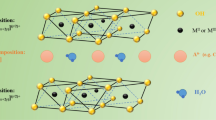
Abstract
Catalytic transformation of renewable biomass into value-added chemicals is an appealing strategy to upgrade biomass resources. Due to the presence of abundant oxygen-containing groups such as hydroxyl and aldehyde, biomass and its derived platform molecules have been served as ideal starting feedstock to synthesize valuable N-containing chemicals through reductive amination. In this mini review, we overviewed the recent advances in the reductive amination of several key bio-platform molecules including hydroxyl carboxylic acids, furfural, 5-hydroxylmethyl furfural and levulinic acid, with a focus on the production of amino acids, furan amines and pyrrolidones using thermocatalysis, electrocatalysis or photocatalysis. Moreover, the functions of active sites and the reaction mechanisms in different catalytic systems are discussed to get insights into the key factors in the reductive amination of biomass resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sustainable production of value-added chemicals and fuels from renewable biomass holds promise to reduce our reliance on fossil resources, and thus has received increasing attention [1,2,3,4,5,6]. Direct routes for biomass transformation include pyrolysis and gasification, which can convert biomass to bio-oils or syngas (CO and H2 mixture). Further upgrading of bio-oils or Fischer–Tropsch reaction of syngas would give desirable fuels or hydrocarbons. However, high temperatures are generally used in these processes, which makes them costly and energy-intensive. Different from pyrolysis and gasification, the catalytic transformation of biomass into particular platform molecules under mild conditions followed by the conversion of platform molecules to target chemicals and fuels has emerged as another attractive route for biomass utilization. Because of the non-harsh reaction conditions, such bio-platform molecule-route may achieve not only low energy consumption, but also high selectivity towards desirable products. To date, a number of key bio-platform molecules such as hydroxyl acids, furfural, 5-hydroxylmethyl furfural (HMF), levulinic acid (LA) have been successfully obtained from the catalytic conversion of biomass [7,8,9,10,11,12,13,14,15,16,17]. This renders the bio-platform molecule-route technologically feasible to produce diverse value-added chemicals.
Nitrogen-containing chemicals are a class of versatile platforms that have broad applications in the manufacture of textiles, pharmaceuticals, medicines, and agricultural compounds [18,19,20]. For example, in medicines, around 85% of commonly used drugs contain at least one or more nitrogen atoms [21]. Currently, N-containing chemicals such as amines are primarily synthesized from fossil hydrocarbons through consecutive reactions including selective oxidation, amination and hydrogenation. Because such process usually involves C–H activation, it is challenging to achieve high selectivity under mild conditions [22, 23]. In contrast, biomass and bio-platform molecules have abundant oxygen-containing groups such as hydroxyl and aldehyde groups, and thus they may serve as alternatives for the construction of C–N bonds via reductive amination. Moreover, water is the only by-product during the amination of bio-platform molecules, [24, 25] which makes the process not only environmentally friendly but also atomically economical. Thus, synthesis of nitrogenous chemicals from biomass-based platform molecules presents an ideal route to enrich the utilization of biomass and contribute to carbon neutrality.
Depending on the structures of bio-platform molecules, a variety of nitrogen-containing chemicals such as amino acids, furan amines, pyrrolidinones can be synthesized (Fig. 1) [1, 26,27,28]. For instance, the reaction of hydroxyl acids with NH3 or amines would generate amino acids or substituted amino acids, [29] while the reductive amination of furan or HMF can afford furan amines [21, 28, 30, 31]. Moreover, the combination of amination and intramolecular dehydrogenation could convert LA to pyrolidinones [32]. So far, several elegant reviews have summarized the amination of biomass and bio-platform molecules, with particular attention on the hydrogen-borrowing-based amination, heterogeneous systems for amination, amination of different bio-platform molecules and so on [19,20,21, 26, 30]. In recent years, the reductive amination of biomass-derived compounds has gained rapid progress in the development of new catalytic systems using thermo-, photo- or electro-catalysis. Herein, this article summarizes the advances in this field with an emphasis on the catalytic transformation of key bio-platform molecules to amino acids, furan amines and pyrrolidinones (Fig. 1). The reaction mechanisms for each kind of nitrogen-containing products have been described. Furthermore, the functions of catalysts and certain reaction conditions are analyzed to understand the key factors governing the reductive amination of biomass.
2 Amino acids from biomass-based hydroxyl and keto acids
Amino acids are essential nutrients for life and can be used as building blocks for cell and tissue repairing, and moreover, they also have wide applications in food, animal nutrients, cosmetics and so on [29, 33]. The global market size for amino acids reached USD 25.19 billion in 2022 and is expected to exceed USD 48.3 billion by 2030, with an annual growth rate of 8.5% [34,35,36]. Currently, amino acids are mainly produced by microbial fermentation of carbohydrates, but this process suffers from the strict operating conditions, low space–time-yield, and high energy consumption. As an alternative, Strecker reaction has been employed as a chemical approach to synthesize amino acids from aldehyde, cyanide, and ammonium salts (Fig. 2a) [29, 37,38,39]. Although the efficiency is high, this process employs highly toxic cyanide to generate carboxylic groups and requires stringent experimental conditions. To overcome the problems of Strecker reaction, Petasis et al. selected more environmental friendly compounds such as boron-containing chemicals, amines, and α-keto acids as the reactants for the synthesis of various α-amino acids (Fig. 2b) [40,41,42,43]. Besides, the reaction of biomass-based hydroxyl/keto acids with NH3 or amines has become another green and sustainable approach to obtain various amino acids (Fig. 2c) [44,45,46].
The conversion of biomass-based keto acids to amino acids normally involves the amination of keto acids with nucleophilic amines to form imine intermediates, and the subsequent reduction of imine intermediates to amino acids. If the reduction of carbonyl group or other reaction occurs prior to the amination, amino acids can be hardly attained. Hence, an appropriate reaction sequence is crucial to the formation of amino acids. By using an acid-stabilized mononuclear iridium hydride complex as catalyst, Fukuzumi and co-workers observed that the pH value of reaction medium played a key role in the amination reaction [47]. Adjusting the pH value in the range of 4–8, the amination of carbonyl carbon was initiated by nucleophilic attack of NH3, and then the hydrogenation of the imine intermediate affords α-amino acid (Fig. 3). However, decreasing pH values to 1–4 led to the protonation of NH3, giving NH4+ species, which could not act as nucleophilic agent to react with keto acids. Thus, the transfer hydrogenation instead of amination occurred and α-hydroxyl acids were formed (Fig. 3) [47]. Such pH dependent phenomena have also been observed in the iron oxyhydroxide-catalyzed amination of pyruvate: the reaction media at alkaline pH promoted the formation of alanine, while neutral or low pH conditions preferred to resulting in the hydrogenation of pyruvate to lactic acid [48]. Besides the pH effects, the concentration of active sites (e.g., Fe(II)) is another key factor to modify the reaction routes and affect the product distribution [48]. It was found that nearly no pyruvate was reacted at an Fe(II) mole fraction of < 30%, and alanine began to form as the Fe(II) fraction was increased to 50%. However, too higher Fe(II) species (> 80%) led to the preferential hydrogenation of carbonyl group in pyruvate, affording lactate [48].
Iridium hydride complexes catalyzed the amination of α-keto acid at different pH values [47]
Electrocatalytic transformation of keto acids is another attractive strategy to produce amino acids. Because reductive reactions are driven at applied potential, the use of external reducing agents such as H2 will be avoided [49]. As shown in Fig. 4, the formation of amino acids follows two possible pathways: one is the reaction of keto acids with ammonia to generate imines, which undergoes a two-electron reduction to give amino acids; the other is the initial formation of oximes, followed by a four-electron reduction on the cathode [50,51,52]. During the electro-reduction process, hydrogen evolution reaction (HER) and the reduction of keto- to hydroxyl-group may occur and compete with the reductive amination. Hence, the electrochemical synthesis of amino acid requires the suppression of these side reactions, while sustaining high activity in the reductive amination. The selection of appropriate electrodes and design of efficient electrocatalysts are crucial to enhance the efficiency of reductive amination.
A successful example is the utilization of titanium-based catalysts for the synthesis of diverse amino acids in aqueous media [50]. In a flow-reactor, Yamauchi and co-workers employed Ti felts covered with anatase TiO2 (TiO2/Ti felt) and nanoscale IrO2 as the cathode and anode, respectively, and investigated the reductive amination of α-keto acids (e.g., pyruvic acid) using NH2OH as a nitrogen resource [50]. The pyruvic acid conversion could reach 89% with a analine Faradaic efficiency of 70% at a applied potential of -2.8 eV, a temperature of 298 K, and using a mixture of 160 mmol/L pyruvic acid and 96 mmol/L (NH2OH)2·H2SO4 as the electrolytes for the cathode. At a low temperature of 273 K, alanine with a Faradaic efficiency was up to 100%. Detailed computation studies suggested that the reductive amination was mediated by a proton-coupled electron transfer process, which significantly lowered down the activation energy compared to the simple hydrogen transfer reaction. Water bridges were found to connect the oxygen atoms of TiO2 facets and the reactants, and facilitated the proton transfer reaction [51]. Moreover, compared to water molecules, pyruvic acid was much easier to adsorb on TiO2 surfaces, which facilitated electron transfer to pyruvic acid and promoted the reductive amination. Due to the high efficiency, such electrochemical system is also able to realize the reductive amination of carboxyl groups in oxalic acid, which produced glycine with a Faradaic efficiency of 28% [53].
Creating defects or incorporation of heteroatoms can modify the catalyst surfaces and thus regulate the formation of amino acids. Through a simple ball-milling treatment for carbon nanotubes (CNT), Yan and co-workers fabricated an electrocatalyst with abundant intrinsic defects for the reductive amination of 2-ketoglutaric acid [54]. This catalyst exhibited 7 times higher activity than the CNT without surface modification, and provided a glutamic acid yield of 60% with a FE of 90% after reaction of 8 h. Mechanism studies indicated that the high-density of defects in the CNTs might serve as active sites for amination, and were beneficial for the electron transfer step during the reduction of imino acid, the rate limiting step, thus accelerating the formation of glutamic acid. It was worthy of note that the nitrogen-doped CNT contained more extrinsic defects in the CNT, but showed relatively low activity and FE compared to the ball-milling CNT. A possible reason is that the nitrogen atoms distorted the graphene lattice and reduced the charge transfer efficiency. Additionally, the high density of pyridinic or pyrrolic nitrogen atoms in the CNT preferred the reduction of C = O bonds instead of C = N bonds, therefore lowering down the formation of glutamic acid [54]. However, a recent study indicates that the introduction of nitrogen and sulfur into carbon nanosheets enhanced the catalytic performance of the electrocatalyst in the reductive amination of pyruvic acid [55]. An alanine selectivity of 99.9% with the formation rate of 1199 μmol h−1 cm−2 could be obtained over the N,S-doped carbon nanosheets. The superior performance was ascribed to the inhibition effect of N,S-doping on the hydrogen evolution reaction. This is quite different from the CNT catalysts bearing intrinsic defects, and thus more studies are still necessary to understand the nature of different strategies for the reductive amination.
Compared to keto acids, hydroxyl acids are a class of more common platform molecules that can be derived from biomass, but the catalytic transformation of hydroxyl acids to amino acids is much more difficult because of its long and complicated reaction route [56,57,58,59]. A study on the amination of lactic acid, a typical cellulose-derived hydroxyl acid, revealed two possible pathways for the alanine (Fig. 5) [56]. One is the indirect path, which involves the first dehydrogenation to a keto acid, and subsequent amination and hydrogenation to form the final product of alanine (path I). The other is SN2 path, in which NH3 attacks the hydroxyl group and then leads to a substitution of the -OH group with an -NH2 group (path II). If there is no α-H in the hydroxyl acid, the pathway I rather than path II is expected to be inhibited due to the unable to form aldehyde intermediate. A systematic study on the amination of α-hydroxyl isobutyric acid (without α-H) and lactic acid showed that on amino acid was observed from α-hydroxyl isobutyric acid, while high selectivity of alanine was produced from lactic acid [56]. This indicates that the amination of hydroxyl acids follows the indirect path (Fig. 5, path I).
Two possible reaction pathways for reductive amination of lactic acid to alanine [56]
Since dehydrogenation is the rate-determining step in the reductive amination of hydroxyl acids, noble metals such as Ru, Pt, and Pd have been widely explored in this reaction. Compared to Pd and Pt, Ru could maintain its dehydrogenation activity when NH3 was presence, thus exhibiting superior performance for the amination [56, 60]. A variety of amino acids including analine, leucine, valine, aspartic acid, and phenylalanine with high yields have been obtained from Ru-catalyzed conversion of hydroxyl acids. For example, carbon nanotubes (CNTs) supported Ru nanoparticles (Ru/CNTs) afforded an alanine yield of 43% in the reaction of lactic acid at 453 K for 2 h [56]. Upon doping N into CNTs (N-CNTs), the strong electronic interaction between N and Ru nanoparticles (NPs) significantly improved the dispersion of Ru NPs, and resulted in an enhancement on the selectivity of amino acids [57]. Under optimal conditions, the yield of alanine on the Ru/N-CNTs reached 70%, higher than that on Ru/CNTs [57]. Additionally, beta zeolite and magnesia also facilitated the dispersion of Ru NPs, and achieved the amination of lactic acid and raw glycerol to alanine [58, 59].
Utilizing solar energy to drive the reductive amination can avoid the problems of high-temperature-induced side reactions and active sites leaching. This unique feature makes photocatalysis a promising alternative for the amination of biomass-derived hydroxyl acids [61]. As displayed in Fig. 6, the photocatalytic amination of hydroxyl acids follows a two-step reaction pathway similar as that in thermo-catalysis. Initially, hydroxyl acids are dehydrogenated to keto acids by the photo-generated holes, and then the reaction of keto acids and ammonia produces imino acid intermediates. A subsequent reduction of the imino acid intermediates by the photogenerated electrons generates amino acids [62]. Cadmium sulfide (CdS), in particular ultrathin CdS, showed high activity in hydroxyl dehydrogenation reaction, the rate-determining step in converting hydroxyl acids to amino acids, and accordingly demonstrated high efficiency for the amination of various biomass-derived hydroxyl acids [62]. Under visible light irradiation, the ultrathin CdS could convert lactic acid to alanine at a formation rate of 10.5 mmol g−1 h−1, which was one order of magnitude higher than that on the conventional semiconductors such as TiO2, ZnO, g-C3N4. Moreover, the ultrathin CdS also outperformed other CdS morphologies such as nanorods, nanowire and nanospheres. The catalytic amination of pyruvic acid, a dehydrogenation product from lactic acid, revealed a negligible difference between the CdS catalysts with different morphologies, indicating that the superior performance of ultrathin CdS could be originated from its excellent ability in the dehydrogenation. An analysis on the dehydrogenation mechanism of lactic acid showed that ultrathin CdS initiated the dissociation of α-hydroxyl group and generated oxygen-centered radicals, the key intermediate of pyruvic acid. However, CdS with morphologies of nanorods, nanowire and nanospheres were inert in the formation of oxygen-centered radicals. Moreover, the ultrathin CdS has a poorer H2 evolution reaction than other CdS with different morphologies, which suggests that less photogenerated electrons were consumed for the reduction of protons on ultrathin CdS, leaving more for the reductive amination [62]. Thus, high alanine formation rate could be obtained on the ultrathin CdS.
Photocatalytic amination of biomass derived α-hydroxyl acids to amino acids [62]
Improving the separation of photogenerated electron–hole pairs is another approach to enhance the photocatalytic efficiency in amination. A hierarchical flower-like CdS/Ti3C2 Schottky junction (MCdS) was synthesized by using CdS and Ti3C2 MXene as hole acceptors and electron acceptors, respectively. This Schottky junction effectively promoted the separation and transfer of photogenerated charges, thereby inhibiting the electron–hole recombination. Furthermore, CdS with reduced surface energy not only preferentially promoted the reduction of imino acid intermediates, but also enabled the reductive amination of pyruvate more thermodynamically favorable than decarboxylation. As a consequence, this MCdS could afford alanine with a selectivity of more than 80% and formation rate of 33.72 mmol g−1 h−1 [63]. By doping Mo into In2O3 semiconductor, Jiang and co-workers prepared a porous rod-shaped Mo- In2O3 catalyst for lactic acid amination [64]. Because the doped Mo species introduced defects below the conduction band of In2O3, and created localized electron trapping sites, the apparent band gap of the catalyst became lowered down, which enhanced the light utilization. Furthermore, the recombination of photogenerated holes and electrons was largely inhibited, and thus the reaction rate was greatly increased. Under the visible-light irradiation, Mo-In2O3 could achieve an alanine selectivity of 91% at the lactic acid conversion of 81%. The alanine production rate reached 0.369 mmol h−1, which was about two times that of In2O3 [64].
Electrocatalysis enables oxidation and reduction reactions to occur simultaneously in a single cell, and thus shows high potential to transform hydroxyl acids to the corresponding amino acids. While the electrocatalytic amination of keto acids has been achieved on the cathode of H-type cells, [49,50,51] the one-pot conversion of hydroxyl acids to amino acids remains challenging. This is because the amination of hydroxyl acids requires oxidation, amination and reduction on both electrodes in a specific sequence, which is hard to control in a H-type cell [49]. A recent solution to overcome this hurdle is to conduct the tandem reactions in a flow cell, in which hydroxyl acids are first oxidized on the anode, and the resulted keto acids immediately transfer to the cathode for subsequent reactions. Before reaching the cathode, the keto acids are rapidly transformed to imino acids by reacting with ammonia that are continuously pumped into the system, and then give amino acids on the cathode (Fig. 7) [65].
A schematic diagram for the electro-catalytic conversion of α-hydroxyl acids to synthesize amino acids [65]
For example, Sun and co-workers selected carbon paper and titanium foil as the anode and cathode, respectively, in a flow cell, and they introduced redox mediators such as N-hydroxyphthalimide (NHPI) and (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) into the anode chamber for the conversion of biomass-derived hydroxyl acids [65]. The use of redox potential controllable NHPI or TEMPO was able to inhibit the decarboxylation of the keto acids, a major side reaction during the formation of amino acids. At a applied potential of 2.7 V, this electrochemical system direct produced alanine, phenylalanine, glycine and leucine, with yields of more than 70% [65]. Furthermore, the flow electro-catalytic system had much higher formation rates of amino acids than bath electrolysis, where each reactions involved in the transformation of hydroxyl acids were performed individually on the electrodes.
Currently, a large number of amino acids have been synthesized from biomass-derived hydroxyl acids using thermo-, photo- or electro-catalysis. Due to the different driven forces between these catalytic systems, quite diverse strategies are needed to enhance the amination efficiency. For thermocatalytic systems, efficient catalysts generally should be active in the activation of reducing agents (e.g., H2) and promoting the hydrogenation of imino acids instead of keto acids under thermal conditions. Different from the thermocatalytic systems, electrochemical amination is performed at applied potentials with much mild conditions (e.g., room temperature). Electrons coupled with protons or water serve as the reducing agents, avoiding the risk caused by using hydrogen gases in the thermocatalysis. However, the hydrogen evolution reaction usually competes with the reductive amination, and efficient electrocatalysts should possess high overpotential for this side reaction. Similar to electrocatalysis, photocatalytic amination can also produce amino acids at mild temperatures. The key to enhance the amination efficiency is to improve the light adsorption and inhibit the recombination of photogenerated electron–hole pairs. Moreover, the hole-induced side reactions such as oxidation of the keto/amino acids should be suppressed as well. Thus, the rational design of catalysts for the reductive amination largely depends on the catalytic systems.
It is also notably that most of the synthesized amino acids are in α-configuration, and few attentions is given to the production of β-amino acids, another group of N-containing chemicals that can be applied in industries. It can be expected that the catalytic systems efficient for α-amino acids may be still effective for the amination of β-hydroxyl acids due to the similar reaction mechanisms for hydroxyl acid conversion. One thing needs to be considered is that the energy barriers of dehydrogenation for α-OH and β-OH groups may be different. Compared to the α-OH, the β-OH has much far distance to the carboxylic group and is less influenced by this group. Thus, the requirements for the amination of β-hydroxyl acids would be slightly different from the β-hydroxyl acids.
3 Amination of furans to furan amines
Furan amines are a class of cycle-like amine compounds which have been used to manufacture pharmaceuticals, pesticides, synthetic resins, and agrochemicals [30]. For example, furfuryl amine can be directly used to synthesize furtrethonium and parasympathomimetic cholinergic drugs, or combined with atropine for medication treatment [66]. Furfuryl amine-derived pyrrolidols are key intermediates for the production of herbicides and insecticides. The market size of furfuryl amine is estimated to reach USD 1.2 billion by 2030, with a compound annual growth rate of 3.5% [66, 67]. As furans (e.g., HMF and furfural) can be obtained from renewable biomass such as cellulose, hemicellulose or their derived monomers, they can serve as ideal feedstock for the synthesis of furan amines. With increasing approaches developed for the synthesis of furans, the conversion of biomass-derived furans into furan amines will be more feasible.
Furfural is one of the simplest furan compounds, and possesses one aldehyde group. The reductive amination of furfural to furfuryl amine is the same as that of keto acid amination (Fig. 8). First, the dehydration of furfural and NH3 affords a reactive furfuryl imine intermediate (2a), which undergoes subsequent hydrogenation to produce furfuryl amine. As a primary amine in the solution, furfuryl amine can act as a new nitrogen source to react with another furfural molecule and give dimeric imine (3a), and further dimeric amine (4a). The intermediate 2a may also undergo self-polymerization, forming trimeric imidazoline (5a, Fig. 8) [68]. Among the formed oligomers (3a, 4a and 5a), 3a could be further transformed to furfuryl amine in the presence of NH3, while 4a and 5a were hardly converted into this target compounds. To enhance the formation of furfuryl amine, the construction of efficient catalytic systems therefore needs to consider not only the rapid hydrogenation of the imine 2a intermediate to prevent the formation of 5a, but also to avoid the generated 3a intermediate being saturated into 4a. Additionally, the hydrogenation of aldehyde or alkene groups will result in compounds 7a or 6a, which also should be inhibited.
Proposed reaction pathways for the reductive amination of furfural to furfuryl amine [68]
Non-noble metals (e.g., Ni and Co) with appropriate hydrogenation ability have been applied in the amination of furfural with ammonia, and most of them show moderate to good furfuryl amine yields [69,70,71,72,73]. For example, over a Raney Ni catalyst, furfural could be converted to furfuryl amine with a yield of 79% using stoichiometric NH3 at 348 K [69]. Due to its high activity in hydrogenation, Ni can easily lead to over-hydrogenation of furfural into furfural alcohol. To overcome this problem, the regulation of the furfural adsorption configuration serves as a solution to improve the selectivity. By modifying Ni nanoparticles with SiO2 and NH3, the furfural adsorption was changed from a plane to linear adsorption configuration, which prevents over-hydrogenation of aldehyde functional groups and improves the reductive amination [70]. The SiO2 supported Ni catalysts achieved much better performances than Raney Ni in the reductive amination of furfural, [71,72,73] and the yield of furfuryl amine could reach up to 95% [65]. In addition, the construction of supported Ni catalysts which can facilitate hydrogen spillover is also conducive to the reductive amination. A recent study revealed that a rod-like Al2O3 supported Ni catalyst with primarily external (111) facets was active in the reductive amination of furfural [74]. This Ni/Al2O3(111) catalyst showed a turnover number of 30.4 h−1 for the production of furfuryl amine, which was 136 times higher than that on Ni/Al2O3 with (110) facets. The crystal surfaces of Al2O3(111) were found to exhibit strong Lewis acidity, which enhanced the H affinity to the support and generated positively charged Ni through electronic interaction. Such strong H affinity and positively charged Ni facilitated the migration of hydrogen from the Ni surfaces to the Al2O3(111) surfaces, thereby preventing the hydrogen-poisoning of Ni sites, and enhancing the adsorption/activation of NH3 and reductive amination of intermediates.
Notably, the non-noble metals have high tendency to interact with NH3, forming complex ions and leading to active site-leaching. A feasible strategy to solve this problem is the incorporation of metal into a graphitic shell or stabilization of the metal by nitrogen atoms. For instance, through assembling cobalt-diamine-dicarboxylic acid metal organic frameworks (MOF) on carbon, followed by pyrolysis under inert atmosphere, a carbon encapsulated Co catalyst can be obtained (Fig. 9) [75]. This catalyst has two forms of cobalt: one is metallic cobalt nanoparticles encapsulated by graphitic shell, and the other is single Co atoms, which are stabilized by nitrogen atoms on the carbon matrix [75]. A further study showed that the pyrolysis temperature played a crucial role in the synthesis of coating layers during the catalyst preparation process. High pyrolysis temperatures could facilitate the growth of graphite shell layers and increase the proportion of Co species in the layers. When the pyrolysis temperature exceeded 1073 K, single atom Co began to form inside the carbon layers, and at the temperature of 1273 K, most of the Co species existed in the form of metallic microcrystals coated with graphite shells. Such structure makes the catalyst highly stable. After consecutive recycling uses, this catalyst maintained its activity in amination reactions. By direct loading cobalt nanoparticles on nitrogen-doped carbon, an efficient and stable Co catalyst could be obtained for the furfural amination [76]. Under the conditions of 26.5 wt% NH3H2O, 403 K and 12 h, a furfuryl amine yield of about 82% was attained over the Co catalyst, and no noticeable deactivation occurred during the reaction.
A scheme for the preparation of graphitic shell-encapsulated Co nanoparticles on carbon using Co-MOFs template [75]
Compared to non-noble metals, noble metals such as Ru, Rh, Ir, and Au are much more stable and efficient in amination reactions [68, 71, 77,78,79,80,81,82,83,84,85,86]. It should be mentioned that the catalytic behavior of the metals might be adjusted by the supports. Taking Ru as an example, when Ru NPs were loaded on Nb2O5, SiO2, and TiO2 for furfural amination, furfuryl amine was formed as the main product, whereas Ru NPs loaded on Al2O3, and MgO mainly gave undesirable by-products (e.g., 3a, and 5a in Fig. 8) [68]. The relatively better performances of Ru NPs on Nb2O5, SiO2, and TiO2 can be attributed to the weak interaction between Ru and these supports, which maintained Ru in metallic state during the amination reaction. Moreover, a weak electron-donating capability of Ru particles on these oxides, in particular Nb2O5, enables the Ru/Nb2O5 catalyst to suppress the reduction of imines (3a in Fig. 8), thereby significantly improving the selectivity of primary amine. The furfuryl amine yield could approach 99% at a full furfural conversion under optimal reaction conditions (363 K, P(NH3) = 1 MPa, 4 h) [68]. This Ru/Nb2O5 catalyst was still able to afford furfuryl amine with a yield of 89% even at a lower reaction temperature (343 K) [77]. If Ru NPs were loaded on ZrO2 followed by reduction at 523 K, metallic Ru and RuO2 would be co-existent and result in both strong Lewis acid sites and metallic hydrogenation sites [79]. RuO2 serves as the Lewis acid sites to promote the activation of carbonyl groups, while metal Ru serves as the hydrogenation sites to promote the hydrogenation reaction of imines. The synergistic effect of the two active sites enhanced the reductive amination of furfural to furfuryl amine.
By coating Ru NPs with nanosized gallium oxide (Ga2O3), followed by dispersing in MgAlGaOx hydrotalcite, a hierarchical Ru/Ga2O3/MgAlGaOx catalyst was constructed for the reductive amination of furfural [87]. The metallic Ru and Al3+ species were responsible for H2 dissociation and intermediate (i.e., N-furfurylidenefurfurylamine) activation, respectively. Since the nanosized Ga2O3 and atomic distributed Ga3+ in MgAlGaOx can function as dual scale hydrogen transfer bridges, the dissociated H species on Ru NPs would rapidly spillover to Al3+ sites, and facilitate the subsequent conversion of intermediate to furfuryl amine. Hence, the Ru/Ga2O3/MgAlGaOx could provide a furfuryl amine yield of 93% and the formation rate of 80.43 gFAM·gRu−1·h−1 after reaction of furfural at 363 K and 2 MPa H2 for 6 h.
Besides metal-based catalysts, the nitrogen sources also have impacts on the amination of furfural. The utilization of hydrous hydrazine (N2H4⋅H2O) instead of ammonia for the synthesis of furfuryl amine would enable the amination reaction at room temperature and quantitatively produce imine intermediates 3b and 4b (Fig. 10) [82]. Such mild temperature can avoid side-reactions such as the polymerization of imide and the hydrogenation of furfural itself. When a boron/nitrogen co-doped carbon (BNC) supported Ru catalyst was combined with N2H4⋅H2O, furfural was first converted to intermediate 3b (Fig. 10) and then to furfuryl amine with a yield of 99% after hydrogenation at 313 K for 4 h [82]. The competitive adsorption of N2H4 and H2 on catalyst surfaces modified the hydrogenation ability of metallic Ru, which suppressed the hydrogenation of unsaturated functional groups, thus improving the selectivity of furfuryl amine [82].
Reaction networks for the reductive amination of furfural using N2H4⋅H2O as the nitrogen source [82]
Without the addition of metal catalysts, the selective synthesis of furfuryl amine can also be achieved by using formamide as the nitrogen source and employing microwave irradiation for heating. Different from the conventional heating mode, which is slow and facilely causes the formation of by-products, microwave irradiation demonstrates high heating rates and is able to significantly accelerate the formation of C-N bond during the amination. Under the catalyst-free conditions, microwave irradiation enables the reaction of furfural with formamide to generate formyl species (e.g., N-formyl carbinolamine, N-formyl imine, and N,N’-(furan-2-ylmethylene)diformamide (FDFAM) in the initial stage, and then formic acid acted both as H-donor and acid to catalyze the hydrogenation of formyl species into 4c compound (Fig. 11) [88]. A further alcoholysis of 4c intermediate with methanol produced furfuryl amine [88]. Due to the rapid heating rate by microwave irradiation, furfural and a variety of aldehydes can be transformed into corresponding amines within a few minutes [88]. As the coupling of intermediate 4c and furfural competes with the formation of furfuryl amine (Fig. 11), adjusting the ratio of different reactants and reaction time needs to be considered to obtain high selectivity of furfuryl amine [88].
Schematic conversion of furfural to amines using formamide as the nitrogen source [88]
In addition to the reductive amination of the aldehyde group to form furfuryl amine, the oxygen atom in the furan ring of furfural molecules can also be substituted with nitrogen to generate pyrrole and its derivatives. For instance, over the combination catalysts of silicate-1 encapsulated Pd (Pd@S-1) and Hβ zeolite, furfural could undergo a decarbonylation reaction to produce furan, which then yielded pyrrole via amination reaction [89, 90]. Subsequently, the carboxylation of pyrrole and CO2 followed by a hydrogenation on Rh/C catalyst could produce proline. This pathway not only diversifies the methods for synthesizing N-containing chemicals from furfural but also efficiently utilizes natural CO2.
Furfuryl alcohol, a hydrogenation product of furfural, can serve as another feedstock to synthesize furfuryl amine. Generally, the dehydrogenation of hydroxyl group is the initial step for furfuryl alcohol amination, which generates one mole of H2 from one mole of furfuryl alcohol (Fig. 12). Because the amount of in situ generated H2 matches that of furfuryl alcohol, it can be directly used in the subsequent reductive amination without addition of an external reducing agent. For example, over a Raney Ni catalyst, furfural alcohol could be converted into furfuryl amine with a yield of 78% in the absence of H2 at 453 K [91]. If excessive H2 (1 MPa) was introduced into this reaction, the hydrogenation of furan ring occurred and mainly produced tetrahydro-furfural alcohol and tetrahydro-furfuryl amine (Fig. 12). The H2-free reaction condition avoids the over-hydrogenation reactions and benefits the formation of furfuryl amine. Nevertheless, the amination reaction requires very long time (e.g., 48 h) to achieve acceptable conversion under the insufficient H2 condition, though the selectivity of furfuryl amine is high [91]. Moreover, Ni catalyst easily deactivated due to the formation of Ni3N species [91].
Proposed reaction pathways for reductive amination of furfuryl alcohol in absence or presence of H2 [91]. FA: furfuryl alcohol; FF: furfural; FAM: furfurylamine; THFF: tetrahydrofurfural; THFA: tetrahydrofurfuryl alcohol; THFAM: tetrahydrofurfurylamine
HMF is a typical furan compound containing both aldehyde and hydroxyl groups. The reductive amination of aldehyde group in HMF is similar to that for furfural amination over metal-based catalysts, which will yield hydroxymethylfuran amine and its derivatives when using different ammonia as nitrogen sources [92,93,94,95,96,97,98,99,100,101]. If the hydroxyl group of HMF is aminated as well, furan diamine compounds such as 2,5-bis(aminomethyl)furan (BAMF) can be attained. In this process, 5-hydroxymethylfurfurylamine (HMFA) is usually formed as a main intermediate, due to the relatively higher reactivity of aldehyde group than hydroxyl group. A consecutive dehydrogenation and amination of HMFA generate BAMF (Fig. 13) [102]. It is notable that the hydroxyl amination is more difficult than aldehyde amination on metal-based catalysts, and thus the conversion of HMFA to BAMF requires much harsh reaction conditions (e.g., high temperature) than the HMF-to-HMFA conversion. However, the harsh conditions would lead to undesirable products caused by the hydrogenation of C = O or C = C bond, decarbonylation, and hydrogenolysis of C − OH bonds.
Possible reaction pathways for the direct reductive amination of 5-HMF into BAMF over Raney Ni [102]. DHMF: 2,5-dihydroxymethylfuran; MFA: 5-methyl furfuryl alcohol; MFAM: 5-methyl furfuryl amine; HMTHFA: 5-hydroxymethyl tetrahydrofurfuryl amine; MTHFA: 5-methyl tetrahydrofurfuryl alcohol; MTHFAM: 5-methyl tetrahydrofurfuryl amine
To enhance the selectivity of BAMF, one strategy is to perform the amination of HMF in an aldehyde-first and then hydroxyl sequence. For example, Raney Co was found to quantitatively convert HMF into HMFA via reductive amination of the aldehyde group in HMF, whereas Raney Ni showed high activity in the reductive amination of the remaining hydroxyl group within HMFA [102]. A combination of Raney Co and Ni could produce BAMF with a high yield of 88% in a stepwise reaction mode, in which the reaction first proceeded on Raney Co at 393 K for 2 h and then the temperature raised to 433 K for 10 h on Raney Ni [102]. Additionally, the use of low H2 concentration was favorable for the formation of BAMF, because H2 was co-produced during the dehydrogenation [103]. Moreover, a low H2 concentration shortened the reaction time that was needed to achieve a high BAMF yield [103]. Very recently, encapsulating Ni nanoparticles into SiO2 demonstrated as another effective strategy to convert furfural to BAMF via the HMFA intermediate. In the SiO2 shell coated Ru catalyst (Ni@SiO2), Ru nanoparticles were designed as the active sites for the reductive amination, while the SiO2 shell could reduce the charge density of Ni surfaces and enhance the adsorption of ammonia and intermediates. Moreover, the appropriate pore sizes of SiO2 only allowed the reactants HMF and HMFA to diffuse to Ni sites, and thus prevented direct hydrogenation of HMF and self-coupling of BAMF and improved the selectivity of BAMF. In addition, the suitable acidity and acidic sites on the SiO2 shell were conducive to the reaction of carbonyl and ammonia, avoiding the direct hydrogenation of carbonyl to alcohols. These features allowed the Ni@SiO2 catalyst to produce BAMF with a yield of up to 99% [104].
The conversion of HMF in a sequence of hydroxyl first and then aldehyde can also produce BAMF. It requires to construct C-N bond from the hydroxyl group and nitrogen sources, or to oxidize the hydroxyl group to aldehyde for further amination. A typical example for the first case is the combination of Ritter reaction and reductive amination of HMF (Fig. 14) [105, 106]. In the Ritter reaction, trifluoromethane sulfonic acid (CHF3O3S) and phosphoric anhydride were employed to promote the substitution of OH group by CH3CN, giving N-acyl-5-aminomethyl furfural (NAMF). After Raney-Ni catalyzed amination and subsequent hydrolysis in a methanol-ammonia solution, NAMF would be easily transformed into BAMF through the N-Acyl-2,5-bis(aminomethyl)furan (NBAF) intermediate (Fig. 14) [105]. By combining aerobic oxidation and reductive amination in a two-step reaction mode, HMF could also be selectively converted to BAMF [107]. The oxidation of HMF on Ru/AC catalyst afforded 2,5-diformylfuran (DFF), and a subsequent reductive amination of DFF by NH3 and H2 finally produced BAMF with a high selectivity up to 95% [107].
4 Amination of LA to pyrrolidinones
Pyrrolidinones are a class of five-membered heterocyclic chemicals, and have broad applications in biology, medicine, solvents, surfactants, chelators, and other fields [32, 108,109,110,111]. Currently, the production of pyrrolidinones relies on the reaction of γ-butyrolactone and amines and/or the reductive amination of butanediol with diverse amines under harsh reaction conditions (> 473 K) [32]. To reduce the use of petroleum-derived feedstock, a sustainable and green approach to synthesize pyrrolidiones is the amination of LA. On one hand, LA is one of the top value-added platform molecules proposed by the US Department of Energy, [112, 113] which can be obtained by acid-catalyzed hydrolysis of lignocellulose [114,115,116,117,118,119,120]. On the other hand, LA contains a γ-carbonyl group and a carboxylic group, and it can undergo reductive amination, producing γ-amino acid, and further be transformed to pyrrolidinone after an intermolecular dehydration reaction between the amino and carboxyl group. The use of different organic amines as nitrogen sources can eventually produce a variety of alkyl substituted pyrrolidinones or pyrrolidines (Fig. 15) [32, 121].
Synthesis of pyrrolidinones and pyrrolidines from LA and its derivatives [32]
During the reductive amination reaction of LA, the carbonyl group may also undergo hydrogenation and intramolecular dehydration with carboxylic group, generating γ-valerolactone. Hence, it is necessary to adjust the structure of the catalyst to give priority to reductive amination rather than reduction reaction. The combination of noble metals (e.g., Ru, Au, Ir, and Pd) with formic acid has been prove to be efficient for the synthesis of pyrrolidinones [121,122,123,124,125,126,127,128]. Noble metals mainly works as active sites for hydrogenation, while formic acid either functions as a reducing agent or acts as a co-catalyst. For example, over a ZrO2 supported Au catalyst, the reaction of LA, NH3·H2O and formic acid resulted in a pyrrolidinone yield of 85% [121]. Using Ru NPs (mean diameter of 3.8 nm) as the catalyst, LA could be transformed to N-cyclohexyl-2-pyrrolidinone with cyclohexane and formic acid, and the pyrrolidinone yield approached 97% [123]. An analysis on the reaction mechanism indicated that amine initially attacked the keto-functional group in LA and formed an imine intermediate A (Fig. 16). This imine would be converted to pyrrolidinone via two parallel routes. One is the hydrogenation of A to a γ-amino acid A1, followed by cyclolization of A1. The other is that A first underwent isomerization to give enamine intermediate, which underwent intermolecular dehydration to generate a pyrrolidione precursor with one C = C bond in the five-membered ring. Then, a subsequent hydrogenation of the precursor yield pyrrolidinone [123]. Ru NPs catalyzed the decomposition of formic acid to generate H2 and facilitated the hydrogenation reactions (Fig. 16). Moreover, formic acid could function as inhibitor for the adsorption of LA on Ru catalyst, which avoided hydrogenation of ketone group in LA, and significantly reduced the selectivity of γ-valerolactone [125]. Therefore, the reductive amination of LA to pyrrolidinone is dramatically enhanced.
Proposed reaction paths for reductive amination of LA [123]
Tuning the support or ligands of noble metals has been utilized in the synthesis of pyrrolidinones [124, 129,130,131,132,133,134,135,136,137,138,139,140]. In particular, introduction of acidic sites into the support would strengthen the interaction between LA and active sites, and accelerate the reductive amination [130, 135, 136]. For example, by oxidizing the mercaptopropyl groups in SiO2 followed by impregnated with Ir NPs, Matinez et al. fabricated a Ir/SiO2-SO3H catalyst bearing acidic SO3H groups for the amination of LA with aniline [130]. Compared to the Ir/SiO2 without any acid functional groups, this Ir/SiO2-SO3H enhanced the yield of pyrrolidinone by one order of magnitude. Diffuse reflectance infrared Fourier transform (DRIFT) studies revealed that the acidic SO3H groups interacted with the -COOH of LA via hydrogen bonding, and benefited aniline nucleophilic attack at the carbonyl group and the formation of imine. Moreover, the adsorbed imine intermediate became easily transformed to pyrrolidinone by consecutive hydrogenation and cyclization. However, the SO3H groups are prone to leach during the reaction and lead to severe deactivation [130]. Han and co-workers reported that porous TiO2 nanosheets (P-TiO2) supported Pt NPs served as a highly efficient and stable catalyst in the amination of LA to pyrrolidinones [135]. Under room temperature, the reaction of LA with n-octylamine on the Pt/P-TiO2 catalyst could give N-octyl-5-methyl-2-pyrrolidones with a yield of up to 97%. On contrast, the Pt NPs loaded on commercial TiO2 or active carbon only demonstrated less than 15% of pyrrolidinone yield. Due to the unique structure (pore and nanosheet) of P-TiO2, more acidic sites could be exposed, making it more acidic than commercial TiO2. The strong acidity of P-TiO2 enabled the carbonyl group in LA strongly interacted with the catalyst, which facilitated the condensation of carbonyl groups and amino groups and the corresponding cyclization, resulting in high activity in the amination of LA [135]. Furthermore, in situ CO–DRIFT analysis disclosed that the electron-donating capability of Pt NPs in Pt/P-TiO2 was much weaker than that in the Pt/TiO2 catalyst. This is caused by the high acidity of P-TiO2 that decreased the electron density of Pt NPs. Such low electron density of the Pt sites was conducive to the desorption of products and therefore enhancing the activity [135].
Adjusting the crystal phases of catalyst supports could tune the acidity, basicity and adsorption ability of the metal-based catalysts, therefore resulting in different catalytic behaviors. For instance, loading Pt on monoclinic ZrO2 (Pt/M-ZrO2) offered a quantitative yield of N-octyl-5-methyl-2-pyrrolidones (OMP) for the reductive amination of methyl levulinate with octylamine at ambient temperature [139]. In contrast, the tetramonal ZrO2 supported Pt (Pt/T-ZrO2) catalyst exhibited an 18-fold lower activity than Pt/M-ZrO2 in terms of turnover frequency. Characterization studies indicate that Pt/M-ZrO2 possessed more acidic and basic sites than Pt/T-ZrO2, which formed abundant frustrated Lewis acid–base pairs (FLP). FLP facilitated the activation of C = N bonds and H2 dissociation, and hence accelerated the reductive amination of methyl leulinate and octylamine. Moreover, Pt/M-ZrO2 had a suitable proportion of Pt0 and Ptn+, which was beneficial to the rapid substrate adsorption and product desorption, whereas Pt/T-ZrO2 contained a large amount of metallic Pt0 species, which led to strong adsorption of H2 and imide intermediates on catalyst surfaces. Thus, the monoclinic ZrO2 displayed better performance than the tetramonal ZrO2.
Due to their high efficiency in the reductive amination of LA with amines, noble metals such as Pd, [141] Au, [142] and Pt [143, 144] have been utilized for the solvent-free conversion of LA. The solvent-free system can avoid the intense energy consumption for solvent-product separation, therefore holding promise in industries [145]. However, the viscosity and strong acid–base features of LA-amine mixtures make the solvent-free amination a challenge. To raise the formation rate of pyrrolidinones, it requires to develop catalysts with more stable and more exposed active sites. Shimizu and co-works prepared a titania supported Pt catalyst and modified it by molybdenum oxide (Pt-MoOx/TiO2) [146, 147]. For the reaction of LA with n-octylamine under solvent-free conditions (3 bar of H2, 373 K, and 20 h), this catalyst exhibited a pyrrolidine yield of 95%, whereas the pyrrolidinone yields were in the range of 4–40 when solvents such as water, dioxane, decane, toluene, and o-xylene were present [145]. Moreover, the turnover number (TON) for the solvent-free catalytic system was one order of magnitude higher than that using water as solvent. The excellent performance of Pt-MoOx/TiO2 can be ascribed to the acidity of MoOx and high dispersion of Pt NPs. MoOx could strongly adsorb the carbonyl group in LA via Lewis acid–base interaction, facilitating its effective polarization. Subsequent reductive amination and cyclic amination to pyrrolidinones became facilely with the assistance of Pt NPs and acid sites.
The amination of LA with nitro compounds over noble metal catalysts can be also achieved in the absence of solvent. In this process, nitro groups in nitro compounds were reduced to amino groups on metal catalysts, followed by the reaction with LA or LA esters (e.g., ethyl levulinate, EL) to form imine intermediates, and then hydrogenation and cyclization of the imine intermediates led to pyrrolidinones (Fig. 17) [143]. Conventional Pt/TiO2 catalyst was very efficient in the hydrogenation of nitro groups into amino groups, but the strong adsorption of nitro compounds on the acid sites would significantly decrease the rates of amination and cyclization, resulting in low yield of pyrrolidiones [143, 144]. By controlling the TiO2 in a nanotube morphology, the surface of TiO2 contained mild acid sites, and such TiO2 nanotube supported Pt (Pt/TiO2-NT) catalyst could exhibit excellent performances in the reductive amination of LA with nitro compounds [143]. Under the solvent-free condition of 393 K and 10 bar H2, the reaction of ethyl levulinate with nitrobenzene over Pt/TiO2-NT afforded 5-methyl-N-phenyl-2-pyrrolidone with a yield of > 90%, while the yield of less than 10% was attained on the Pt/TiO2 catalyst. Compared to Pt/TiO2, the weaker acid sites of Pt/TiO2-NT largely avoided the strong adsorption of nitro groups, which ensured the subsequent reductive amination and cyclization, therefore achieving high yield of pyrrolidiones.
Reaction pathway for pyrrolidones synthesis from EL and nitro compounds [143]
Non-noble metals including Fe [148], Cu [149, 150], Co [151, 152], Ni [153, 154] and Ni2P [155] have been designed to synthesize pyrrolidiones. Similar as their applications in the reductive amination of furfural, non-noble metals are easily leached or sintered during the reaction, leading to catalyst deactivation. Construction of appropriate supports has proved as a promising approach for the protection the metal sites from migration. Pyrolysis of Ni-/Co-containing MOFs in an inert atmosphere could form CoNi alloys inside N-doped porous carbon. Owing to the confinement of porous carbon and anchoring of N atoms, the Co and Ni worked in a synergistic manner for the reductive amination of LA, producing N-benzyl-5-methyl-2-pyrrolidinone with a yield greater than 99% [156]. Moreover, CoNi alloy could maintain its activity after 20 recycling uses. When using reducible CeOx to load Ni nanoparticles, the metal sites would be stabilized by the strong interaction between Ni and the support. Such strong interaction also promoted the reduction of Ce4+ to Ce3+, forming more catalytic active sites and improving the reductive amination efficiency [157].
Li and co-workers reported an atomic layer deposition technology to prepare porous-carbon-coated Ni NPs that pre-loaded on carbon nanotubes (CNFx@Ni@CNTs) [153]. This CNFx@Ni@CNTs catalyst showed a 99% yield of pyrrolidinone in the reductive amination of LA with amines. Furthermore, the recycling uses of the catalyst up to 20 runs did not lead to the leaching and sintering of Ni NPs. Besides the protection of carbon layer, the different reaction route from the noble metal-based system may also contribute to the high stability and efficiency of the catalyst. Reaction mechanism studies indicated that the reaction intermediates were not imines or enamines (Fig. 18) [153]. In this Ni-catalyzed system, amines first reacted with the carboxyl group instead of carbonyl group in LA to form amides intermediate, and then the amides underwent tandem cyclization, intramolecular dehydration, and hydrogenation to pyrrolidinones (Fig. 18) [153]. Because the preferential reaction of amines and acid functional groups avoided the reversible transformation of LA and amines to imines (or decomposition of imine into LA and amines), that usually occurred in noble metal systems, the formation rate of pyrrolidinones were increased. More importantly, the interactions between metals and acidic LA/basic amine were reduced, therefore lengthening the lifespan of the catalyst. Additionally, fabrication of single-atom catalysts (SAC) such as Co-SAC has also proved to be an efficient strategy to stabilize the non-noble metal sites, and over the Co-SAC catalyst, the pyrrolidinone yields could reach up to 95%, and the performance maintained at least 5 runs [152].
Reaction pathways for the reductive amination of LA with amines catalyzed by CNFx@Ni@CNTs [153]
Metal-free catalytic systems for the synthesis of pyrrolidinones have recently received growing attention [158,159,160,161,162,163,164,165]. Ionic liquids, which have tunable cations and ions, high stability, and excellent solubility for various compounds, are ideal candidates in the amination of LA without the addition of metal. Ionic liquids alone have difficulty in the activation of H2, and thus they are combined with strong reducing agents (e.g., hydrosilane) in the reductive amination. For example, Liu and co-workers employed a lactate-based ionic liquid (1-butyl-3-methylimidazolium lactate, [BMIm][Lac]) as the catalyst and triethoxysilane ((EtO)3SiH) as a reducing agent, and obtained diverse pyrrolidinones from the amination of LA with amines in a metal-free system [158]. The mechanism studies on the reaction of LA with aniline suggested that the first step was the formation of a keto-imine A, which reacted with the ionic liquid-activated (EtO)3SiH to give silyl ether A′. After tandem cyclization and reduction by (EtO)3SiH-[BMIm][Lac], this silyl ether was transformed to a five-membered cyclic B or B′, and finally to pyrrolidinone (Fig. 19) [158]. Besides ionic liquids, B(C6F5)3 combined with hydrosilanes is another excellent non-metal catalytic system to catalyze the reductive amination of LA, and pyrrolidinones and pyrrolidines could be selectively attained by adjusting the amounts of hydrosilanes [159]. Moreover, ammonia borane (NH3-BH3) [161], HCOONH4 [162, 163] and formamide (H2NCHO) [164, 165] have also be directly used as both hydrogen and nitrogen sources to realize the metal-free catalytic system for the production of pyrrolidinones from LA amination.
A possible reaction pathway for the reductive amination of LA catalyzed by ionic liquid [BMIm][Lac] [158]
5 Summary and outlook
Reductive amination of oxygen-containing bio-platform molecules such as keto/hydroxyl acids, furan compounds, and levulinic acid has been utilized for the sustainable production of nitrogen-containing compounds including amino acids, furan amines, and pyrrolidiones. While different functional groups exist in the bio-platform molecules, the key reaction mechanisms for their amination are similar. Initially, aldehyde/keto groups react with nitrogen sources (e.g., ammonia, and amines) to form imine intermediates, and a subsequent hydrogenation of the imine intermediates gives amino groups. When hydroxyl groups need to be converted to amino groups, a pre-dehydrogenation reaction is generally required to give aldehyde, which undergoes further reductive amination. In the amination process, how to accelerate the hydrogenation step, while inhibiting the polymerization of imines as well as other side reactions regarding newly generated primary amines and aldehyde groups, holds the key to improving the selectivity of target nitrogen-containing compounds. A number of noble metals (e.g., Ru, Pt, Pd, and Au) and non-noble metals (e.g., Ni, and Co) with unique ability in hydrogenation and dehydrogenation have gained success in the thermo-catalytic reductive amination. To enhance the metal performances, a variety of methods have been developed such as finely tuning of the supports, adjusting of the reaction conditions and selection of appropriate nitrogen sources. It should be noted that different from noble metals, the non-noble metals are prone to leaching or sintering in the presence of amines, thereby leading to catalyst deactivation. Encapsulation of the non-noble metals with supports or construction of single atom catalyst with N doped carbon materials helps to limit the active sites, and therefore is an effective approach to minimize the leaching of metals.
Although the activity and stability of the metal-based catalysts have been improved through various methods, more efforts are still needed to facilitate their large scale applications. Several possible future directions have been suggested for the reductive amination of bio-platform molecules. One is the construction of more efficient and stable metal catalysts with low noble metal loadings, such as fabrication of single-atom metal catalysts, which would reduce the cost of catalysts. Moreover, a large number of the amination catalysts have already been developed for the synthesis of nitrogen-containing compounds, and they can serve as a database for machine learning. The use of artificial intelligence to predict and design active catalysts therefore offers an ideal option to accelerate this progress. On the other hand, metal-free catalytic systems have gained advances in the synthesis of nitrogen-containing compounds such as pyrrolidiones. However, large amounts of strong reducing agents and liquid ions are required to ensure high efficiency, making these processes not cost effective. Avoiding the use of expensive reducing agents or development of new efficient metal-free catalytic systems may be another direction in the reductive amination of bio-platform molecules.
Photo- and electro-catalysis have recently emerged for the reductive amination reactions, in particular for the conversion of keto/hydroxyl acids. Due to their mild operation conditions, photo- and electro-catalysis can avoid many side reactions usually occurred in the thermo-catalytic systems, thereby demonstrating high selectivity in the reductive amination. While most of the photo- and electro-catalytic systems focus on the synthesis of amino acids, there is a notable scarcity of research dedicated to the photo- or electro-chemical transformation of furans and levulinic acid. These compounds possess multiple functional groups and are particularly susceptible to yielding undesired by-products under thermal catalytic conditions. Thus, the development of efficient photo- and electro-catalysts to produce furan amines and pyrrolidinones will be an attractive direction for the amination of bio-platform molecules. Additionally, most of the amination reactions including thermal, photo- and electro-catalytic systems are mainly carried out in batch reactors and the substrate concentration is low, which may hinder their practical applications. Through combining thermo-, photo-, or electro-catalysis with process intensification or membrane engineering, the direct synthesis of nitrogen-containing compounds from concentrated bio-platform molecules and even raw biomass resources can make the process more competitive, and benefit the further scaling up the valorization of biomass.
Availability of data and materials
Not applicable.
References
Zhang Z, Huber GW (2018) Catalytic Oxidation of Carbohydrates into Organic Acids and Furan Chemicals. Chem Soc Rev 47(4):1351–1390. https://doi.org/10.1039/C7CS00213K
Velty A, Iborra S, Corma A (2022) Synthetic Routes for Designing Furanic and Non Furanic Biobased Surfactants from 5-Hydroxymethylfurfural. Chemsuschem 15(13):e202200181. https://doi.org/10.1002/cssc.202200181
Eagan NM, Kumbhalkar MD, Buchanan J, Dumesic JA, Huber GW (2019) Chemistries and Processes for the Conversion of Ethanol into Middle-Distillate Fuels. Nat Rev Chem 3:223–249. https://doi.org/10.1038/s41570-019-0084-4
Turkin AA, Makshina EV, Sels BF (2022) Catalytic Hydroconversion of 5-HMF to Value-Added Chemicals: Insights into the Role of Catalyst Properties and Feedstock Purity. Chemsuschem 15(13):e202200181. https://doi.org/10.1002/cssc.202200412
Wu X, Xie S, Zhang H, Zhang Q, Sels BF, Wang Y (2021) Metal Sulfide Photocatalysts for Lignocellulose Valorization. Adv Mater 33(50):2007129. https://doi.org/10.1002/adma.202007129
Li G, Wang R, Pang J, Wang A, Li N, Zhang T (2024) Production of Renewable Hydrocarbon Biofuels with Lignocellulose and Its Derivatives over Heterogeneous Catalysts. Chem Rev 124(6):2889–2954. https://doi.org/10.1021/acs.chemrev.2c00756
Yan L, Zhang Q, Deng W, Zhang Q, Wang Y (2020) Catalytic Valorization of Biomass and Bioplatforms to Chemicals through Deoxygenation. Adv Catal 66:1–108. https://doi.org/10.1016/bs.acat.2020.09.002
Li S, Deng W, Li Y, Zhang Q, Wang Y (2019) Catalytic Conversion of Cellulose-Based Biomass and Glycerol to Lactic Acid. J Energy Chem 32:138–151. https://doi.org/10.1016/j.jechem.2018.07.012
Li S, Deng W, Wang S, Wang P, An D, Li Y, Zhang Q, Wang Y (2018) Catalytic Transformation of Cellulose and Its Derivatives into Functionalized Organic Acids. Chemsuschem 11(13):1995–2028. https://doi.org/10.1002/cssc.201800440
Gao Z, Ren P, Sun L, Luo N, Wang F (2024) Photocatalysts for Steering Charge Transfer and Radical Reactions in Biorefineries. Nat Synth 3:438–451. https://doi.org/10.1038/s44160-024-00499-4
Jing Y, Guo Y, Xia Q, Liu X, Wang Y (2019) Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass. Chem 5(10):2520–2546. https://doi.org/10.1016/j.chempr.2019.05.022
Wong S, Shu R, Zhang J, Liu H, Yan N (2020) Downstream Processing of Lignin Derived Feedstock into End Products. Chem Soc Rev 49(15):5510–5560. https://doi.org/10.1039/D0CS00134A
Wu X, Luo N, Xie S, Zhang H, Zhang Q, Wang F, Wang Y (2020) Photocatalytic Transformations of Lignocellulosic Biomass into Chemicals. Chem Soc Rev 49(17):6198–6223. https://doi.org/10.1039/D0CS00314J
Rosatella AA, Simeonov SP, Frade RFM, Afonso CAM (2011) 5-Hydroxymethylfurfural (HMF) as A Building Block Platform: Biological Properties. Synthesis and Synthetic Applications Green Chem 13(4):754–793. https://doi.org/10.1039/c0gc00401d
van Putten R, van der Waal JC, de Jong E, Rasrendra CB, Heeres HJ, de Vries JG (2013) Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem Rev 113(3):1499–1597. https://doi.org/10.1021/cr300182k
Mäki Arvela P, Ruiz D, Murzin DY (2021) Catalytic Hydrogenation/Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Chemsuschem 14(1):150–168. https://doi.org/10.1002/cssc.202001927
Mariscal R, Maireles-Torres P, Ojeda M, Sádaba I, López Granados M (2016) Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ Sci 9(4):1144–1189. https://doi.org/10.1039/c5ee02666k
Froidevaux V, Negrell C, Caillol S, Pascault J, Boutevin B (2016) Biobased Amines: From Synthesis to Polymers. Present and Future Chem Rev 116(22):14181–14224. https://doi.org/10.1021/acs.chemrev.6b00486
Gupta NK, Reif P, Palenicek P, Rose M (2022) Toward Renewable Amines: Recent Advances in the Catalytic Amination of Biomass-Derived Oxygenates. ACS Catal 12(16):10400–10440. https://doi.org/10.1021/acscatal.2c01717
Chen X, Song S, Li H, Gözaydın G, Yan N (2021) Expanding the Boundary of Biorefinery: Organonitrogen Chemicals from Biomass. Acc Chem Res 54(7):1711–1722. https://doi.org/10.1021/acs.accounts.0c00842
Chen X, Liu Y, Wang J (2020) Lignocellulosic Biomass Upgrading into Valuable Nitrogen-Containing Compounds by Heterogeneous Catalysts. Ind Eng Chem Res 59(39):17008–17025. https://doi.org/10.1021/acs.iecr.0c01815
Collet F, Dodd RH, Dauban P (2009) Catalytic C-H Amination: Recent Progress and Future Directions. Chem Commun 34:5061–5074. https://doi.org/10.1039/b905820f
Niu F, Xie S, Bahri M, Ersen O, Yan Z, Kusema BT, Pera-Titus M, Khodakov AY, Ordomsky VV (2019) Catalyst Deactivation for Enhancement of Selectivity in Alcohols Amination to Primary Amines. ACS Catal 9(7):5986–5997. https://doi.org/10.1021/acscatal.9b00864
Churro R, Mendes F, Araújo P, Madeira LM, Ribeiro F (2021) Commercial Catalysts Screening for the Direct Amination of Cyclohexanol. J Ind Eng Chem 95:190–202. https://doi.org/10.1016/j.jiec.2020.12.019
Karp EM, Eaton TR, Nogué VSI, Vorotnikov V, Biddy MJ, Tan ECD, Brandner DG, Cywar RM, Liu R, Manker LP, Michener WE, Gilhespy M, Skoufa Z, Watson MJ, Fruchey OS, Vardon DR, Gill RT, Bratis AD, Beckham GT (2017) Renewable Acrylonitrile Production. Science 358(6368):1307–1310. https://doi.org/10.1126/science.aan1059
Bähn S, Imm S, Neubert L, Zhang M, Neumann H, Beller M (2011) The Catalytic Amination of Alcohols. ChemCatChem 3(12):1853–1864. https://doi.org/10.1002/cctc.201100255
Pelckmans M, Renders T, Van de Vyver S, Sels BF (2017) Bio-based Amines through Sustainable Heterogeneous Catalysis. Green Chem 19(22):533–5331. https://doi.org/10.1039/c7gc02299a
Murugesan K, Senthamarai T, Chandrashekhar VG, Natte K, Kamer PCJ, Beller M, Jagadeesh RV (2020) Catalytic Reductive Aminations using Molecular Hydrogen for Synthesis of Different Kinds of Amines. Chem Soc Rev 49(17):6273–6328. https://doi.org/10.1039/C9CS00286C
Xu B, Dai J, Du Z, Li F, Liu H, Gu X, Wang X, Li N, Zhao J (2023) Catalytic Conversion of Biomass-Derived Compounds to Various Amino Acids: Status and Perspectives. Front Chem Sci Eng 17(7):817–829. https://doi.org/10.1007/s11705-022-2254-z
He J, Chen L, Liu S, Song K, Yang S, Riisager A (2020) Sustainable Access to Renewable N-Containing Chemicals from Reductive Amination of Biomass-Derived Platform Compounds. Green Chem 22(2):6714–6747. https://doi.org/10.1039/d0gc01869d
Irrgang T, Kempe R (2020) Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen. Chem Rev 120(17):9583–9674. https://doi.org/10.1021/acs.chemrev.0c00248
Xue Z, Yu D, Zhao X, Mu T (2019) Upgrading of Levulinic Acid into Diverse N-Containing Functional Chemicals. Green Chem 21(20):5449–5468. https://doi.org/10.1039/c9gc02415h
Wendisch VF (2020) Metabolic Engineering Advances and Prospects for Amino Acid Production. Metab Eng 58:17–34. https://doi.org/10.1016/j.ymben.2019.03.008
Yan N, Wang Y (2019) Catalyst: Is the Amino Acid a New Frontier for Biorefineries? Chem 5(4):739–743. https://doi.org/10.1016/j.chempr.2019.03.016
Ge JW, Song Q, Jia YQ, Yang WM (2019) Reaction: A New Option for Producing Amino Acids from Renewable Biomass? Chem 5(4):739–743. https://doi.org/10.1016/j.chempr.2019.03.017
Grand View Research (2022) Amino Acids Market Size, Share and Growth Report, 2030 (https://www.grandviewresearch.com/industry-analysis/amino-acids-market)
Masamba W (2021) Petasis vs. Strecker amino acid synthesis: convergence, divergence and opportunities inorganic synthesis. Molecules. https://doi.org/10.3390/molecules26061707
Zuend SJ, Coughlin MP, Lalonde MP, Jacobsen EN (2009) Scaleable Catalytic Asymmetric Strecker Syntheses of Unnatural α-Amino Acids. Nature 461(7266):968–970. https://doi.org/10.1038/nature08484
Yan H, Suk OhJ, Lee J, Eui Song C (2012) Scalable Organocatalytic Asymmetric Strecker Reactions Catalysed by A Chiral Cyanide Generator. Nat Commun 3(1):1–7. https://doi.org/10.1038/ncomms2216
Helmchen G, Pfaltz A (2000) Phosphinooxazolines A New Class of Versatile, Modular P, N-Ligands for Asymmetric Catalysis. Acc Chem Res 33(6):336–345. https://doi.org/10.1021/ar9900865
Petasis NA, Akritopoulou I (1993) The Boronic Acid Mannich Reaction: A New Method for the Synthesis of Geometrically Pure Allylamines. Tetrahedron Lett 34(4):583–586. https://doi.org/10.1016/S0040-4039(00)61625-8
Petasis NA, Zavialov IA (1997) A New and Practical Synthesis of α-Amino Acids from Alkenyl Boronic Acids. J Am Chem Soc 119(2):445–446. https://doi.org/10.1021/ja963178n
Petasis NA, Zavialov IA (1998) Highly Stereocontrolled One-Step Synthesis of anti-β-Amino Alcohols from Organoboronic Acids, Amines, and α-Hydroxy Aldehydes. J Am Chem Soc 120(45):11798–11799. https://doi.org/10.1021/ja981075u
Deng W, Wang P, Wang B, Wang Y, Yan L, Li Y, Zhang Q, Cao Z, Wang Y (2018) Transformation of Cellulose and Related Carbohydrates into Lactic Acid with Bifunctional Al(III)–Sn(II) Catalysts. Green Chem 20(3):735–744. https://doi.org/10.1039/C7GC02975F
Dusselier M, Van Wouwe P, Dewaele A, Makshina E, Sels BF (2013) Lactic Acid as A Platform Chemical in the Biobased Economy: The Role of Chemocatalysis. Energy Environ Sci 6(5):1415–1442. https://doi.org/10.1039/c3ee00069a
Mäki-Arvela P, Simakova IL, Salmi T, Murzin DY (2014) Production of Lactic Acid/Lactates from Biomass and Their Catalytic Transformations to Commodities. Chem Rev 114(3):1909–1971. https://doi.org/10.1021/cr400203v
Ogo S, Uehara K, Abura T, Fukuzumi S (2004) pH-Dependent chemoselective synthesis of α-Amino acids. Reductive amination of α-Keto acids with ammonia catalyzed by acid-stable iridium hydride complexes in water. J Am Chem Soc 126(10):3020–3021. https://doi.org/10.1021/ja031633r
Barge LM, Flores E, Baum MM, VanderVelde DG, Russell MJ (2019) Redox and pH Gradients Drive Amino Acid Synthesis in Iron Oxyhydroxide Mineral Systems. Proc Natl Acad Sci USA 116(11):4828–4833. https://doi.org/10.1073/pnas.1812098116
Mie Y, Katagai S, Mikami C (2021) Electrochemical Molecular Conversion of α-Keto Acid to Amino Acid at a Low Overpotential Using a Nanoporous Gold Catalyst. Int J Mol Sci 22(17):9442. https://doi.org/10.3390/ijms22179442
Fukushima T, Yamauchi M (2019) Electrosynthesis of Amino Acids from Biomass-Derivable Acids on Titanium Dioxide. Chem Commun 55(98):14721–14724. https://doi.org/10.1039/C9CC07208J
Isegawa M, Staykov A, Yamauchi M (2021) Proton-Coupled Electron Transfer in Electrochemical Alanine Formation from Pyruvic Acid: Mechanism of Catalytic Reaction at the Interface between TiO2 (101) and Water. J Phys Chem C 125(23):12603–12613. https://doi.org/10.1021/acs.jpcc.1c01304
Englezos C, Raman A, Jonker D, Ramos Delgado NA, Altomare M, Gardeniers H, Susarrey A (2024) Alanine Formation in a Zero-Gap Flow Cell and the Role of TiO2/Ti Electrocatalysts. ChemPlusChem 89(6):e202300763. https://doi.org/10.1002/cplu.202300763
Fukushima T, Yamauchi M (2021) Electrosynthesis of Glycine from Bio-Derivable Oxalic Acid. J Appl Electrochem 51(1):99–106. https://doi.org/10.1007/s10800-020-01428-x
Xiao Y, Lim CW, Chang J, Yuan Q, Wang L, Yan N (2023) Electrocatalytic Amino Acid Synthesis from Biomass-derivable Keto Acids over Ball Milled Carbon Nanotubes. Green Chem 25(8):3117–3126. https://doi.org/10.1039/D3GC00265A
Jia S, Tan X, Wu L, Zhao Z, Song X, Feng J, Zhang L, Ma X, Zhang Z, Sun X, Han B (2023) Lignin-derived carbon nanosheets boost electrochemical reductive amination of pyruvate to alanine. iScience 26:107776. https://doi.org/10.1016/j.isci.2023.107776
Deng W, Wang Y, Zhang S, Gupta KM, Hülsey MJ, Asakura H, Liu L, Han Y, Karp EM, Beckham GT, Dyson PJ, Jiang J, Tanaka T, Wang Y, Yan N (2018) Catalytic Amino Acid Production from Biomass-Derived Intermediates. Proc Natl Acad Sci USA 115(20):5093–5098. https://doi.org/10.1073/pnas.1800272115
Xie Z, Chen B, Peng F, Liu M, Liu H, Yang G, Han B (2020) Highly Efficient Synthesis of Amino Acids by Amination of Bio-Derived Hydroxy Acids with Ammonia over Ru Supported on N-Doped Carbon Nanotubes. Chemsuschem 13(21):5683–5689. https://doi.org/10.1002/cssc.202001561
Shah MA, Khalil I, Tallarico S, Donckels T, Eloy P, Debecker DP, Oliverio M, Dusselier M (2022) Catalytic Amination of Lactic Acid Using Ru–Zeolites. Dalton Trans 51(28):10773–10778. https://doi.org/10.1039/D2DT00054G
Wang Y, Furukawa S, Song S, He Q, Asakura H, Yan N (2020) Catalytic Production of Alanine from Waste Glycerol. Angew Chem Int Ed 59(6):2289–2293. https://doi.org/10.1002/anie.201912580
Podolean I, Dogaru M, Guzo NC, Petcuta OA, Jacobsen EE, Nicolaev A, Cojocaru B, Tudorache M, Parvulescu VI, Coman SM (2024) Highly Efficient Ru-Based Catalysts for Lactic Acid Conversion to Alanine. Nanomaterials 14(3):277. https://doi.org/10.3390/nano14030277
Wang W, Liu X, Yang Y, Su W (2013) Reversible Transformation Between α-Oxo Acids and α-Amino Acids on ZnS Particles: A Photochemical Model for Tuning the Prebiotic Redox Homoeostasis. Int J Astrobiol 12(1):69–77. https://doi.org/10.1017/S1473550412000432
Song S, Qu J, Han P, Hülsey MJ, Zhang G, Wang Y, Wang S, Chen D, Lu J, Yan N (2020) Visible-Light-Driven Amino Acids Production from Biomass-Based Feedstocks over Ultrathin CdS Nanosheets. Nat Commun 11(1):1–10. https://doi.org/10.1038/s41467-020-18532-3
Han YW, Ye L, Gong TJ, Fu Y (2023) Surface-Controlled CdS/Ti3C2 MXene Schottky Junction for Highly Selective and Active Photocatalytic Dehydrogenation-Reductive Amination. Angew Chem Int Ed 62(45):e202306305. https://doi.org/10.1002/anie.202306305
Zheng M, Li Q, Liu M, Liu J, Zhao C, Xiao X, Wang H, Zhou J, Zhang L, Jiang B (2022) Creation of Mo Active Sites on Indium Oxide Microrods for Photocatalytic Amino Acid Production. Sci China Mater 65(5):1285–1293. https://doi.org/10.1007/s40843-021-1907-3
Yan K, Huddleston ML, Gerdes BA, Sun Y (2022) Electrosynthesis of Amino Acids from Biomass-Derived α-Hydroxyl Acids. Green Chem 24(13):5320–5325. https://doi.org/10.1039/d2gc01779b
Saini MK, Kumar S, Li H, Babu SA, Saravanamurugan S (2022) Advances in the Catalytic Reductive Amination of Furfural to Furfural Amine: The Momentous Role of Active Metal Sites. Chemsuschem 15(7):e202200107. https://doi.org/10.1002/cssc.202200107
https://industrygrowthinsights.com/report/furfurylamine-market/
Komanoya T, Kinemura T, Kita Y, Kamata K, Hara M (2017) Electronic Effect of Ruthenium Nanoparticles on Efficient Reductive Amination of Carbonyl Compounds. J Am Chem Soc 139(33):11493–11499. https://doi.org/10.1021/jacs.7b04481
Winans CF (1939) Hydrogenation of Aldehydes in the Presence of Ammonia. J Am Chem Soc 61(12):3566–3567. https://doi.org/10.1021/ja01267a102
Wang H, Xiang Q, Niu Y, Wang L, Zhang B, Chu S, Hui Y, Yang J, Qin Y, Song L, Qin S, Zhang J, Gao X, Cao X, Xiao F (2023) Sterically Controllable Adsorption on Nickel Surface for Selective Reductive Amination. Chem Catal 4:100857. https://doi.org/10.1016/j.checat.2023.100857
Zhang J, Yin J, Duan X, Zhang C, Zhang J (2023) Continuous Reductive Amination to Synthesize Primary Amines with High Selectivity in Flow. J Catal 420:89–98. https://doi.org/10.1016/j.jcat.2023.02.017
Dong C, Wu Y, Wang H, Peng J, Li Y, Samart C, Ding M (2021) Facile and Efficient Synthesis of Primary Amines via Reductive Amination over a Ni/Al2O3 Catalyst. ACS Sustainable Chem Eng 9(21):7318–7327. https://doi.org/10.1021/acssuschemeng.1c01456
Manzoli M, Gaudino EC, Cravotto G, Tabasso S, Baig RBN, Colacino E, Varma RS (2019) Microwave-Assisted Reductive Amination with Aqueous Ammonia: Sustainable Pathway Using Recyclable Magnetic Nickel-Based Nanocatalyst. ACS Sustainable Chem Eng 7(6):5963–5974. https://doi.org/10.1021/acssuschemeng.8b06054
Wan Y, Ma P, Lu H, Zhang J, Wang J, Fang W, Song W, Zheng Q, Lai W (2024) External [111] Facets on Nanorod Gamma Alumina Boosts Catalytic Reductive Amination of Carbonyl Compound to Primary Amine. J Catal 429:115285. https://doi.org/10.1016/j.jcat.2023.115285
Jagadeesh RV, Murugesan K, Alshammari AS, Neumann H, Pohl M, Radnik J, Beller M (2017) MOF-Derived Cobalt Nanoparticles Catalyze a General Synthesis of Amines. Science 358(6361):326–332. https://doi.org/10.1126/science.aan6245
Yuan Z, Liu B, Zhou P, Zhang Z, Chi Q (2019) Preparation of Nitrogen-Doped Carbon Supported Cobalt Catalysts and its Application in the Reductive Amination. J Catal 370:347–356. https://doi.org/10.1016/j.jcat.2019.01.004
Deng D, Kita Y, Kamata K, Hara M (2019) Low-Temperature Reductive Amination of Carbonyl Compounds over Ru Deposited on Nb2O5·nH2O. ACS Sustainable Chem Eng 7(5):4692–4698. https://doi.org/10.1021/acssuschemeng.8b04324
Chandra D, Inoue Y, Sasase M, Kitano M, Bhaumik A, Kamata K, Hosono H, Hara M (2018) A High Performance Catalyst of Shape-Specific Ruthenium Nanoparticles for Production of Primary Amines by Reductive Amination of Carbonyl Compounds. Chem Sci 9(27):5949–5956. https://doi.org/10.1039/C8SC01197D
Liang G, Wang A, Li L, Xu G, Yan N, Zhang T (2017) Production of Primary Amines by Reductive Amination of Biomass-Derived Aldehydes/Ketones. Angew Chem Int Ed 56(11):3050–3054. https://doi.org/10.1002/anie.201610964
Dong B, Guo X, Zhang B, Chen X, Guan J, Qi Y, Han S, Mu X (2015) Heterogeneous Ru-Based Catalysts for One-Pot Synthesis of Primary Amines from Aldehydes and Ammonia. Catalysts 5(4):2258–2270. https://doi.org/10.3390/catal5042258
Nishimura S, Mizuhori K, Ebitani K (2016) Reductive Amination of Furfural Toward Furfurylamine with Aqueous Ammonia under Hydrogen over Ru-Supported Catalyst. Res Chem Intermed 42(1):19–30. https://doi.org/10.1007/s11164-015-2334-5
Zou H, Chen J (2022) Efficient and Selective Approach to Biomass-Based Amine by Reductive Amination of Furfural using Ru Catalyst. Appl Catal B: Environ 309:121262. https://doi.org/10.1016/j.apcatb.2022.121262
Chatterjee M, Ishizaka T, Kawanami H (2016) Reductive Amination of Furfural to Furfurylamine using Aqueous Ammonia Solution and Molecular Hydrogen: An Environmentally Friendly Approach. Green Chem 18(2):487–496. https://doi.org/10.1039/c5gc01352f
Martínez JJ, Nope E, Rojas H, Brijaldo MH, Passos F, Romanelli G (2014) Reductive Amination of Furfural over Me/Sio2-SO3H (Me: Pt, Ir, Au) Catalysts. J Mol Catal A: Chem 392:235–240. https://doi.org/10.1016/j.molcata.2014.05.014
Gong H, Wei L, Li Q, Zhang J, Wang F, Ren J, Shi X (2024) Electron-Rich Ru Supported on N-Doped Coffee Biochar for Selective Reductive Amination of Furfural to Furfurylamine. Langmuir 40(17):8950–8960. https://doi.org/10.1021/acs.langmuir.4c00112
Feng H, Liu W, Pang D, Wang L, Zhang M, Zhao S, Zhang X, Li F, Hong S, Yang Y, Wei M (2023) Modulation of coordination environment on atomically dispersed Ir catalysts for highly-efficient reductive amination. J Catal 428:115175. https://doi.org/10.1016/j.jcat.2023.115175
Gao Z, Cai L, Ma H, Zhao Y, Wu H, Liu H, Wang Q, Li D, Feng J (2023) Dual Scale Hydrogen Transfer Bridge Construction for Biomass Tandem Reductive Amination. ACS Catal 13(19):12835–12847. https://doi.org/10.1021/acscatal.3c03486
Li H, Guo H, Su Y, Hiraga Y, Fang Z, Hensen EJM, Watanabe M, Smith RL (2019) N-Formyl-Stabilizing Quasi-Catalytic Species Afford Rapid and Selective Solvent-Free Amination of Biomass-Derived Feedstocks. Nat Commun 10(1):1–13. https://doi.org/10.1038/s41467-019-08577-4
Song S, Fung Kin Yuen V, Di L, Sun Q, Zhou K, Yan N (2020) Integrating Biomass into the Organonitrogen Chemical Supply Chain: Production of Pyrrole and D-Proline from Furfural. Angew Chem Int Ed 59(45):19846–19850. https://doi.org/10.1002/anie.202006315
Ding M, Song S, Li X (2024) A Perspective on Renewable Production of Amino Acids from Biomass through the Chemocatalytic Method. Green Chem 26(8):4468–4476. https://doi.org/10.1039/d4gc00846d
Liu Y, Zhou K, Shu H, Liu H, Lou J, Guo D, Wei Z, Li X (2017) Switchable Synthesis of Furfurylamine and Tetrahydrofurfurylamine from Furfuryl Alcohol over RANEY® Nickel. Catal Sci Technol 7(18):4129–4135. https://doi.org/10.1039/C7CY00981J
Xu Z, Yan P, Liu K, Wan L, Xu W, Li H, Liu X, Zhang ZC (2016) Synthesis of Bis(hydroxylmethylfurfuryl)amine Monomers from 5-Hydroxymethylfurfural. Chemsuschem 9(11):1255–1258. https://doi.org/10.1002/cssc.201600122
Zhu M, Tao L, Zhang Q, Dong J, Liu Y, He H, Cao Y (2017) Versatile CO-Assisted Direct Reductive Amination of 5-Hydroxymethylfurfural Catalyzed by a Supported Gold Catalyst. Green Chem 19(16):3880–3887. https://doi.org/10.1039/C7GC01579H
Chen W, Sun Y, Du J, Si Z, Tang X, Zeng X, Lin L, Liu S, Lei T (2018) Preparation of 5-(Aminomethyl)-2-furanmethanol by Direct Reductive Amination of 5-Hydroxymethylfurfural with Aqueous Ammonia over the Ni/SBA-15 Catalyst. J Chem Technol Biotechnol 93(10):3028–3034. https://doi.org/10.1002/jctb.5661
Nuzhdin AL, Simonov PA, Bukhtiyarov VI (2021) Reductive Amination of 5-Hydroxymethylfurfural by the Hydrogenation of Intermediate Imines in the Presence of a Pt/Al2O3 Catalyst in a Flow Reactor. Kinet Catal 62(4):507–512. https://doi.org/10.1134/s0023158421040091
Nuzhdin AL, Bukhtiyarova MV, Eltsov IV, Bukhtiyarov VI (2021) CuAlOx Catalyst for the Batch-Flow Tandem Synthesis of Amino Acid-Derived Furfurylamines. Mendeleev Commun 31(6):813–814. https://doi.org/10.1016/j.mencom.2021.11.014
Xu Z, Yan P, Xu W, Jia S, Xia Z, Chung B, Zhang ZC (2014) Direct Reductive Amination of 5-Hydroxymethylfurfural with Primary/Secondary Amines via Ru-Complex Catalyzed Hydrogenation. RSC Adv 4(103):59083–59087. https://doi.org/10.1039/c4ra10349a
Zhou K, Liu H, Shu H, Xiao S, Guo D, Liu Y, Wei Z, Li X (2019) A Comprehensive Study on the Reductive Amination of 5-Hydroxymethylfurfural into 2,5-Bisaminomethylfuran over Raney Ni Through DFT Calculations. ChemCatChem 11(11):2649–2656. https://doi.org/10.1002/cctc.201900304
Wu H, Wang Q, Zhao Y, Gao Z, Lin Y, Zheng L, Li D, Feng J (2024) Coupling Cross-Dimensional Ru1–Run Sites in Confined Nanoislands to Overcome the Limitation of Coadsorption and Diffusion in Tandem Reactions. ACS Catal 14(3):1584–1594. https://doi.org/10.1021/acscatal.3c05112
Zhao Y, Wang Q, Wu H, Zheng L, Gao Z, Guo X, Cao X, Li D, Feng J (2024) Boosting Primary Amines Renewable Tandem Synthesis via Defect Engineering of NiCo Alloy. AIChE J 70(7):e18446. https://doi.org/10.1002/aic.18446
Zhu C, Wang K, Gao F, Sun Z, Chen M, Fei J, Chen C, He H, Liu Y, Cao Y (2024) Hybrid Homogeneous/Heterogeneous Relay Catalysis for Efficient Synthesis of 5-Aminomethyl-2-furancarboxylic acid from HMF. Chem Commun 60(58):7483–7486. https://doi.org/10.1039/d4cc02474e
Wei Z, Cheng Y, Zhou K, Zeng Y, Yao E, Li Q, Liu Y, Sun Y (2021) One-Step Reductive Amination of 5-Hydroxymethylfurfural into 2,5-Bis(aminomethyl)furan over Raney Ni. Chemsuschem 14(11):2308–2312. https://doi.org/10.1002/cssc.202100564
Pingen D, Schwaderer JB, Walter J, Wen J, Murray G, Vogt D, Mecking S (2018) Diamines for Polymer Materials via Direct Amination of Lipid- and Lignocellulose-based Alcohols with NH3. ChemCatChem 10(14):3027–3033. https://doi.org/10.1002/cctc.201800365
Liu P, Li X, Zhang H, Zhang Y, Zhao J (2024) One-step Reductive Amination of 5-Hydroxymethylfurfural to 2,5-Bis(ami-nomethyl)furan over A Core-Shell Structured Catalyst. J Catal 429:115291. https://doi.org/10.1016/j.jcat.2024.115291
Wang X, Chen W, Li Z, Zeng X, Tang X, Sun Y, Lei T, Lin L (2018) Synthesis of Bis(amino)furans from Biomass Based 5-Hydroxymethyl Furfural. J Energy Chem 27(1):209–214. https://doi.org/10.1016/j.jechem.2017.06.015
Zhang J, Jia W, Sun Y, Yang S, Tang X, Zeng X, Lin L (2021) An Efficient Approach to Synthesizing 2,5-Bis(N-methyl-aminomethyl)furan from 5-Hydroxymethylfurfural via 2,5-Bis(N-methyl-iminomethyl)furan using a Two-Step Reaction in One Pot. Green Chem 23(15):5656–5664. https://doi.org/10.1039/D1GC01635K
Qi H, Liu F, Zhang L, Li L, Su Y, Yang J, Hao R, Wang A, Zhang T (2020) Modulating trans-Imination and Hydrogenation Towards the Highly Selective Production of Primary Diamines from Dialdehydes. Green Chem 22(20):6897–6901. https://doi.org/10.1039/D0GC02280B
G. Neo A, F. Marcos C, (2018) Selective Synthesis of 3-Substituted Pyrrolidinones by Enol-Passerini and Anomalous Enol-Passerini Condensations. Org Lett 20(13):3875–3878. https://doi.org/10.1021/acs.orglett.8b01462
Roy S, Reiser O (2012) A Catalytic Multicomponent Approach for the Stereoselective Synthesis of cis-4,5-Disubstituted Pyrrolidinones and Tetrahydro-3H-pyrrolo[3,2-c]quinolines. Angew Chem Int Ed 51(19):4722–4725. https://doi.org/10.1002/anie.201107831
Muñoz SB, Daifuku SL, Sears JD, Baker TM, Carpenter SH, Brennessel WW, Neidig ML (2018) The N-Methylpyrrolidone (NMP) Effect in Iron-Catalyzed Cross-Coupling with Simple Ferric Salts and MeMgBr. Angew Chem Int Ed 57(22):6496–6500. https://doi.org/10.1002/anie.201802087
Wang J, Lu X, Guo M, Zhang R, Xiong J, Qiao Y, Yu Z (2023) Reductive Amination of Levulinic Acid to Pyrrolidones: Key Step in Biomass Valorization towards Nitrogen-Containing Chemicals. Chemsuschem 16(24):e202301091. https://doi.org/10.1002/cssc.202301091
Werpy T, Petersen G, Aden A, Bozell J, Holladay J, White J, Manheim A, Elliot D, Lasure L, Jones S, Gerber M, Ibsen K, Lumberg L, Kelley S (2004) Top Value Added Chemicals from Biomas Volume 1 - Results of Screening for Potential Candidates from Sugars and Synthesis Gas. U.S. Department of Energy 2004, Washington
Bozell JJ, Petersen GR (2010) Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates - the US Department of Energy’s “Top 10” Revisited. Green Chem 12(4):539–554. https://doi.org/10.1039/b922014c
Boonyakarn T, Wataniyakul P, Boonnoun P, Quitain AT, Kida T, Sasaki M, Laosiripojana N, Jongsomjit B, Shotipruk A (2019) Enhanced Levulinic Acid Production from Cellulose by Combined Brønsted Hydrothermal Carbon and Lewis Acid Catalysts. Ind Eng Chem Res 58(8):2697–2703. https://doi.org/10.1021/acs.iecr.8b05332
Wang K, Jiang J, Liang X, Wu H, Xu J (2018) Direct Conversion of Cellulose to Levulinic Acid over Multifunctional Sulfonated Humins in Sulfolane-Water Solution. ACS Sustainable Chem Eng 6(11):15092–15099. https://doi.org/10.1021/acssuschemeng.8b03558
Yu F, Zhong R, Chong H, Smet M, Dehaen W, Sels BF (2017) Fast Catalytic Conversion of Recalcitrant Cellulose into Alkyl Levulinates and Levulinic Acid in the Presence of Soluble and Recoverable Sulfonated Hyperbranched Poly(arylene oxindole)s. Green Chem 19(1):153–163. https://doi.org/10.1039/c6gc02130a
Deng W, Zhang Q, Wang Y (2015) Catalytic Transformations of Cellulose and its Derived Carbohydrates into 5-Hydroxymethylfurfural, Levulinic acid, and Lactic Acid. Sci China Chem 58(1):29–46. https://doi.org/10.1007/s11426-014-5283-8
Bucchianico DMD, D, Wang Y, Buvat J, Pan Y, Casson Moreno V, Leveneur S, (2022) Production of Levulinic Acid and Alkyl Levulinates: A Process Insight. Green Chem 24(2):614–646. https://doi.org/10.1039/d1gc02457d
Jiang L, Zhou L, Chao J, Zhao H, Lu T, Su Y, Yang X, Xu J (2018) Direct Catalytic Conversion of Carbohydrates to Methyl Levulinate: Synergy of Solid Brønsted Acid and Lewis Acid. Appl Catal B: Environ 220:589–596. https://doi.org/10.1016/j.apcatb.2017.08.072
Lglesias J, Martínez-Salazar I, Maireles-Torres P, Martin Alonso D, Mariscal R, López Granados M (2020) Advances in Catalytic Routes for the Production of Carboxylic Acids from Biomass: A Step Forward for Sustainable Polymers. Chem Soc Rev 49(16):5704–5771. https://doi.org/10.1039/D0CS00177E
Du X, He L, Zhao S, Liu Y, Cao Y, He H, Fan K (2011) Hydrogen-Independent Reductive Transformation of Carbohydrate Biomass into γ-Valerolactone and Pyrrolidone Derivatives with Supported Gold Catalysts. Angew Chem Int Ed 50(34):7815–7819. https://doi.org/10.1002/anie.201100102
Huang Y, Dai J, Deng X, Qu Y, Guo Q, Fu Y (2011) Ruthenium-Catalyzed Conversion of Levulinic Acid to Pyrrolidines by Reductive Amination. Chemsuschem 4(11):1578–1581. https://doi.org/10.1002/cssc.201100344
Ortiz-Cervantes C, Flores-Alamo M, García JJ (2016) Synthesis of Pyrrolidones and Quinolines from the Known Biomass Feedstock Levulinic Acid and Amines. Tetrahedron Lett 57(7):766–771. https://doi.org/10.1016/j.tetlet.2016.01.018
Sun Z, Chen J, Tu T (2017) NHC-Based Coordination Polymers as Solid Molecular Catalysts for Reductive Amination of Biomass Levulinic Acid. Green Chem 19(3):789–794. https://doi.org/10.1039/C6GC02591A
Bellè A, Tabanelli T, Fiorani G, Perosa A, Cavani F, Selva M (2019) A Multiphase Protocol for Selective Hydrogenation and Reductive Amination of Levulinic Acid with Integrated Catalyst Recovery. Chemsuschem 12(14):3343–3354. https://doi.org/10.1002/cssc.201900925
Wang S, Huang H, Bruneau C, Fischmeister C (2019) Formic Acid as a Hydrogen Source for the Iridium-Catalyzed Reductive Amination of Levulinic Acid and 2-Formylbenzoic Acid. Catal Sci Technol 9(15):477–482. https://doi.org/10.1039/c9cy01019j
Wei Y, Wang C, Jiang X, Xue D, Li J, Xiao J (2013) Highly Efficient Transformation of Levulinic Acid into Pyrrolidinones by Iridium Catalysed Transfer Hydrogenation. Chem Commun 49(47):5408. https://doi.org/10.1039/c3cc41661e
Kar AK, Chauhan A, Srivastava R (2023) A CoPd Nanoalloy Embedded N-doped Porous Carbon Catalyst for the Selective Reduction and Reductive Amination of Levulinic Acid using Formic Acid in Water. Sustain Energ Fuels 7(8):1855–1869. https://doi.org/10.1039/d3se00034f
Zhang T, Ge Y, Wang X, Chen J, Huang X, Liao Y (2017) Polymeric Ruthenium Porphyrin-Functionalized Carbon Nanotubes and Graphene for Levulinic Ester Transformations into γ-Valerolactone and Pyrrolidone Derivatives. ACS Omega 2(7):3228–3240. https://doi.org/10.1021/acsomega.7b00427
Martínez JJ, Silva L, Rojas HA, Romanelli GP, Santos LA, Ramalho TC, Brijaldo MH, Passos FB (2017) Reductive Amination of Levulinic Acid to Different Pyrrolidones on Ir/SiO2-SO3H: Elucidation of Reaction Mechanism. Catal Today 296:118–126. https://doi.org/10.1016/j.cattod.2017.08.038
Wang S, Huang H, Bruneau C, Fischmeister C (2017) Selective and Efficient Iridium Catalyst for the Reductive Amination of Levulinic Acid into Pyrrolidones. Chemsuschem 10(21):4150–4154. https://doi.org/10.1002/cssc.201701299
Xu Z, Yan P, Jiang H, Liu K, Zhang ZC (2017) Iridium-Catalyzed Reductive Amination of Levulinic Acid to Pyrrolidinones under H2 in Water. Chin J Chem 35(5):581–585. https://doi.org/10.1002/cjoc.201600726
Chaudhari C, Shiraishi M, Nishida Y, Sato K, Nagaoka K (2020) One-Pot Synthesis of Pyrrolidones from Levulinic Acid and Amines/Nitroarenes/Nitriles over the Ir-PVP Catalyst. Green Chem 22(22):776–7764. https://doi.org/10.1039/d0gc01725f
Ogiwara Y, Uchiyama T, Sakai N (2016) Reductive Amination/Cyclization of Keto Acids Using a Hydrosilane for Selective Production of Lactams Versus Cyclic Amines by Switching of the Indium Catalyst. Angew Chem Int Ed 128(5):1896–1899. https://doi.org/10.1002/ange.201509465
Xie C, Song J, Wu H, Hu Y, Liu H, Zhang Z, Zhang P, Chen B, Han B (2019) Ambient Reductive Amination of Levulinic Acid to Pyrrolidones over Pt Nanocatalysts on Porous TiO2 Nanosheets. J Am Chem Soc 141(9):4002–4009. https://doi.org/10.1021/jacs.8b13024
Zhang J, Xie B, Wang L, Yi X, Wang C, Wang G, Dai Z, Zheng A, Xiao FS (2017) Zirconium Oxide Supported Palladium Nanoparticles as a Highly Efficient Catalyst in the Hydrogenation-Amination of Levulinic Acid to Pyrrolidones. ChemCatChem 9(14):2661–2667. https://doi.org/10.1002/cctc.201600739
Chakrabortty S, Zheng S, Kallmeier F, Baráth E, Tin S, de Vries JG (2023) Ru-Catalyzed Direct Asymmetric Reductive Amination of Bio-Based Levulinic Acid and Ester for the Synthesis of Chiral Pyrrolidinone. Chemsuschem 16(9):e202202353. https://doi.org/10.1002/cssc.202202353
Meruane-Anich J, Araya-Lopez C, Santiago R, Escalona N, Canales RI (2024) Sustainable Synthesis and Extraction of 5-Methyl-N-phenyl-2-pyrrolidone Produced via Reductive Amination of Levulinic Acid. Sep Purif Technol 338:126531. https://doi.org/10.1016/j.seppur.2024.126531
Saini K, Kumar S, Kaur R, Babu SA, Saravanamurugan S (2023) Accelerated H2 Activation over Pt/M-ZrO2 for the Reductive Amination of Levulinic Acid Esters under Benign Conditions. Catal Sci Technol 13(6):1666–1676. https://doi.org/10.1039/d2cy01550a
Li S, Liu X, Guo Y, Wang Y (2024) Room Temperature Synthesis of N-substituted Pyrrolidone from Levulinic Acid and Nitrobenzene via A Tandem Hydrogenation and Reductive Amination Process. J Catal 429:115191. https://doi.org/10.1016/j.jcat.2023.115191
Louven Y, Haus MO, Konrad M, Hofmann JP, Palkovits R (2020) Efficient Palladium Catalysis for the Upgrading of Itaconic and Levulinic Acid to 2-Pyrrolidones Followed by Their Vinylation into Value-Added Monomers. Green Chem 22(14):4532–4540. https://doi.org/10.1039/D0GC01043J
Muzzio M, Yu C, Lin H, Yom T, Boga DA, Xi Z, Li N, Yin Z, Li J, Dunn JA, Sun S (2019) Reductive Amination of Ethyl Levulinate to Pyrrolidones over AuPd Nanoparticles at Ambient Hydrogen Pressure. Green Chem 21(8):1895–1899. https://doi.org/10.1039/C9GC00396G
Vidal JD, Climent MJ, Corma A, Concepcion P, Iborra S (2017) One-Pot Selective Catalytic Synthesis of Pyrrolidone Derivatives from Ethyl Levulinate and Nitro Compounds. Chemsuschem 10(1):119–128. https://doi.org/10.1002/cssc.201601333
Vidal JD, Climent MJ, Concepcion P, Corma A, Iborra S, Sabater MJ (2015) Chemicals from Biomass: Chemoselective Reductive Amination of Ethyl Levulinate with Amines. ACS Catal 5(10):5812–5821. https://doi.org/10.1021/acscatal.5b01113
Li H, Huang F, Gao H, Zhou Z, Hong X, Yi W, Wang S (2023) Calcium(II)-Catalyzed Reductive Amination of Biomass-Derived Keto Acids to Functionalized Lactams under Solvent-Free Conditions. Org Lett 25(14):2504–2508. https://doi.org/10.1021/acs.orglett.3c00676
Touchy AS, Hakim Siddiki SMA, Kon K, Shimizu K (2014) Heterogeneous Pt Catalysts for Reductive Amination of Levulinic Acid to Pyrrolidones. ACS Catal 4(9):3045–3050. https://doi.org/10.1021/cs500757k
Siddiki SMAH, Touchy AS, Bhosale A, Toyao T, Mahara Y, Ohyama J, Satsuma A, Shimizu K (2018) Direct Synthesis of Lactams from Keto Acids, Nitriles, and H2 by Heterogeneous Pt Catalysts. ChamCatChem 10(4):789–795. https://doi.org/10.1002/cctc.201701355
Metzker G, Dias RMP, Burtoloso ACB (2018) Iron-Catalyzed Reductive Amination from Levulinic and Formic Acid Aqueous Solutions: An Approach for the Selective Production of Pyrrolidones in Biorefinery Facilities. ChemistrySelect 3(2):368–372. https://doi.org/10.1002/slct.201702580
Boosa V, Varimalla S, Dumpalapally M, Gutta N, Velisoju VK, Nama N, Akula V (2021) Influence of Brønsted Acid Sites on Chemoselective Synthesis of Pyrrolidones over H-ZSM-5 Supported Copper Catalyst. Appl Catal B: Environ 292:120177. https://doi.org/10.1016/j.apcatb.2021.120177
Cao P, Ma T, Zhang H, Yin G, Zhao J, Zhang Y (2018) Conversion of Levulinic Acid to N-Substituted Pyrrolidinones over a Nonnoble Bimetallic Catalyst Cu15Pr3/Al2O3. Catal Commun 116:85–90. https://doi.org/10.1016/j.catcom.2018.07.016
Pan Y, Luo Z, Yang J, Han J, Yang J, Yao Z, Xu L, Wang P, Shi Q (2022) Cobalt-Catalyzed Selective Transformation of Levulinic Acid and Amines into Pyrrolidines and Pyrrolidinones using Hydrogen. Adv Synth Catal 364(16):2830–2836. https://doi.org/10.1002/adsc.202200578
Gao J, Feng L, Ma R, Su B, Alenad AM, Liu Y, Beller M, Jagadeesh RV (2022) Cobalt Single-Atom Catalysts for Domino Reductive Amination and Amidation of Levulinic Acid and Related Molecules to N-Heterocycles. Chem Catal 2(1):178–194. https://doi.org/10.1016/j.checat.2021.12.009
Gao G, Sun P, Li Y, Wang F, Zhao Z, Qin Y, Li F (2017) Highly Stable Porous-Carbon-Coated Ni Catalysts for the Reductive Amination of Levulinic Acid via an Unconventional Pathway. ACS Catal 7(8):4927–4935. https://doi.org/10.1021/acscatal.7b01786
Amarasekara AS, Lawrence YM (2018) Raney-Ni Catalyzed Conversion of levulinic Acid to 5-Methyl-2-pyrrolidone Using Ammonium Formate as the H and N Source. Tetrahedron Lett 59(19):1832–1835. https://doi.org/10.1016/j.tetlet.2018.03.087
Wang Y, Nuzhdin AL, Shamanaev IV, Bukhtiyarova GA (2021) Flow Synthesis of N-Alkyl-5-methyl-2-pyrrolidones over Ni2P/SiO2 Catalyst. Mol Catal 515:111884. https://doi.org/10.1016/j.mcat.2021.111884
Wang Y, Chen M, Zhang K, Wu H, Wang J, Cheng Y, Liu Y, Wei Z (2023) CoNi Alloy Nanoparticles Confined in N-Doped Porous Carbon as an Efficient and Versatile Catalyst for Reductive Amination of Levulinic Acid/Esters to N -Substituted Pyrrolidones. ACS Catal 13(19):12601–12616. https://doi.org/10.1021/acscatal.3c03854
Guo J, Li F, Chu Y, Zou P, Li C (2023) Conversion of Levulinic Acid and its Esters to 1,5-dimethyl-2-Pyrrolidone over a Nonnoble Metallic Ni@CeOx Catalyst. Chemsuschem 16(21):e202300754. https://doi.org/10.1002/cssc.202300754
Wu C, Zhang H, Yu B, Chen Y, Ke Z, Guo S, Liu Z (2017) Lactate-Based Ionic Liquid Catalyzed Reductive Amination/Cyclization of Keto Acids under Mild Conditions: A Metal-Free Route to Synthesize Lactams. ACS Catal 7(11):7772–7776. https://doi.org/10.1021/acscatal.7b02231
Wang T, Xu H, He J, Zhang Y (2020) Investigation Towards the Reductive Amination of Levulinic Acid by B(C6F5)3/Hydrosilane System. Tetrahedron 76(36):131394. https://doi.org/10.1016/j.tet.2020.131394
Wei Y, Wang C, Jiang X, Xue D, Liu Z, Xiao J (2014) Catalyst-Free Transformation of Levulinic Acid into Pyrrolidinones with Formic Acid. Green Chem 16(3):1093–1096. https://doi.org/10.1039/C3GC42125B
Zhao W, Meier S, Yang S, Riisager A (2021) Insights into Ammonia Borane-Enabled Green Synthesis of N-Substituted Lactams from Biomass-Derived Keto Acids and Amines. ACS Sustainable Chem Eng 9(12):4377–4382. https://doi.org/10.1021/acssuschemeng.1c00211
Li H, Wu H, Zhang H, Su Y, Yang S, Hensen EJM (2019) A Facile Direct Route to N-(Un)substituted Lactams by Cycloamination of Oxocarboxylic Acids without External Hydrogen. Chemsuschem 12(16):3778–3784. https://doi.org/10.1002/cssc.201901780
Ledoux A, Sandjong Kuigwa L, Framery E, Andrioletti B (2015) A Highly Sustainable Route to Pyrrolidone Derivatives-Direct Access to Biosourced Solvents. Green Chem 17(6):3251–3254. https://doi.org/10.1039/C5GC00417A
Wu H, Yu Z, Li Y, Xu Y, Li H, Yang S (2020) Hot Water-Promoted Catalyst-Free Reductive Cycloamination of (Bio-)Keto Acids with HCOONH4 toward Cyclic Amides. J Supercrit Fluid 157:104698. https://doi.org/10.1016/j.supflu.2019.104698
Wu H, Dai W, Saravanamurugan S, Li H, Yang S (2019) Quasi-Catalytic Approach to N-Unprotected Lactams via Transfer Hydro-amination/Cyclization of Biobased Keto Acids. ACS Sustainable Chem Eng 7(12):10207–10213. https://doi.org/10.1021/acssuschemeng.9b00412
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 22172127 and U23A20123), the Fundamental Research Program of Qingyuan Innovation Laboratory (grant number 00522004).
Funding
Supported by the National Natural Science Foundation of China (Nos. 22172127 and U23A20123), the Fundamental Research Program of Qingyuan Innovation Laboratory (grant number 00522004).
Author information
Authors and Affiliations
Contributions
YLF, WGY, XDS, and ZY collected and analyzed the date, YLF and DWP conceived and designed analysis, and wrote the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All authors certify that the manuscript is original and not submitted to other journals.
Consent for publication
All authors agree with the manuscript to be submitted for publication.
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, L., Wang, G., Xiang, D. et al. Reductive amination of bio-platform molecules to nitrogen-containing chemicals. Carb Neutrality 3, 24 (2024). https://doi.org/10.1007/s43979-024-00098-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43979-024-00098-4