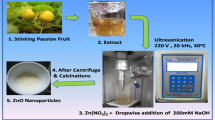
Abstract
This study investigated the use of zinc oxide nanoparticles (ZnO NPs) as an adsorbent for removing total phenols from textile wastewater. The ZnO NPs were synthesized by reducing Zn(NO3)2⋅6H2O using an extract from Neem leaves (Azadirachta indica). Characterization of the adsorbent was performed using Fourier Transform Infrared (FTIR) spectroscopy to identify functional group modifications, high-resolution scanning electron microscopy (HRSEM) for structural orientation, energy dispersive spectroscopy (EDS) for elemental analysis, and X-Ray diffraction analysis (XRD) for crystallinity, revealing particle crystallinity around 200 nm. Adsorption experiments were conducted over contact times of 20–60 min, with adsorbent loadings between 0.2 and 1 g/100 mL, and temperatures ranging from 30 to 50 °C. Optimal phenol removal, achieving 55.93% (0.67 mg/L), occurred at 43.40 min, 33.70 °C, and an adsorbent dosage of 0.69 g/L of textile wastewater. The phenol adsorption process using ZnO NPs was exothermic, spontaneous, and required low energy, fitting well with the Langmuir isotherm and following a pseudo-second-order kinetic model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Industrial effluents, particularly from the textile sector, are a major source of water pollution, posing significant environmental challenges [1, 2]. According to the World Bank, textile dyeing accounts for approximately 17 to 20% of global wastewater [3]. This wastewater is laden with various chemicals, including hydrogen peroxide, surfactants, metal soaps, alkalis, acids, dyes, starch, and aromatic compounds, all of which contribute to its high toxicity and ecological impact [4]. Phenol, a common contaminant in textile wastewater, has a permissible limit of 1 mg/L set by the EPA, USA, and must be treated to avoid damaging municipal wastewater treatment plants [5]. The release of such effluents introduces hazardous substances into the environment, necessitating stringent management practices [2]. Researchers have been exploring improved methods for pollutant separation from textile wastewater due to the high operational costs associated with existing techniques [6]. Traditional phenol removal methods, such as chemical oxidation and activated carbon adsorption, face challenges like high costs and the production of secondary pollutants. Adsorption techniques are widely studied for phenol removal because of their efficiency and potential for pollutant recovery [7]. However, the high cost of activated carbon has driven the search for alternative, low-cost adsorbents [8].
Over the last two decades, nanotechnology has advanced considerably, finding uses in many scientific and technological sectors. Nano-sized adsorbents have been developed for water pollutant removal, effective even at low concentrations (µg/L) under varied conditions [9]. Different nanomaterials, like metal nanoparticles, carbon nanotubes, and graphene oxide-based composites, have proven highly effective as photocatalysts for organic pollutant degradation [10]. Their large surface areas enhance catalytic activity, enabling efficient dye and nitrophenol removal through chemical reduction [11]. Additionally, nanotechnology supports simultaneous pollutant removal, including pathogens and inorganic compounds, offering a cost-effective, environmentally friendly alternative to traditional water treatment methods [12]. Techniques like nanocatalysis, nanofiltration, and nanoadsorption have successfully eliminated persistent organic pollutants (POPs) from wastewater, despite some cost challenges [13]. Therefore, developing more efficient, selective, economical, eco-friendly, and rapid water treatment technologies remains essential.
In recent years, nanoparticle synthesis for environmental remediation has gained considerable interest. Zinc oxide (ZnO) nanoparticles are particularly notable for their superior adsorptive properties and environmental compatibility. Traditional methods of ZnO nanoparticle synthesis, however, involve hazardous chemicals and energy-intensive processes, diminishing their environmental benefits. An eco-friendly alternative is the green synthesis of ZnO nanoparticles using plant-based materials. Extracts from pineapple peels [14], Ruta chalepensis leaves [15], and date pulp waste [16] have been effective as reducing and capping agents. These green-synthesized ZnO nanoparticles display uniform particle size distribution, hexagonal wurtzite structure, and enhanced photocatalytic activity compared to chemically synthesized nanoparticles. They efficiently degrade dyes such as Malachite Green, Methylene Blue, and Eosin Yellow in wastewater treatment and possess antibacterial properties against pathogenic bacteria [16]. The green synthesis process is simple, cost-effective, and environmentally friendly, avoiding the use of hazardous solvents and high-pressure conditions required in traditional methods [17]. Neem (Azadirachta indica) leaves, known for their rich phytochemical content, have been explored for the biosynthesis of ZnO nanoparticles [18]. These biogenic nanoparticles not only reduce the reliance on toxic chemicals but also enhance the adsorption efficiency due to their unique surface properties. Despite the growing interest in green-synthesized ZnO nanoparticles, comprehensive studies on their application for phenol removal from textile wastewater remain limited. There is a need for detailed research that integrates the synthesis, characterization, and practical application of these nanoparticles in real wastewater treatment scenarios.
This study aims to address the gap by synthesizing ZnO nanoparticles using Neem leaf extract and evaluating their effectiveness in removing phenol from textile wastewater. The research will focus on the synthesis and characterization of the nanoparticles, and analyzing their adsorption performance under various conditions. This integrated approach will provide valuable insights into the potential of green-synthesized ZnO nanoparticles for wastewater treatment applications, thereby contributing to the development of sustainable and efficient remediation technologies.
2 Materials and methods
2.1 Materials and collection of samples
The textile wastewater analyzed in this study was collected from the Kaykab Textile Industry treatment plant in Ilorin, Nigeria. The solution was stored in prewashed 5-L kegs and then refrigerated. Zinc nitrate (Zn(NO3)2⋅6H2O) was procured from Lab Trade in Ilorin, while fresh Neem (Azadirachta indica) leaves were obtained from Neem trees at the University of Ilorin, Ilorin, Kwara State, Nigeria.
2.2 Preparation of Azadirachta indica leaf extract
The neem leaves were carefully washed under running water and then thoroughly rinsed with distilled water to ensure they were completely clean. The cleaned leaves were air-dried at room temperature before being ground into a fine powder using an electric grinder. Approximately 20 g of the powdered leaves were added to 100 mL of distilled water and boiled for 20 min [19]. After boiling, the mixture was allowed to cool to room temperature and then filtered using Whatman filter paper. The resulting leaf extract was stored in a refrigerator at 4 °C until needed for use.
2.3 Determination of the total phenolic content
The phenol content of the textile wastewater was estimated using the Folin-Ciocalteu method [19]. Approximately 0.5 mL of the textile wastewater sample was measured into a clean test tube using a micropipette. Additionally, 2.5 mL of Folin C reagent was poured into the sample, followed by the addition of 2.0 mL of Na2CO3. The mixture was allowed to settle for 30 min. After this settling period, absorbance readings were taken using a UV spectrophotometer at a wavelength of 760 nm. These absorbance values were then plotted against the corresponding phenol concentrations to create a calibration curve. Using this calibration curve, the phenol levels were determined. The total phenol concentration in the raw textile wastewater was calculated to be 1.193 mg/L.
2.4 Synthesis of zinc oxide nanoparticles (ZnO NPs)
Approximately 414 mL of Azadirachta indica aqueous extract from the stock solution was transferred into a 1500 mL glass beaker. Then, 41.4 g of anhydrous zinc nitrate (Zn (NO3)2.6H2O) was added and thoroughly mixed using a magnetic stirrer until the zinc salt was completely dissolved. Once dissolved, the solution was heated and continuously stirred at 70 °C using a magnetic stirrer until a brown-like paste formed. To sequester all the nitrates and moisture, the paste was then transferred into a crucible and calcined at 400 °C for 2 h [20].
2.5 Characterization of the adsorbent
X-ray diffraction (XRD) was utilized to assess the crystallinity of the developed zinc oxide nanoparticles (ZnO NPs). Scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) were employed to investigate the structural and compositional characteristics of the ZnO NPs. Fourier-transform infrared spectroscopy (FTIR) was used to identify the functional groups present in the adsorbent [19]. These analyses were conducted on the ZnO NPs both before and after their application in the degradation of phenol in waste water.
2.6 Batch adsorption process
Adsorption studies were conducted by mixing 100 mL of textile wastewater with the adsorbent in closed 250 mL conical flasks, followed by constant shaking in a thermostatic water bath shaker at 150 rpm. The experimental parameters for the adsorption investigation included contact time (20–60 min), adsorbent dosage (0.2–1.0 g), and temperature (30–50 °C). The percentage removal of the targeted pollutant were then estimated using Eq. 1.
where; \({C}_{o}\) and \({C}_{f}\) (mg/L) are the concentrations of phenol in the aqueous solution at the initial stage and final respectively.
The equilibrium amount (\({Q}_{e}\)) of phenol removed per mass of adsorbent was evaluated using Eq. (2):
where; \({Q}_{e}\)(mg g−1) represents the quantity of phenol adsorbed per unit mass of adsorbent at equilibrium. V (L) represents the volume of the solution, \({C}_{e}\) (mg/L) is the concentration at equilibrium and W (g) denotes the mass of the ZnO NPs used. Each of the experiments was conducted three times, and the average values were taken into account for analysis.
2.7 Determination of the effect of contact time
Approximately 0.6 g of ZnO nanoparticles and 100 mL of textile wastewater were placed in a 250 mL conical flask, which was then placed in a shaker at 40 °C for intervals ranging from 30 to 60 min at 10-min increments. At the end of each specified contact time, the suspension was filtered, and the filtrate was analyzed for phenol concentration. This process was repeated three times, and the average phenol concentration was calculated. These values were used to compute the percentage removal of phenol.
2.8 Determination of the effect of adsorbent dosage
To determine the optimal dosage of adsorbent, the amount of ZnO NPs was varied from 0.2 g to 1.0 g in 100 mL of textile wastewater, maintaining a contact time of 40 min at 40 °C. The resulting mixtures were filtered, and the filtrate was analyzed for phenol concentration. This procedure was repeated three times, and the average phenol concentration was used to calculate the percentage removal of adsorbed phenol.
2.9 Determination of the effect of temperature
Using the optimal time and dosage obtained, the temperature was varied from 30 to 50 °C under these conditions. The resulting mixture was filtered, and the filtrate was analyzed for phenol concentration. This process was repeated three times to ensure accuracy, and the average phenol concentration was used to calculate the percentage removal of adsorbed phenol.
2.10 Optimization studies
Initial experiments indicated that contact time (min), adsorbent loading (g), and temperature (°C) are key experimental factors influencing the synthesis of ZnO NPs. The interactive effects of these variables were analyzed using the central composite design (CCD) method [21]. This approach helped capture the impact of contact time, adsorbent dosage, and temperature on the adsorption process, as detailed in Table 1.
2.11 Adsorption isotherms, thermodynamics, and kinetics of the study
2.11.1 Adsorption isotherms of the process
The equilibrium relationship between the concentration of the adsorbate in the mixture and its presence on the adsorbent surface under specific conditions was elucidated using adsorption isotherm models. This analysis is particularly relevant for understanding the adsorption behavior of phenol onto ZnO NPs, determined at an initial phenol concentration of 1.193 mg/L in raw textile wastewater. The equilibrium characteristics of this study were described using the Langmuir and Freundlich isotherm models.
2.11.2 The Langmuir isotherm model
This model suggests that adsorption occurs in monolayers on uniform surfaces with a finite number of adsorption sites. The linearized version of this model, as proposed by Langmuir [22], is expressed as follows.
where; \({q}_{max}\) (mg/g): maximum monolayer adsorbent capacity and \({k}_{L}\) (L/mg) is the Langmuir constant related to the energy of adsorption.
2.11.3 The Freundlich isotherm model
This model postulates heterogeneous adsorption on the surface. The linearized version of the Freundlich model, as represented by ref. [23] is expressed as follows:
where; n and \({k}_{f}\) (mg/g) represent the model parameters reflecting the intensity and capacity of the adsorption, respectively. The adsorption process can be categorized as physical, chemical, or linear whenever n > 1, n < 1 or n = 1. Nevertheless, findings show that n > 1 is favorable for adsorption [24].
2.11.4 The Temkin isotherm model
This model assumes that the heat of adsorption of all the molecules in the layer decreases linearly with coverage of the adsorbent surface [25] and is given as
where; \(\beta =\frac{RT}{b}\), R (8.314 J/mol K−1) refers to the universal gas constant, T (K) is the temperature, α (L/mol) is the Temkin constant or equilibrium binding energy connected with the maximum binding energy, and \(\beta\) refers to the Temkin constant connected to the adsorption heat.
2.11.5 Process thermodynamics
The thermodynamics of the process were studied and are given as follows:
Where; ∆G is Gibb’s Free energy, \({K}_{T}\) is the apparent equilibrium constant, and \(\Delta \text{H}\) is the enthalpy \(\text{and }\Delta \text{S}\) is the entropy.
2.11.6 Adsorption kinetics
The rate and mechanism of adsorption of phenol onto ZnO NPs were analyzed using the pseudo-first-order and pseudo-second-order kinetic models.
2.11.7 The pseudo-first-order kinetic model
The pseudo-first-order model, as proposed by Lagergren [26] is linearly expressed as follows:
where; \({K}_{1}\) (min−1) is the pseudo-first order rate constant, and \({q}_{e}\) (mg/g) and \({q}_{t}\) (mg/g) are the quantities of phenol adsorbed per unit mass of the ZnO NP adsorbent at equilibrium and at time t (min), respectively.
2.11.8 The pseudo-second-order kinetic model
The pseudo-second-order model, in its linearized form, is represented by the following expression, as postulated by Ho et al. [27]:
where; \({K}_{2}\) (g/mg min) refers to the model rate constant.
3 Results and discussion
3.1 Determination of the properties of neem leaf extract (NE)
FTIR measurements of the neem extract (NE), depicted in Fig. 1, indicated the presence of various functional groups. The peaks observed at 775, 1020, 1264, 1727, and 2927 cm−1 correspond to –NH, –C≡N, C–OH, –C=O, and –CH groups, respectively. Additionally, the sharp peaks at 1500 and 3688 cm−1 are attributed to –C=C and –OH groups, respectively [28]. These findings are consistent with previous analyses of neem leaves [29]. Furthermore, the presence of the C=O carboxylic group suggests the occurrence of alkaloids [30].
3.2 Characterization of textile wastewater (TWW)
The textile wastewater (TWW) was analyzed to determine the concentration of phenol, as shown in Table 2. The total phenol concentration was assessed using the Folin-Ciocalteu (F–C) method, which yielded an estimated concentration of 1.193 mg/L. This level is notably higher than the Standard Organization of Nigeria's (SON) limit of 0.25 mg/L and slightly exceeds the World Health Organization's (WHO) standard of ≤ 1.0 mg/L. High phenol concentrations pose significant health risks to aquatic life if discharged untreated. Although the developed ZnO NPs did not reduce the phenol concentration to meet the Nigerian standard, they were effective enough to align with the WHO guidelines.
3.3 Fourier Transform Infrared (FTIR) Spectroscopy Analysis
Figure 2 show the FTIR of adsorbent before adsorption (ABA) and adsorbent after adsorption (AAA) respectively. They exhibited similar peaks at 1050 (–N=O), 1552 (–C–N), 2354(C≡C), and 2821 cm−1 (–C–H) except that the peaks were sharper for ABA [31]. The band at 3160 cm−1 is possibly attributed to the aliphatic hydroxyl (OH) [32] while the bands at 3651 and 3400 cm−1 may also conform to the OH vibrations of the hydroxyl layers. In addition, the characteristic set of stretching vibrations attributed to nitrate anions became visible at 1050 cm−1 [33].
3.4 X-ray diffraction (XRD) analysis
Figure 3 presents the XRD patterns of the adsorbent, revealing highly crystalline material, indicated by the presence of sharp peaks. A review conducted by Agarwal et al. [34] affirmed that Azadirachta indica, a member of the Meliaceae family, is frequently utilized for the synthesis of ZnO nanoparticles (ZnO NPs).
3.5 Scanning electron microscopy and energy dispersive X-ray spectroscopy analysis (SEM–EDS)
Scanning electron microscope (SEM) analysis was conducted to examine the surface morphology of the ZnO nanoparticles (ZnO NPs). Figure 4 (a)-(d) displays the SEM images of the ZnO NPs before (ABA) and after (AAA) adsorption at various magnifications. These images confirmed the structure and approximate spherical shape of the nanoparticles. The energy-dispersive spectroscopy (EDS) results before (ABA) and after (AAA) adsorption are shown in Fig. 5, revealing the presence of elements such as zinc (Zn), sodium (Na), calcium (Ca), potassium (K), chlorine (Cl), aluminum (Al), magnesium (Mg), phosphorus (P), oxygen (O2), silicon (Si), titanium (Ti), iron (Fe), nitrogen (N2), sulfur (S), and carbon (C). The composition of these elements varied, with 77.77% Zn detected before adsorbent usage, decreasing to 58.45% after usage.
3.6 Optimization studies
The central composite design (CCD) was utilized to optimize three variables related to textile wastewater treatment. Table 3 presents the percentage of phenol removal for each experimental run, with the highest removal efficiency recorded at 54.35%. Statistical analysis was peroformed for phenol adsorption process, and the results are summarized in the analysis of variance (ANOVA) in Table 4. The phenol uptake by the ZnO NP model is described using a second-order polynomial, as shown in Eq. (9).
In the model, P, Q, and R represent the contact time (min), adsorbent loading (g), and temperature (°C), respectively. The model’s R2 value of 0.90 indicates a good fit. The predicted R2 value of 0.7034 is reasonably close to the adjusted R2 value of 0.8136, with a difference of less than 0.2. The analysis of variance (ANOVA) for the model is shown in Table 4.
The Model F-value of 10.21 suggests that the model is significant [35]. Additionally, P, Q, PQ, and Q2 are significant model terms. Thus, individual factors such as contact time, and adsorbent dosage, as well as their interactions (contact time and adsorbent dosage, and adsorbent dosage with itself), play a significant role in phenol adsorption using ZnO NPs.
Figure 6 displays the plot between the predicted and actual values of the percentage phenol removal. The numerical optimization yielded an optimum adsorption capacity of 55.93% (0.67 mg/L) for phenol removal at a contact time of 43.397 min, adsorbent loading of 0.689 g, and a temperature of 33.701 °C, with a desirability of 1.000.
3.7 Batch adsorption studies
3.7.1 Effect of contact time
The removal of phenol using ZnO as an adsorbent is influenced by the contact time, as illustrated in Fig. 7. The data reveals that over 50% of phenol is removed within the first 40 min of the adsorption process. This suggests that phenol adsorption on ZnO occurs rapidly during the initial stages, likely due to the high diffusion rate of phenol molecules from the aqueous solution to the active sites on the adsorbent [20]. However, after the 40-min mark, the percentage of phenol removed begins to decrease. This reduction could be attributed to the decreasing availability of active sites or pores on the ZnO adsorbent over time. These results are consistent with those found in previous studies [32].
3.7.2 Adsorbent dosage effect
The phenol removal efficiency from the solution increased with the adsorbent dose, rising from 0.2 to 0.6 g/100 mL (Fig. 8). This trend is due to the increased number of available phenol adsorption sites on the adsorbent surfaces as the dosage increases [19]. However, further increases in the adsorbent dosage resulted in a decrease in phenol removal, suggesting that the adsorbent sites were fully occupied and could no longer adsorb additional phenol. The optimal phenol removal was observed at a dosage of 0.6 g/100 mL. These findings align with those reported by Jabar, Owokotomo, Ayinde, Alafabusuyi, Olagunju and Mobolaji [28].
3.7.3 Effect of the adsorption temperature
Temperature was also found to affect the adsorption process as shown in Fig. 9. The maximum phenol removal was obtained at 40 °C, and a further increase in temperature caused a decrease in the efficiency of phenol removal. This inverse connection may reveal that the process is exothermic [19].
3.7.4 Adsorption isotherm studies
The removal of phenol by ZnO NPs was effectively described using Langmuir, Freundlich, and Temkin isotherms. Table 5 summarizes the data obtained from these isotherms. Among them, the Langmuir model provided the best fit for the adsorption process, as indicated by the R2 value of 0.9944, which is closest to unity.
3.7.5 Adsorption thermodynamic studies
From the graph shown in Fig. 10 and Table 6, the negative value for the change in enthalpy (ΔH) of this process suggests that the adsorption of phenol onto ZnO NPs is an exothermic process. Additionally, the negative entropy change (ΔS) indicates that this adsorption process is less random. Finally, the negative Gibb’s free energy (ΔG) value confirms that the reaction is spontaneous and feasible.
3.7.6 Adsorption kinetic studies
The adsorption process of phenol was analyzed using kinetic models, and the results are presented in Table 7, which lists the parameters for both the pseudo-first-order and pseudo-second-order models. The pseudo-second-order model demonstrated a better fit, as the calculated Qe values closely matched the experimental Qe values. Moreover, the correlation coefficient (R2) for the pseudo-second-order model was significantly closer to 1.000 compared to that of the pseudo-first-order model. These findings confirm that the adsorption of phenol on ZnO NPs is best described by the pseudo-second-order kinetic model.
4 Conclusions
The Neem leaf extract demonstrated effective reducing activity, converting zinc nitrate salts to zinc oxide nanoparticles (ZnO NPs). These ZnO NPs proved to be effective in adsorbing phenols from textile industry wastewater. Specifically, the initial phenol concentration of 1.193 mg/L in the untreated wastewater was reduced by 55.93% after 43.40 min when using an adsorbent dosage of 0.69 g/L at 33.70 °C. The kinetic study was best described by the pseudo-second-order model, while the Langmuir isotherm provided the best fit for the adsorption process. Thermodynamic analysis indicated that the adsorption process was spontaneous and exothermic.
Data availability
Data availability statements The authors confirm that the data supporting the findings of this study are available within the article.
References
Nathanson JA. Pollution. In: Encyclopedia Britannica. vol. 11. 2021. https://www.britannica.com/science/pollution-environment. Accessed 20 Jan 2024.
Varjani SJ, Gnansounou E, Pandey A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere. 2017;188:280–91. https://doi.org/10.1016/j.chemosphere.2017.09.005.
Kant R. Textile dyeing industry an environmental hazard. 2011. https://doi.org/10.4236/ns.2012.41004.
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB. A critical review on textile wastewater treatments: possible approaches. J Environ Manage. 2016;182:351–66. https://doi.org/10.1016/j.jenvman.2016.07.090.
Özbelge TA, Özbelge ÖH, Başkaya SZ. Removal of phenolic compounds from rubber–textile wastewaters by physico-chemical methods. Chem Eng Process. 2002;41:719–30. https://doi.org/10.1016/S0255-2701(01)00189-1.
Villegas LGC, Mashhadi N, Chen M, Mukherjee D, Taylor KE, Biswas N. A short review of techniques for phenol removal from wastewater. Curr Pollut Rep. 2016;2:157–67. https://doi.org/10.1007/s40726-016-0035-3.
Issabayeva G, Hang SY, Wong MC, Aroua MK. A review on the adsorption of phenols from wastewater onto diverse groups of adsorbents. Rev Chem Eng. 2018;34:855–73. https://doi.org/10.1515/revce-2017-0007.
Ahmaruzzaman M. Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv Coll Interface Sci. 2008;143:48–67. https://doi.org/10.1016/j.cis.2008.07.002.
Nzilu DM, Madivoli ES, Sujee Makhanu D, Otenda BV, Kareru PG, P. kinoti Kairigo, T. Tuhkanen,. Environmental remediation using nanomaterial as adsorbents for emerging micropollutants. Environ Nanotechnol Monit Manag. 2023;20:100789. https://doi.org/10.1016/j.enmm.2023.100789.
Aslam S, Ahmad M. Nanoparticles for degradation of organic pollutants. In: Information Resources Management Association, editor. Research anthology on synthesis, characterization, and applications of nanomaterials. Hershey: IGI Global; 2021. p. 1184–210. https://doi.org/10.4018/978-1-7998-8591-7.ch049.
Saxena M, Jain K, Saxena R. Role of nanomaterials in catalytic reduction of organic pollutants. Indian J Biochem Biophys. 2022;59:415–30. https://doi.org/10.56042/ijbb.v59i4.61932.
Hlongwane GN, Sekoai PT, Meyyappan M, Moothi K. Simultaneous removal of pollutants from water using nanoparticles: a shift from single pollutant control to multiple pollutant control. Sci Total Environ. 2019;656:808–33. https://doi.org/10.1016/j.scitotenv.2018.11.257.
Boulkhessaim S, Gacem A, Khan SH, Amari A, Yadav VK, Harharah HN, Elkhaleefa AM, Yadav KK, Rather S-U, Ahn H-J. Emerging trends in the remediation of persistent organic pollutants using nanomaterials and related processes: a review. Nanomaterials. 2022;12:2148. https://doi.org/10.3390/nano12132148.
Mirgane NA, Shivankar VS, Kotwal SB, Wadhawa GC, Sonawale MC. Waste pericarp of ananas comosus in green synthesis zinc oxide nanoparticles and their application in waste water treatment. Mater Today Proc. 2021;37:886–9. https://doi.org/10.1016/j.matpr.2020.06.045.
Kumar MA, Ravikumar C, Nagaswarupa H, Purshotam B, Gonfa B, Murthy HA, Sabir FK, Tadesse S. Evaluation of bi-functional applications of ZnO nanoparticles prepared by green and chemical methods. J Environ Chem Eng. 2019;7:103468. https://doi.org/10.1016/j.jece.2019.103468.
Rambabu K, Bharath G, Banat F, Show PL. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater. 2021;402:123560. https://doi.org/10.1016/j.jhazmat.2020.123560.
Raut SB, Thorat DPV. A review on preparation, characterization and application of zinc oxide (ZnO) nanoparticles by green synthesis method. 2015.
Supin KK, Vasundhara M. Green synthesis of ZnO nanoparticles from Neem and Eucalyptus leaves extract for photocatalytic applications. Mater Today Proc. 2023;92:787–95. https://doi.org/10.1016/j.matpr.2023.04.343.
Mustapha SI, Aderibigbe FA, Adewoye TL, Mohammed IA, Odey TO. Silver and titanium oxides for the removal of phenols from pharmaceutical wastewater. Mater Today Proc. 2021;38:816–22. https://doi.org/10.1016/j.matpr.2020.04.669.
Aderibigbe FA, Adewoye TL, Mustapha SI, Mohammed IA, Saka HB, Amosa MK, Adejumo AL, Owolabi RU, James SB. PHENOL removal in refinery wastewater using mixed oxides prepared by green synthesis. J Eng Res. 2023;11:2307–1877. https://doi.org/10.36909/jer.13917.
Ibrahim S, Ahmad Z, Manzoor MZ, Mujahid M, Faheem Z, Adnan A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci Rep. 2021;11:770. https://doi.org/10.1038/s41598-020-80805-0.
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–403.
Hutson ND, Yang RT. Synthesis and characterization of the sorption properties of oxygen-binding cobalt complexes immobilized in nanoporous materials. Ind Eng Chem Res. 2000;39:2252–9. https://doi.org/10.1021/ie9908433.
Garba ZN, Bello I, Galadima A, Lawal AY. Optimization of adsorption conditions using central composite design for the removal of copper (II) and lead (II) by defatted papaya seed. Karbala Int J Mod Sci. 2016;2:20–8. https://doi.org/10.1016/j.kijoms.2015.12.002.
Temkin MJ, Pyzhev V. Recent modifications to Langmuir isotherms. Acta Physico-Chimica Sinica. 1940;12:217–22.
Lagergren S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar. 1898;24(4):1–39.
Ho Y-S, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34:451–65. https://doi.org/10.1016/S0032-9592(98)00112-5.
Jabar JM, Owokotomo IA, Ayinde YT, Alafabusuyi AM, Olagunju GO, Mobolaji VO. Characterization of prepared eco-friendly biochar from almond (Terminalia catappa L.) leaf for sequestration of bromophenol blue (BPB) from aqueous solution. Carbon Lett. 2021. https://doi.org/10.1007/s42823-020-00214-1.
Bhattacharyya KG, Sharma A. Adsorption of Pb (II) from aqueous solution by Azadirachta indica (Neem) leaf powder. J Hazard Mater. 2004;113:97–109. https://doi.org/10.1016/j.jhazmat.2004.05.034.
Seriana I, Akmal M, Darusman D, Wahyuni S, Khairan K, Sugito S. Phytochemicals characterizations OF neem (Azadirachta indica A. Juss) leaves ethanolic extract: an important medicinal plant as male contraceptive candidate. Rasayan J Chem. 2021;14:343–50. https://doi.org/10.31788/RJC.2021.1415899.
Jabar JM, Odusote YA, Ayinde YT, Yılmaz M. African almond (Terminalia catappa L.) leaves biochar prepared through pyrolysis using H3PO4 as chemical activator for sequestration of methylene blue dye. Results Eng. 2022;14:100385. https://doi.org/10.1016/j.rineng.2022.100385.
Jabar JM, Odusote YA. Removal of cibacron blue 3G-A (CB) dye from aqueous solution using chemo-physically activated biochar from oil palm empty fruit bunch fiber. Arab J Chem. 2020;13:5417–29. https://doi.org/10.1016/j.arabjc.2020.03.020.
Biswick T, Jones W, Pacuła A, Serwicka E, Podobinski J. The role of anhydrous zinc nitrate in the thermal decomposition of the zinc hydroxy nitrates Zn5(OH)8(NO3)2·2H2O and ZnOHNO3·H2O. J Solid State Chem. 2007;180:1171–9. https://doi.org/10.1016/j.jssc.2007.01.012.
Agarwal H, Kumar SV, Rajeshkumar S. A review on green synthesis of zinc oxide nanoparticles—an eco-friendly approach. Resour Effic Technol. 2017;3:406–13. https://doi.org/10.1016/j.reffit.2017.03.002.
Mondal NK, Roy S. Optimization study of adsorption parameters for removal of phenol on gastropod shell dust using response surface methodology. Clean Technol Environ Policy. 2016;18:429–47. https://doi.org/10.1007/s10098-015-1026-6.
Acknowledgements
The authors wish to thank the Department of Chemical Engineering, University of Ilorin, Nigeria for granting access to laboratory facilities.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, editing, resources, and supervision were contributed by Fatai Alade Aderibigbe; Experimental design, validation, and writing of the original manuscript and resources by Sherif Ishola Mustapha; Supervision, and resources were done by Ishaq Alhassan Mohammed; Formal analysis and review by Tunmise Latifat Adewoye and Esther Olubunmi Babatunde; Investigation and writing of original manuscript draft by Habeebllah Ifeoluwa Aminullah; Editing and result validation by Kabiru Bab Muritala. All authors have read and agreed with the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no financial conflicts of interest or personal relationships that could have influenced the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aderibigbe, F.A., Mustapha, S.I., Mohammed, I.A. et al. Green synthesis of zinc oxide nanoparticles for the removal of phenol from textile wastewater. Discov Chem Eng 4, 24 (2024). https://doi.org/10.1007/s43938-024-00061-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-024-00061-w