Abstract
An eco-friendly and easy ultrasound-assisted liquid phase microextraction approach using deep eutectic solvent (UA-DES-LPME) was established to preconcentrate and separate trace amount of nickel (Ni(II)) in various environmental samples before flame atomic absorption spectrometric estimation. In this method, Ni(II) was complexed with 2-(benzothiazolyl azo) orcinol reagent. The impacts various parameters on the microextarction of Ni(II) was investigated. The calibration graph is linear in the range of 1–500 µg L−1 and limits of detection and quantification were determined as 0.27 and 0.90 μg L−1, respectively. The RSD% and preconcentration factor were 2.30% and 100, respectively. The analysis of certified reference materials demonstrated the validity of the established procedure. The microextraction method provided here simple, rapid, cheap, green and was effectively used to determine nickel levels in a variety of environmental samples with recoveries ranged of 95.0–98.54%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nickel (Ni(II)) is harmful to living things at certain concentrations. Nickel enters the body via the air, polluted food and drink, and the smoke of cigarettes. Ni(II) compounds have been classified as carcinogens, and excessive exposure to Ni(II) compounds has been linked to a variety of chronic respiratory issues, lung cancer, and skin dermatitis [1]. As a result, estimating trace Ni(II) in diverse samples using unique and sensitive approaches is a critical goal [2, 3]. Because the Ni(II) concentration is lower than the detection limit of certain devices, such as GFAAS or FAAS, and because matrix ions are inert, a preconcentration and separation procedure is required before measurements to overcome these constraints by increasing responsiveness and improving accuracy.
Multiple approaches to preconcentrate and separate Ni(II) have lately been published in the literature, including cloud point extraction [4,5,6,7,8,9,10,11,12], solid-phase extraction [13,14,15,16], membrane filteration [17], and co-precipitation [18,19,20].
Using dispersive liquid-liquid microextraction (DLLME), the toxicity of extraction solvents has been reduced or eliminated. The DLLME technique has many advantages, including simplicity, speed, cheap cost, ease of use, green solvent use, and high enrichment factors. Many improved liquid-phase microextraction (LPME) methods for Ni(II) separation and microextraction from diverse sources have recently been developed and described [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
To alleviate the growing environmental issues, nontoxic solvents are necessary. Researchers have been looking for a better series of minimal, healthy, and environmentally acceptable solvents to offset the high cost and toxic effects of ionic liquids (ILs). They developed creative, ecologically friendly, and cost-effective deep eutectic solvents (DESs) as a result of their research [36].
DESs are essentially prepared by mixing two or more inexpensive constituents that can join through hydrogen bonding [37]. DESs are often achieved by using cheap, safe, and biodegradable choline chloride (Vitamin B4, ChCl) (through hydrogen bond donors (HBDs)). DESs produced from ChCl have a number of advantages, including being cheap, having simplicity in the synthesis, requiring no further pretreatment, being biodegradable, and being environmentally friendly [38]. When it comes to measuring hazardous metal ions in diverse environmental materials, many instrumental approaches such as ETAAS, FAAS, electro-analytical, and ICP-OES were applied [39,40,41,42,43]. Therefore, the use of the UA-DES-LPME method in conjunction with FAAS offers several advantages including ease of use, cost savings, a lower detection limit, a better preconcentration factor, and environmental friendliness.
At room temperature, some DESs, including the DES employed in the study being presented, are in a liquid state. This provides an advantage for usage of DESs in microextraction studies. Our goal was to develop a green UA-DES-LPME method for preconcentrating and precisely assessing Ni(II) in a variety of environmental samples using FAAS. The effect of numerous variables on the developed method's performance was optimized. The validity of the procedure was tested using certified reference materials.
2 Experimental
2.1 Apparatus
The Ni concentration was determined using an Agilent 55B AA spectrometer (Agilent Technologies Inc., Santa Clara, USA) with an air-acetylene flame burner and Ni-hollow cathode lamp (231.1 nm). The sample introduced a FAAS nebulizer utilising micro-injection method. The pH of the buffer solutions was determined using an AD1000 pH-meter (Adwa Instruments Kft., Szeged, Hungary). To speed up phase separation, a centrifuge (Isolab, GmbH, Germany) was used. A Grant ultrasonic water bath (LabGear, Australia) was used to facilitate analyte separation from sample matrices and the formation of a hazy solution. To produce bidistilled water, Milli-Q (Millipore, USA) was utilised. Before the experiment, glass wares were immersed in HNO3 (5.0% v/v) nightly and cleaned numerous times with bidistilled water. The samples were digested utilising microwave digestion systems from Milestone Ethos UP (Milestone, Sorisole, Italy) up to a maximum temperature of 300 °C and 1450 psi maximum pressure.
2.2 Reagents and solutions
All chemicals and reagents were acquired from the companies (Merck, Darmstadt, Germany) and (Sigma Aldrich, St. Louis, USA).
A Ni(NO3)2·6H2O (Fluka Chemie AG, Basel, Switzerland) with purity 98% was used to make the Ni(II) stock standard solution (1000 µg mL−1) and standardisation by EDTA [44]. Daily dilutions of the stock standard solution resulted in a diluted Ni(II) working solution.
Acetate (CH3COONa–CH3COOH) pH (3.0–5.5), phosphate (Na2HPO4–NaH2PO4) pH (6.0–7.0), ammoniacal (NH3-NH4Cl) solution pH 8.0, and borate (sodium tetraborate and boric acid) pH (9.0–10) are some of the specific buffer series that have been utilised. According to the literature [45], HCl and NaOH are employed in particular to modify pH values.
The 2-(benzothiazolyl)-azo orcinol reagent (BTAO) was prepared according to the protocol described [6]. In a 100 mL measuring flask, a suitable amount of reagent was dissolved in ethanol (purity, 95%) to make a BTAO stock solution (0.2%, w/v).
2.3 DESs preparation
In a glass bottom flask on a water bath at 80 °C for 5.0 min with continuous agitation, four different types of DES were created by combining choline chloride (ChCl) as a hydrogen bond acceptor and hydrogen bonding donors (urea (U), oxalic acid (Ox), lactic acid (LA), and ethylene glycol (EG) at a 1:2 molar ratio.
2.4 Recommended procedure
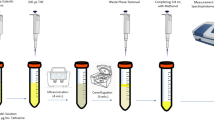
An aliquot of Ni(II) solution (50 mL) containing 1–500 µg L−1 was combined with phosphate buffer (pH 7.0) (3.0 mL) in a centrifuge tube. Posteriorly, BTAO (0.2% w/v) solution (0.5 mL) and 400 μL DES were added, then vortexes were run for 30 s to make a homogeneous solution, and then 300 μL THF was injected rapidly and the mixture was transferred into an ultrasonication bath for 3.0 min. The solution was centrifuged for 5.0 min at 4000 rpm to speed up phase separation. The aqueous phase was removed with a syringe. Finally, the residual DES-rich phase was diluted with acidic ethanol to 500 μL, and the Ni(II) concentration was determined using FAAS.
2.5 Application to real samples
Tap, mineral, sea, well, and waste water samples from Saudi Arabia were passed via a cellulose membrane filter (0.45-μm pore size) and diluted HNO3 was used for acidification, then stored in a polyethylen container in darkness at 4.0 °C. Then, the proposed approach was successfully implemented on water samples. Also, the proposed approach has been used with the reference materials (TMDA-51.3 and TMDA-53.3 fortified water) developed by (National Water Research Institute of Environment Burlington, Canada). The calibration graph was used to calculate the concentration of Ni(II).
Various fresh food samples and cigarette tobacco samples collected from the markets in Saudi Arabia. The samples were dried at 90 °C overnight in an oven before being homogenised in an agate porcelain mortar. Firstly, SRM 1570a spinach leaves or SRM 1573a tomato leaves (0.2 g) (National Institute of Standard Technology, Gaithersburg, MD, USA), food (1.0 g), and cigarette tobacco (0.5 g) samples were weighted in a glass beaker, then processed with 10 mL of a concentrated HNO3–H2O2 (2:1, v/v) combination and placed into Teflon tubes. The microwave digestion procedure has been applied for sample preparation after requiring dilution and pH adjustments [32]. The digested samples were then put through the UA-DES-LPME process.
3 Results and discussion
3.1 Effect of pH
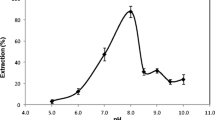
pH affects both the extraction recovery and the metal-chelate complex creation. As a result, the effect of pH was investigated at pH values ranging from 3 to 9. The recovery of the complex enhanced by rising pH, and highest quantitative recoveries were obtained at pH range of 6.0–8.0 as presented in (Fig. 1). At low pH values, the extraction of Ni(II) ions not effective, due to the hydronium ions disturbance on DES functional groups. Because of the production of the corresponding Ni(II) hydroxides at pH values over 8.0, the extraction recovery also falls. Therefore, pH 7.0 of phosphate buffer solution (3.0 mL) was used in following studies.
3.2 Effect of amount of BTAO reagent
In order to acquire quantifiable results, the amount of reagent utilized has a considerable impact on the Ni(II)-BTAO complex recovery. Various quantities of BTAO (0.2%, w/v) were tested in the range of 0.1–1.0 mL, and the curves of the findings are presented in (Fig. 2). By raising the BTAO volume to 0.5 mL, the recovery value was raised, and larger quantities of BTAO had no influence on the recovery value. As a result, in subsequent investigations, 0.5 mL of BTAO (0.2%, w/v) was used as the optimal amount.
3.3 Effect of DES type, composition and volume
DES type used is an important factor in the quantitative microextraction of analytes [31,32,33,34,35]. As extraction solvents, four distinct DES with varying compositions were produced (see Table 1). Quantitative recoveries were achieved, as demonstrated in Table 1. The (ChCl with U) DES solution was chosen for future investigation based on the results obtained.
The extraction solvent volume is a significant criterion that must be adjusted. In the range of 100–800 µL of ChCl: U (1:3 mol ratio), the influence of DES solution volume on the extractability of Ni(II) was investigated. Figure 3 shows that with raising the DES volume, the recovery of Ni(II) ions increased up to 400 μL. Then larger than 400 μL the recovery decreased due to dilution. Hence, DES volume (400 μL) was used for further experiments.
3.4 Effect of the molar ratio of DES
After choosing the optimum DES solvent (ChCl with U), various molar ratios were investigated in the developed UA-DES-LPME method at 1:2, 1:3, and 1:4 (Table 1). The results in Table 1 revealed that raising the U ratio enhanced the quantitative recovery. Because phase separation is apparent at a 1:3 molar ratio and the findings obtained from this location are quantitative, 1:3 was chosen as the best ChCl:U ratio and utilized for all tests.
3.5 Effect of volume of THF
Tetrahydrofuran (THF) is employed as an emulsifier solvent in liquid phase microextraction procedures. When THF is introduced to an aqueous sample solution, the DES phase begins to emulsify, the DES clumps becomes water/THF insoluble. Then, extraction of the analyte from the aqueous phase to the DES-rich phase. THF was chosen for its high extraction efficiency and ability to separate Ni(II)-DES-rich phases precisely. Furthermore, varied volumes of THF (100–600 μL) at a constant volume of DES (400 μL) were utilized to investigate the influence of THF volume (Fig. 4). Because of the solubility of the ChCl: U molecule, no extraction phase was collected in the absence of THF. However, increasing the THF volume increased the volume of the extraction phase. With the addition of 200–400 μL of THF, quantifiable findings for nickel(II) were achieved. So, 300 μL of THF was selected as the optimal volume.
3.6 Effect of sonication time
The duration of ultrasonic radiation exposure has a considerable impact on the developed method's efficiency and performance. The optimal ultrasonication time was found to be between 1.0 and 6.0 min (Fig. 5). The recovery was raised up to 3.0 min, according to the results. There was no notable change in recovery beyond this time until 4.0 min. At time more than 4.0 min, the recovery slightly decreased due to increase the temperature of solution. As a result, the optimum sonication time for future experiments was determined to be 4.0 min.
3.7 Study of centrifuging conditions
In the ranges of 1000–5000 rpm and 2–15 min, the influence of centrifugation rate and time on Ni(II) extraction efficiency was investigated. The rate was raised to 4000 rpm, which was determined to be the best rate. Also, to guarantee complete phase separation, the quantitative recovery was resulted at 5.0 min. When the centrifuging period exceeded 5.0 min, the recovery decreased due to generation of heat which may enhance the dissolving the metal complex into the aqueous phase. For additional research, the optimal centrifuge rate and time were determined to be 4000 rpm and 5.0 min, respectively.
3.8 Effect of sample volume
The sample volume is a key component for calculation the preconcentration factor (PF) for metal ion preconcentration. The PF is the ratio of the original sample volume to the final dilution volume. Over the range of 10–100 mL model solutions, the effect of sample volume on extraction efficiency was tested (Fig. 6). The results revealed that Ni(II) ion recoveries in volumes greater than 50 mL were not quantifiable. As a result, in all future tests, 50 mL of Ni(II) solution was selected as the largest volume. As a result, PF was set to 100.
3.9 Effect of matrix
The effect of certain cations and anions on Ni(II) ions recovery was examined. The highest tolerance limits for Ni(II) ions are shown in Table 2. There was no evidence of matrix ion interference in the estimation of Ni(II) ions under the experimental settings, demonstrating the usability of the developed approach for Ni(II) assessment in a variety of real environmental samples.
3.10 Analytical features and validation of the UA-DES-LPME method
Table 3 shows the analytical features and characteristics of the developed method with the optimised parameters. Linearity was obtained in the range 1–500 µg L−1. LOD and LOQ were computed as 3Sb/m and 10Sb/m, respectively, where Sb is the standard deviation from blank solution measurements (n = 10) and m is the calibration graph slope with preconcentration. The ratio between the slopes of calibration curves with and without preconcentration is known as the sensitivity enrichment factor (EF). The consumptive index (CI) is the ratio of the analyte solution volume to EF. The RSD% of ten determinations of 300 µg L−1 Ni(II) solution in the same day (intraday) and various days (inter-day) was used to assess the repeatability and precision of the proposed approach. The RSD% intra-day and inter-day were determined to be 2.30 and 2.0%, respectively, demonstrating the high method's precision.
3.11 Method validation
Detrmination of Ni(II) content in four standard reference materials (TMDA-51.3 fortified water, TMDA-53.3 fortified water, SRM 1570a Spinach leaves, and SRM 1573a Tomato leaves) was used to validate the accuracy of the UA-DES-LPME approach. The results represented in Table 4 revealed that the found values were in a good accordance with the reported certified values.
Analysis of 100, 200, and 300 μg L−1 Ni(II) (n = 3) on the same day and on five subsequent days were used to estimate intra-day and inter-day precisions. As indicated in Table 5, the RSD% and recovery values were ≤ 3.50% and ≥ 95.30, respectively, respectively. The satisfactory results revealed that the developed method had good potential to detect Ni(II) ions in various samples.
3.12 Analysis of environmental samples
The developed UA-DES-LPME preconcentration method was tested for its ability to recognize and separate Ni(II) ions in a variety of genuine water and food and cigarette tobacco samples. To validate the devised procedure reliability and accuracy, certain amounts of Ni(II) ions were spiked to the sample solutions utilizing addition/recovery test and the recoveries and RSD% were determined. The Ni(II) analyte had excellent quantitative recoveries, ranging from 95.0–98.0% with RSD% ≤ 1.70% for water samples (Table 6) and 95.15–98.54% with RSD% ≤ 1.49% for food and cigarette tobacco samples (Table 7). As a result of these findings, the method may be used to separate, preconcentrate, and analyse Ni(II) in real environmental samples at trace levels.
3.13 Comparison with other approaches
The UA-DES-LPME approach was compared to various extraction strategies described earlier for preconcentration of Ni(II). The comparison provides for a more thorough examination of the suggested method's benefits in comparison to other alternatives. The low limit of detection, extensive working ranges, excellent PF, greater reliability, good precision and the use of green DES, were the method's primary advantages, as shown in Table 8. As a result of these findings, the proposed UA-DES-LPME methodology for analysing Ni(II) in different environmental samples could be successfully executed.
4 Conclusions
This study used the green and efficient UA-DES-LPME approach for preconcentration Ni(II) ions in environmental samples prior to FAAS assessment. The suggested approach has several advantages, including small LOD (0.27 µg L−1), a wide linear range, a large PF (100), simplicity, less operational costs, and small reagent and sample consumption. Reproducibility and repeatability are satisfactory (RSD% < 2.5). The suggested approach has good analytical performance, indicating that it is dependable, and used successfully for trace Ni(II) ions measurement in environmental samples.
Data availability statement
This manuscript has associated data in a data repository. [Authors’ comment: All data included in this manuscript are available upon request by contacting the corresponding author.]
References
Schaumlöffel D (2012) Nickel species: analysis and toxic effects. J Trace Elem Med Bio 26:1–6
Alharthi SS, Al-Saidi HM (2022) Designing a simple semi-automated system for preconcentration and determination of nickel in some food samples using dispersive liquid–liquid microextraction based upon orange peel oil as extraction solvent. Arab J Chem 15:104094
Sahragard A, Alahmad W, Varanusupakul P (2022) Application of electrocolorimetric extraction for the determination of Ni(II) ions in chocolate samples: a green methodology for food analysis. Food Chem 382:132344
Altunay N, Elik A, Bulutlu C, Gürkan R (2018) Application of simple, fast and eco-friendly ultrasound-assisted-cloud point extraction for pre-concentration of zinc, nickel and cobalt from foods and vegetables prior to their flame atomic absorption spectrometric determinations. Int J Environ Anal Chem 98:655–675
Shah A, Keerio FA, Memon SQ, Memon GZ (2019) Cloud point extraction for the determination of different metal ions by using Bis(2-acetyl pyridine 4-phenyl 3-thiosemicarbazone) as complexing reagent. Pak J Sci Ind Res A: Phys Sci 62:76–81
El Sheikh R, Gouda AA, Mostaf AH, Salah EN (2015) Development of efficient cloud point extraction method for preconcentration and spectrophotometric determination of nickel in water samples using 2-(benzothiazolyl azo) orcinol. Int J Pharm Pharm Sci 7:176–184
El Sheikh R, Shaltout M, El Nabawy K, Gouda AA (2020) A green enrichment method of copper, manganese and nickel in water samples via cloud point extraction. Anal Bioanal Chem Res 7:49–60
Khudhair AF, Hassan MK, Alesary HF, Abbas AS (2019) A simple pre-concentration method for the determination of Nickel(II) in urine samples using UV–Vis spectrophotometry and flame atomic absorption spectrometry techniques. Indones J Chem 19:638–649
Han Q, Huo Y, Yang L, Yang X, He Y, Wu J (2018) Determination of trace nickel in water samples by graphite furnace atomic absorption spectrometry after mixed micelle-mediated cloud point extraction. Molecules 23:2597–2607
Temel NK, Sertakan K, Gürkan R (2018) Preconcentration and determination of trace nickel and cobalt in milk-based samples by ultrasound-assisted cloud point extraction coupled with flame atomic absorption spectrometry. Biol Trace Elem Res 186:597–607
Semmoud R, Didi MA (2019) An efficient cloud point extraction of mixed organic-inorganic pollutants using an ionic liquid as extractant: separation of the red Bemacid dye from nickel (II) in saline medium and optimization through factorial design methodology. Desalination and Water Treat 152:393–400
Benabdallah N, Youcef MH, Reffas H, Bendraoua A (2021) Evaluation and optimization of mixed-micelle mediated cloud point extraction of nickel(II) from concentrated chloride medium with Triton X-114-amphiphilic Schiff bases. Sep Purif Technol 56(14):2407–2425
Arslan Y, Kabak B, D Trak, Kenduzler E (2018) Separation and preconcentration of nickel(II) from drinking, spring, and lake water samples with amberlite CG-120 resin and determination by flame atomic absorption spectrometry. Anal Sci 34:1143–1147
Shirkhanloo H, Merchant K, Mobarake MD (2019) Ultrasound-assisted solid-liquid trap phase extraction based on functionalized multiwall carbon nanotubes for preconcentration and separation of nickel in petrochemical wastewater. J Anal Chem 74:865–876
Santos LB, Barreto JA, de Assis RS, de Souza CT, Ferreira SLC, Novaes CG, Lemos VA (2020) Solid-phase extraction and detection by digital image directly in the sorbent: determination of nickel in environmental samples. Water Air Soil Pollut 231:476–484
Kojidi MH, Aliakbar A (2019) Synthesis of graphene oxide-based poly(p-aminophenol) composite and its application in solid phase extraction of trace amount of Ni(II) from aquatic samples. Environ Monit Assess 191:145–157
Bansod PG, Bhutada DS, Kodape SM (2020) Influence of water soluble polymer on nano-filtration membrane for separation of nickel ion. Int J Sci Technol Res 9:1726–1730
Thubkhun N, Tangtreamjitmun N (2018) Determination of nickel by flame atomic absorption spectrometry after preconcentration by coprecipitation with aluminum hydroxide. Anal Sci 34:849–851
Moreira LS, Sá ÍP, Machado RC, Nogueira ARA, da Silva EGP, Amaral CDB (2020) Coprecipitation magnesium(II) hydroxide as a strategy of pre-concentration for trace elemental determination by microwave-induced plasma optical emission spectrometry. Spectrochim Acta Part B At Spectrosc 169:105899
Gouda AA, El Sheikh R, Hassan WS, Gouda N, Khadrajy HA (2020) A new carrier element-free coprecipitation method with 3-benzyl-4-p-nitrobenzylidenamino-4,5-dihydro-1,2,4-triazole-5-thiol for separation, preconcentration, and determination of some metal ions in water and food samples. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1807971
Sanchez-Rojas F, Bosch-Ojeda C (2017) Preconcentration of nickel in waters by vortex assisted dispersive liquid-liquid microextraction. Sample Perp 3:11–17
Altunay N, Elik A, Gurkan R (2019) Vortex assisted-ionic liquid based dispersive liquid liquid microextraction of low levels of nickel and cobalt in chocolate-based samples and their determination by FAAS. Microchem J 147:277–285
Moallaa SMN, Amin AS (2015) An ionic liquid-based microextraction method for highly selective and sensitive trace determination of nickel in environmental and biological samples. Anal Methods 7:10229
Wang Y, Zhang J, Zhao B, Du X, Ma J, Li J (2011) Development of dispersive liquid–liquid microextraction based on solidification of floating organic drop for the determination of trace nickel. Biol Trace Elem Res 144:1381–1393
Jamali MR, Madadjo A, Rahnama R (2014) Determination of nickel using cold-induced aggregation microextraction based on ionic liquid followed by flame atomic absorption spectrometry. J Anal Chem 69:426–431
Sorouraddin MH, Khoshmaram L (2010) Combination of dispersive liquid-liquid microextraction with flame atomic absorption for determination of trace Ni and Co in water samples and vitamin B12. J Chin Chem Soc 57:1346–1352
Rad AS, Rahnama R, Zakeri M, Jamali MR (2019) Dispersive liquid–liquid microextraction based on green type solvents-"deep eutectic solvents"-for highly selective separation and efficient preconcentration of nickel in water samples. J Iran Chem Soc 16:1715–1722
ALOthman ZA, Habila MA, Yilmaz E, Soylak M, Alfadul SM (2016) Ultrasonic supramolecular microextration of nickel (II) as N,N'-Dihydroxy-1,2-cyclohexanediimine chelates from water, tobacco and fertilizer samples before FAAS determination. J Mol Liq 221: 773–777
Sorouraddin SM, Farajzadeh MA, Qarajeh HN (2019) Effervescence-assisted dispersive liquid-liquid microextraction for trace analysis of Co(II) and Ni(II) from aqueous sample based on phthalic acid as a complexing agent and co-disperser. Anal Bioanal Chem Res 6:365–380
Dadfarnia S, Shabani AMH, Bidabadi MS, Jafari AA (2010) A novel ionic liquid/micro-volume back extraction procedure combined with flame atomic absorption spectrometry for determination of trace nickel in samples of nutritional interest. J Hazard Mater 173:534–5538
Erbas Z, Soylak M, Yilmaz E, Dogan M (2019) Deep eutectic solvent based liquid phase microextraction of nickel at trace level as its diethyldithiocarbamate chelate from environmental samples. Microchem J 145:745–750
Abo Taleb S, Antonious M, El Sheikh R, Youssef, Gouda AOA (2021) An eco-friendly ultrasound-assisted emulsification dispersive liquid–liquid microextraction of nickel in environmental samples coupled with spectrophotometry. Egypt J Chem 64: 1877–1888
Alacakoç B, Tekin Z, Unutkan T, Çetin G, Bakirdere S (2019) Determination of trace nickel in spinach samples using the combination of vortex-assisted deep eutectic solvent-based liquid phase microextraction and slotted quartz tube flame atomic absorption spectrometry. Atom Spectros 40:233–237
Tavakoli M, Jamali MR, Nezhadali A (2021) Ultrasound-assisted dispersive liquid liquid microextraction (DLLME) based on solidification of floating organic drop using a deep eutectic solvent for simultaneous preconcentration and determination of nickel and cobalt in food and water samples. Anal Lett 54:2863–2873
Sorouraddin SM, Farajzadeh MA, Okhravi T (2020) Development of dispersive liquid-liquid microextraction based on deep eutectic solvent using as complexing agent and extraction solvent: application for extraction of heavy metals. Sep Sci Technol 55:2955–2966
Jagirani MS, Soylak M (2022) Deep eutectic solvents-based adsorbents in environmental analysis. TrAC - Trends in Anal Chem 157:116762
Wang J, Jing W, Tian H, Liu M, Yan H, Bi W, Chen DDY (2020) Investigation of deep eutectic solvent-based microwave-assisted extraction and efficient recovery of natural products. ACS Sustain Chem Eng 8:12080–12088
El Achkar T, Greige-Gerges H, Fourmentin HS (2021) Basics and properties of deep eutectic solvents: a review. Environ Chem Lett 19:3397–3408
Elahi F, Arain MB, Ali KW, Ul HH, Khan A, Jan F, Castro-Muñoz R, Boczkaj G (2022) Ultrasound-assisted deep eutectic solvent-based liquid–liquid microextraction for simultaneous determination of Ni (II) and Zn (II) in food samples. Food Chem 393:133384
Schaeffer N, Martins MA, Neves CM, Pinho SP, Coutinho JA (2018) Sustainable hydrophobic terpene-based eutectic solvents for the extraction and separation of metals. Chem Commun 54:8104–8107
Ul HH, Bibi R, Balal AM, Safi F, Ullah S, Castro-Muñoz R, Boczkaj G (2022) Deep eutectic solvent (DES) with silver nanoparticles (Ag-NPs) based assay for analysis of lead (II) in edible oils. Food Chem 379:132085
Altunay N, Tuzen M (2022) A simple and green ultrasound liquid–liquid microextraction method based on low viscous hydrophobic deep eutectic solvent for the preconcentration and separation of selenium in water and food samples prior to HG-AAS detection. Food Chem 364:130371
Tavakoli M, Jamali MR, Nezhadali A (2021) Ultrasound-assisted dispersive liquid-liquid microextraction (DLLME) based on solidification of floating organic drop using a deep eutectic solvent for simultaneous preconcentration and determination of nickel and cobalt in food and water samples. Anal Lett 54:2863–2873
Jeffery GH, Bassett J, Mendham J, Denney RC (1989) Vogel’s textbook of quantitative chemical analysis, 5th edn. Wiley, New York
Britton HTS (1952) Hydrogen Ions, 4th edn. Chapman and Hall, London
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests declared by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hameed, A.M. An eco-friendly ultrasound-assisted deep eutectic solvent-based liquid–phase microextraction method for enrichment and quantification of nickel in environmental samples. J.Umm Al-Qura Univ. Appll. Sci. 8, 57–68 (2022). https://doi.org/10.1007/s43994-022-00009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-022-00009-2