Abstract
Briquettes made from carbonized agricultural residues present sustainable material alternatives to wood charcoal and firewood for commercial and industrial applications. However, these briquettes are plagued by property weaknesses including low drop strength and thermal efficiency. Therefore, this study focuses on enhancing the physical, mechanical and thermal properties of composite briquettes produced from carbonized banana peels and waste glass. Composite briquettes comprised of banana peels biochar and waste glass powder (0%, 5%, 10%, 20%, 30%, 40%, and 50%) were developed, characterized, and evaluated using thermo-gravimetric analysis and bomb calorimetry to determine thermo-physical properties and higher heating values, respectively. The thermal efficiency and emissions (CO, CO2, and PM2.5) were assessed using the water boiling test and an emissions monitoring system. Proximate analysis revealed that moisture content, volatile matter, fixed carbon, and ash content of the developed briquettes ranged from 2.5 to 9.7%, 19.2 to 37.2%, 28.7 to 55.6%, and 7.2 to 44.9%, respectively. Drop strength for the briquettes was 84% without waste glass in the composite, increasing to 94–98% with waste glass included in the composite matrix. Higher heating values ranged from 20.1 to 35.8 MJ/kg. Thermal efficiency rose from 22% with no waste glass powder to 40% with 50% waste glass powder addition, while CO and CO2 emissions decreased from 41 to 11 ppm; and 50 to 15 ppm, respectively. PM2.5 remained constant across all banana peel biochar waste glass composites. Notably, even a modest 10% waste glass composition significantly improved drop strength and thermal efficiency, but higher waste glass percentages correlated with elevated ash values and reduced higher heating values. Therefore, the developed composite briquettes can be used in commercial and industrial applications including in some industrial boilers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The world is struggling with the effects of environmental pollution, global warming, and climate change because of fossil fuel conversion processes. There is an urgent need to develop sustainable material alternatives to meet industrial and commercial energy demand of an ever-growing world population [1]. Despite the unsustainable nature of dependency on fossil-based energy resources, most developing countries have limited alternatives to meet the growing energy demand. This has resulted in increased importation of fossil fuels by countries with limited reserves. For example, Uganda’s petroleum importation volume increased from 2,103,939 in 2018 to 2,198,739 m3 in 2019, with this upward trend only expected to increase due to increased demand associated with a relatively high population growth rate of 3% [2]. However, fossil fuel utilization is associated with emissions of enormous amounts of greenhouse gases which are major contributors to global warming and climate change [3].
Additionally, in sub-Saharan Africa, there is also an over-dependence on firewood and charcoal for commercial, industrial and domestic applications. In Uganda, this dependency remains notably high at over 85% [2, 4]. As a result, Uganda as a country has seen 25.4% decline in the country’s forest cover between 2010 and 2015 [2]. This has resulted in the Government of Uganda, through the National Environmental Management Authority and the National Forestry Authority, imposing strict restrictions on the felling of hardwood trees [5, 6]. Agricultural residues are a possible alternative for the generation of solid energy sources and other composites [7,8,9,10]. Several agricultural wastes such as rice husk, sugarcane bagasse, sawdust, biogas digestate, banana peels, and coconut waste have been employed for generating fuel carriers in the form of char and briquettes [4, 8,9,10,11,12]. Banana peels are abundantly available in Uganda as bananas constitute one of the country’s major agricultural products, with 8.3 million metric tons produced in 2019 alone [2, 13]. Organic waste-derived carbonized briquettes have been identified as a more sustainable alternative to charcoal and wood fuel [8,9,10,11,12, 14, 15]. They also directly contribute to the reduction of the energy resource density per unit household, which is in line with SDG 7 [16].
Various studies have explored the conversion of banana peels into biochar or charcoal briquettes as a renewable energy resource. Selvarajoo et al. employed pyrolysis to produce biochar from banana peels yielding a calorific value of 25.9 MJ/kg [17]. Similarly, Kapen et al. developed environmentally friendly charcoal briquettes using banana peels, with the highest heating value recorded at 16.64 MJ/kg [18]. Wilaipon et al. developed moderated die-pressure banana peel briquettes with highest heating value recorded at18.89 MJ/kg [19]. Other studies have focused on the valorization of banana peels as precursor materials in the development of sustainable environmental briquettes. Kabenge et al. characterized banana peel wastes as potential slow pyrolysis feedstock resulting in an average calorific value of 16.15 MJ/kg [20]. Putra et al. hydrothermally carbonized biomass waste, including banana peels, under low-temperature conditions resulting in a higher heating value of 20.09 MJ/kg [21]. Serma-Jimenez et al. valorized banana peel waste as a precursor material for different renewable energy systems [22]. Despite all these studies, briquettes have failed to gain traction within the energy sector in sub-Saharan Africa due to their shortcomings including low burning duration, significantly low drop strength, and lower energy efficiency when compared to charcoal and fuel wood [16]. Briquettes are also susceptible to crumbling when exposed to shock or impact during packaging, storage, handling, and transportation between the processing facility and final utility points [23]. Briquettes lack natural particle bonds that are strong enough to withstand mechanical agitation during handling and temperature increments during cooking [8, 9].
Therefore, for carbonized briquettes to gain a comparative advantage and preference over charcoal and wood fuel, considerable effort should be placed on development of composite carbonized briquettes with improved mechanical, thermal, and physical properties. To achieve this, efforts have been made to develop composite briquettes with beneficial properties from each component material additive in the composite briquette matrix [24,25,26,27]. One possible material is waste glass. Waste glass, in the form of broken pieces of float-glass panels, is one of the major wastes posing disposal challenges in Uganda. It constitutes about 0.3% of all municipal solid waste collected [28,29,30]. Moreover, waste glass is openly dumped on roadsides or landfills due to inadequate waste glass recycling initiatives in the country [31]. Glass is an inorganic non-crystalline material manufactured by a melt quenching process of soda-lime silicate via the float method [32,33,34,35,36,37,38]. It is generally inert to chemical reactions, especially in mild substances such as biochar, as it has a high melting point ranging between 1400 and 1600℃. This implies that glass powder incorporated in the development of composite briquettes will not chemically alter the components of the combustible matter in the briquettes. Furthermore, since glass has a high density (of approximately 2500 kg/m3), it is not expected to have a major contribution to the particulate matter (PM) generated during the use of the briquettes [39, 40].
Glass has the potential to act as a fluxing agent during combustion. This enhances the potential melting and fusion of briquette particles, resulting in improved burning efficiency, reduced ash formation and increased drop strength due to its natural hardness [33,34,35,36,37,38]. Also, when added in appropriate proportions, glass could contribute to the mechanical strength and durability of biomass briquettes by acting as a binder that holds the briquette together, reducing the risk of disintegration during handling, transportation, and storage [38]. Therefore, in this study composite briquettes were developed from banana peels and waste glass. The chemical, thermal and mechanical properties of the developed composite briquettes have been investigated. An emissions study on the developed composite briquettes was also conducted. This was done to determine the potential of the developed composites as solid fuels in commercial and industrial application such as industrial boilers.
2 Methods
2.1 Briquette development
Fresh banana (Musa acuminata) peels totaling to approximately 640 kg were obtained from markets in Kampala, Uganda. The banana peels were sorted to remove all debris and dirt. Sun-drying involved spreading the collected banana peels on a plastic mat for 14 days at an average daily temperature of 27.4 ℃ until their moisture content reduced to less than 10% (See Fig. 1). The dried banana peels have a thickness of 2 mm and as such did not require further size reduction before carbonization. The dried banana peels were carbonized into biochar using a locally fabricated cylindrical mild steel reactor of thickness 2 mm, diameter 50 cm, and height 75 cm at a carbonization temperature of 400 °C. Details of the developed carbonizer and carbonization process have been explained in detail in our previous work [8, 9]. Approximately 10 kg of biochar were obtained for every 80 kg of fresh banana peels after the carbonization process. Approximately 20 kg of waste-glass were collected from glass-mart shops and construction sites. The waste glass was mainly broken pieces of float glass panels commonly used in windows and doors in the construction industry. The waste glass was pulverized using a metallic mortar and pestle, and sieve analysis was used to determine the particle size of the resulting waste glass powder.
Sieve analysis was used to determine exact particle sizes of the fine glass waste powder. Pulverized waste glass powder was sieved using a mechanical shaking approach through a column of sieves with varying graded mesh sizes. The particle size of the resulting waste glass powder ranged between 63 and 250 µm (See Fig. 1).
To investigate the effect of the amount of waste glass on each property of the resulting composite briquettes consisting of banana peels biochar and waste glass. Seven categories of briquettes were developed with 0%, 5%, 10%, 20%, 30%, 40%, and 50% waste glass as partial replacement of banana peels biochar base material. Cassava starch was used as binder. For every 1000 g (banana peels char-glass mixture) (See Table 1), 100 g of cassava flour was measured and mixed with approximately 500 ml of water. The resulting paste was brought to a boil before pouring into the char-glass mixture. The blended material was hand-fed into the molds of a briquetting machine and a compaction pressure of ≤ 10 MPa was applied [8, 9, 16]. The developed briquettes were dried under direct sunlight for two days at an average temperature of 28 °C to reduce moisture content to less than 10%. The resulting composite briquettes are shown in Fig. 2.
2.2 Thermogravimetric analysis and bomb calorimetry
Proximate analysis was done using an Eltra Thermostep non-isothermal Thermogravimetric analyzer at a heating rate of 15 ℃/min from room temperature (25 ℃) to 915 ℃. TGA was used to determine the proximate analysis (moisture content, ash content, fixed carbon and volatile matter of the developed composite briquettes at aa heating rate of 15 \(^\circ{\rm C} \)/min. High purity compressed air was used to clean all crucibles and chamber prior to experimentation. Nitrogen was used as the purge gas for pyrolysis [8, 9, 16].
The data attained from the thermogravimetric analysis offered a basis for the determination of ignition temperature and burnout temperature. The ignition and combustion indexes of the developed composite briquettes were determined using Eqs. (1) and (2) [39, 40]. The ignition temperature was determined at the onset of the second peak of the DTG curve, which indicates the end of the dehydration phase and the beginning of the devolatilization phase on the TGA-DTG curve.
where DTGmax stands for the maximum weight loss rate; DTGavg is the average of the weight loss between ignition and burnout temperatures; Ti stands for ignition temperature; Tf stands for burnout temperature; Tmax stands for maximum weight loss temperature.
The ignition index indicates the rate at which a composite briquette reaches self-sustainable combustion, whereas the combustion index is indicative of the combustion performance of the fuel. The higher the ignition index the faster the fuel shall be ignited and the higher the combustion index the more the amount of oxygen required to sustain the combustion of a fuel [39, 40]. Higher heating values of the developed briquettes were determined using an oxygen bomb calorimeter (Parr instrument company; Model 6772) [9].
2.3 Drop test and bulk density
The mechanical integrity of the developed composite briquettes was evaluated using the drop test. In this test, the composite briquettes were released to fall by gravity from a measured height of 2 m from the ground and the weight of the piece intact was recorded. This experiment was repeated three times, and the average shatter index or drop strength was reported. The purpose of the drop test on the composite briquettes was to enable a better understanding of their durability in terms of resistance to mechanical agitation during handling and transportation [8, 16]. Bulk density was determined as the ratio of the mass of the briquette to the volume of the briquette. These dimensions of the briquettes were determined using a digital external micrometer screw gauge and Vernier caliper while the mass was determined using a calibrated weighing scale with a resolution of 0.01 g. The shatter indices/drop strength and bulk densities of the composite briquettes were determined using Eqs. (3) and (4), respectively [8, 16].
2.4 Water boiling test
The water boiling test was performed following the ISO WBT 4.2.3 PROTOCOL [16, 42]. It was used to determine the thermal characteristics and the emissions of the developed briquettes. A traditional cook stove was used in the test [41, 42].
2.5 Fourier transform infra-red (FTIR) spectroscopy
The chemical characteristics of the developed composite briquettes were done using Fourier Transform Infra-red spectroscopy (Bruker Alpha II) with a spectral range from 400 cm−1 to 4000 cm−1 and resolution of 2 cm−1. FTIR analysis was used to determine the elemental and functional group compositions of the developed composite briquettes [16]. Absorptions were categorized into group regions (above 1500 cm−1) and fingerprint regions (below 1500 cm−1).
3 Results and discussions
3.1 Proximate analysis results
A total of 170 briquettes were developed (see Fig. 2). The developed briquettes were in seven different categories containing 0%, 5%, 10%, 20%, 30%, 40%, and 50% waste glass-powder to banana peel char. Despite the difference in the glass composition, there was no visible physical difference in the physical appearance of all developed composite briquettes except for the shining glass particles distributed across the composite briquette surface for especially the composite briquettes with 40% glass and 50% waste glass. Results from the proximate analysis for the developed briquettes are shown in Table 1. Briquettes developed with 0% glass powder (i.e., consisting purely of 100% banana peels biochar) showed significantly lower values of moisture content at approximately 2.55% compared to the other composite briquettes that had glass powder incorporated into their matrices. The addition of glass powder in the development of the composite briquettes inhibited open air drying. Furthermore, the moisture content of the composite briquettes decreased as the percentage of glass powder increased in the briquettes. This is explained by the hydrophobic nature of glass particles which affects moisture content with their increase in the composite matrix [37, 38].
Volatile matter results for composite briquettes were generally lower than the volatile matter results for briquettes developed with no waste glass, except for composite briquettes with 10% waste glass (i.e. 90:10 banana peel biochar to waste glass) where an increase in volatile matter was observed. Fixed carbon results for the composite briquettes were all lower than for the briquettes developed with no waste glass in the matrix (See Table 1). The effect of waste glass powder on the ash content of the composite briquettes was directly proportional. Therefore, as the composition of the glass powder increased, so did the percentage composition of the ash. This is expected because the thermal decomposition of glass to ash takes place much higher temperatures than that of the banana peels bio-char material in the composite briquette matrix [43, 44]. Generally, development of briquettes with high ash content are not desired due to the challenges they pose in ignition, reduced overall energy content and ash clogging channels in combustion chambers [8,9,10].
3.2 TGA results
TGA-DTG plots for the composite briquettes are shown in Fig. 3. TGA-DTG derived combustion parameters including the ignition index and combustion index are shown in Table 2. TGA-DTG plots depict the thermal decomposition of cellulose, hemicellulose, and lignin in the banana peel glass powder composite briquettes. The TGA-DTG curves show that thermal decomposition occurs in three temperature zones [45,46,47]. The first zone is the dehydration zone. It ends at 155 °C. Dehydration occurs at a relatively low mass loss rate as water in the biomass is lost through evaporation. Devolatilization occurs in the second zone at about 275 °C and ends at about 555 °C. During the devolatilization phase decomposition of hemicellulose begins at a much faster rate than dehydration. The low decomposition temperature range of hemicellulose is due to the presence of acetyl groups in its amorphous and random structure made of ordered micro fibrils [7]. Lignin degradation and decomposition begins at about 610 °C and ends at about 918 °C and accounts for a mass loss of about 4% on a dry basis. The structure of lignin consists of a complex network of cross-linked aromatic molecules that are difficult to decompose [48].
Ignition and maximum temperatures for briquettes developed with 0% glass powder were 234.74 ℃ and 319.96 ℃, respectively. A 19.67% weight loss was experienced in drying these briquettes. The total weight lost at the time of ignition was 25.98% of the original weight of the composite briquette. Char aggregation begins at 511.01 ℃ which is the combustion of the solid matter and ends when the combustible matter is depleted, leaving ash. The total weight lost during the thermal decomposition of these briquettes (0% glass powder) was 49.65%. The ignition temperature for the composite briquettes with 5% glass powder was 222.50 ℃, with a maximum temperature of 300.54 ℃. For these composite briquettes the first peak was between 51 and 130 ℃ and it corresponded to the drying of the briquette resulting in a 7.98% weight loss. Total weight lost at the time of ignition for composite briquettes with 5% glass powder was 11.63% of the original weight of the composite briquette. The char aggregation phase begins at 551.72 ℃. Overall, the total weight lost during the thermal decomposition of the composite briquette (5% glass powder) was 33.05%. TGA-DTG curve for the 10% glass powder composite briquettes indicated these briquettes had an ignition temperature of 223.87 ℃ and a maximum temperature of 286.81 ℃. The drying phase for these composite briquettes resulted in a 10.11% weight loss. Char aggregation phase begins at 587.44 ℃. The total weight lost during the thermal decomposition of these composite briquettes (10% glass powder) was 33.91%. The ignition temperature for composite briquettes (20% glass powder) was 222.98 ℃ and a maximum temperature of 294.43 ℃ was attained. During the drying phase of these briquettes a 9.92% weight loss was observed. Total weight lost at the time of ignition was 12.33%. Char aggregation phase begins at 576.49 ℃. Overall, the total weight lost during the thermal decomposition of the composite briquette (20% glass powder) was 31.21%. Ignition and maximum temperatures for composite briquettes developed with 30% glass powder were 219.68 ℃ and 287.61 ℃, respectively. A 6.38% weight loss was observed during the drying (dehydration) phase in the decomposition of these composite briquettes. The total weight lost at the time of ignition was 11.47%. The char aggregation for these composite briquettes (30% glass powder) begins at 609.08 \(^\circ{\rm C} \). Total weight lost during the thermal decomposition of the composite briquette was 28.6%. The TGA-DTG curve for the composite briquettes (40% glass powder) showed that for these briquettes’ ignition temperature was 214.42 ℃ and maximum temperature attainable was 299.21 ℃. Drying of these composite briquettes resulted in an 11.77% weight loss. The total weight lost at the time of ignition was 13.49% of the original weight of the composite briquette. The char aggregation phase begins at 583.99 ℃. The total weight lost during the thermal decomposition of the composite briquette was 36.44%. For composite briquettes developed with 50% glass powder, ignition and maximum temperatures were 213.67 ℃ and 262.51 ℃. Weight loss of 15.59% was recorded during the drying phase and by the time of ignition weight loss was 18.59% of the original. The first peak was between 45 and 126 ℃ and it corresponded to the drying of the sample resulting in a 15.59% weight loss as calculated at the end of the first DTG peak from the TG curve. The total weight lost at the time of ignition was 18.59% of the original weight of the composite briquette. The char aggregation phase begins at 619.22 ℃. Overall, the total weight lost during the thermal decomposition of the composite briquette was 30.12%.
Ignition and maximum temperatures versus the percentage of waste glass powder in the composite briquette matrix are shown in Fig. 4. Generally, an increase in glass powder composition led to a reduction in the ignition temperature and an increase in burnout temperature respectively. The reduction in ignition temperature is a desirable effect because it implies less energy is required to initiate combustion of the developed composite briquettes. This also implies that the developed composite briquettes will have higher thermal efficiency. Higher burnout temperature is an indicator of fewer combustible components left in the fuel [8, 9].
Figure 5 shows the curves of the ignition and combustion indexes of the developed briquettes. From the curves, composite briquettes developed with 10% glass powder in the matrix resulted in highest values of both ignition and combustion indexes at 6.5 × 10–5 wt%min−1℃−2 and 6.1 × 10–8 wt%min−2℃−3, respectively, while the 30% glass briquettes produced the lowest values, 1.0834 × 10–5 wt%min−1℃−2 and 0.7960 × 10–8 wt%min−2℃−3 respectively. Higher values of ignition and combustion indexes are an indication of ignition and combustion performance of a fuel, respectively [14, 39, 49, 50]. Beyond glass powder percentages of 10%, ignition and combustion indexes drop drastically due to the influence on volatile matter in the banana peels. Composite briquettes with a high composition of glass will therefore require more initial energy input when igniting and less combustion potential due to the lower mass of combustible material as revealed by the higher heating values results.
3.3 FTIR analysis of raw materials and the developed briquettes.
FTIR spectra for the glass powder, cassava starch binder, banana peels biochar, typical composite briquettes, and banana peels powder are shown in Fig. 6. Figure 7 shows the evolution of the FTIR spectra as waste glass powder increased in the composite matrix of the composite briquette. Absorption peaks in the range of 3620 to 3295 cm−1 are attributed to hydroxyl (–OH) groups, which are commonly found in various biomolecules such as carbohydrates, alcohols, and organic acids [51]. This broad peak is absent in the glass powder and disappears in the banana peels biochar. The disappearance of these peaks in biochar after carbonization of the banana peels is due to thermal decomposition and conversion of the organic compounds into carbonaceous materials. The high temperatures during carbonization cause cleavage of chemical bonds and the release of water and other volatile compounds, leading to the reduction or complete removal of hydroxyl groups. Due to the loss of oxygen-containing functional groups during carbonization, graphitic carbon structures are formed, and aromatic compounds are degraded. Carbonization involves subjecting biomass to high temperatures in the absence of oxygen, resulting in the removal of volatile components and the formation of carbon-rich residues. Peaks are noticed between 1550 to 1650 cm−1 in all materials (cassava starch binder, banana peels biochar, banana peels powder, and in the composite briquettes) except in the glass powder. These peaks are associated with the presence of carbonyl (C=O) and aromatic (C=C) bonds mainly as ketones, aldehydes, carboxylic acids, esters, and aromatic compounds [52].
3.4 Mechanical properties
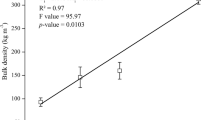
The amount of material content of a briquette is indicative of the compressive pressure applied during densification, and this can also translate into the energy density of the briquette [53]. The bulk density of the developed composite briquettes ranged between 569.2 and 735.2 kg/m3. Bulk density expectedly increased with an increase in glass powder composition, due to the glass being denser than banana peels biochar (See Fig. 8a) [54, 55]. The increase in bulk density can also be attributed to the reduction of the level of porosity within the briquette material as the glass composition is increased due to lower particle sizes (≤ 250 µm) of the glass powder particles. These lower particle sizes of the glass powder enabled enhanced agglomeration with the banana peels biochar and increased the overall amount of material per unit volume of the composite briquettes [43, 44, 56, 57].
Drop strength of briquettes is a regularly used mechanical property to characterize the mechanical integrity of developed briquettes and is extremely important for the determination of storage and transportation ability of the briquettes. It was noted that for the composite briquettes developed, drop strength generally increased with an increase in the composition of glass powder in the briquettes (See Fig. 8b). Drop strength ranged from 93.5 to 97.4% for the developed composite briquettes. These values of drop strength were much higher than the drop strength values of 84.1% recorded for briquettes developed from 100% banana peels biochar. Therefore, the inclusion of glass powder in the matrix of the developed composite briquettes clearly imparted a positive effect towards the drop strength of these composite briquettes. This increase in hardness or resistance to mechanical agitation can be directly attributed to the hardness of the glass powder in the matrix [43, 44, 54].
3.5 Thermal properties
Higher heating values (HHV) results for the developed composite briquettes showed that HHV reduced from 35.8 MJ/kg (when no waste glass powder was added to the briquette) to 20.2 MJ/kg (when the composite briquette consisted of 50% glass powder) (See Fig. 9) [58, 59]. The results of HHV for the developed briquettes show a reducing pattern as the waste glass composition is increased. This agrees with the results shown earlier for the combustion and ignition indexes, which showed that the briquettes with the higher composition of glass, i.e., above 30%, had lower ignition and combustion index values (see Fig. 4). It can therefore be deduced that the higher the amount of waste glass in a briquette, the lower its ability to ignite and maintain the flame and the lower the potential to give off heat energy per unit mass of fuel. This implies that a trade-off must be made between enhanced mechanical properties and reduced heating values when these briquettes are being utilized for environmental sustainability applications. As a result of waste glass powder in the matrix of the composite briquettes it is expected that the thermal efficiency for these briquettes will increase due to the requirement for more time and heat expended during combustion involving biochar (with high carbon content) and non-combustible waste glass powder (See Table 3). It is therefore expected that this will allow the combustion flame to delay its progression along the composite briquette allowing for a longer duration of heat supplied and energy, and hence greater thermal efficiency (See Fig. 9). During ignition, the waste glass present in the composite briquettes behaves endothermically absorbing heat. It retains this heat and releases it over a longer period even after the combustible matter (banana peels biochar) of the briquette is used up hence the increased duration of burning [55]. Results for ignition time, time to boil, burning rate, firepower and thermal efficiency are shown in Table 3. The time taken to boil 1 L of water based on cold start of the developed briquettes was observed to increase with an increase of glass powder in the composite matrix. The firepower in the developed composite briquettes was observed to generally decrease as waste glass powder is increased in the composite matrix. This is because the waste glass powder material is not carbonaceous and therefore does not contribute to the combustion process (See Table 3). Instead, the char that is present in the briquette heats up to the point of self-sustaining combustion after ignition and contributes to the boiling process [43, 54, 55, 60, 61].
3.6 Emissions
Figure 10 illustrates the trends of emissions of Carbon monoxide (CO), Carbon dioxide (CO2), and particulate matter 2.5 \(\upmu \)m or less (PM2.5) from the developed briquettes during the water boiling test, a crucial examination for determining the pollution potential of these briquettes. The CO2/CO emission curves depicted in Fig. 10 reveal three distinct phases. In the initial phase (Phase 1), there were no notable emission levels recorded. This absence of significant emissions is attributed to the fact that the briquettes had not yet ignited, and any trace levels noted during this phase originated from the ignition source [20, 62]. The subsequent phase, also known as the ignition phase (Phase 2), witnessed a sudden rise in both CO2 and CO emissions across all developed briquettes. This surge can be attributed to the intense combustion of the volatile matter present in the briquettes [63, 64]. Previous research has established that carbon-rich matter is more readily ignited and combustible in a gaseous state than in a solid form [65]. This explains the abrupt increase in the detection of CO and CO2 emissions, immediately succeeded by a decline in their detection. This decline can also be elucidated by the depletion of the volatile matter, which, after a few minutes, had been entirely burned out, leaving insufficient volatile matter to sustain the production of CO and CO2 at a noteworthy rate. Yiga et al. noted that the notable increase in CO concentration during ignition suggests incomplete combustion, linked to moisture evaporation from the briquettes' surface [16]. They further attributed this increase to factors such as low burning temperature, inadequate oxygen supply, ineffective fuel mixing with combustion air, and a brief residence time of combustion gases in the combustion zone [16]. The third phase of CO2 emission curve began at the 20th and 17th minute for 0% waste glass and composite briquettes, respectively, showing a gradual decline in CO2 emissions for all briquettes. This decline correlated with the depletion or burning out of combustible matter, as confirmed by the TGA curves, where no further reduction in mass occurred toward ash formation.
Overall, the longest average duration of CO emission, approximately 1 h, occurred in the third phase. It was highest for the 0% waste glass briquettes at 41 ppm, while the composite briquettes ranged between 11 and 36 ppm. These values are below the Occupational Safety and Health Administration's (OSHA) permissible exposure limits (PEL) of 50 ppm for an 8-h exposure. The World Health Organization (WHO) recommends an even lower limit of 10 ppm over the same duration but allows for higher levels in shorter exposures (35 ppm for 1 h and 100 ppm for no more than 15 min) [66, 67]. During the study, as the proportion of waste glass in the briquettes increased, there was a reduction in both CO and CO2 concentration levels. This outcome aligned with the predicted behavior outlined in the introduction. The waste glass did not undergo reaction during the water boiling test, and thus, none of its chemical components decomposed to generate additional CO or CO2 [43]. Instead, all recorded CO2 and CO emissions originated from the combustion of the char and cassava binder components of the briquettes. Consequently, as the char content decreased at the expense of waste glass in the briquettes, there was a corresponding reduction in the amounts of CO2 and CO [42]. Particulate matter (PM2.5) emissions remained consistent between the 0% waste glass briquettes and all composite briquettes developed. This suggests that the inclusion of waste glass had no discernible impact on the quantity of particulate matter released during the testing phase.
3.7 Analysis of variance for results in study
The ANOVA for selected responses and results in the study are shown in Table 4. The properties of volatile matter, fixed carbon, ignition temperature, HHV, burning rate, and firepower had high negative Pearson correlation coefficients. Therefore, these properties have an inversely proportional relationship with the waste glass powder composition in the composite briquettes developed. This implies that these properties always decrease as the waste glass composition is increased, as shown in the results. Properties that had a high positive Pearson correlation, including ash content, thermal efficiency, time to boil, and drop strength, showed a proportional effect on the composition of waste glass powder in the briquettes. Pearson correlation coefficient only provided information concerning the direction and strength of the relationship between the properties and glass powder composition. The p-value was, therefore, important for determining the confidence in stating the significance of the effect of waste glass powder on the properties of the composite briquettes developed. The null hypothesis was that waste glass powder affects the properties of carbonized banana peelings waste glass powder composite briquettes. The results presented in the study were all statistically significant, with a p-value less than 0.05, except for moisture content, fixed carbon, burning rate, ignition time and drop strength. P-value not being less than 0.05 for these properties is due to uniformity in the particle sizes of the banana peel biochar and waste glass powder, which affects the determination of values for these parameters.
4 Conclusions
The main limitations affecting the industrial and commercial utilization of composite briquettes e.g. in industrial boilers, are low drop strengths or shatter indices and limited thermal efficiency. Therefore, in this study composite briquettes were developed from banana peels biochar and waste glass powder varying from 0 to 50% and characterized for their proximate, mechanical and thermal properties. Banana peels biochar was obtained after carbonization at 400 \(^\circ{\rm C} \), while waste glass powder was obtained after pulverization of float glass construction waste. Proximate analysis results showed that ash content increased significantly for composite briquettes with waste glass powder. Fixed carbon and volatile matter were observed to generally decrease for the composite briquettes compared to briquettes developed with banana peels biochar only. Drop strengths or shatter indices ranged from 93.5 to 97.4% for the developed composite briquettes. Higher heating values (HHV) reduced with increase in waste glass addition in the composite matrix. However, thermal efficiency for the composite briquettes increased with an increased in glass powder in the matrix. These results indicate that a compromise must be made between mechanical and thermal properties in using these briquettes. The CO, CO2 and PM2.5 results were within WHO recommended guidelines. Therefore, the developed composite briquettes can be used in commercial and industrial applications e.g. in industrial boilers. It is recommended that more emission and ash generation studies be done to extend applicability of the developed composite briquettes.
Data availability
Data is provided within the manuscript.
References
Ibrahim ID, Hamam Y, Alayli Y, Jamiru T, Sadiku ER, Kupolati WK, Ndambuki JM, Eze AA. A review on Africa energy supply through renewable energy production: Nigeria, Cameroon, Ghana and South Africa as a case study. Energ Strat Rev. 2021;2021(38): 100740. https://doi.org/10.1016/j.esr.2021.100740.
UBOS. Statistical Abstract, 2019. Uganda Bureau of Statistics, 1, 38–40. 2020. http://www.ubos.org/onlinefiles/uploads/ubos/pdf.
Qasim U, Osman AI, Al-Muhtaseb AH, Farrell C, Al-Abri M, Ali M, Vo D-VN, Jamil F, Rooney DW. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: a review. Environ Chem Lett. 2021;19:613–41. https://doi.org/10.1007/s10311-020-01090-x.
Bamwesigye D, Darkwah SA, Hlavackova P, Kupcak V. Firewood and charcoal production in Uganda. Int Multidiscip Sci GeoConf. 2017;17:521–7.
Government of Uganda. The National Environment Act 2019.
National Environmental Management Authority (NEMA). National State of Environment Report; (2018–2019). 2019.
Olupot PW, Candia A, Menya E, Walozi R. Characterization of rice husk varieties in Uganda for biofuels and their techno-economic feasibility in gasification. Chem Eng Res Des. 2016;107:63–72. https://doi.org/10.1016/j.cherd.2015.11.010.
Lubwama M, Yiga VA. Characteristics of briquettes developed from rice and coffee husks for domestic cooking applications in Uganda. Renew Energy. 2018;118:43–55. https://doi.org/10.1016/j.renene.2017.11.003.
Lubwama M, Yiga VA. Development of groundnut shells and bagasse briquettes as sustainable fuel sources for domestic cooking applications in Uganda. Renew Energy. 2017;111:532–42. https://doi.org/10.1016/j.renene.2017.04.041.
Ogwang I, Kasedde H, Nabuuma B, Kirabira JB, Lwanyaga JD. Characterization of biogas digestate for solid biofuel production in Uganda. Sci Afr. 2021;12: e00735. https://doi.org/10.1016/j.sciaf.2021.e00735.
Bot BV, Sosso OT, Tamba JG, Lekane E, Bikai J, Ndame MK. Preparation and characterization of biomass briquettes made from banana peels, sugarcane bagasse, coconut shells and rattan waste. Biomass Convers Biorefinery. 2023;13:7937–46. https://doi.org/10.1007/s13399-021-01762-w.
Sanchez PDC, Aspe MMT, Sindol KN. An overview on the production of bio-briquettes from agricultural wastes: methods, processes and quality. J Agric Food Eng. 2022;1:0036. https://doi.org/10.37865/jafe.2022.0036.
Akankwasa K, Marimo P, Tumuhimbise R, Asasira M, Khakasa E, Mpirirwe I, Kleih U, Forsythe L, Fliedel G, Dufour D, Nowakunda K. The East African highland cooking bananas ‘Matooke’ preferences of farmers and traders: implications for variety development. Int J Food Sci Technol. 2021;56(3):1124–34. https://doi.org/10.1111/ijfs.14813.
Chen L, Xing L, Han L. Renewable energy from agro-residues in China: solid biofuels and biomass briquetting technology. Renew Sustain Energy Rev. 2009;13(9):2689–95. https://doi.org/10.1016/j.rser.2009.06.025.
UNECA, AU, ADB, UNDP. Assessing progress in Africa toward the Millennium Development Goals. 2015.
Yiga VA, Nuwamanya A, Birungi A, Lubwama M, Lubwama HN. Development of carbonized rice husks briquettes: synergy between emissions, combustion, kinetics and thermodynamic characteristics. Energy Rep. 2023;9:5977–91. https://doi.org/10.1016/j.egyr.2023.05.066.
Selvarajoo A, Muhammad D, Arumugasamy SK. An experimental and modelling approach to produce biochar from banana peels through pyrolysis as potential renewable energy resources. Model Earth Syst Environ. 2020;6:115–28. https://doi.org/10.1007/s40808-019-00663-2.
Kapen PT, Tenkeu MN, Yadjie E, Tchuen G. Production and characterization of environmentally friendly charcoal briquettes obtained from agricultural waste: the case of Cameroon. Int J Environ Sci Technol. 2022;19:5253–60. https://doi.org/10.1007/s13762-021-03497-7.
Wilaipon P, Trirattansirichai K, Tangchaichit K. Moderate die-pressure banana-peel briquettes. J Renew Energy Smart Grid Technol. 2014;2(1):50–5.
Kabenge I, Omulo G, Banadda N, Seay J, Zziwa A, Kiggundu N. Characterization of banana peels wastes as potential slow pyrolysis feedstock. J Sustain Dev. 2018;11:14–24. https://doi.org/10.5539/jsd.v11n2p14.
Putra HE, Damanhuri E, Dewi K, Pasek AD. Hydrothermal carbonization of biomass waste under low temperature condition. MATEC Web Conf. 2018;154:01025. https://doi.org/10.1051/matecconf/201815401025.
Serna-Jimenez JA, Luna-Lama F, Caballero A, Martin MM, Chica AF, Siles JA. Valorisation of banana peel waste as a precursor material for different renewable energy systems. Biomass Bioenerg. 2021;155: 106297. https://doi.org/10.1016/j.biombioe.2021.106279.
Obi OF, Pecenka R, Clifford MJ. A review of biomass briquette binders and quality parameters. Energies. 2022;15(7):2426. https://doi.org/10.3390/en15072426.
Kaliyan N, Morey RV. Natural binders and solid bridge type binding mechanism in briquettes and pellets made from corn stover and switchgrass. Biores Technol. 2010;101:1082–90. https://doi.org/10.1016/j.biortech.2009.08.064.
Ajimotokan HA, Ibitoye SE, Odusote JK, Adesoye OA, Omoniyi PO. Physico-mechanical properties of composite briquettes from corncob and rice husk. J Bioresour Bioprod. 2019;4(3):159–65. https://doi.org/10.12162/jbb.v4i3.004.
Muazu RI, Stegemann JA. Effects of operating variables on durability of fuel briquettes from rice husks and corn cobs. Fuel Process Technol. 2015;133:137–45. https://doi.org/10.1016/j/fuproc.2015.01.022.
Huko D, Kamau DN, Ogola WO. Effects of varying particle size on mechanical and combustion characteristics of mango seed shell cashew nutshell composite briquettes. Int J Eng Sci Invent. 2015;4:32–9.
Komakech AJ, Banadda NE, Kinobe JR, Kasisira L, Sundberg C, Gebresenbet G, Vinnerås B. Characterization of municipal waste in Kampala, Uganda. J Air Waste Manag Assoc. 2014;64(3):340–8. https://doi.org/10.1080/10962247.2013.861373.
Murungi C, van Dijk MP. Emptying, transportation and disposal of feacal sludge in informal settlements of Kampala Uganda: the economics of sanitation. Habitat Int. 2014;42:69–75. https://doi.org/10.1016/j.habitatint.2013.10.011.
Yusuf AA, Peter O, Hassan AS, Tunji LA, Oyagbola IA, Mustafa MM, Yusuf DA. Municipality solid waste management system for Mukono District, Uganda. Proc Manuf. 2019;2019(35):613–22. https://doi.org/10.1016/j.promfg.2019.06.003.
Castellani P, Ferronato N, Torretta V. Setting priorities to achieve Sustainable Development Goals through appropriate waste management systems in Uganda. Environ Dev. 2022;44: 100764. https://doi.org/10.1016/j.envdev.2022.100764.
Yamane M, Asahara Y. Glasses for photonics. Cambridge: Cambridge University Press; 2000. https://doi.org/10.1017/CBO9780511541308.
Ahmad J, Martinez-Garcia R, Algarni S, de Prado-Gil J, Alqahtani T, Irshad K. Characteristics of sustainable concrete with partial substitutions of glass waste as a binder material. Int J Concrete Struct Mater. 2022;16(1):21. https://doi.org/10.1186/s40069-022-00511-1.
Khan MNN, Saha AK, Sarker PK. Reuse of waste glass as a supplementary binder and aggregate for sustainable cement-based construction materials: a review. J Build Eng. 2020;28: 101052. https://doi.org/10.1016/j.jobe.2019.101052.
Vafaei M, Allahverdi A. High strength geopolymer binder based on waste-glass powder. Adv Powder Technol. 2017;28(1):215–22. https://doi.org/10.1016/j.apt.2016.09.034.
Adediran A, Lemougna PN, Yliniemi J, Tanskanen P, Kinnunen P, Roning J, Illikainen M. Recycling glass wool as a fluxing agent in the production of clay-and waste-based ceramics. J Clean Prod. 2021;289: 125673. https://doi.org/10.1016/j.clepro.2020.125673.
Zimmer A, Bragança SR. A review of waste glass as a raw material for whitewares. J Environ Manage. 2019;244:161–71. https://doi.org/10.1016/j.jenvman.2019.05.038.
Lawanwadeekul S, Srisuwan A, Phonphuak N, Chindaprasirt P. Enhancement of porosity and strength of clay brick fired at reduced temperature with the aid of corn cob and waste glass. Constr Build Mater. 2023;369: 130547. https://doi.org/10.1016/j.conbuildmat.2023.130547.
Garcia E, Ejim IF, Liu H. Thermogravimetric analysis of co-combustion of a bituminous coal and coffee industry by-products. Thermochim Acta. 2022;715: 179296. https://doi.org/10.1016/j.tca.2022.179296.
Garrido MA, Conesa JA, Garcia MD. Characterization and production of fuel briquettes made from biomass and plastic wastes. Energies. 2017;10(7):850. https://doi.org/10.3390/en10070850.
Verma P, Shukla SK. Performance evaluation of improved cook stove using briquette as fuel. AIP Conf Proc. 2019;2091: 020001. https://doi.org/10.1063/1.5096492.
Lubwama M, Yiga VA, Lubwama HN, Ssempijja I, Kihedu J. Emissions and emission factors for Dichrostachys cinerea, Morus Lactea, Piliostigma thonningii, Combretum molle, and Albizia grandibracteata firewood species and their charcoals. Biomass Convers Biorefinery. 2023. https://doi.org/10.1007/s13399-023-04005-2.
Hasanuzzaman M, Rafferty A, Sajjia M, Olabi A-G. Properties of glass materials. In Reference module in materials science and materials engineering. 2016. https://doi.org/10.1016/b978-0-12-803581-8.03998-9.
Lin Y, Smedskjaer MM, Mauro JC. Structure, properties, and fabrication of calcium aluminate-based glasses. Int J Appl Glas Sci. 2019;10(4):488–501. https://doi.org/10.1111/ijag.13417.
Guo G, Zhang K, Liu C, et al. Comparative investigation on thermal decomposition of powdered and pelletized biomasses: thermal conversion characteristics and apparent kinetics. Biores Technol. 2020;301: 122732. https://doi.org/10.1016/j/biortech.2020.122732.
Sullivan AL, Ball R. Thermal decomposition and combustion chemistry of cellulosic biomass. Atmos Environ. 2012;47:133–41. https://doi.org/10.1016/j.atmosenv.2011.11.022.
Zeng J, Singh D, Chen S. Thermal decomposition kinetics of wheat straw treated by Phanerochaete chrysosporium. Int Biodeterior Biodegrad. 2011;65:410–4. https://doi.org/10.1016/j.ibiod.2011.01.004.
Menya E, Olupot PW, Storz H. (2020) Optimization of pyrolysis conditions for char production from rice husks and its characterization as a precursor for production of activated carbon. Biomass Convers Biorefinery. 2020;10:57–72. https://doi.org/10.1007/s13399-019-00399-0.
Plis A, Lasek J, Skawinska A. Kinetic analysis of the combustion process on Nannochloropsis gaditana microalgae based on thermogravimetric studies. J Anal Appl Pyrol. 2017;127:109–19. https://doi.org/10.1016/j.jaap.2017.08.017.
Cai Z, Ma X, Fang S, Yu Z, Lin Y. Thermogravimetric analysis of the co-combustion of eucalyptus residues and paper mill sludge. Appl Therm Eng. 2016;106:938–43. https://doi.org/10.1016/j.applthermaleng.2016.06.088.
Prabhu L, Krishnaraj V, Gokulkumar S, Sathish S, Sanjay MR, Siengchin S. Mechanical, chemical and sound absorption properties of glass/kenaf/waste tea leaf fiber-reinforced hybrid epoxy composites. J Ind Text. 2022;51(10):1674–700. https://doi.org/10.1177/1528083720957392.
Boraah N, Chakma S, Kaushal P. Optimum features of wood-based biochars: a characterization study. J Environ Chem Eng. 2023;11(3): 109976. https://doi.org/10.1016/j.jece.2023.109976.
Onyenanu IU, Ilochonwu CE, Atanmo PN. Determining the energy value on different compressions of sawdust briquettes. In Engineering Solutions for Sustainability: Materials and Resources II. 2016; 227–234). https://doi.org/10.1007/978-3-319-48138-8_22.
Kundu V, Dhiman RL, Maan AS, Goyal DR. Structural and physical properties of Fe2O3-B2O3-V2O5 glasses. Adv Condensed Matter Phys. 2008. https://doi.org/10.1155/2008/937054.
Ren S, Liu J, Guo A, Zang W, Geng H, Tao X, Du H. Mechanical properties and thermal conductivity of a temperature resistance hollow glass microspheres/borosilicate glass buoyance material. Mater Sci Eng, A. 2016;674:604–14. https://doi.org/10.1016/j.msea.2016.08.014.
Hirst EA, Taylor A, Mokaya R. A simple flash carbonization route for conversion of biomass to porous carbons with high CO2 storage capacity. J Mater Chem A. 2018;6(26):12393–403. https://doi.org/10.1039/c8ta04409k.
Isahak WNRW, Hisham MWM, Yarmo MA. Highly porous carbon materials from biomass by chemical and carbonization method: a comparison study. J Chem. 2013. https://doi.org/10.1155/2013/620346.
Kimutai SK, Kimutai IK. Investigation of physical and combustion properties of briquettes from cashew nutshell and cassava binder. Int J Educ Res. 2019;7:15–26.
Portilho GR, de Castro VR, de Carneiro CO, Zanuncio JC, Zanuncio AJV, Surdi PG, Gominho J, Araújo SO. Potential of briquette produced with torrefied agroforestry biomass to generate energy. Forests. 2020;11(12):1–10. https://doi.org/10.3390/f11121272.
Ciecińska M, Goj P, Stoch A, Stoch P. Thermal properties of 60P2O5–(40–x)Al2O3–xNa2O glasses. J Therm Anal Calorim. 2020;139(3):1763–9. https://doi.org/10.1007/s10973-019-08606-w.
Simoncelli M, Mauri F, Marzari N. Thermal conductivity of glasses: first-principles theory and applications. NPJ Comput Mater. 2023;9:106. https://doi.org/10.1038/s41524-023-01033-4.
Mibulo T, Nsubuga D, Kabenge I, Wydra KD. Characterization of briquettes developed from banana peels, pineapple peels and water hyacinth. Energy Sustain Soc. 2022. https://doi.org/10.1186/s13705-023-00414-3.
Bilen M. Proximate and ultimate analysis before and after physical and chemical demineralization. IOP Conf Ser Earth Environ Sci. 2019;362: 012092. https://doi.org/10.1088/1755-1315/362/1/012092.
Mansor AM, Lim JS, Ani FN, Hashim H, Ho WS. Ultimate and proximate analysis of Malaysia pineapple biomass from MD2 cultivar for biofuel application. Chem Eng Trans. 2018;63:127–32. https://doi.org/10.3303/CET1863022.
ISO TC 285.The Water Boiling Test Version 4.2.3. Cookstove Emissions and Efficiency in a Controlled Laboratory Setting. 2014. https://cleancooking.org/research-evidence-learning/standards-testing/protocols/.
Arora P, Das P, Jain S, Kishore VVN. A laboratory based comparative study of Indian biomass cookstove testing protocol and water boiling test. Energy Sustain Dev. 2014;21:81–8. https://doi.org/10.1016/j.esd.2014.06.001.
Mopoung S, Udeye V. Characterization and evaluation of charcoal briquettes using banana peel and banana bunch waste for household heating. Am J Eng Appl Sci. 2017;10(2):353–65. https://doi.org/10.3844/ajeassp.2017.353.365.
Acknowledgements
We thank Mr. Wabwire Andrew (Department of Mechanical Engineering, Makerere University), Mr. Godias Tumusiime (Busitema University), and Mr. Agaba Jimmy (CREEC), for their expertise and assistance, especially during experimentation.
Funding
The project was self-funded.
Author information
Authors and Affiliations
Contributions
E.K.N. and H.K. conceptualized, run experiments and participated in manuscript development. J.W., E.J., and V.A.Y. were involved in experimental work and manuscript writing. M.L. was involved in conceptualization, experimentation, manuscript writing and overall project supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyakoojo, E.K., Wakatuntu, J., Jasper, E. et al. Characteristics of composite briquettes produced from carbonized banana peels and waste glass. Discov Mater 4, 29 (2024). https://doi.org/10.1007/s43939-024-00104-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-024-00104-7