Abstract
Although the system water + n-propanol + toluene is important for research and industry, its liquid–liquid equilibria LLE are scarcely covered in literature, with no thermodynamic modeling reported using typical equations of state or activity coefficient models. The current work addresses this gap by presenting the equilibria of the system at 20°C and atmospheric pressure. That includes the binodal curve and twelve tie lines covering the entire miscibility gap, whereby the compositions of the conjugate phases are determined using high-precision refractive index measurements. The quality of the data is verified by applying the techniques of conjugate lines and mass balances, whereas it is shown that empirical correlations cannot be used for that purpose. Three different methods to extrapolate the critical point are then employed and, using the more reliable one, that point is located and added to the dataset. Further thermodynamic correlation of the data is conducted using the activity coefficient models, UNIQUAC and NRTL. While the former did not perform well, the latter was able to model the LLE and adapt to temperature changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the past years, several industrial applications of the ternary system water + n-propanol + toluene have been reported. One example is the liquid scintillation counting of tritium water, whereby the alcohol serves as a consolute liquid between water and toluene [1]. The n-propanol is preferred in this application over methanol and ethanol, due to its superior dielectric and quenching properties and the ability to produce homogeneous toluene-water mixtures with higher toluene concentration [1]. On another hand, the fuel industry blends alkanols and hydrocarbons like toluene with fuels to enhance their performance [2]. Because alkanols are hygroscopic, water may become inadvertently a component of the fuel, leading to potential corrosion problems due to the formation of a water-rich phase [3]. Therefore, the liquid–liquid equilibrium LLE data of water + alkanols + solvents are needed to predict the phase split.
Besides that, it is known that water + acetone + toluene is one of the standard test systems for liquid extraction suggested by the European Federation of Chemical Engineering EFCE [4, 5]. Yet, acetone is quite volatile and its aqueous solutions exposed to the atmosphere show relatively fast decline in ketone concentration. That issue exacerbates when working under typical laboratory ventilation, which eventually renders this system impractical if not isolated. Such a situation indeed occurred in our experiments when the system was applied in extraction research. Accordingly, acetone was replaced with n-propanol whose aqueous mixtures exhibited much better stability even under ventilation, while maintaining analogous thermodynamics as discussed next. Another advantage of this replacement is that n-propanol is considerably less chemically aggressive than acetone, which facilitates the selection of materials. Because there was no specific reason to keep using acetone, substituting it with n-propanol as a solute was favorable.
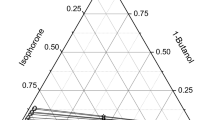
Similar to acetone, the ternary system of n-propanol with toluene and water exhibits a phenomenon called solutropy, first termed by Smith [6]. Such systems, defined as solutropes, are featured by the existence of an intermediate tie line that is horizontal and separates two regions of different affinities [7]. As noted in Fig. 1a, when moving upwards from the bottom binary subsystem to the critical point, tie lines first slope in one direction reflecting higher solute affinity towards one certain phase. With increasing solute concentrations, that preference gradually diminishes until a horizontal tie line is reached. Above it, the tie lines slope reverses and the solute starts to favor the other phase. A universal thermodynamic theory explaining those systems does not exist, perhaps due to different reasons underlying such behavior [8, 9].
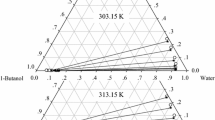
Based on the above, it becomes clear how relevant the system water + n-propanol + toluene is for research and industry, therefore reliable LLE data should be available. To our surprise, in Dortmund Data Bank DDB [10] and NIST [11], only three experimental works were found containing binodal curve and tie lines data, all conducted at the same temperature 25°C. While two studies agree with each other, the third largely deviates [10]; Fig. 1 illustrates the data of the former two. Although the first work sufficiently covers the miscibility gap, Fig. 1a, it does not address the critical point or the immediate surrounding zone [1]. The second study, on the other hand, focuses only on the upper part of the gap with inconsistencies noticed at the crest, Fig. 1b [2]. Also, no data correlation using common thermodynamic models is presented in either work or even found elsewhere in literature. The need becomes clear, therefore, for a new and full dataset of this system, favorably at a different temperature and accompanied by thermodynamic modeling. Tackling those points, the current work presents the liquid–liquid equilibria of water + n-propanol + toluene system at 20°C and atmospheric pressure. This includes the binodal curve and twelve tie lines deliberately chosen to manifest both regions of affinities, detect the slope-changing point, approach the critical point as closely as possible and estimate it. Eventually, two activity coefficient models, NRTL and UNIQAUC, are applied for the thermodynamic correlation of the data. The reported concentrations are mass fractions. All graphics has been created using OriginPro®, except Fig. 2b that is designed in PowerPoint®.
2 Materials and equipment
The distilled water used in the experiments was supplied from the local infrastructure of Ulm University (conductivity < 1 μS/cm). Regarding the organic chemicals, toluene and n-propanol, the specifications and supplier are shown in Table 1. Since the cloud point method was adopted here, binodal curve data were determined via titrations. The analyte liquids to be titrated were prepared gravimetrically using VWR® balance (LSP-4202 ± 0.01 g), in amounts predetermined to reach a total volume around 60 mL at the anticipated endpoint. That volume was chosen as a best trade-off to mitigate as possible the impact of equipment uncertainties while ensuring sufficient agitation in the used vessel. For tie lines determination tests, samples were prepared using a finer analytical balance also from VWR® (SM-1265Di ± 0.01 mg).
Titrations were carried out in a 120-mL double-jacketed glass vessel, Fig. 2a. It was kept essentially isolated during tests, with a minimum relief to avoid overpressure when adding the titrant, Fig. 2b. The stirring was secured by Heidolph® magnetic stirrer MR Hei-Tec, and performed at a constant temperature. The latter was maintained by Julabo® thermostat CP-900F (± 0.03°C) and Ahlborn® temperature sensor PT-100 within overall uncertainty of ± 0.1°C. Titrants were added via standard 1mL syringes from Braun® (Injekt-F) for concentrations ≤ 20 wt% n-propanol, while via 10 and 25mL burettes (± 0.03 mL) for ≥ 30 wt% n-propanol. To prepare the samples for refractive index (n) measurements, they were all first centrifuged in bench-top Sigma® 2–7 centrifuge, then n was measured in the temperature-controlled refractometer DR6300-T from Krüss® (± 0.00002 n, sodium-D wave length of 589.3 nm).
3 Experimental methods
3.1 Binodal curve determination
As mentioned, the binodal curve is determined via the well-known cloud point method, explained extensively in [14, 15] and still widely applied to date [16,17,18,19]. Sketched in Fig. 3a, 19 titrations were planned to cover as possible the entire curvature of the binodal curve. On the left side, the pure toluene (O1) and the mixtures of toluene and n-propanol (O2-9), prepared gravimetrically according to their respective positions in the diagram, were titrated with pure distilled water. Likewise, pure water (A1) and mixtures of water and n-propanol (A2-9) were titrated with pure toluene.
a Titration lines to determine binodal curve; O1-9 are the liquids to be titrated with water (W) while A1-9 with toluene (T) and H is a heterogeneous mixture to be titrated with n-propanol (P). b The planned mixtures T1-10 for tie lines determination tests where the binodal curve is taken from [1]. The red ellipse indicates the potential region of the critical point C as suggested from the tie lines trend in Fig. 1
In all above titrations, the endpoint is considered to be reached when turbidity persists for 10 min of continuous stirring at 20.0°C (± 0.1°C). Our experiments showed that this is the optimal point to end the titration, even though the turbidity may subtly fade within the 10 min. At that end, adding only one more titrant droplet would most likely render the turbidity permanently stable, indicating that the mixture has fully entered the two-phase region. Since binodal curve points are what is sought after, the endpoint should still lie in the one-phase region not inside the miscibility gap. Thus, ending the titrations after ensuring permanent turbidity would lead to points inside the gap within the metastable region. Such points belong to the solubility limit rather than binodal curve as emphasized in [20]. But, because the titrations stopped at the 10-min threshold and the added droplets are very fine relative to the volume of the titrated solutions, the endpoints are considered here to be still on the border of the one-phase region. Thus, they are regarded as binodal curve points rather than solubility limits.
In one additional titration, a heterogeneous mixture of toluene and water as per point H was titrated with pure n-propanol, Fig. 3a. For this particular case, the endpoint is said to be attained when turbidity disappears without reversal after 10 min of stirring. Thereby, all titrations were performed using a pure substance as titrant for ease and better accuracy.
According to the planned n-propanol concentration in the titration, different equipment was utilized to add the titrant. In the region ≤ 20 wt% n-propanol on both sides of the miscibility gap where mutual solubility of toluene and water is still significantly low, 1mL syringes were used to introduce the titrant to detect the endpoint as accurate as possible. The syringes can dispense the titrant in single drops, each weighing 0.005 g (± 0.001). For n-propanol concentrations ≥ 30 wt%, however, the titrant was added via 10 or 25mL (± 0.03 mL) burettes. Once the endpoint of each titration was reached, the mass composition of the mixture was calculated as indicated in [14], where the volume of the added titrant is converted to mass using the density of the pure substance at 20°C (ρw = 0.998 g cm−3 [21], ρP = 0.8045 g cm−3 [21] and ρT = 0.8672 g cm−3 [21]).
As a result of the above procedure, 19 points of binodal curve corresponding to the 19 titrations were determined. At each endpoint, the refractive index (n) of the mixture was additionally measured to enable the interpolation of the composition of the conjugate phases in the further tie lines determination tests. To prepare for n measurements, a portion of 10 mL was taken from the saturated mixture, placed in a sealed 10mL tube and shortly centrifuged to fully ensure the clarity of the liquids; this is to avoid the interference of any remaining turbidity with the measurements. Then, n of the clear sample was measured five consecutive times and averaged.
Overall, the absolute uncertainties in the reported mass fractions of binodal curve points are:
-
In the range ≤ 20 wt% n-propanol, ± 0.009% for any reported mass fraction ≤ 5 wt% and ± 0.04% for any reported mass fraction ≥ 5 wt%.
-
In the range ≥ 30 wt% n-propanol, ± 0.04% for any reported mass fraction.
Those mainly stem from the uncertainty of the used equipment and, in the Supplementary Material, the detailed calculations of uncertainties can be found. To check reproducibility, several titrations were repeated in both concentration regions and they were all reproducible within the reported uncertainties.
3.2 Tie lines determination
For this part, ten heterogeneous mixtures matching the compositions of the points T1-10, Fig. 3b, were prepared. The points are subjectively chosen in this fashion for several reasons: to sufficiently cover both regions of different affinities; to locate the partitioning horizontal tie line; to ensure equal amounts of the conjugate phases at the end of each test for easier analysis; and to approach the critical point C as closely as possible. The latter is deemed to be somewhere within the area of the red ellipse, Fig. 3b, as the trend of the literature tie lines suggests in Fig. 1. The subsequent analysis of results, however, showed that the uppermost tie line T10 was still relatively far from the critical point, so that it could not be extrapolated with reasonable safety. Therefore, two further tie lines were added as described later.
According to each T point, the respective heterogeneous mixture was gravimetrically prepared by way of an analytical balance (± 0.01 mg, see Sect. 2). Around 29 mL of that mixture were formulated in a glass vial of a nominal size of 30 mL in order to minimize evaporation losses. To establish equilibrium, the sealed vial was vigorously stirred for at least 1 h at room temperature using magnetic stirrers. The latter time was chosen based on preliminary tests carried out at similar agitation, which showed equilibrium is quickly reached within several minutes. The vial was then placed in the water bath at 20.00°C (± 0.03) for a minimum of 20 min to thermally equilibrate. To ensure composition homogeneity after temperature changes, the vial was stirred for two more minutes and placed back in the water bath for another 15 min. At the end, the equilibrated mixture splits into two distinct phases inside the water bath.
Carefully using syringes, 10 mL of each conjugate phase was drawn out, transferred to a separate 10mL sealed tube and briefly centrifuged until clarity was fully ensured. The refractive index (n) of each phase was then measured. By means of the n values of binodal curve points recorded earlier, a linear interpolation is performed to determine the mass fraction of each component in the analyzed phase. Only the two points of binodal curve of n values directly below and above the measured one are used for the interpolation. The obtained fractions of the three components are then checked to sum up to 100%, which was always the case. The absolute uncertainty in the interpolated compositions are calculated using error propagation rules, based on the uncertainties reported in Sect. 3.1. Consequently, for any interpolated water mass fraction ≤ 3.63 wt%, n-propanol mass fraction ≤ 4.83 wt% or toluene mass fraction ≤ 4.01 wt%, the absolute uncertainty is ± 0.01%. Otherwise, it is always ± 0.08%. Detailed calculation of uncertainties is presented in the Supplementary Material. To ensure reproducibility, several tie lines in both concentrations’ regions were repeated and the results were consistent.
4 Results and discussion
4.1 LLE data
By connecting the 19 endpoints of titrations, the experimental binodal curve of the studied system is formed, Fig. 4. Endpoints’ compositions along with their respective RI’s are listed in Table 2. The comparison with literature data at 25°C, Fig. 1, shows higher crest of binodal curve at 20 °C, indicating that the miscibility gap enlarges with decreasing temperatures. It is worth mentioning that turbidity appearance/disappearance was not consistent throughout the titrations. Particularly at low n-propanol content on both sides of the miscibility gap, the turbidity appeared like a fine shower of tiny droplets of the undissolved added phase in the titrated solution. Closer to the critical point, however, the added titrant formed a uniform cloudy appearance over the entire mixture.
Additionally, in Fig. 4, the experimental tie lines are shown together with their actual starting mixtures Te1-12, where Te11 and Te12 are the two added tie lines close to the critical point. Obtained by interpolation as explained above, the compositions of the conjugate phases of all tie lines are presented in Table 3 along with the initial mixture Te and the distribution coefficients K. The latter is defined as xP,T/xP,W, where xP,T is the n-propanol mass fraction in the toluene-rich phase and xP,W is that fraction in the conjugate water-rich phase. The refractive indices (n) of the conjugate phases can be found in the Supplementary Material.
It can be seen in Fig. 4 that the tie line Te4 is virtually horizontal, reflecting almost equal affinities of n-propanol toward both phases. Below Te4, n-propanol exhibits more affinity to the water-rich phase, whereby the slope of the tie lines starts from zero at the binary subsystem water-toluene, increases to a maximum around Te2 and declines again to zero close to Te4. On the other hand, in the region above Te4, the slope reverses meaning that the preference turns toward the toluene-rich phase, with the tie lines becoming constantly steeper up to the critical point.
This change in affinity can also be observed in the distribution curve Fig. 5, in which the mass fraction of n-propanol in the toluene-rich phase xP,T is plotted versus that in the conjugate water-rich phase xP,W, rendering each tie line appears as a single point. The curve before Te4 develops below the 45°-line referring to a solute distribution in favor of the water-rich phase (K < 1). Slightly below Te4, the curve intersects with the 45°-line at 15.8 wt% (± 0.1) n-propanol, which represents the concentration at which the intermediate horizontal tie line occurs (K = 1). Comparing to the LLE at 25 °C, Fig. 1a, that tie line seems to occur there below 15 wt%. Then, from Te4 onwards, a sharp inversion in distribution happens toward the toluene-rich phase (K > 1), with K values rapidly increasing to hit a peak at Te8 then declining again to unity at the critical point C. It can be noted that extrapolating C from Te10 might involve high degree of uncertainty due to the relatively far distance from 45°-line. For that reason, Te11 and Te12 were added.
4.2 Tie-lines data quality tests
In many published LLE articles, the quality of the reported data is tested using empirical correlations, sometimes referred to as reliability or consistency tests [20]. Before applying them also to the produced equilibria, it is worthy to draw attention to the soundness of that practice. Last century, several empirical equations were developed, such as Hand [22], Othmer [23, 24], Brancker et al. [25], Bachman [26] and others [9]. As stated by all the cited authors, the motivation behind developing them was to offer a tool to safely interpolate tie lines in a system, for which only very few tie lines are available. In such cases, the interpolation directly from the ternary diagram results in significant inaccuracies, and the only option left to obtain reliable data is to resort back to experiments. Those equations, however, utilize equilibrium data in a way that each tie line gives one point, and when plotted in certain rectangular coordinates, they yield straight lines combining all data. Therefore, only two available tie lines are sufficient to generate the correlated line, from which it becomes much safer to interpolate other unknown tie lines.
Although showing very good linearity for numerous systems, the equations still exhibit varied deviations [27]. Especially for solutropic systems like the one in question, they were not able to produce straight lines [8, 20, 25, 28]. There were even trials to develop correlations specific to such systems [6, 29], but they also fail to produce linearity [8]. Despite the thermodynamic foundation proposed later for Hand equation [8, 30], all the aforesaid correlations are purely empirical without any theoretical basis presented in the original articles. The lack of concrete fundamental theory was explicitly referred to in a critical work focusing on Hand and Othmer correlations [27]. Based on that, such equations cannot be considered as rigorous tools to assess the quality of the LLE data. A thorough review in this regard underlines this common mistake of using them for such a purpose [20]. While well-founded consistency tests exist for VLE, based on Gibbs–Duhem equation, no similar tests for LLE exist in thermodynamic textbooks (interested readers are referred to [20, 27] for further information).
To check the performance of those correlations on the generated data, two of the most common ones are applied, which are Hand equation, Fig. 6a:
and Othmer’s, Fig. 6b:
where W, P and T refers to water, n-propanol and toluene respectively, and xI,J represents the mass fraction of component I in the J-rich phase. As suggested by the formulas, they should result in a straight correlating line when plotted on a logarithmic scale. Yet, by applying them on the presented equilibria, they fail to produce linearity, most likely due to the solutropic nature of the system, Fig. 6. Comparable findings are reported elsewhere on alike systems [8].
However, the quality of the LLE data in this work is examined using the following two methods:
1) Conjugate lines
The International Critical Tables of Numerical Data proposes the so-called conjugate line as a technique to judge the accuracy of LLE [31]. The smoothness of that line and the gentleness of its curvature serve as indicators of the reliability of the data. The typical conjugate line consists of a lower and an upper part, whereby every tie line is represented by a single point in each part. To showcase how to produce it, the tie line Te10, whose two equilibrium phases are O10 and A10, is taken as an example, Fig. 7. Starting with the upper part, the corresponding point of Te10 is U10. It can be located by drawing and intersecting a line from A10 representing the toluene content and another from O10 representing the water content. By repeating the same procedure for all tie lines, the upper part PU12 of the conjugate line results.
Turning to the lower part, the representative point of Te10 becomes B10, which is determined by drawing and intersecting a line of the water content in A10 and another of toluene content in O10. Performing the same steps for all tie lines creates the lower part of the conjugate line that extends below the ternary diagram to a point symmetrizes the vertex P with respect to the bottom side water-toluene. In Fig. 7, only the section from B12 to B9 is shown from the lower part. Other techniques to produce different variants of this line can be found in literature [31, 32]. It is worth mentioning that the conjugate line can also be used to interpolate new tie lines, simply by reversing the aforesaid steps. By examining the smoothness of the entire conjugate line created in Fig. 7 and how gentle its curvature is, the quality of this work’s data can be verified.
2) Mass balance
Due to mass conservation in the ternary diagram, it is known that when two liquids are mixed, the point that represents the resultant mixture lies on the line connecting the points of the two mixed liquids. Such a fact can be used to scrutinize how accurate the LLE data are. Namely, the point that represents each initial heterogenous mixture, i.e. Te1 to Te12 in Fig. 4, should lie on the straight line connecting the two conjugate phases obtained from that particular mixture. What makes this technique practical is that it collectively judges the accuracy of all work steps, from preparation to analysis, without the need to generate more plots. If inaccuracies in any stage exist, the initial mixture would deviate away from its acquired tie line. From Fig. 4, the points Te1-12 position quite well on their respective tie lines, indicating that the mass balances are fulfilled with considerably high precision.
4.3 Critical point estimation
Because of its specific nature, the experimental detection of the critical point is quite challenging. Thus, the typical practice is often to extrapolate it from the generated data, and this is carried out here in three different ways using the above-formulated graphs:
-
From distribution curve
Since the critical point is a reduced tie line where the two phases become identical (K = 1), it belongs to both the distribution curve and the 45°-line. Therefore, by extrapolating the curve to intersect the 45°-line, Fig. 5, point Cext,1 results corresponding to 40.8 wt% (± 0.1) n-propanol.
-
From conjugate line
Connecting the lower and upper parts of the conjugate lines will intersect the binodal curve at the critical point. The closer the ends of both parts to each other are, the more accurate the estimation would be. By performing this connection between B12 and U12 in Fig. 7, the point Cext,2 yields with 41.1 wt% (± 0.1) n-propanol.
-
From Hand plot
In Fig. 6a, only the data of tie lines were plotted using Hand equation, however, the data of binodal curve points can also be added to the same graph. By plotting xP/xT versus xP/xW of each point of the binodal curve, the blue solubility curve in Fig. 8 returns. Since the critical point is, on one hand, a reduced tie line, and on the other, lies on binodal curve, it represents the intersection of Hand graph and the solubility curve. By extrapolating the former, the critical point Cext,3 is thus attained relating to 40.3 wt% (± 0.1) n-propanol.
Extrapolation of the critical point Cext,3 by intersecting Hand plot and the solubility curve drawn using the generated tie lines and binodal curve data, respectively, of the system water (W) + n-propanol (P) + toluene (T) at 20.0°C (± 0.1) and atmospheric pressure. xI,J is the mass fraction of I in J-rich phase
Other extrapolation methods are also available [33]. Although the three techniques give relatively comparable estimations, the first seems to be the most reliable one in light of the consistent slope from Te10 to Te12. The second, however, involves some uncertainty due to the relatively far distance between both parts of the conjugate line, whereas the third appears to be the least trustworthy owing to Hand equation deviations from linearity. Accordingly, the first method is adopted to estimate the critical point Cest in the ternary diagram. By drawing a horizontal line at 40.8 wt% n-propanol from the side water-propanol and intersecting it with the binodal curve, Cest is located. Figure 9 illustrates the complete LLE dataset along with Cest.
5 Thermodynamic correlation of the data
The two thermodynamic models NRTL and UNIQUAC, proven superior for correlating LLE [34], are used to correlate the equilibria of this system. To ensure the model can explain the temperature-dependence of the LLE, both the dataset of this work at 20°C and the literature dataset at 25°C [1] are implemented in the regression, with the maximum likelihood applied as an objective function [35].
With regard to UNIQUAC, it couldn’t converge with reasonable results. Although it could correlate each dataset separately, but applying both sets together led to unacceptable deviations. On the other hand, NRTL was able to model the LLE of this system at both temperatures with accuracy ranging from very good to poor depending on temperature and solute concentration. NRTL model has three parameters that can be fitted for each binary subsystem: the two asymmetric binary interaction parameters τij and τji (τij ≠ τji) and the symmetric non-randomness αij(αij = αji), where i and j denote two different components. τij is typically expressed in commercial simulators as [35]:
where aij, bij, eij, and fij are all asymmetric parameters and T is the temperature. When correlating data, τij and τji are not directly fitted, rather their sub-parameters are, and typically, the first two terms are only used unless a clear necessity exists for the others [35]. Thus, only aij and bij are active while eij and fij are considered zeros. For αij, it is calculated from:
where cij and dij are symmetrical parameters. Similarly, cij is usually fitted while dij is nullified [35]. The chosen root-mean square RMSD deviation for this regression is:
where Texp is the experimental temperature, Tcal is the calculated one using the fitted model, and N is the number of tie lines in the regressed dataset. Since Texp is different among the datasets, each set has its own RMSD that judges the agreement between the experimental and calculated temperatures.
A literature survey shows that when regressing NRTL model, αij has been either fixed at a certain value or fitted along with τij. In this work, both scenarios were applied. αij was first fixed at 0.2 for all binary subsystems as suggested in [36, 37], and in another trial, at 0.3 [38]. For the former case, the obtained solution succeeded in modelling LLE at 20°C but failed at 25°C, while for the latter case, convergence could not be successfully achieved. Therefore, following the suggestion of [35], αij was included in the regression with lower and upper boundaries set for it to avoid unreasonable solutions. The analysis of a large amount of binary equilibria has revealed that αij ranges from 0.2 to 0.47 [38]. Thus, those values were taken as lower and upper bounds, respectively, and this indeed resulted in a successful regression that is adopted in this work and discussed next. It is worthy to mention that wider ranges of αij have been also applied, including a range from 0 (completely randomly distributed molecules in the mixture) to 1 (completely specified distribution) [38, 39]. Despite achieving convergence in some cases, the obtained sets of parameters led to severe calculation errors.
Table 4 shows the obtained values of the fitted NRTL parameters along with RMSD. The absolute values of some bij parameters seem to be high, which might be attributed to the unique thermodynamics of the solutropic system at hand. In Figs. 10 and 11, the experimental and NRTL-modeled data at 20°C and 25°C, respectively, are demonstrated. Apparently, the modeled LLE at either temperature comply well with the experimental one qualitatively.
Comparison between the experimental LLE from this work of the system water (W) + n-propanol (P) + toluene (T) at 20.0°C (± 0.1) and atmospheric pressure with modeled LLE using NRTL at same conditions. An experimental tie line and its respective calculated one are similarly colored for easier comparison
Comparison between the experimental LLE from [1] of the system water (W) + n-propanol (P) + toluene (T) at 25°C and atmospheric pressure with modeled LLE using NRTL at same conditions. An experimental tie line and its respective calculated one are similarly colored for easier comparison
For binodal curve, the agreement remains very good in both cases up to 30 wt% n-propanol, after which anomalies begin to emerge. While the deviations are more pronounced on the toluene-rich branch of binodal curve, the model functions significantly better on the water-rich branch. With regard to tie lines at 20°C, the modeled and experimental data closely match at low n-propanol concentrations up to the slope-changing point. Further up, the accuracy gradually deteriorates to be rather fair to poor especially on the organic side. At 25°C, the situation is rather similar, with relatively poorer performance around the slope-changing point but better at intermediate to high solute content. When comparing the obtained set of parameters in this work with that suggested by Aspen Plus V11®, the former performs much better. The RMSD values, in turn, also indicate acceptable accuracy of NRTL for both temperatures.
6 Conclusions
In this work, the cloud point method is implemented to experimentally produce the binodal curve data of the system water + n-propanol + toluene at 20°C and atmospheric pressure. In addition, twelve tie lines covering the entire length of the miscibility gap are determined depending on refractive index measurements. It was shown that Hand and Othmer empirical equations failed to produce linearity and cannot be used to assess the quality of LLE. Alternatively, the methods of conjugate line and mass balance are applied for that purpose and they both reflect the good accuracy of the generated data.
To estimate the critical point, three different extrapolation techniques were employed, all yielding comparable outcomes. Using the more reliable one, the distribution curve, the critical point was located. The produced dataset was additionally correlated using two thermodynamic models, NRTL and UNIQAUC. To ensure that the produced set of parameters can adapt to temperature changes, the literature dataset carried out at 25°C [1] was additionally included in the regression. While UNIQUAC couldn’t converge with reasonable results, NRTL was able to. The generated set of NRTL parameters could model the LLE at both temperatures while agreeing reasonably with the experimental data. It also well describes the solutropic thermodynamics of the system at both temperatures.
Data availability
All data used in this manuscript is presented within.
Abbreviations
- a, b:
-
Equation parameters in Eq. 1 & 2 [-]
- C:
-
Critical point
- Cest :
-
Final estimated critical point
- Cext :
-
Extrapolated critical point
- K:
-
Distribution coefficient [-]
- n:
-
Refractive index [-]
- P:
-
n-Propanol
- ρ:
-
Density [g cm−3]
- T:
-
Toluene
- Ti :
-
Theoretical heterogenous mixture for tie lines determination
- Tei :
-
Experimental heterogenous mixture for tie lines determination
- W:
-
Water
- xI :
-
Mass fraction of component I
- xI,J :
-
Mass fraction of component I in J-rich phase
References
Baker EM. The ternary system n-propyl alcohol–toluene–water at 25°. J Phys Chem. 1955;59:1182–3. https://doi.org/10.1021/j150533a017.
Letcher TM, Siswana PM. Liquid-liquid equilibria for mixtures of an alkanol + water + a methyl substituted benzene at 25 °C. Fluid Phase Equilib. 1992;74:203–17. https://doi.org/10.1016/0378-3812(92)85062-D.
Letcher TM, Ravindran S, Radloff S. Liquid-liquid equilibria for mixtures of an alkanol + diisopropyl ether + water at 25°C. Fluid Phase Equilib. 1992;71:177–88. https://doi.org/10.1016/0378-3812(92)85012-W.
Mísek T. Standard test systems for liquid extraction. 2nd ed. Rugby: Institution of Chemical Engineers; 1985.
Berger R, Hampe MJ, Schröter J. Neue testsysteme für die Flüssig/Flüssig-Extraktion. Chem Ing Tec. 1992;64:1044–6. https://doi.org/10.1002/cite.330641124.
Smith AS. Solutropes. Ind Eng Chem. 1950;42:1206–9. https://doi.org/10.1021/ie50486a034.
Seader JD, Henley EJ, Roper DK. Separation process principles: chemical and biochemical operations. 3rd ed. Hoboken NJ: Wiley; 2011.
Charles EJ, Morton F. Liquid-liquid equilibrium data for ternary systems containing α-picoline, hydrocarbons and water or triethylene glycol at 20°. J Appl Chem. 1957;7:39–46. https://doi.org/10.1002/jctb.5010070107.
Knypl ET, Wojdylo SZ. Liquid-liquid equilibrium data for the ternary system water-isopropyl alcohol-ethylbenzene at 25°. J Appl Chem. 1967;17:361–3. https://doi.org/10.1002/jctb.5010171206.
Dortmund Data Bank.
Oracz P, Góral M, Wiśniewska-Gocłowska B, Shaw DG, Mączyński A. IUPAC-NIST solubility data series 101 alcohols + Hydrocarbons + Water Part 3. C1–C3 alcohols + aromatic hydrocarbons. J Phys Chem Ref Data. 2016;45:33103. https://doi.org/10.1063/1.4955012.
VWR. https://de.vwr.com/store/product?keyword=84708.290. Accessed on 8 Jan 2024.
VWR. https://de.vwr.com/store/product?keyword=20858.293%20. Accessed on 8 Jan 2024.
Othmer DF, White RE, Trueger E. Liquid-liquid extraction data. Ind Eng Chem. 1941;33:1240–8. https://doi.org/10.1021/ie50382a007.
Treybal RE, Weber LD, Daley JF. The system acetone–water–1,1,2–trichloroethane. Ind Eng Chem. 1946;38:817–21. https://doi.org/10.1021/ie50440a021.
Simić ZV, Radović IR, Kijevčanin ML. Measurement and correlation of liquid-liquid equilibrium data for ternary systems water + C1–C3 alcohols + diethyl adipate at 298.15 K and atmospheric pressure. J Chem Eng Data. 2024. https://doi.org/10.1021/acs.jced.3c00708.
Bezerra JKA, Duarte LJN, de Oliveira HNM, de Medeiros GG, de Barros Neto EL. Experimental study of liquid-liquid equilibrium and thermodynamic modeling for systems containing alcohols + biodiesel + diesel. J Chem Eng Data. 2023;68:1739–47. https://doi.org/10.1021/acs.jced.3c00261.
Gao J, Li Z, Ding C, Sun Z, Ma Y, Xu D, et al. Separation of 1-methoxy-2-propanol and water by organic solvent extraction based on quantum chemistry calculation and liquid-liquid equilibrium experiment. J Chem Thermodyn. 2024;189: 107201. https://doi.org/10.1016/j.jct.2023.107201.
Teymoornejad G, Jabbari M. Thermodynamic insights into phase behavior a new aqueous two-phase system at different temperatures: experimental equilibria, data correlation and modeling. J Mol Liq. 2023;375: 121282. https://doi.org/10.1016/j.molliq.2023.121282.
Arce A, Rodriguez O. Revising concepts on liquid-liquid extraction: data treatment and data reliability. J Chem Eng Data. 2022;67:286–96. https://doi.org/10.1021/acs.jced.1c00778.
VDI Heat Atlas. Berlin. Heidelberg: Springer, Berlin Heidelberg; 2010.
Hand DB. Dineric distribution. J Phys Chem. 1930;34:1961–2000. https://doi.org/10.1021/j150315a009.
Othmer DF, Tobias PE. Liquid–liquid extraction data—toluene and acetaldehyde systems. Ind Eng Chem. 1942;34:690–2. https://doi.org/10.1021/ie50390a011.
Othmer D, Tobias P. Liquid-liquid extraction data—the line correlation. Ind Eng Chem. 1942;34:693–6. https://doi.org/10.1021/ie50390a600.
Brancker AV, Hunter TG, Nash AW. Tie lines in two-liquid-phase systems. Ind Eng Chem Anal Ed. 1940;12:35–7. https://doi.org/10.1021/ac50141a012.
Bachman I. Tie lines in ternary liquid systems. Ind Eng Chem Anal Ed. 1940;12:38–9. https://doi.org/10.1021/ac50141a013.
Carniti P, Cori L, Ragaini V. A critical analysis of the hand and Othmer-Tobias correlations. Fluid Phase Equilib. 1978;2:39–47. https://doi.org/10.1016/0378-3812(78)80003-X.
Treybal RE. Liquid extraction. 1st ed. New York: McGraw-Hill; 1951.
Cumming APC, Morton F. Separation of benzene and n -hexane by means of ethylenediamine as solvent: molar solutropy. J Appl Chem. 1953;3:358–66. https://doi.org/10.1002/jctb.5010030806.
Treybal RE. Ternary liquid equilibria. Ind Eng Chem. 1944;36:875–81. https://doi.org/10.1021/ie50418a003.
Washburn, Edward W. International critical tables of numerical data, physics, chemistry and technology 1st ed. New York etc.: Pub. for the National research council by the McGraw-Hill book company inc; 1928.
Sherwood TK. Absorption and extraction. 1st ed. New York, London: McGraw-Hill book company inc; 1937.
Perry RH, Green DW. Perry’s chemical engineers’ handbook. 8th ed. New York: McGraw-Hill; 2008.
Sørensen JM, Magnussen T, Rasmussen P, Fredenslund A. Liquid—liquid equilibrium data: their retrieval, correlation and prediction part II: correlation. Fluid Phase Equilib. 1979;3:47–82. https://doi.org/10.1016/0378-3812(79)80027-8.
Gmehling J. Chemical thermodynamics for process simulation. Weinheim, Germany: Wiley-VCH; 2019.
Gebreyohannes S, Neely BJ, Gasem KAM. Generalized nonrandom two-liquid (NRTL) interaction model parameters for predicting liquid-liquid equilibrium behavior. Ind Eng Chem Res. 2014;53:12445–54. https://doi.org/10.1021/ie501699a.
Renon H, Prausnitz JM. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968;14:135–44. https://doi.org/10.1002/aic.690140124.
Prausnitz JM, Lichtenthaler RN, de Azevedo EG. Molecular thermodynamics of fluid-phase equilibria. 3rd ed. Upper Saddle River N.J.: Prentice Hall PTR; 1999.
Velho P, Barroca LR, Macedo EA. A geometric approach for the calculation of the nonrandomness factor using computational chemistry. J Chem Eng Data. 2023. https://doi.org/10.1021/acs.jced.3c00532.
Acknowledgements
We thank DAAD (Deutscher Akademischer Austauschdienst) and German Scholars Organization GSO for the financial support of our project. Open access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Zaid Alkhier Hamamah: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision, Funding acquisition. Thomas Grützner: Conceptualization, Methodology, Resources, Data Curation, Writing—Review and Editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamamah, Z.A., Grützner, T. Measurement and thermodynamic correlation of liquid–liquid equilibria of water + n-propanol + toluene at 20°C and atmospheric pressure. Discov Chem Eng 4, 20 (2024). https://doi.org/10.1007/s43938-024-00057-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-024-00057-6