Abstract
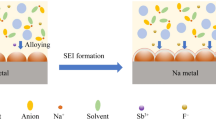
The implementation of sodium metal batteries (SMBs) is known for their low cost and high energy density. However, a major concern in SMBs is the formation of dendrites on the Na metal anode, which can potentially cause short circuits and compromise safety. Herein, to address this issue, we propose a novel approach to create a protective layer by decorating Na surface with NaI particles. This protective layer exhibits a high Young’s modulus and excellent sodium ion transference ability. As a result, the lifespan of the Na/NaI||Na/NaI cell is significantly extended to 850 h at 0.5 mA cm−2/1 mAh cm−2. Furthermore, when the Na/NaI anode is combined with a Na3V2(PO4)3 (NVP) cathode, the full cell retains 83 mAh g−1 (approximately 94% of its initial capacity) even after 1500 cycles at 5 C. Overall, this work presents a simple and effective method for establishing a protective layer on the Na surface, thereby enabling the realization of long lifespan and stable SMBs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The demand for energy has rapidly increased due to the burgeoning development of society. Fossil fuels have been widespread used in industry and daily life [1,2,3], but the limited resources and inevitable emissions of carbon dioxide push people to seek alternative sources of clean energy, such as solar energy, wind energy and tidal energy. It is worth noting that the intermittent nature of clean energies poses challenges in terms of storage [4,5,6,7,8,9,10,11]. Although the application of lithium-ion batteries (LIBs) helped resolve the aforementioned dilemma, the gradual rising cost of LIBs is sustainable for long-term market demands [12,13,14,15,16,17,18,19]. As an alternative, the replacement of lithium with sodium presents several advantages. Sodium not only displays a high theoretical specific capacity (1166 mAh g−1) and negative equilibrium potential (− 2.71 V vs. SHE), but it also possesses economic benefits due to its abundant resources and low price [20,21,22,23]. In addition, replacing the Cu current collector with Al for sodium batteries can further reduce the cost of cell. Nevertheless, the highly reactive Na inclines to form an uneven and fragile solid electrolyte interface (SEI) on Na surface, which cannot withstand the expansion of Na and induce the rupture of SEI during cycling process, resulting in the accumulation of Na and the growth of Na dendrites, thereby increasing the risks of thermal runaway [24,25,26,27,28,29,30,31].
In hopes of alleviating the side-reactions and stemming the growth of Na dendrites, the conception of building a protective layer on Na surface has been proposed. For instance, Cui et al. developed a SEI containing Na2O and NaF through the spontaneous reaction between Na and FEC additives. The introduction of Na2O and NaF not only improves the chemical stability of SEI, but also mitigates Na dendrites to achieve uniform deposition [26]. Huang et al. built a SEI layer of Na-Sn and NaCl by SnCl2 additive, which provides a superiority in protecting Na metal from the corrosion of electrolyte and accelerating Na ion transport. As a result, the lifespan of modified sodium metal batteries (SMBs) was prolonged to 450 h under 0.5 mA cm−2/1 mAh cm−2 when tested in carbonate electrolytes [32]. Moreover, Archer et al. utilized C3H7Br as an additive to create a NaBr layer on Na surface by a spontaneous reaction with electrolyte. The assembled cell, with a NaBr-protected electrode, could operate for around 250 h at 0.5 mA cm−2 (or 1 mA cm−2). The designed SEI of NaBr is helpful to reduce the diffusion barrier and enhance the cycling stability of SMBs. Considering the aforementioned research, it has been found that the involvement of inorganic NaX (X = F, Cl and Br) is helpful to protect the sodium surface from electrolyte corrosion. However, it should be noted that the highly reactive Na has a tendency to react with organic solvents to yield many organic components in SEI, which show low rigidity and are unable to meet the requirements for long-term cycling (e.g., > 600 h).
An ideal SEI should possess three key characteristics, i.e., greater mechanical strength, remarkable ion transference ability, and less reactivity [29, 33,34,35]. The greater mechanical strength of the SEI helps to mitigate dendrites propagation, thereby improving the durability and safety of the cell. Additionally, the excellent ion transportation capability and lower activity play an essential role in realizing uniform deposition and preventing harmful reactions between Na and electrolyte. To achieve these goals, the idea of building an artificial SEI layer with high Young’s modulus is a wonderful way of suppressing the Na dendrite growth. Furthermore, the employment of an artificial SEI has a positive impact on reducing the chemical reactivity of Na and minimizing the side reactions between Na and electrolyte.
As mentioned in current research, NaI displays a small diffusion barrier, which is beneficial to boost homogeneous Na deposition [36]. Based on the consideration of high mechanical strength and fast ion transportation ability. Herein, we proposed to develop an artificial SEI layer of NaI, which possesses a high Young’s modulus and fast kinetic, aiming to address the issue of poor stability of SMBs. As expected, the lifespan of the NaI-modified electrode is improved at both low current density (0.5 mA cm−2) and high current density (1 mA cm−2). Furthermore, the full cell demonstrates favorable rate performance and delivers high capacity of 41 mAh g−1 at 30 C. This work sheds light on the importance of designing an artificial SEI on the Na surface, rather than focusing solely on electrolyte optimization, to achieve a stable anode for high-performance SMBs.
2 Results and discussion
To enhance the passivation of the interface between Na and the electrolyte, a protective layer of artificial NaI was constructed on the Na surface, and the detailed preparation process is illustrated in Figure S1. The fabrication process offers the advantage of efficient and easy control, making it suitable for large-scale promotion. Upon decorating NaI particles (Figures S2 and S3) on the surface of Na, the surface color undergoes a noticeable transformation to silver white (Figure S4). To investigate whether the surface chemical composition was altered after decorating NaI on Na surface, X-ray diffraction (XRD) analysis was conducted. As displayed in Fig. 1a, the crystal structure of NaI and Na remains intact. Moreover, the surface X-ray photoelectron spectroscopy (XPS) analysis of the Na/NaI electrode reveals that the peaks at binding energies of 631.2 and 619.7 eV correspond to 3d3/2 and 3d5/2 states of iodine ion, respectively (Fig. 1b) [37]. The peaks observed in the Na 1s spectrum at 1071 and 1072.8 eV are related to Na metal and NaI, respectively (Fig. 1c) [38, 39]. These results indicate that a protective layer of NaI was successfully formed on the Na surface, and importantly, no new substances were generated during the coating process, suggesting that NaI particles maintain their individuality when in contact with Na metal. The scanning electron microscopy (SEM) analysis illustrates that the surface of the Na/NaI electrode is smooth (Fig. 1d), and the enlarged image reveals that the protective layer is made from the accumulation of particles (Fig. 1e). Element mapping, as exhibited in Figure S5, demonstrates that Na and I elements are homogeneous distributed on the Na surface. Moreover, the side-view observation of the Na/NaI electrode indicates that the NaI protective layer adheres closely to the Na surface, and the thickness of such layer is determined to be about 85 μm (Fig. 1f).
The protective effect of a NaI layer on the Na plating /stripping was investigated through fixed capacity measurements with different current densities using 2032-coin cells. As shown in Fig. 2a, the voltage curves of Na/NaI symmetric cell displays a low overpotential of 33 mV over a period of 400 h under the condition of 0.5 mA cm−2/1 mAh cm−2. Then, the overpotential undergoes a gradual increase in subsequent cycles, but remains below 80 mV (Figure S6). In contrast, the overpotential of the symmetric pristine Na cell remains consistently high (around 90 mV) until it reaches a short circuit after approximately 200 h. Thanks to the introduction of NaI electrode, the lifespan of Na/NaI||Na/NaI extends to 850 h, which is far greater than that of pristine Na symmetric cell. Moreover, the difference in overpotential between the pristine Na and Na/NaI symmetric cells has shown notable progress when the current density reached 1 mA cm−2 (Fig. 2b). From the voltage profiles, it is evident that the overpotential of the pristine Na electrode is up to 180 mV and experiences a sharp decline after operating for 65 h. Interestingly, the local voltage profiles displayed in Figure S7 illustrates that the Na/NaI symmetric cell possesses impressive durability for over 430 h with a small overpotential of ~ 80 mV, and the curves remains relatively flat without significant fluctuations. Additionally, the Na/NaI symmetric cell displays remarkable rate performance, as illustrated in Fig. 2c. As the rate increases from 1 to 2 and 3 mA cm−2, its overpotential remains consistently low at 160 and 210 mV, respectively. Moreover, its limiting current density can arrive 8 mA cm−2 (Figure S8). In contrast, the pristine Na symmetric cell shows a higher overpotential of 183 and 270 mV under the same conditions (Figure S9). Furthermore, the Na/NaI symmetric cell exhibits a small nucleation overpotential of 30 mV at the current density of 0.5 mA cm−2, which is much lower than that of the pristine Na symmetric cell (70 mV).
To better understand the regulation mechanism of the Na/NaI electrode, the nucleation behavior and surface morphology change were further examined at a current density of 1 mA cm-2 with various sodium plating/stripping capacities. Figure 3a exhibits that the nucleation overpotential of the pristine Na symmetric cell (190 mV) is nearly five times higher than that of the Na/NaI symmetric cell (40 mV) in the initial stage. This indicates that the employment of a Na/NaI protection layer has a positive effect on regulating homogeneous Na deposition and retarding harmful parasitic reactions at high current density. On the other hand, at different electrochemical conditions as illustrated in Fig. 3b, the surface morphology of electrodes was analyzed. Figure 3c-e and Figure S10 depict the surface feature of the electrode with a NaI protective layer under various sodium plating and stripping conditions. Its surface morphology remains flat and well-preserved after charging/discharging. In contrast, the surface of the pristine Na electrode is rough (Fig. 3f and Figure S11a), which tends to cause the enrichment of Na and accelerate the development of dendrites. As shown in Fig. 3g and Figure S11b, the surface of pristine Na indeed undergoes a significant change, with a large amount of sharp Na dendrites visible. And the problem of dendrite propagation becomes more entrenched after the sodium stripping (Fig. 3h and Figure S11c). Based on the analysis of the surface morphology change, it can be concluded that the durability of the Na/NaI electrode is superior to that of the pristine Na electrode, which not only demonstrates the ability to withstand the huge volume expansion of Na, but also plays an important role in suppressing dendrite development. Furthermore, the crystal stability of the Na/NaI electrode was confirmed through XRD measurement. The presence of the NaI peak and the absence of impurity peaks in the patterns obtained at a plating of 1 mAh cm-2 and a stripping of 1 mAh cm-2 (Figure S12). Moreover, the SEM, XRD and XPS analysis of long cycled Na/NaI electrode further reveals the exceptional stability throughout the entire process (Figures S13 and S14).
a The nucleation overpotential of Na/NaI and pristine Na symmetric cells at 1 mA cm−2. b The voltage profiles of Na deposition and stripping for symmetric Na/NaI and pristine Na cells during the first cycle. SEM images of (c-e) Na/NaI and (f–h) pristine Na electrodes collected at Na plating of (c, f) 0 mAh cm−2 and (d, g) 1 mAh cm−2, as well as (e, h) Na stripping of 1 mAh cm−2
To further probe the role of the NaI protective layer in mitigating Na dendrites, AFM measurement was conducted to assess its strength. As displayed in Fig. 4a, the Young’s modulus of the Na/NaI electrode can up to 12.5 GPa, which is much higher than that reported in previous studies for pristine and artificial Na electrodes (Table S1) [40], indicating that the introduction of the NaI protective layer can enhance the mechanical strength of the electrode and further suppress the propagation of dendrites. In addition, the deposition behavior of Na on different electrode surfaces can be accurately simulated using finite element simulation in CMSOL multiphysics (Fig. 4b). It is evident that, for the SEI of pristine Na electrode, the Na+ concentration varies significantly at different depths, leading to an exacerbation of heterogeneous deposition. In contrast, the Na+ concentration in the Na/NaI SEI is relatively low and gradually decreases without obvious fluctuations, which illustrates that the addition of a NaI layer facilitates the transfer of Na+. Moreover, Tafel plot analysis was employed to verify the promotion of charge-transfer kinetics with the NaI layer. As showed in Fig. 4c, the exchange current density (j0) of pristine Na is 0.25 mA cm-2, which is lower than that of Na/NaI (0.68 mA cm-2). The charge transfer impedance of the Na/NaI symmetric cell at different temperatures displays smaller values (Figure S15). The fitting results, based on ln (1/Rct) versus 1000/T, reveal that the activation energy (Ea) of pristine Na and Na/NaI symmetric cells after 3 cycles is 44 and 33 KJ mol-1, respectively (Fig. 4d). The above results clearly reveal that the beneficial effect of introducing a NaI protective layer on the transport of Na ions. Figure 4e provides a clearly in-situ optical observation of sodium deposition behaviors of pristine Na and Na/NaI electrodes. Initially, both of them appear flat. However, as the plating time increases to 10 min, noticeable differences start to emerge. Tiny particles begin to form on the Na surface and gradually grow over time. If left unchecked, these particles can lead to the formation of Na dendrites, which can negatively impact the lifespan of batteries in practical applications. In contrast, with the protection by the NaI layer, the surface remains flat without severe dendrites even after 60 minutes of plating (Figure S16).
a Young’s modulus of the Na/NaI electrode. b A finite element simulation of Na+ concentration for the pristine Na and Na/NaI electrodes. c Tafel plots and (d) activation energy of the pristine Na and Na/NaI electrodes measured after 3 cycles at 1 mA cm−2/1 mAh cm−2. e In-situ optical observation of Na deposition on the bare Na and Na/NaI electrodes
The feasibility of using a Na/NaI electrode in practical applications can be confirmed by assembling a full cell with Na3V2(PO4)3 (NVP) cathode. From Fig. 5a, it can be observed that the difference between NVP||Na/NaI and NVP||pristine Na cells increases as the rate gradually intensifies. Although the capacity of NVP||pristine Na is higher than that of NVP||Na/NaI at current densities of 1, 2 and 5 C, the capacity of NVP||Na/NaI surpasses that of NVP||pristine Na at 10 C. Furthermore, under 20 C, it remains at 54 mAh g-1 while the capacity of NVP||pristine Na sharply declines to almost zero. Even when the current density is further increased to 30 C, the capacity of NVP||Na/NaI still possesses 41 mAh g-1. In addition, the corresponding voltage-capacity curves, as displayed in Figure S17, exhibit that the polarization of NVP||Na/NaI is much less than that of NVP||pristine Na, which can be accounted for its remarkable rapid dynamic ability. Moreover, the resistance of NVP||Na/NaI before (20 Ω) and after (52 Ω) cycling is both much lower than that of NVP||Na (90 and 269 Ω), further demonstrating superior electrochemical kinetics.
The decrease of charge transfer resistance suggests that the introduction of NaI promotes the transport of Na+. Inspired by the remarkable rate performance of NVP||Na/NaI, the cycling stability of NVP||Na/NaI at high rate was further investigated. As expected, NVP||Na/NaI can operate for 1500 cycles with a high-capacity retention rate of 94% at 5 C (Fig. 5b). Their voltage-capacity curves, as exhibited in Fig. 5c and Figure S19, reveal that the capacity fluctuation for NVP||Na/NaI is minimal, while the capacity of NVP||pristine Na exhibits a greater change at different cycles, and even declines close to zero during the 700th cycle. Compared to the pristine Na full cell, the CE of NVP||Na/NaI also maintains flat at around 100% even after 1500 cycles (Figure S20). Therefore, the exceptional rate performance and durability of NVP||Pristine Na suggest that the addition of a NaI protective layer can be considered as a potential anode for achieving better performance in SMBs.
3 Conclusion
In this study, a convenient method for decorating NaI particles on Na surface is developed to create a protective layer. Through AFM, infinite element simulation and electrochemical measurement, it is evident that the sodium anode coated with the NaI protective layer acts as a barrier for dendrite formation and facilitates the transfer of Na ions. As a result, the symmetric cell with NaI as the covering layer exhibits an increased lifespan of 850 h at 0.5 mA cm-2 and 430 h at a high current density of 1 mA cm-2. Furthermore, the NVP||Na/NaI full cell remains a favorable capacity of 83 mAh g-1 after 1500 cycles at 5 C. This work successfully demonstrates the creation of a NaI coating layer with high mechanical strength and low diffusion barrier NaI coating layer, leading to dendrite-free and durable SMBs.
Availability of data and materials
Data and materials are available upon reasonable request.
Abbreviations
- LIBs:
-
Lithium-ion batteries
- SMBs:
-
Sodium metal batteries
- SHE:
-
Standard hydrogen electrode
- SEI:
-
Solid electrolyte interface
- NVP:
-
Na3V2(PO4)3
- XRD:
-
X-ray diffraction
- XPS:
-
X-ray photoelectron spectroscopy
- SEM:
-
Scanning electron microscopy
- AFM:
-
Atomic force microscopy
References
Zhao C, Ju S, Xue Y, Ren T, Ji Y, Chen X (2022) China’s energy transitions for carbon neutrality: challenges and opportunities. Carb Neutrality 1(7):7. https://doi.org/10.1007/s43979-022-00010-y
Hu Y, Yin H, Wang F (2022) The dilemma for China’s national carbon trading market: minimizing carbon abatement costs or maximizing net social benefits? Carb Neutrality 1(22). https://doi.org/10.1007/s43979-022-00023-7
Wang H, Matios E, Luo J, Li W (2020) Combining theories and experiments to understand the sodium nucleation behavior towards safe sodium metal batteries. Chem Soc Rev 49:3783–3805. https://doi.org/10.1039/D0CS00033G
Song R, Ge Y, Wang B, Lv Q, Wang F, Ruan T, Wang D, Dou S, Liu H (2019) A new reflowing strategy based on lithiophilic substrates towards smooth and stable lithium metal anodes. J Mater Chemist A 7:18126–18134. https://doi.org/10.1039/C9TA05503G
Chen C, Jiao F, Lu B, Liu T, Liu Q, Jin H (2023) Challenges and perspectives for solar fuel production from water/carbon dioxide with thermochemical cycles. Carb Neutrality 2(9). https://doi.org/10.1007/s43979-023-00048-6
Lai X, Peng J, Cheng Q, Tomsia AP, Zhao G, Liu L, Zou G, Song Y, Jiang L, Li M (2021) Cover picture: bioinspired color switchable photonic crystal silicone elastomer kirigami. Angew Chem Int Ed 60:14197–14197. https://doi.org/10.1002/anie.202105322
Zhang X, Feng L, Li X, Xu Y, Wang L, Chen H (2023) Economic evaluation of energy storage integrated with wind power. Carb Neutrality 2(16). https://doi.org/10.1007/s43979-023-00054-8
Zhao R, Sun N, Xu B (2021) Recent advances in heterostructured carbon materials as anodes for sodium-ion batteries. Small Struct 2:2100132. https://doi.org/10.1002/sstr.202100132
Ferrari S, Falco M, Muñoz-García AB, Bonomo M, Brutti S, Pavone M, Gerbaldi C (2021) Solid-state post li metal ion batteries: a sustainable forthcoming reality? Adv Energy Mater 11:2100785. https://doi.org/10.1002/aenm.202100785
Obama B (2017) The irreversible momentum of clean energy. Science 355:126–129. https://doi.org/10.1126/science.aam6284
Tang C, Min Y, Chen C, Xu W, Xu L (2019) Potential applications of heterostructures of TMDs with MXenes in sodium-Ion and Na–O2 batteries. Nano Lett 19:5577–5586. https://doi.org/10.1021/acs.nanolett.9b02115
Yang J-L, Zhao X-X, Li W-H, Liang H-J, Gu Z-Y, Liu Y, Du M, Wu X-L (2022) Advanced cathode for dual-ion batteries: Waste-to-wealth reuse of spent graphite from lithium-ion batteries. eScience. 2:95–101. https://doi.org/10.1016/j.esci.2021.11.001
Gao H, Grundish NS, Zhao Y, Zhou A, Goodenough JB (2021) Formation of stable interphase of polymer-in-salt electrolyte in all-solid-state lithium batteries. Energy Mater Adv 2021(2021):1932952. https://doi.org/10.34133/2021/1932952
Yu H, Chen D, Zhang T, Fu M, Cai J, Wei W, Ji X, Chen Y, Chen L (2022) Insight on the double-edged sword role of water molecules in the anode of aqueous zinc-ion batteries. Small Structures 3:2200143. https://doi.org/10.1002/sstr.202200143
Zhang Q, Shen X, Zhou Q, Li K, Ding F, Lu Y, Zhao J, Chen L, Hu YS (2022) Large scale one-pot synthesis of monodispersed Na3(VOPO4)2F cathode for na-ion batteries. Energy Mater Adv 2022(2022). https://doi.org/10.34133/2022/9828020
Xia X, Chen K, Xu S, Yao Y, Liu L, Xu C, Rui X, Yu Y (2023) Robust artificial interlayer with high ionic conductivity and mechanical strength toward long-life na-metal batteries. Small Sci 3:2300038. https://doi.org/10.1002/smsc.202300038
Yang Z, Zhang J, Kintner-Meyer MCW, Lu X, Choi D, Lemmon JP, Liu J (2011) Electrochemical energy storage for green grid. Chem Rev 111:3577–3613. https://doi.org/10.1021/cr100290v
Zhang X, Chen S, Zhu J, Gao Y (2023) A critical review of thermal runaway prediction and early-warning methods for lithium-ion batteries. Energy Mater Adv 4:0008. https://doi.org/10.34133/energymatadv.0008
Li M, Li Y, Cu Q, Li Y, Li H, Li Z, Li M, Liao H, Li G, Li G, Wang X (2023) Hollow and hierarchical CuCo-LDH nano catalyst for boosting sulfur electrochemistry in Li-S batteries. Energy Mater Adv 4:0032. https://doi.org/10.34133/energymatadv.0032
Xia X, Xu S, Tang F, Yao Y, Wang L, Liu L, He S, Yang Y, Sun W, Xu C, Feng Y, Pan H, Rui X, Yu Y (2023) A multifunctional interphase layer enabling superior sodium-metal batteries under ambient temperature and −40 °C. Adv Mater 35:2209511. https://doi.org/10.1002/adma.202209511
Jiang Y, Yang Y, Ling F, Lu G, Huang F, Tao X, Wu S, Cheng X, Liu F, Li D, Yang H, Yao Y, Shi P, Chen Q, Rui X, Yu Y (2022) Artificial heterogeneous interphase layer with boosted ion affinity and diffusion for Na/K-metal batteries. Adv Mater 34:2109439. https://doi.org/10.1002/adma.202109439
Lv X, Tang F, Xu S, Yao Y, Yuan Z, Liu L, He S, Yang Y, Sun W, Pan H, Rui X, Yu Y (2023) Construction of inorganic/organic hybrid layer for stable Na metal anode operated under wide temperatures. Small 19:2300215. https://doi.org/10.1002/smll.202300215
Xia X, Yang Y, Chen K, Xu S, Tang F, Liu L, Xu C, Rui X (2023) Enhancing interfacial strength and wettability for wide-temperature sodium metal batteries. Small 19:2300907. https://doi.org/10.1002/smll.202300907
Tarascon J-M, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367. https://doi.org/10.1038/35104644
Zhao Y, Liu B, Yi Y, Lian X, Wang M, Li S, Yang X, Sun J (2022) An anode-free potassium-metal battery enabled by a directly grown graphene-modulated aluminum current collector. Adv Mater 34:2202902. https://doi.org/10.1002/adma.202202902
Seh ZW, Sun J, Sun Y, Cui Y (2015) A highly reversible room-temperature sodium metal anode. ACS Cent Sci 1:449–455. https://doi.org/10.1021/acscentsci.5b00328
Zheng Y, Pan Q, Clites M, Byles BW, Pomerantseva E, Li CY (2018) High-capacity all-solid-state sodium metal battery with hybrid polymer electrolytes. Adv Energy Mater 8:1801885. https://doi.org/10.1002/aenm.201801885
Zhu X, Wang Y, Wang W, Wu K, Zhu M, Wang G, Xu G, Wu M, Liu H-K, Dou S-X, Wu C (2022) Stable sodium metal anodes enabled by an in-situ generated mixed-ion/electron-conducting interface. Chem Eng J 446:136917. https://doi.org/10.1016/j.cej.2022.136917
Liu W, Liu P, Mitlin D (2020) Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv Energy Mater 10:2002297. https://doi.org/10.1002/aenm.202002297
Cao K, Ma Q, Tietz F, Xu BB, Yan M, Jiang Y (2021) A robust, highly reversible, mixed conducting sodium metal anode. Sci Bull 66:179–186. https://doi.org/10.1016/j.scib.2020.06.005
Wu Y, Wu L, Wu S, Yao Y, Feng Y, Yu Y (2021) Status and challenges of cathode materials for room-temperature sodium-sulfur batteries. Small Sci 1:2100059. https://doi.org/10.1002/smsc.202100059
Zheng X, Fu H, Hu C, Xu H, Huang Y, Wen J, Sun H, Luo W, Huang Y (2019) Toward a stable sodium metal anode in carbonate electrolyte: a compact, inorganic alloy interface. J Phys Chem Lett 10:707–714. https://doi.org/10.1021/acs.jpclett.8b03536
Wang H, Wang C, Matios E, Li W (2018) Facile stabilization of the sodium metal anode with additives: unexpected key role of sodium polysulfide and adverse effect of sodium nitrate. Angew Chem Int Ed 57:7734–7737. https://doi.org/10.1002/anie.201801818
Bao C, Wang B, Liu P, Wu H, Zhou Y, Wang D, Liu H, Dou S (2020) Solid electrolyte interphases on sodium metal anodes. Adv Func Mater 30:2004891. https://doi.org/10.1002/adfm.202004891
Wang T, Hua Y, Xu Z, Yu JS (2022) Recent advanced development of artificial interphase engineering for stable sodium metal anodes. Small 18:2102250. https://doi.org/10.1002/smll.202102250
Tian H, Shao H, Chen Y, Fang X, Xiong P, Sun B, Notten PHL, Wang G (2019) Ultra-stable sodium metal-iodine batteries enabled by an in-situ solid electrolyte interphase. Nano Energy 57:692–702. https://doi.org/10.1016/j.nanoen.2018.12.084
Xu J, Chen J, Ao Y, Wang P (2021) 0D/1D AgI/MoO3 Z-scheme heterojunction photocatalyst: highly efficient visible-light-driven photocatalyst for sulfamethoxazole degradation. Chin Chem Lett 32:3226–3230. https://doi.org/10.1016/j.cclet.2021.04.003
L.P. V., R. Vijayaraghavan (2017) Chemical manipulation of oxygen vacancy and antibacterial activity in ZnO. Mater Sci Eng C. 77:1027–1034. https://doi.org/10.1016/j.msec.2017.03.280
Arena A, Marco GD, Lanza M, Patané S, Saitta G (1997) Spectroscopic study of Na–TCNQ in a poly (ethylene oxide) matrix (TCNQ = 7, 7′, 8, and 8′ tetracyanoquinodimethane). J Mater Res 12:1405–1409. https://doi.org/10.1557/JMR.1997.0191
Yang H, He F, Li M, Huang F, Chen Z, Shi P, Liu F, Jiang Y, He L, Gu M, Yu Y (2021) Design principles of sodium/potassium protection layer for high-power high-energy sodium/potassium-metal batteries in carbonate electrolytes: a case study of Na2Te/K2Te. Adv Mater 33:2106353. https://doi.org/10.1002/adma.202106353
Acknowledgements
Thanks for the support from the Clean Energy and Nanomaterials Research Groups of University of Science and Technology of China and Guangdong University of Technology.
Funding
Open access funding provided by Shanghai Jiao Tong University. This work was supported by the National Natural Science Foundation of China (Grant No. 52222210, 51925207, U1910210, 52161145101, 51872277, 51972067, 51902062, 52002083 and 52302260), the “Transformational Technologies for Clean Energy and Demonstration” Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDA21000000), the National Synchrotron Radiation Laboratory (KY2060000173), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (Grant No. YLU-DNL Fund 2021002), the Fundamental Research Funds for the Central Universities (WK2060140026), and the Natural Science Foundation of Shandong Province (Grant No. ZR2023QB011).
Author information
Authors and Affiliations
Contributions
KZC: Methodology, Data curation, Investigation, Writing – original draft. HYH: Investigation, Data curation, Validation. STX: Data curation, Validation. ZSY: Theoretical calculation. YY: Theoretical calculation. YY: Data curation, Validation. XHZ: Data curation, Validation. XHR: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. YY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors here declare that they have no competing financial interests or personal relationships that could have appeared to influence the reported work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, K., Huang, H., Xu, S. et al. Durable sodium iodide interphase stabilizing sodium metal anodes. Carb Neutrality 3, 7 (2024). https://doi.org/10.1007/s43979-024-00082-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43979-024-00082-y