Abstract
Removal of heavy metal pollutants from wastewater is critical for preserving a clean environmental setting and guaranteeing universal well-being. Physical, chemical, and biological approaches have been devised for the removal of heavy metals from various wastewater sources. Methods used include electrocoagulation (EC) and adsorption (AD). Although both methods are reported to be efficiently applied in wastewater treatment, the EC method is impeded by high energy consumption, particularly when treating concentrated effluents, as it demands substantial current for coagulant formation. Alternatively, the AD process is hindered by adsorbent saturation and the competitive effects of specific chemicals. Here, we only found limited studies on integrated EC-AD combined process, where the AD and EC were either used in separate or combined system vessels to augment the removal efficiency of heavy metal ions from wastewater or synthetic solutions. It is imperative to conduct more studies on synergistic approaches that combine adsorption with other wastewater treatment methods to address current limits and optimize removal processes. The review identified current density, pH, time, temperature, and adsorbent dosages as factors influencing the EC-AD process in heavy in the removal of heavy metals from wastewater. Although electrocoagulation combined with adsorption has been explored in several studies which have been confined to synthetic effluents, limiting their relevance to real-world scenarios. Therefore, this review proposes for development and design of EC-AD combined technologies to exploit their strengths and minimize associated limitations. Overall, the combined strategies proved more effective and economical compared to individual adsorption and electrocoagulation methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals are naturally occurring micro-elements that are essential for life, and when present beyond the recommended threshold by regulatory bodies (Table 1), they become hazardous to humans and other organisms [1,2,3,4,5]. Sources of heavy metal pollutants from wastewater include (but are not limited to) industrial, mining, agricultural activities, and waste disposal [6,7,8,9]. Membrane filtration, ion exchange, biological methods, and chemical precipitation are conventional methods used for the exclusion of heavy metal pollutants from wastewater. However, most of these techniques come with high costs, incomplete ion removal, sludge generation, and the extra cost of sludge disposal and membrane fouling [10]. Therefore, alternative advanced wastewater treatment approaches devised include adsorption, electrochemical, membrane, and advanced oxidation processes-based wastewater treatment approaches [11,12,13,14,15,16]. Selection of these methods relies on various factors, including concentration and heavy metal types present, wastewater volume, and the desired level of treatment efficiency among others. The effectiveness and long-term viability of heavy metals removal methods are being improved by ongoing studies and technological developments, opening the door to more effective and ecologically friendly wastewater treatment options.
Studies have shown that combining two methods for the removal of pollutants from wastewater offers several significant advantages such as high efficiency, synergistic effects, lower energy consumption, reduces chemical usage and operational costs [17,18,19]. Integrated approaches enhance the overall efficiency of the treatment process by leveraging the strengths of each method. For instance, EC effectively destabilizes and coagulates metal particles, while AD provides a high surface area for capturing these particles, leading to more comprehensive pollutant removal [20]. The dual approach in wastewater treatment not only provides greater flexibility in handling varying wastewater compositions but also enhances the robustness and resilience of the treatment system to fluctuations in pollutant loads. Therefore, the combined use of EC and AD represents a promising strategy for more efficient and sustainable heavy metal removal from wastewater.
As reviewed extensively elsewhere, adsorption (AD) and electrocoagulation (EC) are key methods applied separately to treat different wastewater [21,22,23,24]. The EC and AD systems have applications in wastewater treatment processes, albeit existence of certain drawbacks. Particularly, primary challenge of the EC lies in its energy consumption, particularly when treating highly concentrated effluents that require a substantial current to generate sufficient coagulant for treatment. Although, adsorption, is known for its adaptable design and operational flexibility, the process may also encounter limitations. For example, adsorbent saturation and the competitive effects of certain compounds are among the adsorption bottlenecks. A viable solution to address these constraints and leverage the strengths of both processes is their integration with other methods, providing an effective approach to wastewater treatment [25]. In this mini-review work, we have given the overview of adsorption, electrocoagulation and their simulations combination for the removal heavy metal contaminants from wastewater sources.
1.1 Electrocoagulation technique
The electrochemical processes can be primarily categorized into electrocoagulation (EC), electroflotation (EF), Electrooxidation (EO), and ion exchange (IE) [21]. Research show that these methods can be coupled with other wastewater treatment approaches either sequentially, concurrently or consequentially to augment electro-adsorption processes. Indeed, as reviewed extensively, electrocoagulation has been combined with adsorption to remove organic pollutant from wastewater [27,28,29,30,31,32]. Noteworthy, most of these studies are narrowed towards synthetic effluents, constraining their applications in real-life settings [33]. Electrochemical process as such electrocoagulation technique uses electric currents to remove suspended, dissolved pollutants, or emulsified from water [34, 35].
In the EC method, steel (iron) or aluminum electrodes are used as electrodes given their reliability and non-toxicity properties, and the mechanism involved is illustrated by Eqs. (1) (2) and (3) [36]. The passing of an electric current through a solution containing heavy metals results to precipitation that can be easily removed as sludge [37,38,39]. This technique has shown promise in the removal of heavy metal contaminants due to its capacity to generate coagulants in situ and efficiency [40,41,42]. More prominently, electrocoagulation offers several advantages over conventional chemical coagulation or chemical flocculation. Firstly, as no chemicals are introduced during the process, there exist zero risk of secondary pollution from chemical concentrations, unlike in chemical coagulation or chemical flocculation methods. Additionally, the gas bubbles generated in EC aid in lifting pollutants to the surface, simplifying their collection. The operational simplicity of EC equipment enables easy automation of the process. Moreover, EC-treated wastewater yields clear, colorless, and odorless water, while the flocs produced are larger and more stable than those from chemical coagulation or chemical flocculation, facilitating their separation during filtration. Electrocoagulation also results in significantly less sludge volume, which is both more stable and non-toxic [36] Furthermore, even the smallest colloidal particles are effectively removed by EC due to the accelerated collision facilitated by the applied electric current. However, EC presents certain drawbacks that include the requirement for frequent replacement of sacrificial anodes due to their dissolution into the solution, potentially leading to increased operational costs [43, 44]. Additionally, the occurrence of cathode passivation can diminish the efficiency of the EC process and the high current required generating sufficient coagulant for the treatment [45], impacting its overall effectiveness. Moreover, in regions with limited access to affordable electricity, the operational expenses associated with EC can become prohibitive, posing a challenge to its widespread adoption [36]. To overcomes some of these limitations, electrode passivation which entails adding aggressive ion, alternating current operation, polarity reversal operation, ultra-sonication, chemical and mechanical cleaning of electrodes, and hydrodynamic scouring. Still, each pathway has hitches, such as being costly, generating hazardous materials and sludge accumulation [46]. Hence, the application of EC procedure in the removal of pollutant from wastewater is still not well developed for large scale applications. More importantly, exploring into alternative solutions is an important scientific goal in wastewater treatment.
Cations released by anode destabilize colloidal particles and as well generate hydroxide complex metal ions (coagulants), which then react with negatively charged pollutants existing in wastewater as:
Several studies have been brought forward to describe wastewater treatment via electrocoagulation processes (Table 2). Numerous studies have used the electrocoagulation technique (EC) to successfully remove soluble heavy metals pollutants from various wastewaters [38, 47, 48], such as zinc [49], nickel [50], mercury [51] among others. Specifically, the usefulness of electrocoagulation in the removal of heavy metals is demonstrated by a number of recent studies. An extensively reviewed, Cd(II) and Pb(II) could be removed from wastewater using an EC system using a Fe-Al anode and a stainless steel cathode, whereas, Cu(II) and Zn(II) could be removed from wastewater using an aluminum electrode at high efficiency rates [48]. Also, studies on the application of a three-dimensional graphene-modified paper electrode for the removal of Cr(VI) has been explored in an effort to improve the efficacy of electrocoagulation technology [52]. This study reported reduced energy consumption compared to traditional EC systems.
1.2 Adsorption techniques
Although a number of wastewater processes have been documented, adsorption is still simple, adaptable, practical, insensitive to obnoxious substances, and commercially viable approach. Numerous low-cost adsorbents (Table 3), such as nanomaterial [59, 60] activated carbon [61], natural zeolites [62, 63] and agro-waste biochar from banana peel [64], corncob [65], grapefruit peel [66], sugarcane bagasse [67], potato peel [68], rice straw [69], orange peel [70], wheat stem [71] and among others. Overall, absorbents can also be classified as polymeric based adsorbents which offer a versatile approach to heavy metal ion removal, utilizing synthetic materials with tailored functional groups such as poly(acrylic acid) (PAA), poly(amidoxime), and poly(hydroxamic acid) to selectively bind metal ions through chelation and ion exchange mechanisms [72]. Similarly, industrial by-product adsorbents, including metal hydroxide sludge, fly ash, and red mud, repurpose waste materials for cost-effective heavy metal removal. Additionally, natural minerals based adsorbents like silica, zeolite, and montmorillonite offer inherent adsorption properties, providing sustainable solutions for environmental remediation. Chitosan, a natural biopolymer, showcases potential for water purification through chelation of heavy metal ions. Each category presents unique advantages, contributing to effective and sustainable methods for heavy metal ion removal in various applications [72]. Notably, adsorption processes are hindered by costs, adsorbent saturation, selectivity and the competitive effects of specific chemicals.
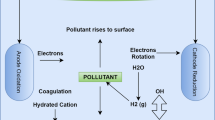
The most often used adsorbent is activated carbon, which produces the greatest results but is very costly in terms of production and regeneration [73, 74]. In adsorption, a solution becomes adsorbed on the surface of an adsorbent and occurs in two ways: physio-sorption, in which adsorbate attaches to adsorbent via van der Waals forces, which is a weak, reversible, and endothermic process, and chemi-sorption, which is an irreversible, selective, and exothermic process [75]. Adsorption mechanisms for removal of heavy metals from wastewater are illustrated in Fig. 1 [76,77,78,79].The adsorption process is described by isotherms which represent the estimated amount the solute that is adsorbed on the surface of the adsorbent per unit mass as a function of equilibrium concentration at a constant temperature. The adsorption process is described by the most widely used isotherms, namely Freundlich and Langmuir, Tables 4 and 5 [22].
Adsorption processes has been used to remove of heavy metal ions from waste water, such as adsorptive removal of Cr (IV) from aqueous solutions by adsorption using adsorbents from hazelnut shell [89]. Compared to other similar studies, this was found to be more efficiency than coconut shell and wood adsorbents[90] with removal of between 58.5–87.6 mg/g, respectively. As reviewed by Hussain et al. [61], activated carbon prepared from wood saw dust to remove Cr (VI) from wastewater exhibited adsorption capacity of Cr(VI) 44 mg/g at an optimum pH 2.0, compared to activated carbon from coconut shell, sugarcane bagasse, coconut tree saw dust. Based on most reviewed literature and studies on the separate application of adsorption and electrocoagulation, we report that adsorbents have limited efficiency compared with the use of electrochemical method in the removal of heavy metal pollutants. Generally, the combination of electrocoagulation and adsorption methods is a potential method for eliminating heavy metal contaminants from wastewater, and the mechanism involve in adsorption (Fig. 1) is very crucial.
1.2.1 Isotherms and kinetics
Previous studies have proposed numerous isotherms and kinetic [91,92,93,94] however, only Langmuir and Freundlich adsorption isotherms have been described in EC-ADS systems. In practice, both Langmuir and Freundlich models are used, often in combination, to interpret experimental data and gain insights into the adsorption behavior of different systems. Aware of the reported limitations associated with isotherm parameters [95,96,97], the assessment of other parameters that affect EC-ADS process could be very scientifically interesting.
The following Langmuir [98], and Freundlich [99, 100] adsorption isotherms (Tables 4, 5) have been used to describe in the removal of heavy metals by EC-ADS process.
The parameters qe and qm (mg/g) correspond to the quantity of adsorbed ions per unit mass of adsorbent and the optimum amount of ions per unit mass necessary for a full layer on the surface of the adsorbent, respectively. The Ce mirrors the adsorbate's titre at equilibrium (mg/L). KL denotes the Langmuir equilibrium factor (L/mg), whilst n and KF are Freundlich constant which describe the extent of adsorption and the non-linear link between metal concentrations in solution and adsorption.
In this setting, qe and qt (mg/g) describe the equilibrium and time-dependent functions of heavy metal adsorption, accordingly. k1 signifies the pseudo-first-order rate constant (1/min), whereas k2 depicts the pseudo-second-order rate constant (g/mg.min). The Elovich equation's coefficients are β and α. β denotes the desorption constant (g/mg) and α defines the initial sorption rate. Kid clarifies the intra-particle diffusion rate constant (g/mg min1/2), whilst c is the intercept from the plot of qt versus t1/2.
1.3 Main application of EC-AD combined processes in the removal heavy metal pollutants from wastewater
Recently, interest in electrochemical-adsorption combined process have generated interest as evidently shown by registered patents in last decade [101]. Electrical potential is applied during the adsorption process in the EC-AD combined process. This combined approach, which uses a device with several electrodes coupled to an external circuit and combines adsorption with electrochemical concept design. Through electrical links, the potentiostat supply the working, reference, and counter electrodes with electrical impulses [102], serving as source of the current and voltage to the electrolytic cells. The electrolyte solution's constituent parts are adsorbed onto the working electrode during this process. By creating a double layer contact between the electrode and the solution, electro-adsorption works. This process is driven by a number of interactions, such as dipole and electrostatic interactions, to increase the rate and capacity of adsorption. Surface features, area, microstructure, pore size distribution, solution state, and electrode material properties are some of the variables that affect the EC-AD capacity. The technique lowers operating costs by prolonging the adsorbent regeneration life[101].The combined electrochemical-adsorption is a modified EC system introduced to enhance removal of chemical oxygen demand (COD), inorganic salts, antibiotics, dyes, colloidal particles, and turbidity pollutants [103]. Moreover, our literature review identified limited studies that have employed electrochemical-adsorption methods to remove heavy metals. For example, a study extensively reconnoitered the use of manganese oxide (birnessite-type) for synergistic removal of Cd(II) and As(V) from wastewater, shown the possibility of using electrosorbents in EC-AD process [104]. Orescanin et al. [105] suggested a novel electroplating wastewater treatment approach via integration of EC with Fe and Al electrodes with ozonation system, resulting to high efficiency (~ 97%) in the removal of Pb. In another scenario, Ferniza-García et al. [106] explored the elimination of heavy metal contaminants from simulated mining water by integrating EC with aluminum electrodes with phytoremediation supported by a conventional power supply. As we observed, limited attention has been directed towards understanding integrated electrochemical-adsorption proces, particularly when coupled with low-cost organic absorbents (Table 3). Currently, different adsorbents including AC, carbon nanotubes, bio-sorbents and low-cost adsorbents (agricultural wastes, industrial by-products, sands and clays) may be employed [107]. The effect of a few adsorbent types on EC-AD approaches on kinetics including pseudo-first order, pseudo-second order, and intra-particle diffusion have been reported [108]. Therefore, our work outline the application and selected parameters that affect electrocoagulation–adsorption methods. In the EC-AD combined system, the AD and EC can be used simultaneously, either in separate or combined system vessels.
Coupling of AD and EC processes refer to the solution for solving their limitations while advantaging from their potential. Moreover, the combination of these two processes offers the benefits of process intensification, such as a smaller unit footprint, increased versatility, and greater mobility. Additionally, the integration reduces operating costs and energy consumption, and the use of low-cost materials lowers investment costs. These advantages make this process particularly attractive for small industries. The EC coupled with adsorption has been used to remove target pollutants [109], however no inclusive literature has been devoted to understand its application in removal of heavy metal ions in wastewater treatment settings. A variety of electrode and adsorbent combinations have been employed in combined EC and AD processes to treat diverse wastewaters (Table 6). This process’ characteristic, its versatility increase in dealing with specific pollutant problems’ removal.
Narayanan and Ganesan, [110] in their study used granular activated carbon adsorption coupled with batch electrocoagulation (EC) using an Al–Fe electrode pair to remove Cr(VI) from synthetic effluents. The key mechanisms in the removal of Cr(VI) were found to be the combined effects of chemical precipitation, co-precipitation, sweep coagulation, and adsorption. Comparing the results to the traditional EC technique, it was found that the use of an adsorbent improved the Cr(VI) removal efficiency, requiring lower current intensities and shorter operating time spans.
Ali et al. [1] used a combined EC-AD system for the removal of heavy metal pollutants from industrial wastewater was developed based on a bipolar configuration. This system used Al as the electrode and slag as the adsorbent. The removal efficiency was recorded as 99%, 91%, and 99% for Fe, Zn, and Cu, accordingly. The synergistic effects of electrokinetic treatment and adsorption were responsible for this efficiency. Analysis via XRF, SEM, FTIR, and PSA confirmed the enhanced removal efficiency attributed to EC. Freundlich isotherms were observed for Fe and Zn, while Cu removal did not follow a specific isotherm. Chemisorption was identified as the predominant removal mechanism for all metals. As a single-step, low-cost treatment method for removing trace heavy metals from wastewater that adheres to circular economy principles, this creative integrated approach has a lot of promise.
Hussin et al. [111] removed Pb(II) from aqueous solutions using an integrated method that included solar combined EC-AD procedures with a removal efficiency of 99.88%. The following settings were found to be ideal for maximal Pb(II) removal (99.88%): pH 6.01, starting Pb(II) concentration of 15.00 mg/L, and adsorbent dosage of 2.50 mg/L compared with single methods. Due to the low adsorbent usage, this combination method offers great removal efficiency and cost-effectiveness, surpassing individual electrocoagulation and adsorption treatments. Using low-cost and sustainable solar energy, it shows robustness and success in treating wastewater contaminated with heavy metals. Although further study is required to optimize and scale-up the process for industrial use, continuous operation utilizing solar energy has promise for large-scale applications.
Electrocoagulation combined with adsorption processes holds immense promise for efficiently treating wastewater contaminated with diverse pollutants, leveraging the synergistic effects of electrochemical coagulation and adsorption. However, as these processes are scaled-up, a spectrum of opportunities and challenges emerges. Enhanced treatment efficiency and versatility in heavy metal pollutant removal mark key opportunities, alongside the potential for cost-effectiveness and environmental sustainability through reduced chemical usage, low energy cost and sludge generation. For example, Hussin et al. [111], integrated the use solar in EC-ADS processes for Pb removal from aqueous solution. The energy consumption of the combined system, estimated at 0.045 kWh/m3, underscores its sustainability and cost-effectiveness, supported by previous studies [112, 113]. However, challenges such as maintaining electrode and adsorbent stability, managing energy consumption, optimizing processes, and ensuring regulatory compliance remain a bottleneck in the large scale-up application of EC-ADS process. Albeit the presence of a literature review on EC-ADS, There is a clear need to bridge the gap in addressing these challenges through the deployment of robust technological innovations and operational strategies. This is crucial for effectively implementing this technology on a larger scale and making substantial contributions to sustainable wastewater management practices across diverse industrial sectors.
1.4 Parameters that affect the EC-ADS efficiency in the removal of heavy metals from wastewater
1.4.1 Adsorbent dosages
Adsorbent doses influence the removal efficiency of heavy metal contaminants during the ADS/EC combined process. For example, in AGWTR experiments, the efficiency removal for Cu2+ and Ni2+ is augmented by the adsorbent dosage increase [102]. The ions in the solutions interacted with the ADS-binding sites resulting to higher removal efficiencies as previously studied [1, 117,118,119]. Hussin et al. [111] described that the higher adsorbent dosages increased Pb(II) removal efficiency, aligning with the premise that active sites of adsorbent may effectively adsorb more pollutants from the solution.
1.4.2 Energy considerations
The electric current density is a critical parameter in the EC-AD approaches, as it influences the generation of coagulant dosage rate, gas bubbles, and floc growth and size, which are essential for effective wastewater treatment [120, 121]. Current density has a major impact on mass transfer at the electrodes and solution mixing, which are both essential for the efficient removal of pollutants. Increasing the current density improves the mass transfer rates and improves mixing, which leads to higher removal efficiencies of Fe2+, Zn2+, and Cu2+ by the EC-AD process [1]. Also, This improvement may be explained by variations in floc size and development, as well as, higher rates of coagulant and bubble generation [42]. In the ADS/EC (AGWTR) system, current density has a major effect on solution mixing and mass transfer at the electrodes. Cu2 + and Ni2 + removal is reduced and anode dissolution is modest as a result of the low current density. On the contrary, owing to the faster generation of hydroxides through co-precipitation and enhanced dissolution of the iron electrode material, stronger current densities result in higher removal efficiencies for both metal ions [102].
1.4.3 Electrolysis time
Electrolysis time affects the efficiency of water and wastewater treatment, and inferred from contact period between the metal ions and the adsorbents in ADS/EC methods, The removal efficiency of heavy metals varied with time, the longer treatment periods improve the efficacy of removal by increasing metal hydroxide formation [42]. Similarly, in the exclusion of pollutant by AGWTR system, the longer adsorption contact times led to increased removal of copper (Cu2 +) and nickel (Ni2 +) due to active surface sites on the adsorbent material. For instance at 120 min, 1 g of adsorbent removed 73.51% of Cu2 + and 66.01% of Ni2 + . Additionally, the coagulant generated from iron electrodes in ADS coupled with EC increases with reaction time, enhancing removal efficiencies through sweep coagulation and co-precipitation. The peak removal efficiency for Cu2 + and Ni2 + occurs at 30 min, where 100% and 99.98% of respective ions were removed, facilitated by iron (III) hydroxide "sweep flocs" with large surface areas conducive to the adsorption of pollutants from wastewater. Overall, the Cu2+ and Ni2+ removal was directly proportional to the adsorption contact time which is clarified by the high availability of active surface sites on the adsorbents [102, 122].
1.4.4 Concentration of hydrogen ions (pH)
The pH influence the removal of heavy metal pollutants during EC-AD process [102]. For example, the pH value of 2–8 favored the removal of Cu2+ from simulated wastewater in the EC-AD simultaneous process. Specifically, the highest removal efficiency was observed at pH 4.0 due to the presence of Fe(OH)3, which is influenced by the pH and Fe3+ concentration. The removal efficiency of Cu2+ was higher than Ni2+ in both AD and AD/EC processes. Maintaining a low initial pH, such as 4.0, was found to enhance the Cu2+ and Ni2+ removal without the need for additional chemicals. In another study, the Pb2+ precipitate in solutions with a pH > 7, implying that the removal efficiency of these pollutants was similarly influenced by pH [1]. This underscores the importance of controlling pH in the treatment of wastewater.
1.4.5 Adsorption isotherms
This review indicates that when Cu2+ and Ni2+ were removed from synthetic wastewater using AGWTR coupled with AD and EC, the adsorption isotherms indicated that Langmuir had a superior fit with higher correlation coefficients than Freundlich. On the AGWTR surface, monolayer adsorption with maximum adsorption capabilities was suggested using the Langmuir model. Overall, the computed adsorption isotherms revealed that heavy metals could be readily adsorbed during the EC-AD process [102]. In the removal of Pb2+ by combined solar EC-AD process, pseudo-first-order kinetics analysis produced a fit experimental data [111]. The results indicated that Pb(II) is adsorbed and removed from aqueous solutions more quickly in the combined treatment procedure than in individual treatments. This implies that the combined system of solar EC-AD outperforms processes that were carried out individually.
From literature review, the study suggests a deeper consideration of process parameters concerning potential scale-up for the EC-AD (Electrocoagulation and Adsorption) method in removing heavy metals from wastewater. For examples, most study used model solution which may not give true about the applicability of this method although promising. Our study found that the efficacy of EC-AD is influenced by different parameters: adsorbent dosages, current density, electrolysis time, and pH. Generally, the study found that adsorbent dosages affect removal efficiency by increasing interaction with metal ions, while current density influences mass transfer rates and mixing, crucial for pollutant removal [1]. Electrolysis time affects removal efficacy through prolonged contact periods. While, pH plays a significant role, with optimal values favoring metal ion removal. Adsorption isotherms, particularly Langmuir, suggest efficient heavy metal adsorption during the EC-AD process, although recent work have reported flaws associated with isotherms parameters [95,96,97]. In summary, we suggest, a thorough re-evaluation of these parameters to optimize the process for potential scale-up is necessary for ensuring effective and efficient heavy metal removal from wastewater. The main challenges to the scale-up of integrated electrocoagulation-adsorption strategies for the removal of heavy metal pollutants from wastewater include optimizing the design and operation of large-scale electrocoagulation reactors to ensure efficient removal of heavy metals while minimizing operational costs. Additionally, achieving consistent and reliable adsorption performance over extended periods poses a challenge, as factors such as adsorbent regeneration and stability need to be addressed. Furthermore, the scalability of these integrated systems may be hindered by the variability of wastewater composition and heavy metal concentrations, necessitating robust monitoring and control mechanisms. Moreover, addressing potential environmental impacts and ensuring compliance with regulatory standards at scale are important considerations. Finally, economic viability and the availability of suitable adsorbents in large quantities can also influence the successful scale-up of electrocoagulation-adsorption strategies for heavy metal removal from wastewater.
2 Conclusion
This mini-review explores the application and influencing factors of the AD-EC combined process for heavy metal ion removal from different wastewaters. Wastewater treatment is a critical aspect of environmental protection. This review explores the integration of the electrocoagulation-adsorption process as an effective and sustainable approach for heavy metal ion removal. Adsorption using various adsorbents provides extraordinary surface area and affinity for heavy metal ions, whereas electrocoagulation ensures efficient coagulation and subsequent separation of the formed flocs. One of the approaches to sidestep the limitations of individual wastewater treatment methods is the integration of EC with AD processes, allowing optimal removal of pollutants from wastewater. This review highlights that combined AD-EC approaches exhibit high efficiency in heavy metal removal from diverse wastewater sources, although the design, optimization, and scaling up of this method for broader application in wastewater treatment remains a topic for further exploration. Future studies should aim to bridge the gap between laboratory-scale experiments and real-world applications. While many studies have established the efficacy of EC with AD processes in controlled settings, there is a need for more research conducted in actual wastewater treatment plants or field sites. This would provide valuable insights into the scalability, reliability, and cost-effectiveness of these technologies in practical scenarios.
Data availability
The data sharing are not applicable as there are no new data created in this review.
References
Ali AA, Ouda M, Naddeo V, Puig S, Hasan SW. Integrated electrochemical-adsorption process for the removal of trace heavy metals from wastewater. Case Stud Chem Environ Eng. 2021;4:100147. https://doi.org/10.1016/j.cscee.2021.100147.
Brevik EC, et al. Soil and human health: current status and future needs. Air Soil Water Res. 2020;13:1178622120934441.
Elbasiouny H, et al. Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: a review. Environ Monit Assess. 2021;193(7):449. https://doi.org/10.1007/s10661-021-09236-2.
Hu X, et al. Effects of heavy metals/metalloids and soil properties on microbial communities in farmland in the vicinity of a metals smelter. Front Microbiol. 2021. https://doi.org/10.3389/fmicb.2021.707786.
Tahoon MA, Siddeeg SM, Alsaiari NS, Mnif W, Rebah FB. Effective heavy metals removal from water using nanomaterials: a review. Processes. 2020;8(6):645. https://doi.org/10.3390/pr8060645.
Bora AJ, Dutta RK. Removal of metals (Pb, Cd, Cu, Cr, Ni, and Co) from drinking water by oxidation-coagulation-absorption at optimized pH. J Water Process Eng. 2019. https://doi.org/10.1016/j.jwpe.2019.100839.
Abdulraheem FS, Al-Khafaji ZS, Hashim KS, Muradov M, Kot P, Shubbar AA. Natural filtration unit for removal of heavy metals from water. IOP Conf Ser Mater Sci Eng. 2020;888(1): 012034.
Zhang K, Luo X, Yang L, Chang Z, Luo S. Progress toward hydrogels in removing heavy metals from water: problems and solutions—a review. ACS ES&T Water. 2021;1(5):1098–116. https://doi.org/10.1021/acsestwater.1c00001.
Alloway BJ. Sources of heavy metals and metalloids in soils. In: Alloway BJ, editor. Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Dordrecht: Springer Netherlands; 2013. p. 11–50. https://doi.org/10.1007/978-94-007-4470-7_2.
Saleh TA, Mustaqeem M, Khaled M. Water treatment technologies in removing heavy metal ions from wastewater: a review. Environ Nanotechnol Monit Manag. 2022;17: 100617. https://doi.org/10.1016/j.enmm.2021.100617.
Górecki R, Reurink DM, Khan MM, Sanahuja-Embuena V, Trzaskuś K, Hélix-Nielsen C. Improved reverse osmosis thin film composite biomimetic membranes by incorporation of polymersomes. J Membr Sci. 2020;593: 117392.
Tang S, et al. Degradation of anticancer drug capecitabine in aquatic media by three advanced oxidation processes: mechanisms, toxicity changes and energy cost evaluation. Chem Eng J. 2021;413: 127489.
Mishra S, Singh RP, Rout PK, Das AP. Membrane bioreactor (MBR) as an advanced wastewater treatment technology for removal of synthetic microplastics. In: Development in wastewater treatment research and processes. Amsterdam: Elsevier; 2022. p. 45–60. https://doi.org/10.1016/B978-0-323-85583-9.00022-3.
Khan AH, et al. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J Clean Prod. 2020;269: 122411.
Völker J, Stapf M, Miehe U, Wagner M. Systematic review of toxicity removal by advanced wastewater treatment technologies via ozonation and activated carbon. Environ Sci Technol. 2019;53(13):7215–33.
Tang CY, Yang Z, Guo H, Wen JJ, Nghiem LD, Cornelissen E. Potable water reuse through advanced membrane technology. Environ Sci Technol. 2018. https://doi.org/10.1021/acs.est.8b00562.
Almukdad A, Hafiz M, Yasir AT, Alfahel R, Hawari AH. Unlocking the application potential of electrocoagulation process through hybrid processes. J Water Process Eng. 2021;40: 101956. https://doi.org/10.1016/j.jwpe.2021.101956.
Asfaha YG, Tekile AK, Zewge F. Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: a review. Clean Eng Technol. 2021;4: 100261. https://doi.org/10.1016/j.clet.2021.100261.
Asfaha YG, Zewge F, Yohannes T, Kebede S. Application of hybrid electrocoagulation and electrooxidation process for treatment of wastewater from the cotton textile industry. Chemosphere. 2022;302: 134706. https://doi.org/10.1016/j.chemosphere.2022.134706.
Genethliou C, Tatoulis T, Charalampous N, Dailianis S, Tekerlekopoulou AG, Vayenas DV. Treatment of raw sanitary landfill leachate using a hybrid pilot-scale system comprising adsorption, electrocoagulation and biological process. J Environ Manage. 2023;330: 117129. https://doi.org/10.1016/j.jenvman.2022.117129.
Qasem NAA, Mohammed RH, Lawal DU. Removal of heavy metal ions from wastewater: a comprehensive and critical review. NPJ Clean Water. 2021;4(1):36. https://doi.org/10.1038/s41545-021-00127-0.
Hussain A, Madan S, Madan R. Removal of heavy metals from wastewater by adsorption. In: Nazal MK, Zhao H, editors. Heavy metals—their environmental impacts and mitigation. IntechOpen; 2021. https://doi.org/10.5772/intechopen.95841.
Ye Q, Li Q, Li X. Removal of heavy metals from wastewater using biochars: adsorption and mechanisms. Environ Pollut Bioavail. 2022;34(1):385–94. https://doi.org/10.1080/26395940.2022.2120542.
Hegazi HA. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013;9(3):276–82. https://doi.org/10.1016/j.hbrcj.2013.08.004.
Yenkie KM. Integrating the three E’s in wastewater treatment: efficient design, economic viability, and environmental sustainability. Curr Opin Chem Eng. 2019;26:131–8.
Das TK, Poater A. Review on the use of heavy metal deposits from water treatment waste towards catalytic chemical syntheses. Int J Mol Sci. 2021;22(24):13383.
Elhouda MN, Chabani M, Bouafia-Chergui S, Touil AH. Removal of chemical oxygen demand from real petroleum refinery wastewater through a hybrid approach: electrocoagulation and adsorption. Chem Eng Process Process Intensif. 2024; 109680.
Dehghani Y, Honarvar B, Azhdarpour A, Nabipour M. Treatment of wastewater by a combined technique of adsorption, electrocoagulation followed by membrane separation. Adv Environ Technol. 2021;7(3):171–83.
Myllymäki P, Lahti R, Romar H, Lassi U. Removal of total organic carbon from peat solution by hybrid method—electrocoagulation combined with adsorption. J Water Process Eng. 2018;24:56–62.
Wang Y, Yang Q, Dong J, Huang H. Competitive adsorption of PPCP and humic substances by carbon nanotube membranes: effects of coagulation and PPCP properties. Science Total Environ. 2017;619–620:352–9. https://doi.org/10.1016/j.scitotenv.2017.11.117.
Goyal H, Mondal P. Life cycle assessment (LCA) of the arsenic and fluoride removal from groundwater through adsorption and electrocoagulation: a comparative study. Chemosphere. 2022;304: 135243.
Ouaissa YA, Chabani M, Amrane A, Bensmaili A. Integration of electro coagulation and adsorption for the treatment of tannery wastewater—the case of an Algerian factory, Rouiba. Proc Eng. 2012;33:98–101.
Nigri EM, Santos AL, Rocha SD. Removal of organic compounds, calcium and strontium from petroleum industry effluent by simultaneous electrocoagulation and adsorption. J Water Process Eng. 2020;37: 101442.
Hakizimana JN, et al. Electrocoagulation process in water treatment: a review of electrocoagulation modeling approaches. Desalination. 2017;404:1–21. https://doi.org/10.1016/j.desal.2016.10.011.
Garcia-Segura S, Eiband MMSG, de Melo JV, Martínez-Huitle CA. Electrocoagulation and advanced electrocoagulation processes: a general review about the fundamentals, emerging applications and its association with other technologies. J Electroanal Chem. 2017;801:267–99. https://doi.org/10.1016/j.jelechem.2017.07.047.
Moussa DT, El-Naas MH, Nasser M, Al-Marri MJ. A comprehensive review of electrocoagulation for water treatment: potentials and challenges. J Environ Manage. 2017;186:24–41.
Safwat SM, Mohamed NY, El-Seddik MM. Performance evaluation and life cycle assessment of electrocoagulation process for manganese removal from wastewater using titanium electrodes. J Environ Manag. 2023;328: 116967. https://doi.org/10.1016/j.jenvman.2022.116967.
Al-Shannag M, Al-Qodah Z, Bani-Melhem K, Qtaishat MR, Alkasrawi M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: kinetic study and process performance. Chem Eng J. 2015;260:749–56. https://doi.org/10.1016/j.cej.2014.09.035.
Chen Y, Ji S, Chen C, Peng Q, Wang D, Li Y. Single-atom catalysts: synthetic strategies and electrochemical applications. Joule. 2018;2(7):1242–64.
Gatsios E, Hahladakis JN, Gidarakos E. Optimization of electrocoagulation (EC) process for the purification of a real industrial wastewater from toxic metals. J Environ Manag. 2015;154:117–27. https://doi.org/10.1016/j.jenvman.2015.02.018.
Bhagawan D, et al. Effect of operational parameters on heavy metal removal by electrocoagulation. Environ Sci Pollut Res. 2014;21(24):14166–73. https://doi.org/10.1007/s11356-014-3331-8.
Bazrafshan E, Mohammadi L, Ansari-Moghaddam A, Mahvi AH. Heavy metals removal from aqueous environments by electrocoagulation process—a systematic review. J Environ Health Sci Eng. 2015;13(1):74. https://doi.org/10.1186/s40201-015-0233-8.
Mu R, Liu B, Chen X, Wang N, Yang J. Hydrogel adsorbent in industrial wastewater treatment and ecological environment protection. Environ Technol Innov. 2020;20: 101107.
Zaied B, Rashid M, Nasrullah M, Zularisam A, Pant D, Singh L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci Total Environ. 2020;726: 138095.
Graça NS, Rodrigues AE. The combined implementation of electrocoagulation and adsorption processes for the treatment of wastewaters. Clean Technol. 2022;4(4):1020–53.
Ingelsson M, Yasri N, Roberts EP. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—a review. Water Res. 2020;187: 116433.
Rincon GJ, La Motta EJ. Simultaneous removal of oil and grease, and heavy metals from artificial bilge water using electro-coagulation/flotation. J Environ Manage. 2014;144:42–50.
Shahedi A, Darban A, Taghipour F, Jamshidi-Zanjani A. A review on industrial wastewater treatment via electrocoagulation processes. Curr Opin Electrochem. 2020;22:154–69.
Kobya M, Demirbas E, Dedeli A, Sensoy M. Treatment of rinse water from zinc phosphate coating by batch and continuous electrocoagulation processes. J Hazard Mater. 2010;173(1–3):326–34.
Dermentzis K, Valsamidou E, Lazaridou A, Kokkinos N. Nickel removal from wastewater by electrocoagulation with aluminum electrodes. J Eng Sci Technol Rev. 2011;4(2):188–92.
Chaturvedi SI. Mercury removal using Fe–Fe electrodes by electrocoagulation. Int J Mod Eng Res (IJMER). 2013;3(1):101–8.
Yao J, et al. Graphene-modified graphite paper cathode for the efficient bioelectrochemical removal of chromium. Chem Eng J. 2021;405: 126545.
AlJaberi FY, Hawaas ZA. Electrocoagulation removal of Pb, Cd, and Cu ions from wastewater using a new configuration of electrodes. MethodsX. 2023;10: 101951.
Kim T, Kim T-K, Zoh K-D. Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J Water Process Eng. 2020;33: 101109. https://doi.org/10.1016/j.jwpe.2019.101109.
Un UT, Ocal SE. Removal of heavy metals (Cd, Cu, Ni) by electrocoagulation. Int J Environ Sci Dev. 2015;6(6):425.
Ayub S, et al. Removal of heavy metals (Cr, Cu, and Zn) from electroplating wastewater by electrocoagulation and adsorption processes. Desalin Water Treat. 2020;179:263–71.
El-Ashtoukhy ESZ, Amin NK, Fouad YO, Hamad HA. Intensification of a new electrocoagulation system characterized by minimum energy consumption and maximum removal efficiency of heavy metals from simulated wastewater. Chem Eng Process Process Intensif. 2020;154: 108026. https://doi.org/10.1016/j.cep.2020.108026.
Sharma D, Chaudhari PK, Prajapati AK. Removal of chromium (VI) and lead from electroplating effluent using electrocoagulation. Sep Sci Technol. 2020;55(2):321–31.
Saritha D. A concise review on the removal of heavy metals from wastewater using adsorbents. Mater Today Proc. 2022;62:3973–7.
Arora R. Nano adsorbents for removing the arsenic from waste/ground water for energy and environment management-a review. Mater Today Proc. 2021;45:4437–40.
Hussain I, Qi J, Sun X, Wang L, Li J. Melamine derived nitrogen-doped carbon sheet for the efficient removal of chromium (VI). J Mol Liq. 2020;318: 114052.
Tasić Ž, Bogdanović G, Antonijević M. Application of natural zeolite in wastewater treatment: a review. J Min Metal A Min. 2019;55(1):67–79.
Velarde L, Nabavi MS, Escalera E, Antti M-L, Akhtar F. Adsorption of heavy metals on natural zeolites: a review. Chemosphere. 2023;328: 138508. https://doi.org/10.1016/j.chemosphere.2023.138508.
Negroiu M, et al. Novel adsorbent based on banana peel waste for removal of heavy metal ions from synthetic solutions. Materials. 2021;14:3946. https://doi.org/10.3390/ma14143946.
Juang R-S, Wu F-C, Tseng R-L. Characterization and use of activated carbons prepared from bagasse for liquid-phase adsorption. Colloids Surf A Physicochem Eng Aspects Colloid Surf A. 2002;201:191–9. https://doi.org/10.1016/S0927-7757(01)01004-4.
Zou W, Zhao L, Zhu L. Adsorption of uranium (VI) by grapefruit peel in a fixed-bed column: experiments and prediction of breakthrough curves. J Radioanal Nucl Chem. 2013;295:717–27.
Oliveira JA, Cunha FA, Ruotolo LAM. Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. J Clean Prod. 2019;229:956–63.
Singh M, et al. Characterization of organophosphate pesticide sorption of potato peel biochar as low cost adsorbent for chlorpyrifos removal. Chemosphere. 2022;297: 134112.
Wu Y, et al. Functionalized agricultural biomass as a low-cost adsorbent: utilization of rice straw incorporated with amine groups for the adsorption of Cr (VI) and Ni (II) from single and binary systems. Biochem Eng J. 2016;105:27–35.
Akinhanmi TF, Ofudje EA, Adeogun AI, Aina P, Joseph IM. Orange peel as low-cost adsorbent in the elimination of Cd (II) ion: kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour Bioprocess. 2020;7(1):1–16.
Jalali A, Mirnezami F, Lotfi M, Shafiee M, Mohammadi AH. Biosorption of lead ion from aqueous environment using wheat stem biomass. Desalin Water Treat. 2021;233:98–105.
Zaimee MZA, Sarjadi MS, Rahman ML. Heavy metals removal from water by efficient adsorbents. Water. 2021;13(19):2659.
Azam K, et al. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J Environ Chem Eng. 2020;8(5): 104220.
Liu Q, Zhou Y, Lu J, Zhou Y. el cyclodextrin-based adsorbents for removing pollutants from wastewater: a critical review. Chemosphere. 2020;241: 125043.
Hussain A, Madan S, Madan R. Removal of heavy metals from wastewater by adsorption. Heavy metals—their environmental impacts and mitigation, 2021. https://doi.org/10.5772/intechopen.95841.
Liu Z, et al. Preparation, characterization and application of activated carbon from corn cob by KOH activation for removal of Hg (II) from aqueous solution. Biores Technol. 2020;306: 123154.
Zhou G, Luo J, Liu C, Chu L, Crittenden J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018;131:246.
Ku Y, Jung IL. Photocatalytic reduction of Cr (VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001;35(1):135.
Zhang H, et al. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere. 2017;185:351–60. https://doi.org/10.1016/j.chemosphere.2017.07.025.
Sabri MU, Qayyum AA, Akhtar M, Munawar Z. Adsorption kinetics of iron (II) from waste/aqueous solution by using potato peel as carbonaceous material. Int J Biosci. 2018;13:212–20.
Mutongo F, Kuipa O, Kuipa PK. Removal of Cr (VI) from aqueous solutions using powder of potato peelings as a low cost sorbent. Bioinorg Chem Appl. 2014. https://doi.org/10.1155/2014/973153.
Kyzas GZ, Mitropoulos AC. Zero-cost agricultural wastes as sources for activated carbons synthesis: lead ions removal from wastewaters. 2018; 2: MDPI, 11 ed., 652.
Abdelfattah I, Ismail AA, Al Sayed F, Almedolab A, Aboelghait KM. Biosorption of heavy metals ions in real industrial wastewater using peanut husk as efficient and cost effective adsorbent. Environ Nanotechnol Monitor Manag. 2016;6:176–83.
Banerjee M, Basu RK, Das SK. Cu (II) removal using green adsorbents: kinetic modeling and plant scale-up design. Environ Sci Pollut Res. 2019;26:11542–57.
Weng C-H, Lin Y-T, Hong D-Y, Sharma YC, Chen S-C, Tripathi K. Effective removal of copper ions from aqueous solution using base treated black tea waste. Ecol Eng. 2014;67:127–33.
Wen T, et al. Production of a generic magnetic Fe3O4 nanoparticles decorated tea waste composites for highly efficient sorption of Cu (II) and Zn (II). J Environ Chem Eng. 2017;5(4):3656–66.
Mi H, Yi L, Wu Q, Xia J, Zhang B. Preparation and optimization of a low-cost adsorbent for heavy metal ions from red mud using fraction factorial design and Box-Behnken response methodology. Colloids Surf, A. 2021;627: 127198.
Park J-H, et al. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci Total Environ. 2020;740: 140205.
Kobya M. Removal of Cr (VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: kinetic and equilibrium studies. Bioresour Technol. 2004;91(3):317–21.
Selomulya C, Meeyoo V, Amal R. Mechanisms of Cr (VI) removal from water by various types of activated carbons. J Chem Technol Biotechnol Int Res Process Environ Clean Technol. 1999;74(2):111–22.
Xu R, et al. Simultaneous and efficient removal of multiple heavy metal (loid) s from aqueous solutions using Fe/Mn (hydr) oxide and phosphate mineral composites synthesized by regulating the proportion of Fe (II), Fe (III), Mn (II) and PO43–. J Hazard Mater. 2022;438: 129481.
Li Y, Wen J, Xue Z, Yin X, Yuan L, Yang C. Removal of Cr (VI) by polyaniline embedded polyvinyl alcohol/sodium alginate beads—extension from water treatment to soil remediation. J Hazard Mater. 2022;426: 127809.
Singh R, Datta B. Banana peel powder as an effective multilayer adsorbent of ammonium ions. Ind Eng Chem Res. 2022;61(50):18464–74.
Kumar M, Mukherjee S, Thakur AK, Raval N, An AK, Gikas P. Aminoalkyl-organo-silane treated sand for the adsorptive removal of arsenic from the groundwater: immobilizing the mobilized geogenic contaminants. J Hazard Mater. 2022;425: 127916.
Chu KH, Hashim MA, da CostaSantos YT, Debord J, Harel M, Bollinger J-C. The Redlich-Peterson isotherm for aqueous phase adsorption: pitfalls in data analysis and interpretation. Chem Eng Sci. 2024;285: 119573.
Tran HN, Bollinger J-C, Lima EC, Juang R-S. How to avoid mistakes in treating adsorption isotherm data (liquid and solid phases): some comments about correctly using Radke-Prausnitz nonlinear model and Langmuir equilibrium constant. J Environ Manage. 2023;325: 116475.
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq. 2019;273:425–34.
Ain QU, et al. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb (II), Cd (II), and crystal violet from aqueous solution. J Clean Prod. 2020;247: 119088.
Mokhtar A, Abdelkrim S, Hachemaoui M, Adjdir M, Zahraoui M, Boukoussa B. Layered silicate magadiite and its composites for pollutants removal and antimicrobial properties: a review. Appl Clay Sci. 2020;198: 105823.
Moghaddam SAE, Harun R, Mokhtar MN, Zakaria R. Kinetic and equilibrium modeling for the biosorption of metal ion by Zeolite 13X-Algal-Alginate Beads (ZABs). J Water Process Eng. 2020;33: 101057.
da Costa JGR, Costa JM, de Almeida Neto AF. Recent advances and future applications in electro-adsorption technology: an updated review. J Environ Chem Eng. 2021;9(6): 106355. https://doi.org/10.1016/j.jece.2021.106355.
Jean Claude N, Shanshan L, Khan J, Yifeng W, Dongxu H, Xiangru L. Waste tea residue adsorption coupled with electrocoagulation for improvement of copper and nickel ions removal from simulated wastewater. Sci Rep. 2022;12(1):3519.
Linares-Hernández I, Barrera-Díaz C, Roa-Morales G, Bilyeu B, Ureña-Núñez F. A combined electrocoagulation–sorption process applied to mixed industrial wastewater. J Hazard Mater. 2007;144(1–2):240–8.
Yang X, et al. Synergistic adsorption of Cd (II) and As (V) on birnessite under electrochemical control. Chemosphere. 2020;247: 125822.
Orescanin V, Kollar R, Nad K. The application of the ozonation/electrocoagulation treatment process of the boat pressure washing wastewater. J Environ Sci Health, Part A. 2011;46(12):1338–45.
Ferniza-García F, Amaya-Chávez A, Roa-Morales G, Barrera-Díaz CE. Removal of Pb, Cu, Cd, and Zn present in aqueous solution using coupled electrocoagulation-phytoremediation treatment. Int J Electrochem. 2017;2017:1–11. https://doi.org/10.1155/2017/7681451.
Wang J, Liu F, Wei J. Enhanced adsorption properties of interpenetrating polymer network hydrogels for heavy metal ion removal. Polym Bull. 2011;67(8):1709.
Macías-García A, Gómez Corzo M, Alfaro Domínguez M, Alexandre Franco M, Martínez Naharro J. Study of the adsorption and electroadsorption process of Cu (II) ions within thermally and chemically modified activated carbon. J Hazard Mater. 2017;328:46–55. https://doi.org/10.1016/j.jhazmat.2016.11.036.
Sorayyaei S, Raji F, Rahbar-Kelishami A, Ashrafizadeh SN. Combination of electrocoagulation and adsorption processes to remove methyl orange from aqueous solution. Environ Technol Innov. 2021;24: 102018.
Narayanan NV, Ganesan M. Use of adsorption using granular activated carbon (GAC) for the enhancement of removal of chromium from synthetic wastewater by electrocoagulation. J Hazard Mater. 2009;161(1):575–80.
Hussin F, Aroua MK, Szlachta M. Combined solar electrocoagulation and adsorption processes for Pb(II) removal from aqueous solution. Chem Eng Process Process Intensif. 2019;143: 107619. https://doi.org/10.1016/j.cep.2019.107619.
García-García A, Martínez-Miranda V, Martínez-Cienfuegos IG, Almazán-Sánchez PT, Castañeda-Juárez M, Linares-Hernández I. Industrial wastewater treatment by electrocoagulation–electrooxidation processes powered by solar cells. Fuel. 2015;149:46–54.
Nawarkar C, Salkar V. Solar powered electrocoagulation system for municipal wastewater treatment. Fuel. 2019;237:222–6.
Xie S, et al. An electrochemical adsorption method for the reuse of waste water-based drilling fluids. Nat Gas Ind B. 2018;5(5):508–12.
Elabbas S, et al. Eggshell adsorption process coupled with electrocoagulation for improvement of chromium removal from tanning wastewater. Int J Environ Anal Chem. 2022;102(13):2966–78.
Ait Ouaissa Y, Chabani M, Amrane A, Bensmaili A. Removal of Cr (VI) from model solutions by a combined electrocoagulation sorption process. Chem Eng Technol. 2013;36(1):147–55.
Ibrahim AG, Saleh AS, Elsharma EM, Metwally E, Siyam T. Chitosan-g-maleic acid for effective removal of copper and nickel ions from their solutions. Int J Biol Macromol. 2019;121:1287–94.
Liu S, et al. Facile synthesis of Cu (II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Sci Total Environ. 2017;592:546–53.
Wang FY, Wang H, Ma JW. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—bamboo charcoal. J Hazard Mater. 2010;177(1):300–6. https://doi.org/10.1016/j.jhazmat.2009.12.032.
Barhoumi A, Ncib S, Bouguerra W, Hamrouni B, Elaloui E. Combining adsorption on activated carbon with electrocoagulation process for copper removal from used water. Desalin Water Treat. 2017;83:212–21.
Ziouvelou A, Tekerlekopoulou AG, Vayenas DV. A hybrid system for groundwater denitrification using electrocoagulation and adsorption. J Environ Manage. 2019;249: 109355.
Ramesh TN, Kirana DV, Ashwini A, Manasa TR. Calcium hydroxide as low cost adsorbent for the effective removal of indigo carmine dye in water. J Saudi Chem Soc. 2017;21(2):165–71.
Acknowledgements
PT conceived the concept idea and YW revised the final manuscript. The authors show a special appreciation to the School of Environment, Northeast Normal University, China.
Funding
This work was financially supported by School of Environment, Northeast Normal University, China.
Author information
Authors and Affiliations
Contributions
Pontien Twizerimana wrote the manuscript, Yang Wu prepared the manuscript figures and tables and both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Twizerimana, P., Wu, Y. Overview of integrated electrocoagulation-adsorption strategies for the removal of heavy metal pollutants from wastewater. Discov Chem Eng 4, 14 (2024). https://doi.org/10.1007/s43938-024-00053-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-024-00053-w