Abstract
Aiming at the classic problem of dyeing of polyester–cotton blended fabric one-bath one-step dyeing of PC blends with disperse dye after surface modification of cotton were studied. Surface modification of cotton was carried out using fibrous acetylation methods. The optimum value for surface modification was obtained with a concentration of acetylation agent 16% and time of reaction 2.5 h, gave a percent acetylation of 34. Surface chemistry and thermal decomposition were studied by using FTIR spectra and TGA. The tear strength crease recovery, pilling and abrasion resistance were evaluated. The experiment result of dyeing showed that the optimum value was obtained with dye concentration above 1% at a temperature of 120 °C warp tensile strength decreased by 12% and weft tensile strength was decreased by 9% from the control half-bleached fabric. Results of this study showed that one-step one bath dyed modified PC blend with disperse dye fabric presents good fastness property and color strength values compared with conventional two-bath dyed fabric.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polyester/cotton fabrics are widely used in the clothing industry and other application areas due to their complementary properties [1, 2]. Polyester fibers deliver crease recovery, dimensional stability, abrasion resistance, tensile strength and easy-care properties, while cotton fibers give humidity absorption and anti-static characteristics and diminish pilling rolls. This makes polyester/cotton blends are famous due to their aesthetic value, user-friendly performance and limitations of both fibers are balanced adequately by blending these two fibers making a perfect blend [3, 4].
Hundreds of fabric blends are available on the market today, but polyester/cotton fabrics in various proportions are the most common for printed clothes [5, 6]. Polyester/cotton blends are utilized in everything from bedding to shirts [7]. Dyeing of polyester/cotton blends poses some challenges to dyer as polyester shows a hydrophobic character while cotton shows a hydrophilic character making it inevitable to dye them with a chemically different class of dyes [8, 9]. Muralidharan and Laya [10], found that Polyester/cotton blended fabrics are often coloured using a two-bath or one-bath two-step dyeing process. It discusses a new method for dyeing polyester/cotton blends utilizing dispersion and reactive dyes in a one-bath procedure that uses an azeotropic ternary mixture of organic solvents as a pretreatment. In these cases, the polyester/cotton blended surface-modified fabrics dye uptake and dye-fiber bond formation is increase with improvement in dimensional, surface, fastness and wrinkle properties. This improvement is due to a large increase in intersurface area by swelling and greater segmental mobility of polymer molecules. Singha [11], also has described the challenges of dyeing the polyester and cotton blend fabric (P/C fabric or PCF) due to its variation in color value, shed depth, tensile strength and surface residual weight loss. All of these flaws can be mitigated by choosing the right dye-fiber mix, which determines the dyed blend fabric's qualities. Many studies have been conducted in recent years to dye polyester/cotton blends in a one-bath dyeing method using conventional dispersed dyes and newly developed reactive dyes that can be dyed at acidic or neutral temperatures around 100–130 °C and are added simultaneously to the same bath after modifying polyester/cotton fabrics [11,12,13].
For a polyester/cotton blend dyeing, two-bath or one-bath two-step dyeing techniques using appropriate dyes and agents for each fiber have often been utilized [13,14,15]. These dying techniques, on the other hand, are time-consuming and difficult. The process of the one-bath two-step dyeing process is shorter than the two-bath method but its disadvantage is lower dyeability from migration and poor reproducibility since two different sorts of dyes are utilized in an equivalent bath [10]. The one-bath one-step dyeing process of polyester/cotton blends with disperse/ reactive dyes has advantages over the conventional dyeing processes on reducing the dyeing cycle as well as energy consumption [16].
Surface modification of the polymeric fibers without changing the bulk properties has been a classical research topic [17]. Surface modification of textile fiber enhances and increase their dyeability, because many functional groups bond to the surface such as hydroxyl, ester, carboxyl, and carbonate groups, instead of one desired functional group [18,19,20]. Chemical modification of cellulose fibers at the surface without appreciably changing their native crystallinity and morphology, which can be achieved by esterification reactions changing the surface properties through modification of fiber surface chemistry except to decrease the natural hydrophilic character of cellulose fibers [16, 21].
Acetylation is an esterification reaction, which plasticizes natural fibers by introducing acetyl groups (CH3CO−) [22, 23]. In this reaction, acetic acid (CH3COOH) is produced as a by-product that must be separated from the reaction mixture to prevent damage to the fibers. When treated with anhydride (CH3–C(=O)–O–C(=O)–CH3), it substitutes the hydroxyl groups existing within the cell membrane polymers with acetyl groups, making the fibers hydrophobic in nature [24, 25].
This work involves one-bath one-step dyeing of polyester/cotton (PC) blends with disperse dye after acetylation of cotton to reducing the dyeing cycle as well as energy consumption. The effect of acetic anhydride and time on percent acetyl content at room temperature was studied. Modified PC was carried out in HTHP dyeing machine incorporates with different dye concentrations and dyeing temperature.
2 Materials and methods
2.1 Materials and reagents
Half bleach polyester/cotton (PC) fabrics with a blend ratio of 65:35 samples at the specification of 90 picks per inch and 152 ends per inch and 201 GSM (gram per meter square). The chemicals used were obtained from Almeda Textile PLC and EiTEX laboratory respectively. Acetic acid, acetic anhydride, zinc chloride, C.I. Disperse dye yellow (C.I. Disperse Yellow 3) and reactive dye yellow S-8G were purchased from chemo scientific trading in Addis Ababa Ethiopia. These substances are utilized without being purified in any way. Yellow disperse dyes (C.I. Disperse Yellow 3) are bright yellow dyes with good crystalline characteristics and dispersion behavior that forms depending on the crystal structure (Fig. 1). It is an azo dye (4-(2-hydroxy-5-methylphenylazo) acetanilid) with a structure consisting of acetanilide substituted on the 4-position of the phenyl group with a 6-hydroxy-m-tolylazo group [26, 27]. It is a monocarboxylic acid amide and a member of azobenzenes. It exhibits great dispersion and fabric compatibility, resulting in good color qualities [28]. The energy type of disperse dye used for the modified PC blend is medium energy (SE type) due to its moderate molecular weight, moderate polarity, moderate dyeing rate and moderate sublimation fastness [29].
2.2 Methods
2.2.1 Acetylation process
Conventionally, acetylation is carried out by the reaction of glacial acetic acid as a solvent with acetic anhydride as the acetylating agent in the presence of zinc chloride as a catalyst. Acetylation using zinc chloride as a catalyst causes a reduction in fabric strength compared to using sulphuric acid according to [30]. The reaction conditions depend on several factors including time of reaction and concentration of acetic anhydride that keeps varying to determine the optimum conditions for the acetylation. The concentration of acetic anhydride used for acetylation were 10 to 20% and 6gpl of zinc chloride as a catalyst at MLR of 1:10 and the time of reaction was 1–4 h at room temperature due to temperature effects tensile strength of the fabric [30, 31]. The combination of glacial acetic acid at 1:2 ratios to sample weight was used for the acetylation method. The acetylation was done using a central composite design in 13 runs.
2.2.1.1 Percent acetylation
Percent acetylation is the percentage of the acetyl group that has replaced the hydroxyl group in the pretreated sample using titration [32]. The estimation of acetyl content was administered, using an aqueous caustic soda solution to hydrolyze the cellulose acetate and back estimating the surplus caustic soda with a typical acid (hydrochloric acid) solution.
Two grams of the sample were taken and then exactly 40 ml of 0.5 normality of sodium hydroxide solution was added into the flask to hydrolyze the acetylated cotton and warmed at 45° for 15 min. Afterward, the flask was kept for 24 h to complete hydrolysis at room temperature. Then excess sodium hydroxide was back titrated against 0.5 normality hydrochloric acid solution using phenolphthalein as an indicator (Eq. 1) [32, 33].
whereas B is the normality of hydrochloric acid (0.5) used to titrate blank; S is the required amount of hydrochloric acid (ml) used to titrate a sample; N is the normality of hydrochloric acid and sodium hydroxide (0.5) used for the titration; M is the molecular weight of the acetyl group (CH3CO) is 4.3 g/mol; W is the mass of the sample in grams (dry basis).
2.2.2 Dyeing method
After modifying the cotton part with disperse dye in one bath with a high temperature and high-pressure (HTHP) machine at various dyeing temperatures, the HTHP machine was used to identify the optimum dyeing temperature of this blend with the least amount of tensile strength loss in the cotton component. The PC blend was dyed using both the conventional dyeing method and the one-bath one-step dyeing techniques. Dyeing was carried out with disperse dye, after modification of cotton part dyed in HTHP dyeing machine at MLR 1:20. pH was maintained at 5–6 using acetic acid. The dye bath temperature was varying from 100 to 140 °C for 60 min. The dyeing was rinsed and then reduction cleared in an aqueous solution of 2gpl sodium hydroxide (NaOH) and 2gpl sodium hydrosulphite (Na2S2O4) at 60 °C for 15 min.
When dyeing PC blend modified with disperse dye, Design Experiment 11 with 29 runs were used to determine the effect of dye concentration and temperature to find the best dyeing temperature at a minimum loss of strength along warp and weft direction.
2.3 Characterization of modified PC blends
2.3.1 Instrumentation
All the acetylation reactions of the cotton cellulose were carried out in a 500 ml laboratory beaker in a Crest Ultrasonic cleaner (Model No. P230 with 115 V). The surface chemistries of untreated and acetylated cotton fabrics were evaluated by using Fourier Transform Infrared spectroscopy (Perkin Elmer FTIR) according to ASTM 7575 test standard methods. The results were recorded in the frequency range of 4000–400 cm−1 by using 20 scans. Thermal analysis was performed using TGA (Perkin Elmer) instrument (Model No. TGA 4000) to distinguish the thermal stability of blend fabric before and after modification. Measurements were conducted from ambient temperature to 500 °C at a heating rate of 20 °C/min in a nitrogen atmosphere. The result of TGA was used to study the denaturation properties of modified and unmodified polyester/cotton blends. The tensile strength test was done according to the ASTM D-5034-textile grab test method using Tensolab 100 (Mesdan Lab, Italy). The breaking force and elongation were recorded.
2.3.2 Measurement of moisture regains
Moisture regains of the modified PC blended fabrics were determined as per the ASTM standard test method 2654. The modified blended fabrics were conditioned in a standard testing atmosphere of 20 °C and 65% relative humidity for 24 h before actual testing (Eq. 2).
where %a is the proportion of fiber ‘a’, %; and %b is the proportion of fiber ‘b’, %
2.3.3 Color strength measurements
A spectrophotometer was used to determine the color strength of dyed cloth samples (Gretag Macbeth color eye 3100). The test is done by the procedure set by the AATCC 6-2003 test standard methods. The color strength of dyed fabric samples was measured from the reflectance value at a maximum wavelength from which surface color strength using K/S value. K/S is a function of color depth and is represented by the equation of Kubelka–Munk (K–M) as shown in Eq. 3 [34, 35].
where R is the reflectance of the dyed fabric; K is the sorption coefficient, and S is the scattering coefficient. The spectrophotometer was standardized for a 1-inch diameter specimen viewing aperture in reflectance–specular included mode. Illuminant D65 and the CIE 10-degree observer were used.
2.3.4 Fastness properties
Before fastness properties determination the dyed samples are dried followed by curing at a temperature of 140 °C for 5 min to modify the crystalline structure, resistance of wet creasing, dimensional stability and avoidance of shade variation on treated fabrics for polyester parts to provide better sublimation fastness. Applying heat on the modified fabrics also helps to the thermomigration of dye molecules inside the fiber surface. Dyed modified PC blended fabric was tested for colorfastness to rubbing according to AATCC standard test method 116-2001 using Crock-meter (Mesdan Lab, Italy). Colorfastness to washing was done according to the AATCC standard test method 61-2001 using Laundry-O-Meter (Mesdan Lab, Italy). Colorfastness to light was determined according to ISO105-B02 standard test method using SOLAR BOX (Italy), 2013. Colorfastness to perspiration was determined according to AATCC standard test method 15-1997 (AATCC, 2001). Change in the shade was evaluated using the AATCC Gray Scale for Evaluating Change in Color and color transfer was evaluated using the AATCC Gray Scale for Evaluating Staining (AATCC, 2001). Finally, the crease recovery was determined according to AATCC-128 standard test methods using Shirley crease recovery tester.
3 Results and discussion
3.1 Optimization of the acetylation
In this study, the acetylation process was made for surface modification of cotton parts in PC blends. Different parameters are considered during the acetylation process such as concentration, time and percent of acetylation (Table 1). The response percent acetylation is used to evaluate the degree of the acetyl group that has been substituted in place of the hydroxyl group of cotton cellulose. The experimental design was used to study the relationship between the variables acetic anhydride concentration, and reaction time at room temperature. Analysis of variance ANOVA determines whether the variables acetic anhydride concentration and time are significantly affecting the percent acetylation.
Figure 2 shows the plots of the normality test of the residuals for the degree of substitution, warp way tensile strength and weft way tensile strength respectively. It is observed that in all the graphs, the residuals lie on the straight line, which indicates that the distribution of residuals for percent acetylation is normal. From the results, the assumption of constant variance is confirmed and the suggested model is suitable.
Table 2 shows P-values less than 0.0500 and an F-value of 33.67 implies the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise. The concentration of acetic anhydride (A and A2) and B2 (square of time) are significant model terms. Values greater than 0.1000 indicate the model terms are not significant. The Lack of Fit F-value of 3.04 implies there is a 15.36% chance that a Lack of Fit F-value could occur due to noise. Therefore, this ANOVA table indicates the process is significant and the model is fit.
3.2 Effect of acetic anhydride concentration on percent acetylation
The solvent, glacial acetic acid, acts as a cotton swelling agent by enhancing the accessibility of the cotton hydroxyl groups to the acetylating agent (acetic anhydride) [32, 36]. It was proposed that the reaction was heterogeneous at first, with –OH groups on the surface of solid cellulose being preferentially acetylated, and that as acetylation advanced, cellulose acetylation gradually continued with dissolving in the reaction medium (Fig. 3). The extent of esterification was found to increase with the increase in esterifying agents. Maximum esterification of 35% was obtained when the amount of acetic anhydride was increased from 7 to 15%. Further increasing the concentration of acetic anhydride from 15 to 22% shows a decrease in acetylation percentage (Table 1). A high concentration of acetic anhydride reduce the percent of acetyl content, acid-catalyzed hydrolysis of the ester is responsible for the reduction of percent acetylation and water molecules will react with acetic anhydride beyond 15% of acetic anhydrides and a high concentration of acetic anhydride can cause degradation of carbohydrates which results in a decrease in weight percentage gain. At the fibrous stage, without destroying the fibrous structure the maximum percent acetylation is 32–35% [37].
3.3 Effect of time on percent acetylation
For all of the studies conducted, the extent of percent acetylation increased when the reaction time was increased from 1 to 4.62 h. Further increase in the reaction time from 2.5 to 4.5 h, the percent acetylation was found to decrease from 35 to 32% (Table 1). The maximum value of acetate formation was observed to be 35% at a reaction time of 2.5 h. Longer reaction times result in the hydrolysis of the ester groups or cellulose acetate which causes a reduction of percent acetylation. Figure 4 shows the effect of reaction time on acetate formation. It can be concluded from the time variation study that a maximum level of acetylation was reached by continuing the reaction for the optimum time under the set of reaction conditions. After acquiring the optimum extent of esterification, the cellulose molecule did not undergo further electrophilic displacement with the acyl pyridinium complex. As a result, the establishment of equilibrium between the products and the reactants under the chosen set of reaction circumstances can be interpreted as the leveling-off of the esterification value. The maximum values were achieved for the percent acetylation after the optimum time (2.5 h) duration and at 16% concentration were 34%.
3.4 Fourier transform infrared spectroscopy (FTIR) analysis
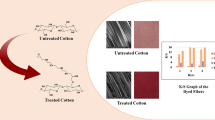
Since the properties of cotton cellulose acetylated are one of the significant factors contributing to the dyeability of the material, differences between the cotton-acetylated sample and cotton cellulose were evaluated to understand the chemical change that has taken place in acetylation (Fig. 5). Acetylated cotton is characterized by FTIR to ensure the reaction of hydroxyl groups with acetic anhydride. A typical loss in transmittance band for the acetylated cotton is observed in the range 3600 cm−1 to 3300 cm−1 that is assigned to the CH3CO− of the acetyl group as also reported by [38]. In this region, the stretch isn't as broad or stronger as it was in cotton OH-stretch. The transmittance band from 3400 to 3000 cm−1 is very broad in the untreated cotton which signifies the large numbers of –OH groups in the cotton [39]. The intensity of the –OH absorption band that the hydroxyl group contents in the cotton fabric were reduced after the reaction. The ester carbonyl absorption peaks at 1712 cm−1 and the C–H stretch at 1242 cm−1 to form an acetyl group [40]. The strong sharp peak at 1250 cm−1 and 1300 cm−1 may be due to the C–O stretching of the polymer backbone. The observed strong sharp peaks were due to the cellulosic component of the fiber materials.
3.5 Moisture regain of modified PC blend
Moisture regains and content of PC blend fabric were calculated by Eq. 2 [41]. As Fig. 6 shows the average test result of four pre-treated polyester/cotton blended samples moisture regain and contents are 2.176% and 1.033% respectively. From the result obtained, moisture of PC blends after acetylation is reduced due to the hydrophilic group of cotton cellulose is replaced the by acetyl group with a medium degree of substitution. Since cellulose fibres or fabric can be directly acetylated without destroying their fibrous structure [42].
3.6 Thermogravimeteric analysis (TGA)
The TG curves of the PC blends and surface-modified cotton fabrics are shown in Fig. 7. The TG curves were separated into three sections. At first, the weight loss in the temperature range of 100 °C to 150 °C could be assigned to the evaporation of water. The weight loss is a direct measure of the volatiles formed during decomposition, and the rate of weight loss is an indication of the thermal decomposition kinetics. In the range of 250 °C to 300 °C, there appeared a minor thermal decomposition of residues. The TG curve revealed that all main decompositions occur between 350 and 500 °C for control PC blends and 354 °C to 554 °C for modified polyester/cotton blends, respectively. Maximum thermal decomposition of control and modified PC blends are 714.87 °C and 718.12 °C as well as the rate of weight loss at this temperature is 92.75% and 88.99% respectively from TGA data. The residual mass percentage beyond this temperature corresponds to the combined ash and fixed carbon percentages. When comparing the samples, it was discovered that the major decomposition temperature of modified PC blend fabric was higher than normal fabric. The result suggests that the increase of thermoplasticity and heat resistance property of cotton was caused by the acetylation [43, 44]. This increment of thermal resistance is required in use for technical textiles and home furniture application area of this blends fabric.
3.7 Crease recovery of modified PC blends
Crease recovery angle of control and modified PC blends after 5-min load application was assessed. The crease recovery angles obtained in warp directions were higher than the crease recovery angles obtained in weft directions for both control and modified PC blends. The crease recovery of control samples is less than modified samples in both warp (52° and 57°) and weft (37° and 42°) direction respectively as shown in Fig. 8. Because the esterification process improves anti-pilling and wrinkle recovery properties of cotton fabrics due to hydroxyl groups of cellulose are replaced by flexible acetyl groups through an esterification reaction. The crease action applied on the fabric was shared by a higher number of yarns in the warp direction which resulted in a better crease recovery. PC blend fabric was pretreated and acetylation is an esterification reaction, which plasticizes cotton by introducing acetyl functional groups. These results provide a tool for the occurrence of esterification reactions and also manifest the morphological changes incurred in the blend substrate which provides a better crease recovery angle [23, 35].
3.8 Dyeing of modified PC blends
Analysis of variance (ANOVA) was used to determine whether the variables like dye concentration and temperature are significantly affecting the response values of color strength and tensile strength in warp and weft directions. Normal % probability vs. residual showed on Fig. 9 for color strength and tensile strength in warp and weft direction lies on straight lines which indicates the distribution of residuals for all test results are normal and the assumption of constant variance is confirmed and suggests the model is suitable. The normal probability plot of the residuals is approximately linear supporting the condition that the error terms are normally distributed in both color strength and tensile strength.
3.9 Effect of dye concentration on colour strength
The dye concentration affects greatly the K/S value of the dyed material. In the dyeing procedure, the dye concentration influences both dye fixation and the color strength of the dyed fabric. As the dye concentration rises, so does the availability of dye molecules in the dye bath, allowing more dye molecules to permeate from the dye bath to the fiber [45]. As a result, the higher the dye exhaustion, the more dye is attached to and diffuses into the fiber, and the more dye is inside and outside the fiber, the better the chance of fixing a larger amount of dye on the fiber. Then the color strength of the fabric becomes increases due to the high concentration of the dye. Table 3 and Fig. 10 show that increasing concentration has no effect on tensile strength in the warp and weft directions. Dye concentration causes significant effects on rubbing fastness properties of both treated and untreated fabrics than other parameters.
3.10 Effect of temperature on warp and weft way tensile strength
The effect of concentration and dyeing temperature on the responses’ color strength was shown in Fig. 10a. According to the results, both dye concentration and dyeing temperature greatly increase color strength, as shown in Table 3, and there is no significant change in fabric tensile strength throughout the warp and weft directions when the temperature is varied. As shown in Fig. 10b, c and Table 3, increasing the temperature had a substantial effect on tensile strength in the warp and weft directions, although there was no change in strength as dye concentration increased. The desirability was found to be 0.7 for the preferred criteria of optimization are 1% shade at optimum dyeing temperature 120 °C and minimum loss of tensile strength in the warp (1143 N) and weft (624 N) direction were observed. Tensile strength in the warp and weft directions was reduced by 13% and 10%, respectively, under these conditions compared to control fabric samples. In case of fabric hand properties, there was no difference between the modified and unmodified PC blend samples examined in the assessment of fabric PC blends (by touch of hand). Apart from that, acetylation improves the quality of modified samples by forming an even distribution on the treated fabric's surface [46].
3.11 Effect of temperature on colour strength of modified PC blends
As shown in Fig. 10a K/S value reflects that dyeing temperature has a great impact on dye uptake for both fabrics. A dramatic change occurs in the fiber polymer system when the temperature is applied as a medium of energy. At lower temperature, 105 to 110 °C color strength of dyed modified PC blends are low. From the result, blend composition has a higher proportion of polyester. The disperse dyeing of polyester at this temperature is difficult due to the crystal structure of polyester were not open at this temperature [47]. It results in lower diffusion of dye into fibre structure. The color strength of modified PC blends was determined at 120, 130 and 134 °C which results in good color strength as shown in Table 3. With increasing dye fixation, the glass transition temperature of the polyester portion is lowered and its structure is opened, allowing significant diffusion of dispersed dye into the fiber structure. Rising the temperature from 120 to 134 °C increases the color strength of the dyed fabric, in opposite it reduces the tensile strength of fabric along the warp and weft direction. The optimized dyeing condition of modified PC blend with disperse dye in one-bath one-step method is found at a concentration of 1% and a temperature of 120 °C, with tensile strengths of 1142 N and 624 N in the warp and weft directions, respectively, as shown in Table 3.
3.12 Colourfastness properties
In general, the fastness properties of modified PC blend fabrics are dyed with disperse-yellow dye were studied with different dye concentrations (1–3% o.w.f). Table 4 indicates that the color fastness to washing of a one-bath one-step dyed PC blend with disperse dye after surface modification is comparable to a standard two-bath two-step dyed fabric sample. The hydrophobic nature of disperse dye results in the good wash fastness property of dyed fabric [48]. In general, the fastness properties of the samples examined are moderate to good.
As shown in Table 4, the color fastness to washing (color change) of one bath one-step dyed polyester/cotton blend were show good fastness property compared to conventional two-bath two-step dyed sample. This is due to disperse dye is hydrophobic in nature the tendency of hydrolyzed with water during washing less and disperse dye form a strong mechanical bonding with an acetyl group and ester functional group of both polyester portion as well as acetylated cotton. Particulate disperse dye molecules accumulate at the surface of the dyed substrate because of the dye’s low aqueous solubility, which impairs the brightness of the dyeing and during reductive clearing process all unreacted disperse dye is removed by reductive clearing agents and only dye fiber bond formation remained and hydrophobic in nature of disperse dye results good wash fastness property.
As shown in Table 5 the samples dyed in one-bath one-step disperse dye after surface modification of PC blend have good dry/wet rubbing and lightfastness compared to conventional two-bath dyed samples [8].
Color fastness to perspiration (acid and alkaline) refers to a dyed fabric’s capacity to resist fading and staining when perspired, and it is one of the most used color fastness testing items for textiles. When the garments which come into contact with the body have high fastness to perspiration, they may suffer serious local discoloration. The results in Table 6 were intended to determine the resistance of color removal from dyed textile by the action of acidic and alkaline conditions. From the result, good fastness to perspiration was determined for modified PC blend under acid and alkali conditions compared to conventional two-bath dyed fabrics.
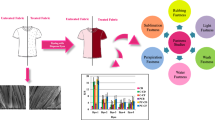
Figure 11 shows the color strength of modified PC blends dyed in the one-bath one-step dyeing method. From the result obtained K/S values indicate the depth of shade reflected from the dyed fabric surface. The result represents good color strength is determined and the K/S values were within the acceptable limit for practical dyeing. Based on the test results disperse dye has better affinity and dyeability with fibers ester or acetyl functional groups.
3.13 Dyeing mechanism
In the mechanism of the aqueous phase transfer of disperse dyes, a greater proportion of dye is present in bulk dispersion within the dye bath, while a small amount of dye dissolves and forming an aqueous solution. Dye molecules from the aqueous solution bind to the fiber surface and diffuse from there to the interior of the substrate. Transfer of dye molecules from the external aqueous phase to the surface and interior of the substrate up to its saturation equilibrium corresponds to the diffusion process. Dyeing of disperse dye with hydrophobic fiber categorized under a free volume model thermodynamic dye diffusion principles, in which the diffusion of a dye molecule, which has been initially adsorbed onto a polymer chain, through a polymeric material such as fiber can only occur when the segmental mobility of the composite molecular chains is such that sufficient free volume is formed that can accommodate both the dye molecule and chain segment [49, 50]. After surface modification of cotton parts blended polyester and cotton forms ester and acetyl hydrophobic chemical structure which conforms to free volume thermodynamic dyeing diffusion. An increase in temperature will result in a corresponding increase in the mobility of the molecular chains within the fiber, which are leads to both thermal expansion and reduced density fiber which results in an increase in dye diffusion through the open structure of the fiber. From Table 7 temperature and K/S of dyed samples were increased.
Polyester/cotton blend dyed exhibit one tone color effect since partially acetylation carried out on cotton parts, which do not show high color strength than polyester parts and considering the strength of cotton parts optimized temperature of dyeing these blends done at 120 °C, this condition also enhance the leveled color strength of both cotton and polyester parts.
The tensile strength of PC blend dyed in one-bath one-step methods with disperse dye after surface modification of cotton has slightly lower than conventional two-bath dyed samples. Table 7 shows the tensile strength along warp and weft directions are decreased in 13% and 10% respectively in one-bath one-step dyed when compared with dyed control samples. This is due to acetylation conditions and dyeing temperature reduces the tensile strength of PC blends. Cotton’s strength was reduced during the acetylation process due to the use of acidic media in the acetylation bath [51]. This result shows cellulose has a low tendency to resist both weak and strong acids. Only the dyeing temperature affects fabric tensile strength in two-bath dyeing [47]. From the result as temperature increased the strength of PC blend samples is reduced due to high-temperature high-pressure dyeing of polyester affect the strength of cotton and acidic media of disperse dyeing of polyester portion also considered for reduction of cotton strength. The difference of strength loss between the two methods is not causing considerable loss of strength whereas acetylation methods and selected catalyst made a slight effect on the tensile strength of the fabric.
The tear strength of PC blends dyed with disperse dye in one-bath one-step methods results is almost similar compared to conventional two-bath dyed samples. Table 7 shows that one-bath one-step dyed sample of modified PC blend tear strength along warp and weft direction is decreased in 8% and 7% respectively when compared with control samples because acetylation condition and dyeing temperature reduces in both tensile strength and tear strength of PC blends.
From experimental results, modification of PC blend can be carried out to increase the hydrophobic character of cotton by reactions that covalently bind bulky aryl residues to the fibers by acetylation process. From the result of fastness tests and abrasion resistance, we understood that good color strength was determined.
3.14 Pilling assessment
The samples are then given a rating of between 1 and 5 by using photographic assessment. The visual assessment table is given below: [ISO 12945-1] (Table 8).
Pilling test was accomplished by ISI pilling box machine obeying ISO 12945-1:2000 standard method where four specimens were tumbled in pilling box for 11,500 numbers of revolutions. Pilling of polyester/cotton blend was high since the higher presence of polyester fiber on blended fabric affects the pilling attitude of the fabric to downgrade. The main reason is that although cotton hairiness breaks during the pilling process, the broken cotton hairiness is tightly wrapped by polyester hairiness due to the entanglement of the strong polyester hairiness. Polyester anchor hairiness makes the pills difficult to fall off, resulting in an increase in the number of pills. Therefore, the polyester–cotton blended woven fabric is easily prone to pilling. The esterification process improves the anti-pilling and wrinkle recovery properties of cotton and the acetylation process reduces the strength of cotton fibers not responsible to enhance the more pilling effect on the fabric surface.
In comparison to the traditional two-bath approach, there is a substantial change in fastness qualities. This one-bath one-step dyeing procedure conserves water and chemical, as well as energy and time. In this study, it was discovered that compared to the traditional two-bath dyeing procedure, one dying approach saves cost and time [14]. When compared to the traditional two-bath dyeing procedure, the one-bath one-step dyeing approach for PC blend cloth is more cost-effective and environmentally beneficial. If the processes are accepted, it will be profitable commercially [52].
The development of more effective dyes that can be fixed fiber with higher efficiency, reducing losses in tailings waters and reducing the amount of dye required in the dyeing process, would be an alternative to minimize the problems associated with the treatment of textile effluents, and would undoubtedly improve the effluent’s cost and quality [46]. The textile services industry is dedicated to a long-term business model that is as environmentally conscious as possible. Increasing the usage of polyester/cotton blends in textile production helps to mitigate environmental challenges and greenhouse gas emissions, contributing to a more sustainable future [53].
4 Conclusion
PC blend dyed with disperse dye in one-bath one-step dyeing methods after surface modification of cotton by acetylating methods is studied. Using fibrous acetylation methods, the surface of cotton fabric was modified. The best value for acetylation was obtained using a 16 percent acetylation agent concentration and reaction duration of 2.5 h, yielding a percent acetylation of 34. The experiment result of dyeing showed that the optimum value was obtained by dye concentration above 1% at temperature 120 °C, warp tensile strength decreased by about 12% and weft tensile strength was decreased by 9% from the control half-bleached fabric. Therefore, techniques must be devised and studied to introduce a chemical surface modification of PC blends without significantly affecting other physical properties. The method would have a significant potential for industrial application as the one-bath one-step dyeing methods relatively safe, environmentally friendly technique, with less requirement of energy, water, time, and resource as PC blends dyed with one dye. Results of this study showed that a one-step one-bath dyed modified PC blend with disperse dye fabric presents good fastness property, abrasion resistance and tear strength value compared with conventional two-bath dyed fabric. The one-bath one-step dyeing methods of PC blends with disperse dye also showed level dyeing having good color strength values properties and the methods offer the option of cost-effectiveness.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from all authors on reasonable request.
References
Rowe HD. Biotechnology in the textile/clothing industry—a review. J Consum Stud Home Econ. 1999;23(1):53–61.
Zou Y, Reddy N, Yang Y. Reusing polyester/cotton blend fabrics for composites. Compos B Eng. 2011;42(4):763–70.
Chattopadhyay D, Shaikh T. Dyeing of polyester/cotton blends—an overview. Man Made Text India. 2002;45(12):456–69.
Maradiya HR, Patel VS. Dyeing of hydrophobic fabrics with disperse dyes. J Serb Chem Soc. 2001;66(6):367–76.
Uddin F. Introductory chapter: textile manufacturing processes. In: Textile manufacturing processes. London: IntechOpen; 2019.
Zhang W, et al. One-bath one-step low-temperature dyeing of polyester/cotton blended fabric with cationic dyes via β-cyclodextrin modification. Text Res J. 2019;89(9):1699–711.
Khanzada H, Khan MQ, Kayani S. Cotton based clothing. In: Cotton science and processing technology. Singapore: Springer; 2020. p. 377–91.
Najafi H, et al. One bath method dyeing of polyester/cotton blend fabric with sulphatoethylsulphonyl disperse/reactive dyes treatment by chitin biopolymer. Afr J Biotechnol. 2009;8(6).
Maeda S, et al. One-bath dyeing of polyester/cotton blends with reactive disperse dyes in supercritical carbon dioxide. Text Res J. 2004;74(11):989–94.
Muralidharan B, Laya S. A new approach to dyeing of 80: 20 polyester/cotton blended fabric using disperse and reactive dyes. Int Sch Res Not. 2011. https://doi.org/10.5402/2011/907493.
Singha K. Characterization of dyeing P/C blends fabric: a thermodynamic view. Int J Text Sci. 2013;2(1):1–6.
Kim M, et al. Dyeing of cotton and polyester/cotton blend with disperse dyes using sodium 2-(2, 3-dibromopropionylamino)-5-(4, 6-dichloro-1, 3, 5-triazinylamino)-benzenesulfonate. Fibers Polym. 2006;7(4):352–7.
Elsherbiny AS, Kaukab M. One-bath one-step dyeing of a polyester/cotton blend using the pad-dry-fixation process. Fibres Text East Eur. 2016;2(116):113–9.
Prasad RK, Shahid MA, Dulal M. A comparative study between one bath dyeing method for polyester cotton (PC) blended fabric over conventional two bath dyeing method. Eur Sci J. 2015;11(33).
Wen-chan C. One-bath-two-step dyeing process of T/C fabric with disperse/reactive dyes. Dyeing Finish. 2013;3.
Meena C, et al. One-bath dyeing process for polyester/cotton blend using physical mixtures of disperse/reactive dyes. Eur Int J Sci Technol. 2013;2(2):6–16.
Shahidi S, Wiener J, Ghoranneviss M. Surface modification methods for improving the dyeability of textile fabrics. In: Eco-friendly textile dyeing and finishing, vol. 10. London: InTechOpen; 2013. p. 53911.
Shahidi S, Wiener J, Ghoranneviss M. Surface modification methods for improving the dyeability of textile fabrics. In: Eco-friendly textile dyeing and finishing. London: InTechOpen; 2013. p. 34–50.
Tausif M, et al. A comparative study of mechanical and comfort properties of bamboo viscose as an eco-friendly alternative to conventional cotton fibre in polyester blended knitted fabrics. J Clean Prod. 2015;89:110–5.
Awal A, et al. Surface modification of cotton fabric by wet chemical treatments to impart hydrophobicity. Res J Text Appar. 2010;14(1):65–74.
Baiardo M, et al. Surface chemical modification of natural cellulose fibers. J Appl Polym Sci. 2002;83(1):38–45.
Lisperguer J, et al. The effect of wood acetylation on thermal behavior of wood-polystyrene composites. J Chil Chem Soc. 2007;52(1):1073–5.
Ashori A, et al. Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohydr Polym. 2014;102:369–75.
Hill C, et al. Kinetic and mechanistic aspects of the acetylation of wood with acetic anhydride. Holzforschung Int J Biol Chem Phys Technol Wood. 1998;52(6):623–9.
Saha P, et al. A brief review on the chemical modifications of lignocellulosic fibers for durable engineering composites. Polym Bull. 2016;73(2):587–620.
Zhang L, et al. Relating electron donor and carboxylic acid anchoring substitution effects in azo dyes to dye-sensitized solar cell performance. ACS Sustain Chem Eng. 2013;1(11):1440–52.
Gür M, Kocaokutgen H, Taş M. Synthesis, spectral, and thermal characterisations of some azo-ester derivatives containing a 4-acryloyloxy group. Dyes Pigm. 2007;72(1):101–8.
Adeel S, et al. Sustainable dyeing of microwave treated polyester fabric using disperse yellow 211 dye. J Mex Chem Soc. 2018. https://doi.org/10.29356/jmcs.v62i1.580.
Ketema A, Worku A. Review on intermolecular forces between dyes used for polyester dyeing and polyester fiber. J Chem. 2020. https://doi.org/10.1155/2020/6628404.
El Nemr A, Ragab S, El Sikaily A. Testing zinc chloride as a new catalyst for direct synthesis of cellulose di-and tri-acetate in a solvent free system under microwave irradiation. Carbohydr Polym. 2016;151:1058–67.
Anbu N, et al. Acetylation of alcohols, amines, phenols, thiols under catalyst and solvent-free conditions. Chemistry. 2019;1(1):69–79.
Bello A, et al. Acetylation of cotton stalk for cellulose acetate production. Am Sci Res J Eng Technol Sci (ASRJETS). 2016;15(1):137–50.
Bello-Pérez LA, Contreras-Ramos SM, Jìmenez-Aparicio A. Acetylation and characterization of. Acta Cient Venez. 2000;51:143–9.
Gesesse GD, et al. On the analysis of diffuse reflectance measurements to estimate the optical properties of amorphous porous carbons and semiconductor/carbon catalysts. J Photochem Photobiol A Chem. 2020;398:112622.
Pottier F, et al. Simulating the composition and structuration of coloring layers in historical painting from non-invasive spectral reflectance measurements. C R Phys. 2018;19(7):599–611.
Mantanis G, Young R, Rowell R. Swelling of compressed cellulose fiber webs in organic liquids. Cellulose. 1995;2(1):1–22.
Frisoni G, et al. Natural cellulose fibers: heterogeneous acetylation kinetics and biodegradation behavior. Biomacromol. 2001;2(2):476–82.
El Nemr A, et al. Synthesis of cellulose triacetate from cotton cellulose by using NIS as a catalyst under mild reaction conditions. Carbohydr Polym. 2015;130:41–8.
Stenstad P, et al. Chemical surface modifications of microfibrillated cellulose. Cellulose. 2008;15(1):35–45.
Zhuang J, et al. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl Sci. 2020;10(12):4345.
Hussain U, et al. Comfort and mechanical properties of polyester/bamboo and polyester/cotton blended knitted fabric. J Eng Fibers Fabr. 2015;10(2):155892501501000200.
Yan L, Kasal B, Huang L. A review of recent research on the use of cellulosic fibres, their fibre fabric reinforced cementitious, geo-polymer and polymer composites in civil engineering. Compos B Eng. 2016;92:94–132.
Hill CA, Khalil HA, Hale MD. A study of the potential of acetylation to improve the properties of plant fibres. Ind Crops Prod. 1998;8(1):53–63.
Adebajo M, et al. Raman spectroscopic investigation of acetylation of raw cotton. Spectrochim Acta A Mol Biomol Spectrosc. 2006;64(2):448–53.
Shahid MA, et al. Effect of different dyeing parameters on color strength & fastness properties of cotton-elastane (ce) and lyocell-elastane (le) knit fabric. Int J Text Sci. 2016;5(1):1–7.
Chequer FD, et al. Textile dyes: dyeing process and environmental impact. Eco-friendly Text Dyeing Finish. 2013;6(6):151–76.
Chakraborty J. Fundamentals and practices in colouration of textiles. Boca Raton: CRC Press; 2015.
Micheal M, Tera F, Ibrahim S. Effect of chemical modification of cotton fabrics on dyeing properties. J Appl Polym Sci. 2002;85(9):1897–903.
McKenna GB. Glass formation and glassy behavior. Pergamon Press plc Compr Polymer Sci Synth Charact React Appl Polym. 1989;2:311–62.
Padbury RP. Bulk property modification of fiber forming polymers using vapor phase techniques. Raleigh: North Carolina State University; 2014.
Onyekwere O, Igboanugo A, Adeleke T. Optimisation of acetylation parameters for reduced moisture absorption of bamboo fibre using Taguchi experimental design and genetic algorithm optimisation tools. Niger J Technol. 2019;38(1):104–11.
Sarker F, et al. Scope of dyeing polyester cotton (PC) blended fabric in single bath process for water, energy and time saving. IOSR J Polymer Text Eng. 2015;2(3):12–6.
Notten P. Sustainability and circularity in the textile value chain: global stocktaking. 2020.
Acknowledgements
The authors would like to acknowledge the Ethiopian Institute of Textile and Fashion Technology (EiTEX) finance office.
Funding
This research was funded by the Ethiopian Institute of Textile and Fashion Technology (EiTEX), Bahir Dar University, Ethiopia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GKumsa, GG and GKetema. The first draft of the manuscript was written by GKumsa and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumsa, G., Gebino, G. & Ketema, G. One-bath one-step dyeing of polyester/cotton (PC) blends fabric with disperse dyes after acetylation of cotton. Discov Mater 1, 19 (2021). https://doi.org/10.1007/s43939-021-00019-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-021-00019-7