Abstract
This paper discusses the rate of hydration of magnesia (CCM1 and CCM2) during the formation of magnesium hydroxide with magnesium acetate and distilled water. The influence of magnesium acetate and the reactivity of the two types of caustic calcined magnesia were studied by thermogravimetric analysis, differential scanning calorimetry, mass spectrometry, particle size detection and pH. Also, both citric acid and acetic acid test were done to measure the reactivity of magnesium oxide powder. The results indicate that the hydration rate of both oxides in magnesium acetate system are vigorously exothermic compared to the water system. The study shows mechanistically that magnesium acetate enhanced the degree of hydration of magnesium oxide due to the presence of acetate ions and Mg2+ ions when it compared to water. Mathematical models confirm the findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Magnesium hydroxide has become of high importance in the field of powder technology. It is used in many industrial applications as filler in the paper industry, flame retardants added to the polymer matrix, antibacterial agent and neutralizer of acidic water pollutants, water wastes and gases. Magnesium hydroxide can be used as catalyst and catalyst support in wastewater treatment [1,2,3] also common application uses are a source material to produce magnesium oxide [4, 5]. The formation of ultra-superfine magnesium hydroxide powder led to improvement in the mechanical properties of the material such as a high thermal stability, high fire retardant and very low toxicity [6]. The studies performed by Dong et al. [7] showed that nano-sized magnesium hydroxide can be used as an antibacterial agent as well [8]. The origin of magnesium hydroxide is either natural as a mineral or synthetically produced from seawater or brines, or from the hydration of magnesium oxide. Magnesium oxide is an inorganic compound that occurs in nature as the mineral periclase and can be also obtained through heating of the various forms of magnesium carbonate. The reactivity of magnesium oxide depends on the temperature and the duration of heat treatment of the magnesium compounds during the production of magnesium oxide. The rate of MgO hydration depends on its particle size, specific surface area and purity. Mg(OH)2 generated in water is located on the surface of MgO particles, therefore particle size is increasing [9, 10].
The reaction of magnesium oxide in water occurs according to the reaction Eq. (1)
During the hydration reaction Smithson and Bakhshi [11] observed that there is a growth in the particle size of the powder. The magnesia hydration mechanism is considered similar to the one on the magnesium oxide surface where water molecules absorbed chemically forming a surface layer of magnesium hydroxide [12].
The chemical adsorption of water on metallic oxide surfaces is given by Eq. (2):
Filippou et al. [13, 14] Reported that the hydration process is a process of dissolution–precipitation of magnesium oxide. The acetate ions in magnesium acetate solution enhanced the hydration process behavior of magnesium oxide. Magnesium acetate dissociation represented in Eq. (3):
Then followed by magnesia dissolution Eq. (4):
Or can react directly by acetic acid formed in solution [Eq. (5)]:
Finally, dissociation of magnesium complexes and magnesium hydroxide precipitation is due to supersaturation as shown in Eq. (6) [15]:
The reactivity of caustic calcined magnesia can be measured by citric acid test or acetic acid test where the time needed for the magnesium oxide sample to neutralize the citric acid/acetic acid solution is measured. Values less than 60 s indicate a highly reactive magnesium oxide. Medium reactive magnesium oxide gives measurements between 180 and 300 s for the standardized test. A low reactivity magnesium oxide gives a value of more than 600 s [16]. The aim of this paper is to study the hydration process in a comparative manner in two different media with two different types of magnesia. The effects of thermogravimetric analysis, differential scanning calorimetry, mass spectrometry, particle size and pH on the degree of hydration were studied. The results give insight on the influence on the amount produced.
2 Materials and methods
The experiments for the hydration process included two types of commercially available caustic calcined magnesia, namely CCM1 and CCM2. The hydration of two types of magnesia in different media of magnesium acetate and water is studied. The composition of the raw material is shown in Table 1.
CCM1 and CCM2 are commercial grades with approx. purity more than 96% MgO content.
2.1 Citric acid reactivity test
For the determination of the reactivity 2.0 g of untreated MgO powder were introduced into 100 mL of 0.4 N citric acid solution under stirring and monitored measurement of pH. The time to reach a pH value of 8.6 is recorded and noted as a measure for the reactivity of the caustic magnesia.
2.2 Acetic acid reactivity test
For comparison, also the reactivity vs. acetic acid was investigated. Therefore 5.0 g of caustic magnesia was added to 100 mL of 1.0 M acetic acid under stirring condition and the time to reach neutralization (pH = 7) was checked with a pH meter.
2.3 Particle size and pH measurements
Particle size distribution of the solid particles within the suspensions was determined using a Malvern Mastersizer 2000. Particle size measurement range: 0.02–2000 Microns with 1000 times/s scanning speed. The measurements were conducted in water and several drops of suspension were introduced into the measurement chamber until the signal to noise ratio was within the acceptable limits. The pH value was recorded at room temperature.
2.4 Transmission electron microscope (TEM)
The morphology and the particle size of the prepared samples were examined by using a transmission electron microscope JEOL JEM-1400 120 kV TEM for both types of caustic calcined magnesia “CCM1 and CCM2”. Using a hydrophobically modified copper grid sample holder.
2.5 Calorimetry
Isothermal calorimetric investigation was conducted on a TAM AIR thermometric calorimeter from TA Instruments. MgO samples were hydrated with tap water and with 0.2 M magnesium acetate solutions as hydrating agent. Each sample contained 1.6 g of solid and 6.4 g of water and magnesium acetate, respectively. Firstly, the samples were mixed with a Vortex test tube shaker for 60 s and then placed in the calorimeter for 24 h measurements of heat evolution.
2.6 Mathematical models
To predict properties of the hydration process, the model tree (MT) method is used, M5 model rules. The comparison parameters are the Mean Absolute Error (MAE), the correlation coefficient (R2) and the Root Mean Squared Error (RMSE).
2.7 Pilot scale hydration temperature and torque recording
Lab scale hydration was conducted within a closed metallic stirred vessel (diameter approximately 22 cm) with temperature and torque recording. Temperature and torque readings were taken in 10 s intervals for ~ 24 h. Stirring was accomplished by a Visco-Jet-type stirrer with diameter of 12 cm and stirring rate of 250 rpm. In each hydration process, 560 g of magnesium oxide were placed in 2240 g water or magnesium acetate (0.2 M) solution. Starting temperature for the hydration was ~ 40 °C.
2.8 Thermogravimetric analysis, differential scanning calorimetry, mass spectrum
The measurements were conducted on a “STA 449 F3 Jupiter” from Netzsch using atmospheric air at 60 mL/min and Al2O3 crucibles starting at room temperature to 700 °C with heating rate of 10 °C/min. Recordings of TGA and DSC, coupled with MS (mass spectrometer) was used to determine the thermal decomposition curves and endothermic as well as exothermic behavior together with the species that are separated from the matrix. Mass fragments recorded were m/z = 18 for water release and m/z = 44 for the release of carbon dioxide. Samples were hydrated for 30 min and 24 h, respectively, prior to measurement. For instance, 30 min samples were hydrated for 15 min under stirring, then centrifuged for 15 min at 8500 rpm (Biofuge centrifuge) to strip off the water part and then the sample was resuspended in acetone, then again centrifuged for 15 min to obtain the solid part and finally dried at 120 °C for 2 h. 24 h samples were centrifuged for 15 min at 8500 rpm to release the water portion and resuspended in acetone prior to drying at 120 °C for 2 h.
3 Results and discussion
3.1 Reactivity test
The reactivity of caustic calcined magnesia can be measured by citric acid test or acetic acid test, where the time needed for the magnesium oxide sample to neutralize the citric acid/acetic acid solution is measured. Values less than 60 s indicate a highly reactive magnesium oxide.
Medium reactive magnesium oxide gives measurements between 180 and 300 s for the standardized test. A low reactivity magnesium oxide gives a value of more than 600 s.
3.2 Citric acid reactivity test
The citric acid test for reactivity of the CCM1 and CCM2 samples gives a reactivity value of 79.8 s and 81.6 s, respectively which corresponds to the values given for a highly reactive samples.
3.3 Acetic acid reactivity test
The acetic acid test for reactivity of the first type of the CCM1 sample gives a reactivity value of 19.5 s, which corresponds to the values given for a highly reactive sample. The vigorous reactivity of CCM2 was reflected by a reactivity value of 13 s.
3.4 Effect of particle size and pH measurement
The particle size (D50) of CCM1 and CCM2 were measured with magnesium acetate system and with water system. As shown in Table 1 the particle size of CCM1 in water system is smaller than in magnesium acetate system (8.5 µm and 13.6 µm, respectively), while the particle size of CCM2 in water system and magnesium acetate system were 7.3 µm and 16.7 µm, respectively. The hydroxide formed from the hydration in water had surface area smaller than hydroxide obtained from magnesium acetate. Rapid hydration occurred due to large particle size and high surface area of hydroxide produced from magnesium acetate system, so the dispersion effect is better [14, 17]. The pH values in magnesium acetate system for both oxides are more acidic than in water system. The hydration degree enhanced in acidic media due to higher concentration of H+ ion in the slurry that increased the solubility of MgO as shown in Table 2 [4, 5].
3.5 Transmission electron microscope (TEM)
The morphology and the particle size of the prepared samples were examined by using a transmission electron microscope JEOL JEM-1400 120 kV TEM for both types of caustic calcined magnesia “CCM1 and CCM2” using a hydrophobic modified copper grid holder.
As shown in Figs. 1, 2 very distinct and finely structured features of platelets and hexagonal sheets for the hydrated CCM samples, there is no significant different observed between the two types of caustic calcined magnesia.
3.6 Calorimetry test
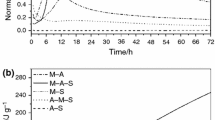
Isothermal calorimetry is a useful technique to monitor the hydration of CCM1 and CCM2 with different hydrating agents, water and magnesium acetate and their impact on the hydration process. The exothermic reaction in the previous two systems is studied over time. As shown in the Fig. 3, after magnesium acetate system is 33 mW, while in water system is 13 mW. The reaction rate decreases with increasing time. The results indicate that the reaction rate of magnesium oxides during the first few hours showed the highest peak in magnesium acetate system, no significant difference between both oxides was observed.
3.7 Lab scale hydration temperature and torque recording
As shown in Fig. 4 this result was confirmed by TGA. The degree of hydration in the magnesium acetate system was higher than that in the water system. As a function of temperature CCM released more heat in magnesium acetate system than that of water in short time range.
The exothermic reaction in magnesium acetate system reached its peak after almost 1 h at 60 °C for CCM1, while 44 °C in water. For CCM2 the exothermic reaction in magnesium acetate system reached 55 °C, while in water it was 40 °C after almost 1.5 h. The two types of magnesium oxide behave the same [12].
The study is reported that the magnesia hydration rates increased with an increase in hydration temperature. An increase in the hydration temperature accelerates the course of hydration. [14].
3.8 Mathematical models
As can be seen in Fig. 5, the Model Tree method applied on the mixtures under investigation revealed that calculated values are in good agreement with experimental data. Despite a slight deviation from the actual heat evolution compared to the experimental data the results of the mathematical models can be used to identify the hydration paths. The use of M5 Model Rules (MT) has shown greater computational efficiency accuracy of the results [18].
3.9 Thermogravimetric analysis, differential scanning calorimetry, mass spectrum
Table 3 summarizes the results obtained. The hydroxide content of CCM1 and CCM2 in magnesium acetate system is significantly higher than that in water system at the same time of hydration. While after 24 h the hydroxide content of both oxides increases. TG results for both oxides (CCM1 and CCM2) show similar tendencies for the two different media [19]. The percentage of hydroxide content after 30 min in magnesium acetate and water system as shown in Table 3: 64.7%, 38.4% respectively, after 24 h the percentage of hydroxide content 85.1%, 76.3% respectively. There is acceleration in the amount of magnesium hydroxide content after 30 min until 24 h [19]. According to stoichiometric considerations, complete hydration of 100% pure material is equal to a mass loss of 30.9%. This assumption is valid only in the case that the magnesium oxide does not contain any other compound in the hydrated form.
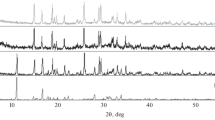
For the comparison of hydration process of magnesia in different slurries representative results from thermogravimetry measurement are depicted in Figs. 6, 7, 8. According to the DSC curve in Fig. 8, it can be observed in the temperature range from 250 to 500 °C, that after 0.5 h of hydration of magnesia the amount of hydroxide produced in magnesium acetate system is much higher than that in water system. While, after 24 h there is no significant difference in the amount of hydroxide produced.
Figure 9a, b showed the emission of carbon dioxide gas and water vapor in the hydration process of magnesia in magnesium acetate and water system at temperature range (250–500) ºC, Fig. 9a reported that, generally the water released (m/z = 18) after 24 h showed higher ion current intensity than that after 0.5 h hydration reaction. No absence of magnesium acetate in hydroxides after 24 h. In Fig. 9b, the carbon dioxide (m/z = 44) emission is enhanced in the hydration reaction that occurred in the magnesium acetate system after 24 h indicating that the ion current signal after 24 h exceeds the signal after 0.5 h of the start of the hydration in the same system. For comparison between the different systems water and magnesium acetate the ion current intensity in magnesium acetate system emits a huge amount of carbon dioxide compared to that of water as shown in Fig. 9b.
a TGA-MS data for m/z = 18 from 250 ºC to 500 °C of two different ccm types hydrated for 30 min and 24 h in presence and absence of MgAc (A: 30 min/water) (B: 30 min/MgAc) (C: 24 h/water) (D: 24 h/MgAc). b TGA-MS data for m/z = 44 from 250 ºC to 500 °C of two different ccm types hydrated for 30 min and 24 h in presence and absence of MgAc (A: 30 min/no MgAc) (B: 30 min/MgAc) (C: 24 h/no MgAc) (D: 24 h/MgAc)
4 Conclusion
Experimental data show increasing magnesia hydration rates in presence of magnesium acetate and with increasing temperature. In this study, there is no significant difference observed between the two types of caustic magnesia powder. On the other hand, hydration rate was influenced by the different hydrating media. From the results it seems that after 30 min hydration process is not completed because after 30 min the percentage of magnesium hydroxide accelerated. The hydration rate increased in magnesium acetate system more than that of water due to the additional acetate ions in the solution. In thermal gravimetric analysis the degree of hydration improved from hydration time of 0.5–24 h in water and magnesium acetate from 40 to 74.7% and from 65.7 to 84.1%, respectively.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Gulková D, Šolcová O, Zdražil M. Preparation of MgO catalytic support in shaped mesoporous high surface area form. Microporous Mesoporous Mater. 2004;76:137–49. https://doi.org/10.1016/j.micromeso.2004.07.039.
Climent MJ, Corma A, Iborra S, Mifsud M. MgO nanoparticle-based multifunctional catalysts in the cascade reaction allows the green synthesis of anti-inflammatory agents. J Catal. 2007;247:223–30. https://doi.org/10.1016/j.jcat.2007.02.003.
Gao C, Zhang W, Li H, Lang L, Xu Z. Controllable fabrication of mesoporous MgO with various morphologies and their absorption performance for toxic pollutants in water. Cryst Growth Des. 2008;8:3785–90.
Shand MA. The chemistry and technology of magnesia. Hoboken: Wiley; 2006.
Aphane E, Merwe E, Strydom C. Influence of hydration time on the hydration of MgO in water and in a magnesium acetate solution. J Therm Anal Calorim. 2009;96:987–92.
Li X, Shi T, Chang P, Hu H, Xie J, Liu Y. Preparation of magnesium hydroxide flame retardant from light calcined powder by ammonia circulation method. Powder Technol. 2014;260:98–104.
Dong C, Song D, Cairney J, Maddan OL, He G, Deng Y. Antibacterial study of Mg(OH)2 nanoplatelets. Mater Res Bull. 2011;46:576–82.
Sierra-Fernández A, Gomez-Villalba LS, Milosevic O, Fort R, Rabanal ME. Synthesis and morpho-structural characterization of nanostructured magnesium hydroxide obtained by a hydrothermal method. Ceram Int. 2014;40:12285–92.
Pilarska AA, Klapiszewski Ł, Jesionowski T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: a review. Powder Technol. 2017;319:373–407.
von Hoessle F, Mohamed M, Farid R, Hammouda RM, Kühn FE, Bassioni G. Interfacial phenomena of magnesium hydroxide micro phases. Ain Shams Eng J (2021). https://doi.org/10.1016/j.asej.2021.01.020.
Holub M, Estokova A, Demcak S, Stone C. Analytical methods for the determination of the input material quality for gypsum wallboard production. IOP Conf Ser Mater Sci Eng. 2018;385:012020.
Rocha SDF, Mansur MB, Ciminelli VST. Kinetics and mechanistic analysis of caustic magnesia hydration. J Chem Technol Biotechnol Int Res Process Environ Clean Technol. 2004;79:816–21.
Filippou D, Katiforis N, Papassiopi N, Adam K. On the kinetics of magnesia hydration in magnesium acetate solutions. J Chem Technol Biotechnol. 1999;74:322–8.
Von Hoessle F, Mohamed M, Farid R, Hammouda RM, Kühn FE, Bassioni G. Interfacial phenomena of magnesium hydroxide micro phases. Ain Shams Eng J. 2021. https://doi.org/10.1016/j.asej.2021.01.020.
Kurama H, Hosgun HL. Magnesium hydroxide recovery from magnesia waste by calcinations and hydration processes. Physicochemical Probl Miner Process. 2015;51(1):33–245.
Aphane ME. The hydration of magnesium oxide with different reactivities by water and magnesium acetate. Adelaide: University of South Australia; 2009.
Merwe E, Strydom C. Hydration of medium reactive magnesium oxide using hydration agents. J Therm Anal Calorim. 2006;84:467–71.
Bassioni G, Farid R, Mohamed M, Hammouda RM, Kühn FE. Effect of different parameters on caustic magnesia hydration and magnesium hydroxide rheology: a review. Mater Adv. 2021. https://doi.org/10.1039/D0MA00887G.
Tang X, Lv Q, Yin L, Nie Y, Jin Q, Ji Y, Zhu Y. Pilot scale experiments of magnesia. InAIP Conf Proc. 2017;1864: 020004. https://doi.org/10.1063/1.4992821.
Acknowledgements
This research was supported by the Department of Chemistry at the Technical University of Munich (TUM). Special thanks to researchers and lab engineers who performed some measurements and provided their insight and expertise that greatly assisted this study.
Author information
Authors and Affiliations
Contributions
FvH, RF and MM prepared the main manuscript. The experimental data were generated by RF and MM under direct supervision of FvH in the lab and with his methodology and assistance. RMH, FEK and GB are supervisors of these students. All three professors have edited and corrected versions of this manuscript. GB is a visiting professor hosted by FEK and this work has been mainly generated through long-term scientific collaboration between both institutions. GB and FvH are involved in an industrial collaboration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to correct the heading “Material and methods materials” to “Materials and methods”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Hoessle, F., Farid, R., Mohamed, M. et al. The effect of different hydration media on magnesia. Discov Mater 1, 17 (2021). https://doi.org/10.1007/s43939-021-00017-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-021-00017-9