mg/kg/day, p.o.) [16] and daily SC saline injections for a period of 6 weeks.
-
5.
Group 5, diabetic rats treated with insulin + lactoferrin. Ten rats received daily oral Lf (200 mg/kg/day, p.o.) and 2 U of insulin (Mixtard, Novo Nordisk) by SC injection for 6 weeks.
2.4 Blood sampling and techniques
By using the orbital sinus technique while the rats were sedated with ether toward the finishing of the test, the samples of the blood had been taken from each experimental rat in the morning. By using “Contour TS, Glucometer, Ascensia, Japan”, the fasting blood glucose level was measured. The next assays of the biochemical brain tissue indicators, including Serum Neuron-Specific Enolase (SNE), Serum Brain-Derived Neurotrophic Factor (BDNF), and Tissue Necrosis Factor-Alpha (TNF-Alpha), required the collection and storage of sera at − 80 °C. By using an automated immunofluorescent test on the BRAHMS Krypto (Package insert: BRAHMS NSE Krypto. Thermo Fisher Scientific BRAHMS LLC; Version 20.04.2012), serum levels of SNE were determined in accordance with [17]. In order to quantify the levels of serum BDNF in non-human primates, Takuma, [13] developed the enzyme-linked immunosorbent assay (ELISA) method. TNF-Alpha, has been used measured by Enzyme-linked immunosorbent assay (ELISA) according to the technique of Konstantinou [18].
2.5 Histopathological analysis of brain tissue
At the end of experimental period, experimental rats were sacrificed and brain tissue samples were taken, fixed in phosphate-buffered formalin 10% (v/v), pH 7.4, dried in increasing grades of ethanol, cleaned in toluene, and mounted in molten pararplast (58–62 °C). The following histological sections were each stained with hematoxylin and eosin (H&E). Light microscopy was used to examine the changes in the hippocampus, cerebellum, and brain.
2.6 Comet assay
The cerebrum tissue tests were homogenized in cradle (pH 7.5) before 6 mL of the homogenate was suspended on low dissolving agarose (0.5%) on completely iced slides, which were put away on ice during the polymerization process. Slides were lowered in lysate arrangement. The slides were put in electrophoresis (1 mM Na2EDTA, 0.3 M NaOH, pH = 13) cushion for 10 min at 0 °C after the 0.5% agarose layer had solidified to permit DNA to loosen up. The slides were killed with tris-buffer at pH 7.5 prior to being stained with 20 gm/L ethidium-bromide. Each slide was inspected utilizing a German epifluorescence microscope. On each slide, the programmed computerized investigation framework examined 100 cells.Tail length times DNA content equivalents the tail moment. Tail length and force are estimated utilizing picture investigation tools.
2.7 Statistical analysis
The data from three distinct trials were examined using Graph Prism 9.0 (GraphPad Software Inc., USA). The information is displayed as mean and standard deviation (S.D.). The statistics were performed using one-way ANOVA test in addition to the hoc analysis of Tukey’s post At (P < 0.05), the level of significance was established.
3 Results
3.1 Levels of blood glucose and brain tissue markers
When compared to control rats, glucose levels in diabetic animals were considerably higher during the whole study period (Fig. 1A; P < 0.05). Diabetes-related insulin therapy in rats likewise resulted in an abnormal higher value (P < 0.05). When compared to diabetic untreated rats, treatment with Lf alone or in combination with insulin was related with a substantial reduction in blood glucose levels (P < 0.05).
Additionally, Fig. 1B–D demonstrates that untreated diabetic rats had considerably higher serum levels of the brain tissue biomarkers SNE, BDNF, and TNF-Alpha, than controls. However, although being negligible compared to control rats, it was demonstrated that their levels were raised in diabetic rats treated with insulin alone. Additionally, compared to untreated rats, rats treated with lactoferrin alone or in combination with insulin showed a substantial decline in all investigated biomarkers, with levels approaching control (P < 0.05).
3.2 Histopathological analysis of brain tissue
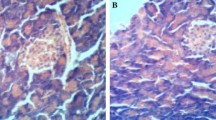
In control groups, the cerebral external granular (G) demonstrated a dense distribution of pyramidal and stellate cells with centrally located nuclei (Fig. 2C and C1). Numerous T2D rats’ neuroglial cells had nuclear chromatin clumping or chromatolysis (also known as caryolysis) (pyknosis). The angiogenesis of blood vessels, a disease-causing feature, appears to be widely distributed throughout the brain tissue. A clustering of glial cells suggests inflammation of the brain tissue. Significantly more necrotic, edematous, and spongiform deteriorated foci appeared (Fig. 2D–D2). On the other hand, treatment with lactoferrin (Fig. 2DL–DL2) alone or with Insulin ameliorates (Fig. 2DIL–DIL2) this damage. Insulin supplementation (Fig. 2DI–DI2) enhanced these alterations.
(C-DIL2). Impact of Lactoferrin on cerebellum histology of T2D animals. A Photomicrograph of histological section staining with H&E of the cerebellum in control group (C-C1), diabetic group (D-D2), T2D + insulin group (DI-DI2), T2D + insulin + Lactoferrin group (DL-DL2), AND T2D + insulin + Lactoferrin group (DIL-DIL2). B Brain weight/body weight (%) and C Image analysis of brain tissue showing decreased expression in T2D group compared to other animals' group. Data expressed as mean ± S.D. (n = 8–10), *p < 0.05, **p < 0.01 versus T2D. M molecular layer, P purkinje cells, G ganglionic layer, red arrows: massive degeneration, black arrows: moderate degeneration
3.3 Comet assay of T2D- rat brain tissue
Around 50 strand breaks for every diploid mammalian cell are the awareness limit. The comet examine was at first evolved to distinguish varieties in DNA harm and fix limit in mammalian cells; this method can be finished in under 24 h [9]. In synapses presented to the stress of diabetes in T2D-treated rodents, the DNA of the mind appeared to have been harmed. On the other hand, rats treated with lactoferrin either with or without insulin displayed reduced harm (Fig. 3A, C, D, DI, DL and DIL).
4 Discussion
4.1 Blood levels of glucose and brain tissue biomarkers
A considerable increase in blood glucose levels was observed in diabetic rats in this investigation. This finding supports the theory that STZ causes diabetes by presumably producing oxidative free particles by destroying beta cells in the pancreas [19]. A pancreatic oxidative state and a single DNA break in pancreatic beta cells are the causes of the elevated glucose levels in STZ-treated mice [20]. Lf treatment of diabetic rats resulted in lower blood glucose levels. This result is consistent with that of [21], who discovered that Lf can lower hyperglycemia, which is accompanied by elevated plasma levels of insulin. They attributed this effect to the increased secretion of glucagon-like peptide-1 (GLP-1) and improved small intestine glucose absorption.
The findings obtained showed that diabetic untreated rats had significantly higher levels of blood brain indices including SNE, BDNF, and TNF-Alpha, than control values. According to Huang et al. [22], illnesses that cause relatively quick neuronal degeneration (hours/days to weeks as opposed to months to years) frequently exhibit higher serum levels of SNE. Therefore, the differential diagnosis of a range of neuron-destructive and neurodegenerative illnesses can be aided by measuring SNE in serum or CSF. Additionally, it was discovered that the BDNF protein participates in the development, maturation (differentiation), and maintenance of nerve cells (neurons), hence promoting the survival of these cells [13]. TNF-Alpha, is a proinflammatory cytokine with a wide range of actions that, according to Yang et al. [23], has a significant impact on the metabolism and operation of adipose tissue. Numerous disorders, including diabetes, have been related to increased circulating TNF-Alpha, levels. Researchers found that mixed cerebrovascular and neurodegenerative lesions brought on by persistent hyperglycemia, insulin resistance, inflammation, and oxidative stress caused by diabetes may have an impact on cognition [24].
In the current investigation, lactoferrin treatment to diabetic rats either alone or in combination with insulin resulted in a considerable drop in the levels of SNE, BDNF, and TNF-Alpha, assessed in brain tissue, approximating their levels to control values. Due to its ability to pass the blood–brain barrier from the peripheral circulation, lactoferrin was discovered to be elevated in rat brain after oral administration [25]. This was thought to be a protective tissue response [16]. Moreover, Studies revealed that intracerebroventricular injections of Lf in rat pups demonstrated reduced brain injury and could modify the effects of inflammatory stressors to brain development. In a mouse model, oral Lf therapy has also been shown to be neuroprotective [26]. As a result, in vitro research has shown that Lf probably inhibits the release of pro-inflammatory cytokines [16]. This protective effect of lactoferrin could result from a reduction in the generation of dangerous free radicals and an ameliorating effect on the oxidative stress condition linked to diabetes mellitus.
Lactoferrin affects the central nervous system (CNS) and has been shown to have anti-nociceptive effects on thermal, visceral, and formalin-evoked nociception in rats after oral, intraperitoneal, and intrathecal treatment. In the current investigation, we found that Lf given orally prevented hyperalgesia in diabetic rats (Figs. 1DL, 2DL and 3DL). According to [27], Lf crosses the blood–brain barrier and enters the CSF by receptor-mediated transcytosis. Following oral or intraperitoneal injection, we also discovered the presence of Lf in rat CSF in the preliminary investigation. A work that found that TNF- Alpha, increased the rate of trans endothelial transport of Lf in a blood–brain barrier model suggests that this macromolecular transcytosis may be amplified in inflammatory situations [28]. Therefore, oral administration of Lf may be more efficient in lowering nociception in inflammatory pain by way of the central opioidergic system. By creating Lf-linked liposomes harboring therapeutic genes paired with neuronal promoters, as shown elsewhere, the brain-specific effect could be increased even further [29]. The use of brain-targeted medicines would be made possible by agents made of Lf and neuroreceptor ligand that do not have negative effects on the body's other organs. Lf's quick brain uptake could be advantageous for a delivery method that targets the brain. Additionally, higher expression of Lf on micro arteries and neurons has been documented, suggesting that the delivery system with Lf attachment may be more effective under some pathological situations, such as the disease of Parkinson [30] and the disease of Alzheimer [31]. Our current findings offer potential neuro-protective strategies for treating diabetic neurodegenerative diseases in light of these facts.
4.2 Histopathological analysis and comet assay
Histopathological analysis of the brain tissue in the current study revealed some sort of histological alteration in untreated diabetic rats including caryolysis of the neuroglial cells, wide distribution of angiogenesis of blood vessels through brain tissue. A clustering of glial cells suggests inflammation of the brain tissue. Significantly more necrotic, edematous, and spongiform deteriorated foci appeared (Fig. 2D–D2). On the other hand, treatment with lactoferrin (Fig. 2DL–DL2) alone or with Insulin ameliorates (Fig. 2DIL–DIL2) this damage. Insulin supplementation (Fig. 2DI–DI2) enhanced these alterations.
The Comet Examine is a strategy in light of gel electrophoresis for assessing DNA harm in synapses. It is adaptable, fast, and simple to execute. Notwithstanding, alterations to the strategy empower the discovery of DNA twofold strand break, base harm, crosslinks, and apoptotic nuclei in addition to DNA single-strand break, which is the focus of many investigations.
Blasiak et al. [31] have reported that the degrees of basal endogenous and oxidative DNA harm in diabetes patients were higher than in control subjects, the outcomes that affirm our discoveries proposing that type 2 diabetes mellitus might be related not just with the raised degree of oxidative DNA harm yet in addition with the expanded weakness to mutagens and the decreased adequacy of DNA repair. Lactoferin administration in diabetic rats, either alone or combined with insulin, reduced DNA damage as compared to diabetic untreated rats. Such protective action could be attributed to the ability of Lf to pass blood–brain barrier protecting brain cells through its antioxidative action. Diabetes patients were found to showed higher defenselessness to hydrogen peroxide and doxorubicin and diminished adequacy of fixing DNA harm actuated by these agents than sound controls [30].
5 Conclusion
The findings of the current research indicated that oral administration of lactoferrin to diabetic rats alone or in combination with insulin treatment for 6 weeks reduced both brain tissue and DNA damages and maintains the normal histological structure of brain tissue. This demonstrates how crucial it is to administer lactoferrin protein to people with diabetes in order to reduce the progression of neurodegenerative disorders.
Data availability
The datasets generated during and/or analysed during the current study are available from the author on reasonable request.
References
Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10(9):819–828. https://doi.org/10.1016/S1474-4422(11)70072-2
McKhann GM et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7(3):263–269. https://doi.org/10.1016/J.JALZ.2011.03.005
Kalaria RN (2009) Neurodegenerative disease: diabetes, microvascular pathology and Alzheimer disease. Nat Rev Neurol. https://doi.org/10.1038/nrneurol.2009.72
Qiu L et al (2010) | SGD. https://www.yeastgenome.org/reference/S000132086 (accessed Oct. 17, 2022)
Debette S et al (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77(5):461–468. https://doi.org/10.1212/WNL.0B013E318227B227
Bartoskova A, Adlerova L, Kudlackova H, Leva L, Vitasek R, Faldyna M (2009) Lactoferrin in canine sera: a pyometra study. Reprod Domest Anim 44(Suppl 2):193–195. https://doi.org/10.1111/J.1439-0531.2009.01409.X
Berlutti F et al (2011) Antiviral properties of lactoferrin–a natural immunity molecule. Molecules 16(8):6992–7012. https://doi.org/10.3390/MOLECULES16086992
Velusamy SK, Poojary R, Ardeshna R, Alabdulmohsen W, Fine DH, Velliyagounder K (2014) Protective effects of human lactoferrin during Aggregatibacter actinomycetemcomitans-induced bacteremia in lactoferrin-deficient mice. Antimicrob Agents Chemother 58(1):397–404. https://doi.org/10.1128/AAC.00020-13
Vesce F et al (2014) E-mail vaginal lactoferrin administration before genetic amniocentesis decreases amniotic interleukin-6 levels. https://doi.org/10.1159/000358877
Tsubota A et al (2008) Bovine lactoferrin potently inhibits liver mitochondrial 8-OHdG levels and retrieves hepatic OGG1 activities in Long-Evans Cinnamon rats. J Hepatol 48(3):486–493. https://doi.org/10.1016/J.JHEP.2007.11.013
Artym J, Zimecki M, Kruzel ML (2003) Reconstitution of the cellular immune response by lactoferrin in cyclophosphamide-treated mice is correlated with renewal of T cell compartment. Immunobiology 207(3):197–205. https://doi.org/10.1078/0171-2985-00233
Fang B, Zhang M, Tian M, Jiang L, Guo HY, Ren FZ (2014) Bovine lactoferrin binds oleic acid to form an anti-tumor complex similar to HAMLET. Biochim Biophys Acta 1841(4):535–543. https://doi.org/10.1016/J.BBALIP.2013.12.008
Takuma H, Arawaka S, Mori H (2003) Isoforms changes of tau protein during development in various species. Dev Brain Res 142(2):121–127. https://doi.org/10.1016/S0165-3806(03)00056-7
El-Beeh ME, El-Badawi AA, Qari SH, Ramadan MF, Filfilan WM (2022) Protective and health-promoting impact of Washingtonia filifera oil on the kidney of STZ-induced diabetic mice. Appl Biol Chem. https://doi.org/10.1186/S13765-022-00713-X
Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51:216–226. https://doi.org/10.1007/s00125-007-0886-7
Zhao X, Kruzel M, Aronowski J (2021) Lactoferrin and hematoma detoxification after intracerebral hemorrhage. Biochem Cell Biol 99(1):97–101. https://doi.org/10.1139/BCB-2020-0116
Simona-Moussa J (2020) The subjective well-being of those vulnerable to poverty in Switzerland. J Happiness Stud 21(5):1561–1580. https://doi.org/10.1007/S10902-019-00143-5/TABLES/9
Konstantinou GN (2017) Enzyme-linked immunosorbent assay (ELISA). Methods Mol Biol 1592:79–94. https://doi.org/10.1007/978-1-4939-6925-8_7
Gupta S, Kataria M, Gupta PK, Murganandan S, Yashroy RC (2004) Protective role of extracts of neem seeds in diabetes caused by streptozotocin in rats. J Ethnopharmacol 90(2–3):185–189. https://doi.org/10.1016/j.jep.2003.09.024
Yamamoto H, Uchigata Y, Okamoto H (1981) Streptozotocin and alloxan induce DNA strand breaks and poly(ADP–ribose) synthetase in pancreatic islets. Nature 294(5838):284–286. https://doi.org/10.1038/294284a0
Maekawa Y, Sugiyama A, Takeuchi T (2017) NOTE lactoferrin ameliorates corticosterone-related acute stress and hyperglycemia in rats. J Vet Med Sci 79(2):412–417. https://doi.org/10.1292/jvms.16-0498
Huang K et al (2015) Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol Cell Endocrinol 399:178–189. https://doi.org/10.1016/J.MCE.2014.08.014
Yang T, Liu G, Wei Y, Zhang X, Dong X (2019) The impact of dual-fairness concerns under different power structures on green-supply-chain decisions. Int J Enterp Inf Syst 15(3):1–26. https://doi.org/10.4018/IJEIS.2019070101
Strachan MWJ, Strachan MWJ, Reynolds RM, Marioni RE, Price JF (2011) Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 108:108–114. https://doi.org/10.1038/nrendo.2010.228
Wang J et al (2017) Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed Pharmacother 95:1669–1677. https://doi.org/10.1016/J.BIOPHA.2017.09.104
Liu H et al (2020) Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J Neurochem 152(3):397–415. https://doi.org/10.1111/JNC.14857
Fillebeen C, Dehouck B, Benaïssa M, Dhennin-Duthille I, Cecchelli R, Pierce A (1999) Tumor necrosis factor-α increases lactoferrin transcytosis through the blood-brain barrier. J Neurochem 73(6):2491–2500. https://doi.org/10.1046/J.1471-4159.1999.0732491.X
Shi N, Pardridge WM (2000) Noninvasive gene targeting to the brain. Proc Natl Acad Sci USA 97(13):7567. https://doi.org/10.1073/PNAS.130187497
Faucheux BA et al (1995) Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc Natl Acad Sci USA 92(21):9603. https://doi.org/10.1073/PNAS.92.21.9603
Kawamata T, Tooyama I, Yamada T, Walker DG and McGeer PL (1993) Lactotransferrin immunocytochemistry in Alzheimer and normal human brain. Am J Pathol 142(5):1574. Accessed: Oct. 19, 2022. Available: /pmc/articles/PMC1886898/?report=abstract
Blasiak J et al (2004) DNA damage and repair in type 2 diabetes mellitus. Mutat Res 554(1–2):297–304. https://doi.org/10.1016/j.mrfmmm.2004.05.011
Funding
The author declares that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The author contributed to the study conception and design. Material preparation, data collection and analysis were performed by IAA-S. The first draft of the manuscript was written by the author and commented on previous versions of the manuscript. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Ethical standards
Rats care and experimentation followed the guidelines of Umm Al-Qura University, Saudia Arabia, for the use of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Sharif, I.A. Protective effect of lactoferrin administration against brain tissue damage in diabetic rats. J.Umm Al-Qura Univ. Appll. Sci. 9, 115–122 (2023). https://doi.org/10.1007/s43994-023-00026-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00026-9