Abstract
A new technical route of organic matter capture and carbon fixation is proposed in response of the increasingly strict emission standards of volatile organic compounds (VOCs) in petrochemical industry and the Chinese national strategic development goals of carbon peak and carbon neutralization. A closed loop from raw materials to adsorbents for gas treatment can be achieved by two key technical characteristics: (1) construct a new mesoporous adsorbent with complete desorption and regeneration function by carbon nanotubes (CNTs); (2) convert gaseous organic matter which cannot be recycled in liquid/gas state to CNTs. It realizes the resource integration of "turning waste into treasure" and maximizes the carbon emission reduction effect of waste gas treatment process without consuming extra precious fossil fuel, compared with the traditional technologies of VOCs treatments, including combustion or catalytic oxidation. What’s more, the increase in supply of various green electricity is expected to change the current situation of large investment and heavy cost burden of environmental protection technology, and make a great contribution to the national carbon peak and carbon neutrality policy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon neutral is an important issue recognized globally for the sustainable development of human society [1, 2]. The current focus is mainly on the controlled reduction of CO2 in huge amount in many industries from fossil fuels by adopting various new technologies and the compensation from biomass (plants) or the trade with carbon tax [3]. Among many processes producing CO2, the treatment of volatile organic compounds (VOCs) is a special one, which involves low concentration of organics in the gas but huge gross amount of the released, as well as arises the undesirable secondary pollution of photo-chemical smog [4, 5]. For instance, the amount of the released VOCs per year across China is estimated to be around 20 million tons, similar to those amounts of \(\mathrm{S}{O}_{x}\) or \(\mathrm{N}{O}_{x}\). In detail, the emission of CO2 in the treatment of VOCs includes the direct emission in several technical routes (i.e., direct combustion or catalytic degradation [6,7,8]) and the indirect emission (by using fossil fuel or grey electricity as the energy supply) in all technical routes (such as combustion, catalytic degradation, adsorption-desorption, cooling etc.). Big challenges lie in that when trying to suppress the concentration of directly released VOCs, in order to reduce direct emission of CO2 and curb secondary pollution, the indirect CO2 emission from energy consumption would increase drastically due to increasing technical difficulties. From this perspective, the fabrication of a near-zero carbon cycle for the treatment of VOCs remains a great challenge, but of high imperative importance.
Storage of CO2 is a key step of carbon capture, utilization and storage (CCUS) for carbon neutral, but full of technique and economical challenge [9, 10] and limitation of available storing space [11, 12]. In this work, we proposed the carbon fixation of organics (from the adsorption-desorption unit) to production of carbon nanotubes (CNTs) by chemical vapor deposition (CVD) method, in which the organic matters decomposed mainly by transition metal-based catalysts under high temperature to CNTs and H2 [2,3,4,5,6,7,8]. In addition, we performed many studies on the CNT-based adsorbents for the treatment of VOCs and organic-containing water previously [13,14,15,16,17,18]. These allowed us to build a cycle of adsorption-desorption-carbon fixation technology route, in which CNT-based adsorbent was used for efficient adsorption and desorption of organic matter, and the desorbed organic matter was then transformed into CNTs by high-temperature catalyst. By this means, the direct carbon emissions were reduced largely and the carbon element was changed from pollution source to new material resource. In addition, the era of carbon neutral called for the use of green electricity (from hydro, solar, wind and photovoltaics power etc.) as an energy supply for the emission reduction, aiming to reduce the indirect carbon emissions mentioned above [19, 20]. In this case, it allowed us to develop an industrial closed loop based on the exhaust gas. For the quantitative comparison, we presented the comprehensive analysis of different technologies (including direct combustion, catalytic oxidization, adsorption-desorption-combustion, and adsorption-desorption-carbon fixation) for the treatment of VOCs from aromatics storage tank from 3000–65,000 mg/m3 to 12 mg/m3. Their energy consumption and carbon emissions varied significantly. The use of green electricity as energy supply would make a great contribution to reduce carbon emissions because the green electricity generating process would not burn coal or other energy sources and produced no CO2. Furthermore, the combination of our technology and green electricity showed the potential to produce a near carbon-zero carbon emissions cycle, as well as large quantities of valuable carbon products. This evaluation would provide a new insight into the carbon emissions of VOCs treatment and offer a new choice for any chemical processes toward carbon zero.
2 A new closed-loop technology route constructed by CNTs and its outstanding carbon reduction effect
2.1 A new technical route of organic matter capture and carbon fixation
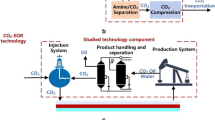
In previous work, we developed a CNT-based adsorbent for revisable adsorption-desorption of organics from VOCs or waste water [13,14,15,16,17,18]. There are also many reports on the conversion of organics into CNTs [21,22,23,24,25,26,27]. At 2020, we, for the first time, integrated these apparatuses together in a skid-mounted equipment for VOCs treatment with the capacity of 900 Nm3/h (Fig. 1a). The equipment mainly included the adsorption tower (packed with CNT-based adsorbent, operating at 5–10 °C), the carbon fixation column (packed with nanosized iron catalyst, operating at 700–800 °C) and the necessary heat exchanger, pump and compressor. The equipment is capable of continuous operation of adsorption-desorption and carbon fixation. According to estimates (S2), at least 6.3 kg of toluene, 1.1 kg of CNT products (about 659 dollars), as well as 0.1 Nm3 CH4 and 2.4 Nm3 H2/900 Nm3 VOCs gas were obtained in the carbon fixation process at low concentration.
Thus, we are able to fabricate a closed loop in which the organic matter was completely captured by a new type of CNT-based adsorbent and converted to CNTs by a new catalytic technology (Fig. 1b). The investment of VOCs treatment would be greatly reduced since the produced CNTs could be used as raw materials to fabricate adsorbent. Most of the carbon in the organic matter in VOCs gas was recycled in liquid form or immobilized in CNT products, reducing the CO2 emission greatly. However, the processes of adsorption-desorption and conversion of gaseous organic matter to CNTs required energy. The carbon fixation process produces methane and hydrogen at the same time as CNTs. Using methane and hydrogen as heating sources can reduce additional energy consumption and further reduce emission of CO2 (Figure S10).
2.2 The performance of adsorption–desorption of VOCs by CNT-based adsorbents
CNTs exhibited the convex pore structure, large mesoporous capacity and high chemical stability (sp2 hybrid carbon), enabling rapid adsorption and desorption for VOCs [13, 15,16,17]. Through the exploration and optimization of the formula and mold methods, we prepared CNT-based adsorbents with high strength, high mesoporous ratio, large specific surface area and high conductivity [14], which behaved rapid desorption rate, high desorption ratio and good recycling performance compared with activated carbon (AC) [13, 15]. In addition, the high thermal and electrical conductivities of CNTs provide an effective heat conduction advantage for cooling and heating operations in the process of adsorption and desorption when used in industrial installations [18, 28, 29].
After the successful adsorption and desorption, macromolecular aromatic substances are the representative refractory substances of microporous AC. The most obvious advantages of the CNT-based adsorbent are excellent cyclic performance and large adsorption capacity. Taking toluene as an example, the adsorption-desorption cycle test of the prepared CNT-based adsorbents was conducted for the gas system of toluene (56,000 mg/m3) and N2. As shown in the cycles in the adsorption isotherm (Fig. 2a), the adsorption has good cyclic performance with an outgas concentration kept below 12 mg/m3. The adsorption rates were all above 99.99%, the desorption rates were near 100%, and the uptake on the adsorbent increased linearly and was far from saturation (Fig. 2b). The outstanding cycle performance provides great possibility of infinite recycling. The far-from-saturation uptake indicate a potential of the adsorbents to work in a primary adsorption process for the treatment of some extremely high concentration gas, providing its usage in another way.
2.3 Conversion of gaseous organics into CNTs
In the process of VOCs treatment, there are two outlets for the concentrated organic matter after desorption. For the macromolecules that are easy to liquefy, they are often recycled in liquid form. Smaller fractions of light hydrocarbons cannot be recycled, which is why many scenarios have to use combustion to convert these light hydrocarbons into CO2 and meet VOCs emission standards. This is not only high in energy consumption, but also very bad for CO2 reduction.
In general, the preparation of CNTs from various carbon sources was very mature [30,31,32,33]. Based on our nano-metal catalysts, CNTs were successfully prepared from the transformation of butane, pentane and other hydrocarbons (such as gasoline, ethanol and acetone, showed in S2) to CNTs efficiently. The technology ensured the total amount of NMHC in tail gas well below the national emission standards. The flameless processing technology was realized.
The conversion of pentane was presented here as an example (Fig. 3a). The convention rate of pentane was 100% during the 6 h reaction. The outlet gas was mainly CH4 and H2 after 1 h reaction. High selectivity for hydrogen and methane was sustained during the entire reaction period (Fig. 3b). Apparently, the amount of NMHC were very few considering the highly efficient conversion with the nanosized metal catalyst in the present work. SEM image of carbon product had characteristic morphology of CNTs (Fig. 3c). TEM image (Fig. 3c inserted) suggested that the as-produced CNTs had an outer diameter of 8–15 nm. The purity (> 90%) (Fig. 3d) was suitable for the fabrication of CNT-based adsorbents, following the method proposed in reference [34].
3 Energy consumption and CO2 emissions of closed-loop technology route compared to traditional VOCs treatment methods
The VOCs in the typical aromatics storage area mentioned above was taken as the object, taking pentane and toluene in nitrogen as the main components, and their concentrations were 1500 mg/m3 and 6500 mg/m3, respectively. Based on the flow and the experimental data, we were able to compare the new adsorption-desorption-carbon fixation process based on CNTs with many conventional VOCs treatment methods (direct combustion process, catalytic oxidation process, and adsorption-desorption-combustion process). The carbon emission, CH4 consumption (CH4 as ancillary fuel for energy supply), air consumption (air as an assisted medium for burning) and electricity consumption (electricity as energy supply in cooling or heating) were calculated based on the mass balance and energy balance (the calculation details of was showed in SI).
As shown in Fig. 4, only the direct combustion used the fuel of CH4 and air in large amount. The preheating of the whole exhaust gas to the burning temperature (800 °C or above) consumed energy in large amount. In comparison, the catalytic oxidation worked at around 300 °C. Only air was needed to oxidize carbon in organic matter. Electricity was considered here for the heating of the system to the oxidation temperature. For the integrated process of adsorption-desorption-combustion, where organics were condensed, the amount of air and electricity (aiming to energy consumption) were both significantly reduced, compared to the direct combustion. Air was needed for the oxidization of organic matter and CH4 which acted as igniter. Electric energy can be used for cooling the adsorption section at -5 °C and for heating at desorption section at 200 °C. And the burning exhausted gases was not used for the heat exchange in desorption section. For the adsorption-desorption-carbon fixation process, electricity was further used in heating for the CNTs growth at 700 °C and the high temperature gas (H2, CH4) was not used as part of energy source for desorption section or carbon fixation section. In this case, the electricity consumption amount was high among these processes. However, if the produced gases (H2, CH4) were burned as energy supply, the amount of electricity would drop drastically from 56.4 kW to 44.1 kW/900 Nm3 VOCs gas, and the air consumption was only 6.5 Nm3/900 Nm3 VOCs gas, far lower than 78.4 Nm3/900 Nm3 VOCs gas in catalytic oxidation, since most of carbon from organics was fixed as CNTs. From the comparison, we would state that the direction combustion processes needed fossil fuel in the most amount, while the adsorption-desorption-carbon fixation process represented the process needing electricity in the most amount. There were plenty of choices to balance the fossil fuel consumption and electricity consumption between these two processes.
The amount of CH4, air, and electricity consumption of different VOCs treatment methods. The process codes were:1#: direct combustion; 2#: catalytic oxidation; 3#: adsorption-desorption-combustion; 4#: adsorption-desorption-carbon fixation; 5#: adsorption-desorption-carbon fixation-supply heat. The same codes were used for all figures
As follows, we calculated the CO2 emission of different processes (Fig. 5). The direct combustion and catalytic oxidation featured the direct emission of CO2 (released by the oxidation of organics). While the adsorption-desorption-combustion process and processes with carbon fixation mainly featured the indirect emission from the energy supply. Conventional treatment methods of VOCs gave high direct CO2 emission which cannot be reduced. Seemingly, the indirect emission in carbon fixation exceeds the direct emission in traditional ways. However, the amount of the indirect emission of CO2 can be decline tremendously if green electricity is applied. In other words, the green potential in carbon fixation process is great, advantage over the other processes. In addition, only the processes with carbon fixation contributed to the valuable solid product (CNTs) with the yield of 1.1 kg/900 Nm3 VOCs gas and valuable gaseous products (H2, CH4), whose value goes far beyond the cost of electricity. Even valuable gaseous products (H2, CH4) are combusted for energy supply, it reduces the indirect emission of CO2 by saving electricity. Direct emission of the adsorption-desorption-carbon fixation process is insignificant. In this case, the present carbon fixation as solid product is better than the CCUS where carbon elements are captured and stored in the form of CO2.
4 The influence of green electricity ratio on CO2 emissions
Considering current electricity supply contain a mixed source of grey electricity from the fossil fuel and green electricity from renewable energy, it is essential to evaluate the possibility of the reduction of indirect emission with the change of electricity ratio. When the green/grey electricity ratio changes, CH4, electric energy, and air consumption by the different processes are stable (Fig. 4), while CO2 emission changes (Fig. 6). The CO2 emission of direct combustion is fixed since it needs no electricity. However, if 100% green electricity is used, the carbon reduction effect of the catalytic oxidation process is obvious. Its CO2 emission of grey electricity will be reduced by 49.9%, and its total CO2 emission is only 27.0% of the direct combustion process. Also, the increase of green electricity ratio will lead to a significant reduction of CO2 emission in the adsorption and desorption processes (Figure S13) in the adsorption-desorption-combustion method, and CO2 emission exists only in the combustion process when green electricity ratio is 100%.
At the same time, the advantages of green electricity are also fully reflected in the process of adsorption-desorption-carbon fixation in which CO2 is mostly emitted indirectly. The CO2 emissions of adsorption, desorption and carbon fixation all tend to be zero when green electricity is used, and the CH4 and H2 produced by this process can be used as raw materials for other chemical process (Figure S14).
In addition, if further increasing the initial concentration of VOCs (for example15000 mg/m3 for pentane and 65,000 mg/m3 for toluene), which represents other tough-treated VOCs, it is able to obtain the associated CO2 emission data (Fig. 7). The CO2 emission trends in the processes of direct combustion and catalytic oxidation are similar. The CO2 emission ratio of high and low concentration is almost steady when direct CO2 emission is dominate (Fig. 7a). In comparison, when indirect CO2 emission is dominate, their CO2 output of high and low concentration decline sharply with the increase of green electricity (Fig. 7b).
It is indicated that whether a process has green potential is the key to CO2 emission reduction in the future. The technology route of adsorption-desorption-carbon fixation which resulted in zero total CO2 emissions is extremely attractive.
From the discussion above, it is clear that a near carbon-zero loop of adsorption-desorption-carbon fixation depends directly on the supply of green electricity. The adsorption-desorption-carbon fixation-supply heat loop would have flexibility when green electricity is in short or unstable supply.
Note that CNTs based adsorbent exhibited long life time. The amount of as-produced CNTs in carbon fixation section for long time operation would far exceed the amount of CNTs used in adsorption section. The situation would result in the significant reduction of the CNTs cost, which, in turn, stimulate the expanded application of CNT-based adsorbent in treatment of VOCs, waste water, and other fields.
5 Conclusions and prospects
The adsorption-desorption-carbon fixation process has great greening potential to effectively reduce the total CO2 emission as well as produce valuable by-products. The CNT products generated in the carbon fixation process can be used as adsorbent raw materials in the adsorption process, and the carbon element closed loop chain is formed with the CNTs based adsorbent as the bridge. By further using CH4 and H2 as the heat suppler, the CO2 emission could be further reduced. As the proportion of green electricity increases, the process of adsorption-desorption-carbon fixation process shows its advantage on carbon emission reduction and becomes a green cycle. Obviously, the concept of adsorption-desorption-carbon fixation process is also applicable to the green and low carbonization treatment of organic wastewater. The trend is to develop some more efficient catalysts which can convert all VOCs to CNTs and hydrogen without methane. This technology embodies the development of the cleanest process and has a bright future. In the context of global carbon emission reduction and China's "dual carbon" era, this process will provide technical support for the reduction of carbon tax/carbon emission trading quota, and further delay the arrival of a negative carbon economy [35].
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- VOCs:
-
Volatile organic compounds
- CVD:
-
Chemical vapor deposition
- CNTs:
-
Carbon nanotubes
- CCUS:
-
Carbon capture, utilization and storage
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscope
References
Gasser T, Kechiar M, Ciais P, Burke EJ, Kleinen T, Zhu D, Huang Y, Ekici A, Obersteiner M (2018) Path-dependent reductions in CO2 emission budgets caused by permafrost carbon release. Nat Geosci 11(11):830–835
Wan L (2021) In-Depth Report on Basic Chemical Industry: Carbon Neutralization in Chemical Industry Series Report II: How Big is The Carbon Emission Pressure in Chemical Industry?, Orient Securities: 22
Giordano P, Watanuki M (2012) A global carbon tax on climate change: policy implications for Latin America, draft preliminary results: 1–37
Zhang X, Gao B, Creamer AE, Cao C, Li Y (2017) Adsorption of VOCs onto engineered carbon materials: a review. J Hazard Mater 338:102–123
Sone H, Fugetsu B, Tsukada T, Endo M (2008) Affinity-based elimination of aromatic VOCs by highly crystalline multi-walled carbon nanotubes. Talanta 74(5):1265–1270
Delimaris D, Ioannides T (2009) VOC oxidation over CuO-CeO2 catalysts prepared by a combustion method. Appl Catal B 89(1):295–302
Zang M, Zhao C, Wang Y, Chen S (2019) A review of recent advances in catalytic combustion of VOCs on perovskite-type catalysts. J Saudi Chem Soc 23(6):645–654
Wang Q, Yeung KL, Bañares MA (2020) Ceria and its related materials for VOC catalytic combustion: a review. Catal Today 356:141–154
Raza A, Glatz G, Gholami R, Mahmoud M, Alafnan S (2022) Carbon mineralization and geological storage of CO2 in basalt: mechanisms and technical challenges. Earth Sci Rev 229:104036
Zhang T, Zhang W, Yang R, Liu Y, Jafari M (2021) CO2 capture and storage monitoring based on remote sensing techniques: a review. J Clean Prod 281:124409
Karvounis P, Blunt MJ (2021) Assessment of CO2 geological storage capacity of saline aquifers under the North Sea. Int J Greenhouse Gas Control 111:103463
Vishal V, Verma Y, Chandra D, Ashok D (2021) A systematic capacity assessment and classification of geologic CO2 storage systems in India. Int J Greenhouse Gas Control 111:103458
Duoni, Di Z, Chen H, Yin Z, Cui C, Qian W, Han M (2018) carbon nanotube-alumina strips as robust, rapid, reversible adsorbents of organics, RSC. Advances 8:10715–10718
Duoni (2018) Research on Rapid, Reversible Carbon Nanotube Adsorbents of Organics in Waste Water Treatment, Department of chemical engineering, Tsinghua University
Chen H, Qian W, Xie Q, Cheng X (2017) Graphene-carbon nanotube hybrids as robust, rapid, reversible adsorbents for organics. Carbon 116:409–414
Yin Z, Duoni, Chen H, Wang J, Qian W, Han M, Wei F (2018) Resilient, mesoporous carbon nanotube-based strips as adsorbents of dilute organics in water. Carbon 132:329–334
Yin Z, Cui C, Chen H, Duoni, Yu X, Qian W (2020) The application of carbon nanotube/graphene-based nanomaterials in wastewater treatment. Small 16:1902301
Yin Z, Shen B, Cui C, Chen H, Duoni, Wang J, Qian W, Zhao L (2021) High-performance graphene/carbon nanotube-based adsorbents for treating diluted o-cresol in water in a pilot-plant scale demo. ACS Appl Mater Interfaces 13(36):43266–43272
Clark CF, Kotchen MJ, Moore MR (2003) Internal and external influences on pro-environmental behavior: participation in a green electricity program. J Environ Psychol 23(3):237–246
Surplus DC, B9 Ships: an Innovative Use of Wind and Bio-Methane Power to End the Reliance on Fossil Fuels. B9 Shipping Ltd, UK: 1–5
Wei F, Zhang Q, Qian W, Yu H, Wang Y, Luo G, Xu G, Wang D (2008) The mass production of carbon nanotubes using a nano-agglomerate fluidized bed reactor: a multiscale space-time analysis. Powder Technol 183(1):10–20
Qian W, Liu T, Wang Z, Wei F, Li Z, Luo G, Li Y (2004) Production of hydrogen and carbon nanotubes from methane decomposition in a two-stage fluidized bed reactor. Appl Catal A 260(2):223–228
Qian W, Liu T, Wei F, Wang Z, Li Y (2004) Enhanced production of carbon nanotubes: combination of catalyst reduction and methane decomposition. Appl Catal A 258(1):121–124
Qian W, Wei F, Liu T, Wang Z, Li Y (2003) What causes the carbon nanotubes collapse in a chemical vapor deposition process. J Chem Phys 118(2):878–882
Qian W, Liu T, Wei F, Wang Z, Wang D, Li Y (2003) Carbon nanotubes with large cores produced by adding sodium carbonate to the catalyst. Carbon 41(13):2683–2686
Qian W, Liu T, Wei F, Wang Z, Yu H (2003) Carbon nanotubes containing iron and molybdenum particles as a catalyst for methane decomposition. Carbon 41(4):846–848
Wen Q, Qian W, Nie J, Cao A, Ning G, Wang Y, Hu L, Zhang Q, Huang J, Wei F (2010) 100 mm long, semiconducting triple-walled carbon nanotubes. Adv Mater 22(16):1867–1871
Baloch KH, Voskanian N, Bronsgeest M, Cumings J (2012) Remote joule heating by a carbon nanotube. Nat Nanotechnol 7(5):316–319
Hu N, Li H, Wei Q, Zhou K, Zhu W, Zhang L, Li S, Ye W, Jiao Z, Luo J, Ma L, Yan Q, Lin C (2020) Continuous diamond-carbon nanotube foams as rapid heat conduction channels in composite phase change materials based on the stable hierarchical structure. Compos B Eng 200:108293
Qian W, Liu T, Wang Z, Yu H, Li Z, Wei F, Luo G (2003) Effect of adding nickel to iron-alumina catalysts on the morphology of as-grown carbon nanotubes. Carbon 41(13):2487–2493
Zhang Q, Qian W, Xiang R, Yang Z, Luo G, Wang Y, Wei F (2008) In situ growth of carbon nanotubes on inorganic fibers with different surface properties. Mater Chem Phys 107(2–3):317–321
Zhu X (2019) Study on the treatment of gasoline wastewater with carbon nanotube adsorbent, Department of Chemical Engineering, Tsinghua University
Prokudina NA, Shishchenko ER, Joo OS, Kim DY, Han SH (2000) Carbon nanotube RLC circuits. Adv Mater 12(19):1444–1447
Cui C, Qian W, Wei F (2011) Water-assisted growth of carbon nanotubes over Co/Mo/Al2O3 Catalyst. Acta Phys Chim Sin 27(10):2462–2468
Bednar J, Obersteiner M, Baklanov A, Thomson M, Wagner F, Geden O, Allen M, Hall JW (2021) Operationalizing the net-negative carbon economy. Nature 596(7872):377–383
Funding
This work was financially supported by National Natural Science Foundation of China (22109085), Sinochem Quanzhou petrochemical Co. Ltd (A042019009) and Sinopec Engineering Group Luoyang R&D center of Technologies (121022).
Author information
Authors and Affiliations
Contributions
ZFY: Writing—original draft, Writing—review & editing, Investigation, Formal analysis, Data curation. CJC: Project administration, Funding acquisition, Writing—review & editing, Conceptualization, Investigation. XY: Investigation, Formal analysis. WHZ, DXL and YZ: Project administration, Resources, Investigation. KL: Resources, Investigation. WZQ: Supervision, Project administration, Writing—review & editing, Conceptualization, Investigation. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The research does not require ethical approval.
Consent for publication
We agree to publish the work.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, Z., Cui, C., Yu, X. et al. Near carbon-zero cycle from VOCs capture to carbon fixation. Carb Neutrality 1, 27 (2022). https://doi.org/10.1007/s43979-022-00028-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43979-022-00028-2