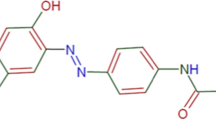
Abstract
Most often, reactive and disperse dyes are utilized separately in two- bath two-step dyeing method to dye polyester-cotton blend fabric. The cost of this conventional dyeing method is very expensive; energy and chemical usage is also quite higher in addition to this, the dyeing cycle is complex as compared to one-bath two-step dyeing methods or single-bath single-step dyeing methods. The single-bath single-step dyeing procedure with a single type of dye class was studied in this research. Following surface treatment of cotton through esterification procedures, polyester-cotton blend fabric dyed in single-bath single-step dyeing processes with disperse dye was examined. The association between time and the percentage of esterification was investigated at room temperature. Varying dye concentrations and dyeing temperatures were utilized to dye surface-treated p/c blend fabric in an HTHP dyeing machine. The color strength of dyed material and their fastness properties and the Surface chemistry by using FTIR were evaluated. The influence of dye concentration and temperature on color strength was investigated. With a 15% esterifying agent concentration and a reaction period of 2.5 h, the optimum value for surface modification was obtained, yielding a percent esterification of 34.95. The optimal value was attained with a dye concentration above 1% at a temperature of 120 °C, according to the dyeing experiment results. The findings of this study revealed that when compared to conventional two-bath dyed fabric, one-step one-bath dyed modified polyester/cotton blend with Disperse dye fabric has good wash fastness, and color strength, and is environmentally friendly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polyester/cotton fabrics are frequently used in garment and other technical textile application areas because of their remarkable and complementary properties [1]. Polyester fibers offer abrasion resistance, tensile strength properties, and dimensional stability, while cotton fibers are more hydrophilic, comfortable, and have more anti-static properties; Because of this polyester/cotton blend, textiles are important for their easy use of aesthetic value. The limitation of both fibers is balanced by combining these two fibers, making a good combination [2].
P/c blend fabric dyeing has various obstacles for the reason that polyester fibers have a hydrophobic nature, although cotton affirms a hydrophilic nature which permits a chemically distinct class of dyes [2]. Single-bath two-stage or double-stage dyeing methods, which usually employ correct dyes and chemicals for each fiber, perform a polyester/cotton blending process. These dyeing processes are however relatively long and complex [3]. The single-stage polyester/cotton dyeing process with disperse/reactive dyes has the advantages over conventional dyeing processes to reduce the dyeing cycle as well as energy efficacy [4].
Polymeric fibers were classically subject to surface modifications without changing bulk properties. As several functionalities are associated with regions like hydroxyl, ester, carboxyl, and carbonate rather than just one functional group, which is the desired modification, surface treatment of textile fibers strengthens and improves dyeability [5, 6]. Chemical modification of cellulose fibers on the surface without alteration of their native crystalline structure and morphology through esterification reactions that modify their surface properties by altering their chemical surface fiber but reduce the cellulose fibers’ natural hydrophilic character [7].
In two-bath processes, polyester/cotton fabrics should be dyed using polyester dyes and other cotton fiber dyes. These methods of dyeing are relatively lengthy and difficult. The single-stage two-bath process is quicker than the double-bath double-step dyeing process, however with multiple dye types used in the same bath, there is a problem with dyeability from migration and poor reproducibility [8]. The single bath shade produced with a single step reduces the process of dyeing and environmental pollution with one dye for both parts [9].
For the one-bath one-step dyeing method, various studies are being conducted utilizing various dyes in acidic or neutral conditions and at various temperatures on polyester/cotton blends [10].
Dyeing of polyester/cotton blends with disperse dyes was studied using sodium 2-(2,3-di-bromopropionylamino)-5-(4,6-di-chloro-1,3,5-tri-azinylamino)-bs modification of cotton using disperse dyes containing amino groups [11]. One-bath one-step dyeing of P/c blend with disperse dye after acetylation of cotton was investigated to reduce the dyeing cycle, as well as energy consumption and also the effect of acetic anhydride and time on percent acetyl content at room temperature was studied [12].
Recently, a novel strategy for dyeing Polyester/cotton blends has been proposed, involving pre-treatment with the biopolymer chitosan and subsequent dyeing using direct dyes [13]. A similar approach has been taken, which includes corona discharge pre-treatment of Polyester/cotton blends to improve the dyeability of both components. Polyester, cotton, and Polyester/cotton blend fabric were used to examine the feasibility of a one-bath one-step dyeing method [12, 13]. However not only have there been few studies on this topic but the butane tetracarboxylic acid surface modification of P/c blend fabrics has not yet been used to assess their dyeability with disperse dye.
This work consists of single-bath single-step dyeing of polyester/cotton blends with disperse dye after cotton fiber esterification because disperse dye has a stronger affinity and dyeability to esterified fibers. The methods shorten the process of dyeing, decrease the dyeing cycle, reduce environmental pollution, save energy, and time, and transmit the process of functional finishing as an easy-care treatment, and flame retardant. Developments of such dyeing method is suitable for polyester-cotton blends used in the cold climate, in particular, technical textiles and for home furniture applications since this fabric’s functionality and performance are considered.
2 Materials and methods
2.1 Materials
Half-bleached polyester/cotton blend fabrics were obtained from the EITEX laboratory with a blend ratio of 65:35 samples at the specifications of 90 picks per inch and 152 ends per inch with a GSM of 200. All the chemicals used were obtained from EiTEX laboratory, Esterification agent’s 1,2,3,4-Butanetetracarboxylic acid (BTCA,99%, CAS#:1703-58-8); citric acid (98%,CAS#:77–92-9);Sodium hypophosphite catalyst (99%, CAS#:7681-53-0); C.I. Disperse dye blue-S-6GF200 (CAS#:13695-46-); Ammonium sulphate (99%, CAS#:7704–34-9); Dispersing agent (CAS#:9002-93-1) Detergent, Sodium hydroxide (NaOH CAS#:1310–73-2, Sodium hydrosulphite (98%, CAS#:7775-14-6).
2.2 Methods
2.2.1 Esterification process
Commonly, butane tetracarboxylic acid (BTCA) and citric acid are used as a solvent, and sodium hypophosphite as a catalyst to facilitate the esterification process. Optimal esterification conditions are determined by a variety of parameters, including reaction duration and BTCA concentration, Taking into consideration the various literature on optimal esterification conditions, the carboxylic acid concentration of 10 to 20 percent and 6gpl of sodium hypophosphite in M.L.R. of 1:20, the time for reactions at room temperature of 1 h to 4 h as the temperature increased for esterification and tensile strength has an impact on the fabric's condition [14,15,16]. Citric acid mixtures have a weight-to-weight ratio of 1:2 for the esterification method. Using a central composite design, the esterification was carried out in 13 runs.
2.2.2 Percent esterification
The esterification percentage has been done by the percentage of the ester group that replaced the hydroxy group with titration [17]. Two grams of samples were added to the flask to hydrolyze the esterified cotton and it was 0.5 typical sodium hydroxide solution that was heated at 40–45 °C for 15 min. The flask was then kept at room temperature for 24 h to complete hydrolysis. Excess sodium hydroxide was then restored to 0.5 normal hydrochloric acids with phenolphthalein as an indication.
Where, B = ml of 0.5 normality of hydrochloric acid used to titrate blank
-
S = ml of 0.5 normality of hydrochloric acid used to titrate the sample
-
N = is 0.5, the normality of hydrochloric acid and sodium hydroxide used for the titration.
2.2.3 Dyeing methods
Dyeing methods have used high temperatures and high-pressure dyeing machines with different dyeing temperatures after modification of the cotton part with dispersed dye in one bath for the optimal dye of this blend with the minimum loss of cotton tensile strength. The polyester/cotton blend in these activities will be dyed using the conventional process and a bath dyeing process. The dyeing of polyester/cotton blend fabric is dyed in an HTHP dyeing machine with an M.L.R of 1:20. pH with acetic acid is maintained at 5–6. The temperature of a dye bath for 60 min is 110,120,130 °C as shown in Fig. 1. The dyeing was rinsed and cleared for fifteen minutes with an aqueous solution of 2 g/l of sodium and 2 g/l of sodium hydrosulfite at 60 °C.
2.2.4 Characterization of Modified PC Blends
2.2.4.1 Fourier transform infrared spectroscopy (FTIR) analysis
A 500 ml lab beaker was used for all of the esterification processes on the cotton fabric (115-V Model No. P230). The surface chemistries of untreated and esterified cotton fabrics were evaluated using Fourier Transform Infrared spectroscopy (Perkin Elmer FTIR) and ASTM 7575 test standard procedures [19, 20].
2.2.4.2 Color strength measurements
Using a spectrophotometer (Gretag Macbeth color eye 3100), the color strength of dyed samples was assessed. The test was carried out by the AATCC 6-2003 test standard method. The color strength of dyed fabric samples was determined using the reflectance value at a maximum wavelength, and the surface color strength was determined using the K/S value. The equation of Kubelka and Munk describes K/S as a function of color depth.
where K stands for the sorption coefficient, S for the scattering coefficient, and R stands for the reflectance of the dyed fabric. In reflectance–specular included mode, the spectrophotometer was calibrated for a 1-inch diameter specimen viewing aperture. The CIE 10-degree observer and Illuminant D65 were utilized [21,22,23].
2.2.4.3 Fastness properties
The Laundry-O-Meter (Italian, Mesdan Lab) was used to examine the colorfastness of dyed material under AATCC standard Test Method 61-2001. It was done with the AATCC Gray range for assessing alters in Color and the AATCC Gray Scale for assessing Staining [24, 25].
2.2.4.4 Tensile strength measurement
The tensile strength test of esterified polyester/cotton blend fabric before and after treatment was done according to the ASTM D5034-textile grab method test using Tensolab 100. When the specimen broke, the braking force and elongation were noted [26, 27].
3 Results and discussions
3.1 Optimization of esterification
In this research, a process was established in polyester-cotton blends for the esterification of cotton components on the surface. During the esterification process, many parameters such as BTCA concentration, time, and percentage of esterification are taken into account (Table 1). The response esterification percentage is used to establish the degree to which the hydroxyl group of cotton has been replaced by an ester group. The experimental design was employed to examine the relationship between BTCA and time. The ANOVA determines if the concentration and time variables of the BTCA affect the % esterification considerably.
Figure 2 shows residual normality test plots for the degree of substitution, where it is observed that residuals are on a straight line in all the graphs, which shows that the percentage of residues distributed to the esterification is normal. The results verify the assumption of regular variance and that the anticipated model is suitable.
Table 2 displays P-values of less than 0.0500 and F-values of 7.57 imply an important model. There is only a 0.96% chance that this large F-value might occur because of noise. The butane tetracarboxylic acid concentrations (A and A2) and (B and B2) were considered in terms of the model. Values above 0.1000 do not indicate significant conditions of the model. The 0.25 fit-value deficiency means that an 86.02 percent chance of an F-value deficiency due to noise could arise. This ANOVA table, therefore, shows that the process is important and that the model is fit.
3.1.1 Effect of butane tetracarboxylic acid concentration on percent esterification
As a solvent, Citric acid works as a cotton inflating agent by increasing the accessibility of cotton hydroxyl groups to the esterifying agent (BTCA) [28]. The esterification of cellulose progressed gradually following the initial suggestion that the reaction was heterogenic, and it eventually disintegrated into the reaction liquid. Carboxylation of OH groups on the surface of solid cellulose is favored as shown in Fig. 3. It was discovered that as the number of esterifying agents rose, so did the amount of esterification. The amount of butane tetracarboxylic acid was increased from 8 to 15% to get the highest esterification of 34.95%. The rate of esterification decreases when butane tetracarboxylic acid concentrations increase from 15 to 22% (Table 1). High butane tetracarboxylic acid concentrations reduce carboxylate percent, esters acid-catalyzed hydrolysis reduces the percentage esterification and water molecules react with BTCA beyond 15% of BTCA, and a high BTCA level may cause carbohydrate degradation, resulting in a reduction in weight gain percentage. The maximal esterification rate at the fibrous stage is 32–35% without degrading a fibrous structure [29].
3.1.2 Effect of time on percent esterification
With the extension of the reaction time from 1 to 4 h for all testing carried out, the amount of percent esterification values increased. The percentage of esterification fell from 35 to 32 percent as reaction times increased from 2.5 to 4.5 h (Table 1). The highest value of ester formation was recorded during a time of 2.5 h, with a maximum value of 35%. Longer reaction periods result from the hydrolysis of ester groups and cellulose, resulting in a decrease in esterification. Figure 4 illustrates the impact on ester formation of reaction time. From the time variance study, it can be concluded that the optimal period of reaction under the set of reaction conditions has reached a maximum esterification level. Thus, the levels of esterification value can be seen under the selected reaction conditions as establishing the equilibrium between products and reactants. After the optimum time (2.5 h), the maximum values of the percent esterification were achieved with a 15% concentration of 35%.
3.1.3 Fourier transform infrared spectroscopy (FTIR) analysis
Cotton cellulose esterifies are one of the major drivers of the material's dyeability, To understand the chemical changes in esterification, the authors compared cotton esterified samples to cotton cellulose samples (Fig. 5). The FTIR spectrum of esterified cotton is analyzed to guarantee that the hydroxy group reacts with the ester function group. A typical loss of the transmission bend in the range of 3600 cm−1 to 3300 cm−1 for the ester group is observed [30]. The stretch of cotton is not as wide or strong in this area as it was in the OH stretch. The absorption of the ester carbonyl peaks at 1714 cm −1 and the C-H at 1236 cm−1 extend to form a group of esters [31]. The strong sharp peak at 1020 cm−1 and 724 cm−1 may be caused by the polymer backbone being stretched in the C-O and C-H directions. The strong, sharp peaks seen were caused by the cellulosic component of the fiber components.
3.2 Dyeing of modified PC blends
The normal percent probability vs. residual in Fig. 6 for color strength is in a straight line, which shows that the residual distribution is normal and that the assumption of constant variance has been confirmed, suggesting that the model is adequate. Concerning the normal distribution of the error terms in color Strength, the normal probability of the residues is about linear.
During the dyeing process, several factors have been taken into account, including dye concentration, dyeing time, and color strength (Table 3). The color strength is used to assess the absorption degree of the esterified polyester/cotton blend material. The experimental design was used for the examination of the relationship between the dye concentration and dyeing temperature in standard dyeing time. ANOVA examines whether variables such as dye concentration and dyeing temperature have a significant impact on color strength.
Tables 4 and 5 shows P-values less than 0.0500 and an F-value of 8.69 implies the model is significant. There is only a 0.65% chance that an F-value this large could occur due to noise. The dye concentration (A and A2) and B are significant model terms. Values greater than 0.1000 indicate the model terms are not significant. The Lack of Fit F-value of 3.90 implies there is an 11.08% chance that a Lack of Fit F-value could occur due to noise. Non- significant lack of fit is good—we want the model to fit.
3.2.1 Effect of dye concentration on color strength
The concentration of the dye has a great influence on the K/S value. The concentration in the dyeing process affects both the dye fixation and the color strength of the dyed fabric. In general, the availability of the dye molecules in the dye bath increases with increased concentrations, as a result of which more dye molecules from the dye bath to the fiber can diffuse [32]. As a result, the more dye exhaustion, the more dye attaches to and diffuses through the fiber, the more dye in and out of the fiber, the bigger the amount of dye in and out of the fiber, the better the benefit of fixing more dye on the fiber as depicted in Fig. 7. Consequently, the color strength will increase due to the increased dye concentration on the fiber.
3.2.2 Effect of temperature on color strength of modified PC blends
Figure 7 displays the K/S value, which shows that both fabrics' dye uptake is significantly influenced by the dyeing temperature. When the temperature is used as a source of energy, the fiber polymer system undergoes a significant transformation. The color strength of modified dyed P/c blends is 105 to 110 °C at lower temperatures. From the result, there is a higher polyester composition of the blend. The disperse dyeing of polyester at this temperature is difficult as the polyester crystal structure at this temperature was not open. This results in less dye diffusion into the fiber. The improved P/c blend's color strength was established at 120, 130, and 134 °C, yielding satisfactory color strength as displayed in Table 3. Since the polyester portion's glass transition temperature was reduced and its structure opened, disperse dye into the fiber structure was significantly diffused with increasing dye fixation. The color strength of the dyed fabric is boosted by the temperature increase between 120 and 134 °C.
The color strength of a one-stage dyeing technique for modified P/c blends at 120 °C is shown in Fig. 8. The shade depth reflected from the surface of the dyed fabric is shown by K/S values from the results. The outcome shows good color strength, and the K/S values for actual dyeing were acceptable. According to the test results, disperse dye has a stronger affinity and dyeability with fibers of the ester function groups.
3.3 Mechanisms of dyeing
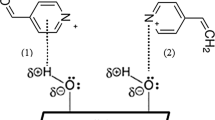
A larger component of the dye is present in bulk dispersion inside the dye bath in the mechanism of aqueous phase transfer of disperse dyes, whereas a little fraction of the dye dissolves and forms an aqueous solution [33]. The water solution's dye molecules adhere to the fiber surface and permeate from there into the substrate's interior. Diffusion is the transfer of dye molecules from the external aqueous phase to the surface and interior of the substrate up to saturation equilibrium. The dyeing of hydrophobic fiber with disperse dye is classified using thermodynamic dye diffusion concepts based on a free-volume model as shown in Fig. 9. A dye molecule that has been adsorbed onto a polymer chain can permeate through a polymeric material like fiber when the composite molecular chains' segmental mobility is such that enough free volume is created to accommodate both the dye molecule and the chain segment [34]. The blended fabric produces ester that conforms to free volume thermodynamic dyeing diffusion after surface treatment of cotton sections as shown in Fig. 9. As a result of both thermal expansion and decreased fiber density brought on by a rise in temperature, the molecular chains inside a fiber become more mobile, increasing dye diffusion across the open structure of the fiber.
3.4 Optimized condition of esterified polyester/cotton blend fabric
Based on the output result, the optimized condition was maintained by adjusting dye concentration,
and the temperature was minimized because the temperature is energy. The color strength was set to be maximum because as much as possible color strength should be maximum to get better dyeability.
The optimal condition gives maximum color strength of 2.90 when there is a 1% dye con and 119 °C as shown in Tables 4 and 5. These conditions are selected because it gives a desirability value Maximum that is near 1. This newly developed optimum condition was then used for color Fastness measurements and tensile strength properties and also for further evaluations.
3.5 Colourfastness properties
The wash fastness properties of treated P/c blend fabric are dyed with a disperse-blue dye through different dye concentrations. Colorfastness to washing in Table 6 demonstrates that single-bath single-step dyed P/c blend fabric with disperse dye following surface treatment is approximately comparable to conventional double- bath double- step dyed fabric samples. The hydrophobic nature of disperse dye results in the good wash fastness property of dyed fabric [35]. In general, the two samples were analyzed as having moderate to good fastness properties.
The outcome advocates that single-bath single-step dyeing color change for the P/C blend fabric is approximately comparable to a conventional double-bath double-step dyed material. This is for the reasons that disperse dyes are non-hydrophilic and the susceptibility to water hydrolyzed during washing is minor and both polyester fabric and esterified cotton fabric have an ester functional group that offers a strong mechanical bond. As a result of the low aqueous solubility of dye which influences the brightness of the dyeing and during a reduction clearing process all reactive dye dispersions are mounted up on the surface of the dyed materials and reduction agents have been booked and only the formation of dye fibers remains and the hydrophobic nature of the dye has produced a good quality of wash fastness.
In distinction to usual double-bath dyed samples, materials dyed in single-bath single-step dyed with disperse dye following surface treatment of Polyester/cotton blend fabric had excellent wet/dry rubbing fastness as verified in Table 7.
As shown in Table 8 the samples dyed in the one-bath one-step method after surface modification of P/c blend has good light fastness compared to conventional two-bath dyed samples [36].
Table 9 shows that the samples dyed in one bath one-step with disperse dye after surface modification of cotton part have good fastness perspiration to acid and alkali compared to conventional two bath dye samples. The differences in the fastness of perspiration between these two methods were small.
3.6 Effect of esterification on the physical properties P/c blend fabric
The tensile strength of P/c blend fabrics dyed using one-bath, one-step techniques using disperse dye differs slightly from samples traditionally dyed in two baths, and also the tensile strength of P/C blend fabrics esterified with BTCA after surface modification of cotton is slightly lower than control samples as indicated in Table 10. This is because dyeing temperatures and esterification conditions lower the tensile strength of P/c fabrics. Cotton's strength decreased during the esterification process since the esterification bath was conducted in acidic media. This finding demonstrates the poor tendency of cellulose to withstand both weak and strong [12, 37].
As a result of high-temperature, high-pressure dyeing of polyester affecting the strength of cotton and acidic media of disperse dyeing of the polyester component also taken into consideration for loss of cotton strength, the strength of P/c blend sample is lowered as temperature increases. The difference in strength loss between the two approaches does not result in a significant loss of strength, whereas esterification techniques and particular catalysts had only a little impact on the fabric's tensile strength [38].
In comparison to the control and traditional two-bath dyed samples, the tear strength of P/c blends treated with BTCA and dyed with disperse dye in one-bath one-step procedures results is nearly identical, as shown in Table 10 [12].
4 Conclusion
Polyester/Cotton blend fabrics dyed with disperse dye in a single-bath single-step dyeing method after esterification processes have been examined in cotton surface treatment. Cotton fabric surface treatment was carried out by esterification methods. The results demonstrated that the optimum esterification value with an esterification concentration of 15% and a reaction time of 2.5 h was determined, and an esterification of 35% was obtained. The experimental results showed that dyeing was obtained at a temperature of 120 °C by a concentration above 1%. Techniques for imparting chemical surface modification of P/c blends should be developed and investigated without influencing other physical properties considerably. The method has a substantial implication for textile industries because one-bath- one-step techniques are relatively safe and environmentally friendly with lower energy, water, time, and resource requirements as P/c blends dyed with one dye. The outcomes from this study demonstrated that a single-bath single-step dyeing method with disperse dye has an excellent fastness property in contrast to the usual dyeing method. The single-stage P/c dyeing methods blended with disperse dyeing also show that the level of dyeing has good values for color strength, and the methods offer the option of economical efficiency.
Data availability
The datasets used and/or analyzed during the current study are available from all authors on reasonable request.
References
Fecker T, et al. Active site flexibility as a hallmark for efficient PET degradation by I. sakaiensis PETase. Biophys J. 2018;114(6):1302–12.
Walter T, et al. Enzymatic degradation of a model polyester by lipase from Rhizopus delemar. Enzyme Microb Technol. 1995;17(3):218–24.
Tokiwa Y, et al. Biodegradation of synthetic polymers containing ester bonds. Washington: ACS Publications; 1990.
Figeroa Y, Hinks D, Montero G. A heterogeneous kinetic model for the cutinase-catalyzed hydrolysis of cyclo-tris-ethylene terephthalate. Biotechnol Prog. 2006;22(4):1209–14.
Gamerith C, et al. Improving enzymatic polyurethane hydrolysis by tuning enzyme sorption. Polym Degrad Stab. 2016;132:69–77.
Gamerith C, et al. Enzymatic recovery of polyester building blocks from polymer blends. Process Biochem. 2017;59:58–64.
Han X, et al. Structural insight into catalytic mechanism of PET hydrolase. Nat Commun. 2017;8(1):1–6.
Hegde K, Veeranki VD. Production optimization and characterization of recombinant cutinases from Thermobifida fusca sp. NRRL B-8184. Appl Biochem Biotechnol. 2013;170(3):654–75.
Herrero Acero E, Wei R, Zimmermann W, Zinn M, et al. Enzymatic surface hydrolysis of PET: Effect of structural diversity 476 on kinetic properties of cutinases from Thermobifida. Macromolecules. 2011;44:4632–40.
Walawska A, Filipowska B, Rybicki E. Dyeing polyester and cotton-polyester fabrics by means of direct dyestuffs after chitosan treatment. Fibres Text East Eur. 2003;11(2):71–4.
Kim M, et al. Dyeing of cotton and polyester/cotton blend with disperse dyes using sodium 2-(2, 3-dibromopropionylamino)-5-(4, 6-dichloro-1, 3, 5-triazinylamino)-benzenesulfonate. Fibers Polym. 2006;7(4):352–7.
Kumsa G, Gebino G, Ketema G. One-bath one-step dyeing of polyester/cotton (PC) blends fabric with disperse dyes after acetylation of cotton. Discov Mater. 2021;1(1):1–16.
Timm DA, Hsieh YL. Solvent-induced structural changes in sulfonated poly (ethylene terephthalate)(SPET) fibers. J Appl Polym Sci. 1994;51(7):1291–301.
Heumann S, et al. New model substrates for enzymes hydrolysing polyethyleneterephthalate and polyamide fibres. J Biochem Biophys Methods. 2006;69(1–2):89–99.
Hu X, et al. Diversity of polyester-degrading bacteria in compost and molecular analysis of a thermoactive esterase from Thermobifida alba AHK119. Appl Microbiol Biotechnol. 2010;87(2):771–9.
Huang Y-C, et al. Heterologous expression of thermostable acetylxylan esterase gene from Thermobifida fusca and its synergistic action with xylanase for the production of xylooligosaccharides. Biochem Biophys Res Commun. 2010;400(4):718–23.
Huson DH, Scornavacca C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol. 2012;61(6):1061–7.
Kawai F, et al. Comparison of polyester-degrading cutinases from genus Thermobifida. In: Green polymer chemistry: biocatalysis and materials II. Washington: ACS Publications; 2013. p. 111–20.
Kavkler K, et al. FTIR spectroscopy of biodegraded historical textiles. Polym Degrad Stab. 2011;96(4):574–80.
Garside P, Wyeth P. Identification of cellulosic fibres by FTIR spectroscopy differentiation of flax and hemp by polarized ATR FTIR. Stud Conserv. 2006;51(3):205–11.
Kawai F, et al. Enzymatic hydrophilization of polyester fabrics using a recombinant cutinase Cut 190 and their surface characterization. J Fiber Sci Technol. 2017;73(1):8–18.
Haji A, Qavamnia SS, Nasiriboroumand M. The use of D-optimal design in optimization of wool dyeing with Juglans regia bark. Industria Textila. 2018;69(2):104–10.
Huong NT, Khanh VTH, Linh NPD. Optimizing content of Pyrovatex CP New and Knittex FFRC in flame retardant treatment for cotton fabric. Industria Textila. 2021;72(3):315–23.
Shahid MA, et al. Effect of different dyeing parameters on color strength & fastness properties of cotton-elastane (ce) and lyocell-elastane (le) knit fabric. Int J Text Sci. 2016;5(1):1–7.
Gun AD, Tiber B. Color, color fastness and abrasion properties of 50/50 bamboo/cotton blended plain knitted fabrics in three different stitch lengths. Text Res J. 2011;81(18):1903–15.
Shet R, Yabani A. Crease-recovery and tensile-strength properties of unmodified and modified cotton cellulose treated with crosslinking agents. Text Res J. 1981;51(11):740–4.
Sricharussin W, et al. Effect of boric acid and BTCA on tensile strength loss of finished cotton fabrics. Text Res J. 2004;74(6):475–80.
Nechwatal A, et al. A contribution to the investigation of enzyme-catalysed hydrolysis of poly (ethylene terephthalate) oligomers. Macromol Mater Eng. 2006;291(12):1486–94.
Perz V, et al. Hydrolysis of synthetic polyesters by Clostridium botulinum esterases. Biotechnol Bioeng. 2016;113(5):1024–34.
Gulrajani ML. Advances in the dyeing and finishing of technical textiles. Elsevier; 2013. https://doi.org/10.1533/9780857097613
Welzel K, Müller RJ, Deckwer WD. Enzymatischer Abbau von Polyester-Nanopartikeln. Chem Ing Tec. 2002;74(10):1496–500.
Whewell C. Advances in the finishing of textile fabrics—a new era in fabric enhancement. Rev Prog Color Relat Top. 1984;14(1):157–65.
Erkan G, Sariisik M. Microencapsulation in textiles. Colourage. 2004;51:61–4.
Pastore C, Kiekens P. Surface characteristics of fibers and textiles. New York: M. Dekker. 2001, viii.
Wei Q, et al. Functionalization of textile materials by plasma enhanced modification. J Ind Text. 2007;36(4):301–9.
Najafi H, et al. One bath method dyeing of polyester/cotton blend fabric with sulphatoethylsulphonyl disperse/reactive dyes treatment by chitin biopolymer. Afr J Biotechnol. 2009. https://doi.org/10.3923/jas.2008.3945.3950.
Huang C, et al. Development of hydrophilic anti-crease finishing method for cotton fabric using alpha-Lipoic acid without causing strength loss and formaldehyde release problem. Prog Org Coat. 2021;151: 106042.
Khanh VTH, Huong NT. Influence of crosslinking agent on the effectiveness of flame retardant treatment for cotton fabric. Industria Textila. 2019;70(5):413–20.
Acknowledgements
The authors are highly grateful for the support received from the Ethiopian Institute of Textiles and Fashion Technology. Thanks to EITEX laboratory assistants especially Mrs. Adanech for her technical support.
Author information
Authors and Affiliations
Contributions
WT and TT did the experimental work and YZ and GK did documentation part. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tegegne, W., Tesfaye, T., Zeleke, Y. et al. One-bath one-dye class dyeing of polyester/cotton blend fabric with disperse dye after esterification of cotton fabric. Discov Mater 2, 14 (2022). https://doi.org/10.1007/s43939-022-00034-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-022-00034-2