Abstract
The unprecedented increase of atmospheric CO2 concentration and the associated climate change calls for the urgent implementation of CO2 mitigation approaches. Among the various proposed measures, CO2 capture from several industrial point sources or directly from air and its subsequent hydrogenation via renewable H2 towards value-added products formation has gained particular attention. Specifically, the production of CO or CH4 is of great importance for the eventual generation of liquid fuels or synthetic natural gas, respectively. Herein, an overview of the state-of-the-art noble and non-noble metal-based catalysts employed for the thermocatalytic CO2 hydrogenation towards CO (reverse water–gas shift reaction, rWGS) or CH4 (Sabatier reaction) is elaborated. A brief description of fundamental considerations is initially provided for each reaction, involving thermodynamic, mechanistic and kinetics considerations. Then, the recent catalytic studies on rWGS and Sabatier reactions over both noble metal and non-noble metal catalysts (e.g., metal oxides, carbides, metal organic frameworks) are discussed from the perspective of structure–property relationships. Lastly, the most important conclusions arising from the comparative analysis of the most promising catalysts are summarized and are complemented with proposed outlooks associated with future directions towards the rational design of highly active and selective catalytic materials for each process.
Highlights
-
Recent advances on the catalytic CO2 hydrogenation to CO or CH4
-
Comprehensive overview and comparison of state-of-the-art catalytic systems
-
Thermodynamic, mechanistic and kinetics considerations
-
Rational design of cost-efficient and highly active CO2 hydrogenation catalysts
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is largely acknowledged that the valorization of the ever-increasing emissions of CO2 is a necessity to avoid the catastrophic climate change consequences on our planet and society. Specifically, the latest report of the Intergovernmental Panel on Climate Change (IPCC), a United Nations entity, elaborately confirms the human-induced global warming over the last century and presents very ominous projections regarding the state of the climate over the next decades [1]. To this end, and given the relatively large time frame required for the decarbonization of anthropogenic activities on a global scale, the scheme of the capture and utilization of carbon in the form of emitted CO2, the so-called Carbon Capture and Utilization, CCU, has attracted significant research interest lately. Whereas several processes have been proposed for an effective and sustainable CCU technology, the exploitation of the intermittently generated renewable energy (i.e., solar or wind energy) as a source for hydrogen production via water electrolysis and its combination with a CO2 capture unit (preferably from an industrial point source such as cement production or power plants) can potentially produce value-added chemicals in a manner that not only decreases the carbon footprint of the parent carbon sources, but also curtails the excess renewable energy, effectively storing it in a stable chemical carrier [2,3,4,5].
Herein, an overview of the advances in the catalytic materials employed for heterogeneous thermocatalytic CO2 hydrogenation is provided instead. Given the significance of the scheme of CO2 hydrogenation, this reaction has attracted immense research interest and there exists a plethora of relevant lab-scale studies and review papers addressing the optimization of catalytic materials. Besides, elucidation of the underlying micro-scale phenomena is a prerequisite with the eventual scope of process scaling-up.
However, the catalyst is nothing but a small part in an integrated CO2 hydrogenation process and catalyst optimization is not by itself sufficient for the development and implementation of flexible and efficient processes on an industrial scale. With this in mind, several chemical engineering aspects must be taken into consideration, such as reaction engineering and reactor design, process modelling, integration, intensification, optimization and lastly even feasibility and life cycle assessments and supply chain modeling. As with any catalytically-driven process, the integration of catalyst development and engineering approaches is essential for the understanding of the dynamic character of the involved reaction or system of reactions as well [6]. In other words, the operation and control of a real CO2 hydrogenation process may very well be influenced by the behavior of the catalyst, thereby modifying the overall reaction pathway. For this reason, it is crucial that, prior to implementation, a detailed assessment and elucidation of possible mechanisms should be conducted. Insights regarding real-scale catalytic behavior enable process engineers to minimize the effect of kinetic limitations of elementary reaction steps, eventually leading to an optimized process (Fig. 1).
General approaches for multi-scale modeling of catalytic reaction processes in terms of relevant space and time frames. Adapted from [6]
As of yet, the most prominent category of the examined CO2 hydrogenation catalysts in the scientific literature involves composite materials based on both noble and non-noble transition metals supported on different oxides. In general, these materials are largely characterized by properties that enhance the overall catalytic activity, selectivity and thermal stability, such as high CO2 and H2 adsorption affinity, reducibility, basicity, electronic properties, resistance to sintering or poisoning and synergy between the active phases and the supports in multi-component catalytic systems.
In particular, metal oxides have shown high activity in CO2 adsorption and activation on the basis of oxygen defects and surface acidity/basicity [7]. In conjunction with these parameters, the modification of specific properties, such as structure, metal particle size, loading and dispersion, the formation of active centers at the metal-support interface can exert a profound influence on the local surface chemistry and metal-support interactions, thus leading to the fabrication of catalytic materials of increased activity and endurance with direct implications on selectivity/stability [8, 9].
In light of the above, in the present work a general overview of the state-of-the-art catalysts in CO2 hydrogenation for CO and CH4 production is given, as these are the main routes of interest in the scope of implementing the hydrogenation at low or even ambient pressure conditions. Firstly, and in order to better contextualize the necessity of the two main schemes examined in this review, a description of the fundamentals as well as the thermodynamic considerations of each involved reaction is presented at the start of each section. Subsequently, since practically all mentioned catalysts involve the presence of a metallic phase, in each category a further subdivision is made based on the type of metal, that is: i) noble metal-based and ii) non-noble metal-based catalysts. Categorization on the basis of the supporting material is not attempted, as the various supports are essentially included in the above analyses. Finally, a brief overview of the various proposed reaction mechanisms regarding the studied products of CO2 hydrogenation is made in an attempt to provide the full picture regarding the trends in the thermocatalytic route for CO2 hydrogenation. Moreover, a thorough comparison under similar reaction conditions is carried out to reveal the most promising catalysts for the two reaction schemes (Sabatier and rWGS reactions), providing the rationale and main principles for the design of cost-efficient and highly active catalysts for CO or CH4 production via CO2 hydrogenation.
2 CO2 hydrogenation to CO
2.1 Fundamentals and thermodynamics
The reduction of carbon dioxide to carbon monoxide through the rWGS reaction (Eq. 1) is of paramount importance, taking into account that CO is a building block for the production of various useful chemicals such as methanol or other long-chain hydrocarbons via the well-established Fischer–Tropsch (F-T) synthesis from syngas (a mixture of COx/H2) [10]. Besides, a single-step process for the production of methanol, dimethyl ether (DME) or hydrocarbons directly from CO2 hydrogenation has not yet been commercialized, predominantly due to the inherently higher stability of carbon dioxide as opposed to the highly active carbon monoxide. However, once CO2 is activated, any subsequent reactions will be energetically favorable and practically proceed to a larger extent, owing to the higher reactivity of the CO molecule. Thus, undesired CO2 in the syngas mixture can be removed by its conversion to CO, rendering the rWGS reaction an intermediate in situ or ex situ process in many CO2 conversion schemes. Therefore, a fundamental understanding of the rWGS reaction is of key importance towards the design of active and highly selective catalysts for syngas production [6].
Although syngas is conventionally produced from fossil fuels and biomass, the growing demand for environmentally friendly fuels and chemical commodities reinforces the significance of designing highly efficient rWGS processes in conjunction with CO2 valorization schemes. Furthermore, rWGS reactors can be advantageously implemented with the current infrastructure in any heavy carbon industry (e.g., cement, steel making, refineries) [11]. Indeed, this process is more feasible compared to the alternative CO2-to-CO technologies and provides additional versatility, by the variable products that may be obtained from the subsequent CO conversion. Every reaction pathway involving syngas, mainly from hydrocarbons partial oxidation, steam or autothermal reforming will produce a gas with a specific H2/CO ratio, which also depends on the feedstock employed. Commonly, syngas composition is modified by adjusting this ratio via the WGS/rWGS reaction or a downstream separation step. Although other oxygenated compounds like DME and alcohols might be produced, COx methanation reactions (Eqs. 2–3) represent the main side reactions that may affect product distribution even under atmospheric pressure. Also, the stoichiometric H2:CO2 ratio is unity (see Eq. 1), the lowest of all the other relevant CO2 hydrogenation processes. This is evidently a benefit of the specific process, especially considering the bottleneck associated with the high cost of RES-produced H2. However, given its endothermicity, this reaction is practically limited in temperatures below 600 °C (Fig. 2a), whereas complete suppression of methane formation occurs only after 700 °C (Fig. 2b), as revealed by the thermodynamic analysis carried out at the stoichiometric ratio of H2:CO2 = 1. Furthermore, given that during rWGS the number of gaseous moles remains unchanged, the effect of pressure is expected to be practically nonexistent, rendering this process operational even at atmospheric pressure.
As mentioned earlier, the production of CO from CO2 proceeds according to Eq. 1. However, several parallel and side reactions practically occur under real CO2 hydrogenation conditions, most notably the exothermic Boudouard reaction (or CO disproportionation), CO and CO2 methanation (Eqs. 2–4);
The above reaction system very often occurs in real applications, thus resulting in mixtures of H2O, CO, CO2, H2 and C, although the formation of solid carbon is demonstrated less frequently. Also, the forward water–gas shift reaction (WGS) takes place to a certain extent under relevant applications, which may limit CO2 and H2 conversion by instead producing them via the forward route, especially below 400 °C.
In general, however, the rWGS reaction showed higher efficiency compared to other CO-producing processes when CO2 is derived from a flue gas stream [12]. Therefore, a major incentive for improving rWGS catalytic activity in the low-temperature regime is the reduction of heat requirements, since this process is designed as intermediate to the eventual F-T synthesis. According to a comprehensive thermodynamic analysis that was conducted using ASPEN Plus by the Gibbs free energy minimization method, it can be seen from Fig. 2c, at temperatures lower than 500 °C, COx methanation reactions are dominant, and significant CO production is expected only at above 600 °C. Moreover, although a higher H2 concentration in the feed favors CO yield (Fig. 2d), this can also lead to enhanced COx methanation activity, along with inducing additional costs for the provision of the excess amount of hydrogen. The most challenging issues regarding the development of efficient rWGS catalysts are associated with low temperature activity/selectivity, metal sintering and carbon deposition. Therefore, an appropriate catalyst is needed in order to address these issues and achieve high activity and selectivity at the medium-to-low temperature regime [13,14,15].
In the sections below mechanistic and reaction kinetics considerations of rWGS reaction are provided on the basis of the recent theoretical and experimental advances in the field.
2.2 Mechanistic and kinetic considerations
2.2.1 Proposed mechanisms
The inherent reaction mechanism for the production of CO from heterogeneous thermocatalytic CO2 hydrogenation has not yet been fully understood. Also, it must be stated that the rWGS does not necessarily proceed via a specific mechanism, but rather than a distinct pathway that is almost completely dependent on the solid catalytic material used. In general, though, given the great amount of literature works for the forward reaction (WGS), valuable mechanistic information can be obtained, especially by combining in situ and/or operando studies with computational methods [16]. As of yet, it is considered that there are two main pathways for the reverse route, essentially referring to the direct participation or not of H2 in the activation of CO2, i.e. the redox and the associative/dissociative mechanism [15, 17,18,19], whose elementary steps are summarized in Scheme 1 and are briefly described below. The symbol “*” denotes adsorption sites or surface adsorbed species and “(g)” refers to gaseous species. In any case, the interested reader can find detailed information and a literature overview of mechanistic studies of the reverse water gas-shift reaction in recent literature reviews [12, 17, 20, 21].
Elementary reactions for the main proposed mechanisms for rWGS. Adapted from [22]
Redox mechanism: In the redox mechanism, as the name suggests, the reaction is carried out via a reduction/oxidation cycle on the catalyst surface. Specifically, gaseous CO2 is firstly activated by its adsorption on an electron donor entity, most typically a metal or an oxygen vacancy in a reducible metal oxide, and subsequently reacts for the formation of intermediate carbonyl species (either metal-bound or support-bound) that are eventually desorbed as gas-phase CO. The participation of H2 lies in the reduction of the oxidized sites by removing oxygen ad-atoms and forming water molecules, leading to their regeneration for another CO2 activation cycle and so on.
Associative/dissociative mechanism: Within the associative mechanism (also referred to as dissociative route), surface-bound CO2 activated species (denoted as *CO2) react with H ad-atoms derived from H2 dissociation (*H) for the formation of intermediate species that eventually decompose to CO and H2O. The main associative reaction path is the one involving the formation of active formate species (*HCOOH), although other routes are proposed, such as formyl and carboxyl. Most commonly, bidentate formate species are produced through the reaction of *CO2 with *H, preferably via the adsorption of O atoms to a metal surface, which then decompose into *HCO and *O and subsequently into *CO and *H. For the carboxyl route, *CO is selectively produced and is eventually desorbed through activation of the C–O bond, followed by H-assisted formation of COOH*.
In principle, the primary active site in a metal-based catalytic material is the metal surface and in unsupported catalysts the metal phase must be able to dissociatively adsorb both CO2 and H2. As for most CO2 activation reactions, CO2 dissociation is considered the rate determining step (rds) due to the thermodynamic stability of the CO2 molecule and the observed dissociative adsorption heat over the metal phase that practically determines the reaction rate [23]. In this regard, in a theoretical study by Nolen et al. [24] on CO2 transformation reactions over close-packed surfaces of transition metal catalysts (Ni, Co, Rh, Ru, Pd, Pt), it was found that the first step of CO2 activation is enabled from a kinetic and thermodynamic point of view on most transition metal surfaces. CO2 is activated through direct dissociation over oxophilic transition metals, such as Ni, Co, Rh, Ru, whereas in less oxophilic transition metals, such as Pd and Pt, the contribution of hydrogen is required. As illustrated in Fig. 3, the selectivity in CO, CH4 and methanol is dependent of the different steps, i.e., CO desorption, C–O dissociation and hydrogenation. Methane formation requires two C-O bond dissociation steps, while the formation of methanol or CO requires only one. CO is the C1 product that is kinetically preferred on all transition metal surfaces (Ni, Co, Rh, Ru, Pd, Pt) [24]. According to the results, the conversion of CO2 progresses through the formation of CO* and the hydrogenation to C1 products (adsorbed species are denoted as *). The formation of CO/CH4 is favored over Ni, Co, Rh, Ru surfaces, while the Pd and Pt surfaces favor the formation of CO/CH3OH. The CH4/CH3OH selectivities are driven by the metal's C-O dissociative ability, whereas CO selectivity by the competition between CO* desorption and surface hydrogenation [24].
CO2 hydrogenation routes on transition-metal surfaces including C-O bond cleavage (–O), C–OH bond cleavage (–OH), and hydrogenation (+ H) to either the carbon or an oxygen atom. Reproduced with permission from [24]
In this point, it should be mentioned that the catalytic conversion of CO2 is strongly associated with the adsorption and dissociation processes of gaseous molecules on transition metal surfaces. Therefore, as shown in Fig. 4, there is a consistency between the changes in the adsorption energy trend and the charge transfer amount from the transition metal surfaces to CO2 [25].
Change trends of adsorption energy and Bader charge of CO2 on different low index transition metal surfaces. Reproduced with permission from [25]
Nevertheless, under reaction conditions, the above reaction pathways may very well occur simultaneously and the dominant intrinsic mechanism depends mainly on the catalytic system and to a lesser extent on reaction conditions such as H2 concentration [22]. This is evident in the great variabilities of the prevailing pathway with the catalyst composition, structural, or electronic properties demonstrated in experimental works, whereas the results from computational studies also show a dependence on the system modelling, i.e., whether extended surfaces or finite clusters are used or whether the consideration of model (well-defined) or non-model surface studies are made [26]. In any case, high CO selectivity values and methane formation suppression can be attained by adjusting catalytic features that enhance a specific reaction mechanism, such as metal size, shape and crystal facets exposure, acid/basic properties, metal-support interactions, among others [15]. The latter can be achieved under a rational design approach of multifunctional composites through the use of advanced synthesis and promotional routes, as recently reviewed in relevant articles [27,28,29] and further discussed below on the framework of state-of-the-art catalytic systems of rWGS reaction.
2.2.2 Reaction kinetics
The elucidation of reaction kinetics is of critical importance in the scheme of process implementation, particularly for catalyst and reactor design, as well as for process simulation. All these can provide valuable insight into the performance of a process at various conditions stemming from changes in configuration and operating conditions, especially in a process involving rWGS and RES-produced H2. Essentially, all reactions that may take place during CO2 hydrogenation (Eqs. 5, 6) are driven by vapor equilibrium, so the equilibrium constant Kc,rWGS, is typically defined by Eq. 5, depending on the partial pressures of reactants and products. Also, the standard free energy difference of the reaction, ΔG, can be derived by Eq. 6;
Thus, as is the case for all chemical reactions, the rWGS reaction is in theory thermodynamically favorable only at the temperature regime for which the value of ΔG is negative. As can be seen from Fig. 2a, this occurs at values higher than ca. 800 °C. This essentially necessitates the use of a catalyst that will render the reaction more favorable kinetically at lower reaction temperatures.
Given its inherent reversibility, the rWGS reaction proceeds through a similar mechanism as reported for the forward WGS reaction, thus most of the kinetic studies are based on the widely employed Cu-based catalyst. Indicatively, CO2 dissociation was identified as the rate determining step for Cu(110), ascribed to the nearly identical activation energies for rWGS and CO2 dissociation [30, 31]. From these studies it was concluded that under over-excess hydrogen conditions, (i.e., H2:CO2 > 10), the reaction order is around 1 for CO2 and 0 for H2, at a range between 2 and 10 the reaction rate diminished due to the formation of the less active Cu entities and for H2:CO2 ratios lower than 2 the rate is limited by the dissociative CO2 adsorption. For commercial CuO/ZnO/AI2O3 a similar behavior was disclosed [32], however, though the rate was also independent of the H2 partial pressure at high and low hydrogen coverages, a dependence of the rate on both reactant partial pressures was seen for intermediate H2:CO2 feed ratio values. The kinetics of rWGS was also studied on Cu nanoparticles supported on SiO2 [33], where it was disclosed that the reaction mainly proceeds through the formation of formate species.
Elsewhere, the effect of CO2 and H2 reaction orders towards product distribution was studied for a variety of catalysts, and it was found that CO2 methanation was largely suppressed and CO generation was instead favored on Fe/Al2O3, MoS2 and WS2, with higher values for partial reaction orders for CO2 than for H2 [34]. Validation of experimental results and kinetics expression using the Langmuir–Hinshelwood-Hougen-Watson (LHHW) model was performed for Cu/ZrO2 and Ga2O3/Cu/ZrO2 [35]. The authors postulated that the reaction rate is almost independent of the total pressure and that based on CO2 dissociation being the rate determining step, a kinetic model was derived accordingly. Also, a kinetics evaluation based on both reaction mechanisms was attempted for Pt/TiO2 and Pt/Al2O3 [36]. Whereas the initial reaction rate assuming both reaction mechanisms was consistent with experimental data under low and high hydrogen coverages, only the surface redox mechanism model was experimentally consistent under intermediate hydrogen coverages.
2.3 Catalysts for rWGS reaction
2.3.1 Noble metal (NM)-based catalysts
Insights from theoretical and experimental studies have showcased that one of the most important criteria in the design of metal/metal oxide rWGS catalysts is the electronic properties of the metallic d-orbital holes and the difference between the dissociation barrier of metal-bound carbonyl and the desorption energy and dissociation barrier of metal carbonyl species [37]. Noble metals are associated with partially filled d-orbitals that generally are a favorable electronic configuration for the adsorption of gaseous reactants and intermediate active species formation onto the catalyst surface, thereby improving the catalytic activity. Also, the dispersion and chemical state of noble metal nanoparticles is crucial for the adsorption behavior of the reactants and the subsequent conversion of reaction intermediates [38]. Thus, they are considered promising catalysts for the rWGS reaction, together with characteristics such as corrosion resistance and strong affinity for H2 dissociation [39]. Research on the noble metal catalysts for rWGS mainly focuses on Pt, Pd and Au-based catalysts, which are generally the most active for the forward reaction. In general, the main product of CO2 hydrogenation over Rh-, Ru-, Co- and Ni-based catalysts is CH4, while Pd-, Pt-, Au-, Fe- and Cu-based catalysts are mostly selective towards CO generation [40], as thoroughly discussed below.
2.3.1.1 Pt-based catalysts
Platinum has been widely studied as a rWGS catalyst and it was found that it largely suppresses CO2 methanation [41]. However, the activity and selectivity of Pt-based catalysts were strongly influenced by the nature of the oxide support. For example, CO yield over Pt/γ-Al2O3 is higher than Pt/CeO2 at similar Pt loadings and CO2 conversion values [42]. Elsewhere [43], a study on mono- and bimetallic Pt catalysts supported on different oxides showed that catalytic activity and selectivity were notably dependent on the support nature. Additionally, the replacement of titania with ZrO2 as support led to intensification of the metal-support interaction, which weakened the C-O and O-bonds of the intermediate species at the Pt/Co-oxide interphase and thus high selectivity toward CO was observed [44]. On a Pt/SiO2 catalyst, oxygen vacancy formation was hindered and CO2 could be weakly adsorbed at the metal-oxide interface; in contrast, the presence of oxygen vacancies due to the presence of reducible TiO2, led to superior CO2 adsorption affinity [45]. The favorable interaction of Pt with titania was also observed in the study by Kim et al. [46], in which it was found that the increased electron donation properties of Pt0 in contact with Ti3+ sites causes strong metal-support interactions and generates new active Pt-Ov-Ti3+ sites for CO formation (Ov denotes oxygen vacancies) (Fig. 5).
Reaction mechanism of CO production from CO2 hydrogenation over Pt/TiO2. Reproduced with permission from [46]
Elsewhere, the effect of CeO2 addition to Pt/TiO2 showed that ceria affected the lattice and pore structure through substitution with TiO2 and optimized the catalyst activity [47]. Notably, CO selectivity is improved even further upon promotion. Huang and co-workers [48, 49] studied the effect of adding K to supported Pt-based catalysts and they showed that even though Pt is able to catalyze both rWGS and CO2 methanation, the addition of potassium highly increases CO yield, ascribed to the strong interaction between Pt nanoparticles and KOx species that weakens the adsorption energy of Pt-bound carbonyl, thus inhibiting its further hydrogenation to methane.
Furthermore, Na promotion favored the CO selectivity of Pt/m-ZrO2 catalyst, as evidenced by Seuser et al. [50], who demonstrated that the presence of sodium resulted in the suppression of on-top platinum sites, consequently dispersing the Pt0 ensembles that contribute to methanation. Another study examined the promotion of a Pt-based catalyst with other transition metals such as Ni and Co [51]. The formation of bimetallic catalysts enhanced the electronic properties of platinum and in turn CO selectivity compared to monometallic Pt. The same trend was observed by Wang et al. [52] who suggested that Fe addition on a Pt catalyst can not only act as a promoter by regulating the surface sites of the catalyst, but also functions as active site for the formation of intermediate species. Expanding further on catalytic synergy, a series of bimetallic Pt-Re/SiO2 catalysts showcased the promotional effect of Re, which favored the adsorption and activation of CO2, while the presence of Pt helped H2 activation, eventually leading to enhanced rWGS activity [53]. Furthermore, Pt addition to α-MoC catalysts resulted to a synergistic effect that enhanced the catalytic activity, where α-MoC can effectively break the C=O bond of carbon dioxide and mitigate the strong *CO adsorption properties while Pt facilitated H2 activation and CO formation occurred on the α-MoC sites [54].
2.3.1.2 Pd-based catalysts
Pd-based catalysts have been also investigated, despite their tendency to be also selective to CH4 [55, 56], since both the Sabatier reaction and the rWGS follow the same initial mechanistic steps and involve similar intermediates. Nevertheless, Qian et al. [57] designed a series of Pd nanoparticles supported on two-dimensional silicon hydride nanosheets, which demonstrated a great synergetic effect between the two components. More specifically, even though Pd nanoparticles exhibit low catalytic activity, SiH nanosheets were able to immobilize Pd nanoparticles, thus leading to a higher surface area and dispersion of the metal, favoring CO selectivity. Wang et al. [58, 59] investigated the effect of Pd loading on Al2O3 and they noted that CO2 is adsorbed on the hydroxyl groups of the alumina surface to form formate species, while H2 is dissociatively adsorbed on the Pd nanoparticles. The difference in CO selectivity between catalysts with high and low Pd loading is determined by the concentration of active sites and their CO adsorption/desorption affinity. In accordance with the aforementioned results, Kwak et al. [60] studied the effect of support on a series of Pd/Al2O3 and Pd/MWCNT catalysts, highlighting the distinctive function of alumina not only as support but also as an active participant in the catalytic configuration due to its ability to effectively activate CO2. The ability to tune Pd tendency to favor CO selectivity was also shown on a Pd/Ga2O3 catalyst by a study combining modulation-excitation spectroscopy with phase sensitive detection [61]. Contrary to alumina, Ga2O3 was reduced by H2 to form Ga-H entities through the spillover effect. In addition, the effect of Pd particle size was explored by Lebarbier et al. [62] on a Pd/ZnO catalyst, who demonstrated higher CO selectivity with a decrease of the particle size, highlighting the structure sensitivity of that system.
2.3.1.3 Au-based catalysts
Given the low CO adsorption energy of gold, Au-based catalysts are also employed as active materials for the rWGS reaction [17]. In particular, gold nanoparticles encapsulated in the metal organic framework UiO-67 selectively produce CO [63] and Au supported on CeO2 was found to be active for both methanol synthesis and rWGS reactions [64]. In another study, it was shown that Au/TiO2 exhibited a higher reaction rate than Au/Al2O3, since titania actively participates in the reaction. More specifically, the presence of oxygen vacancies in the support combined with the enhanced electron mobility of Ti provided enough active sites for CO2 activation while the presence of Au was crucial for the activation of gas-phase H2 and its subsequent spillover [65]. Also, promotion via alkali addition enhances rWGS reaction activity, stemming from the stabilization of Au particles due to the “Metal-O(OH)-Alkali” interphase and leading to increased CO desorption [6]. Moreover, promoting Au/SiO2 catalysts with MoOx led to the creation of under-coordinated Au/MoOx interfacial sites that were highly active for the rWGS reaction even at 300 °C [66]. Similarly, gold nanoparticles (2.2 nm) supported on TiO2 prepared via deposition–precipitation attained high CO yield (50%) at low temperatures (400 °C), ascribed to the synergistic interaction between Au and TiO2 [67].
2.3.1.4 Other noble metal-based catalysts
Several other noble metals have been separately explored as active phases for rWGS reaction, as promoters or even as bimetallic entities. As is the case of Pd, Ru and Rh are also known to strongly adsorb CO species, which is the main reason for their high CO2 methanation activity. However, very small (or atomically dispersed) Ru particles have been shown to favor the production of CO rather than CH4, with the selectivity being very sensitive to Ru particle size [68,69,70]. Also, a small amount of Ru (0.5 wt.% Ru) enhanced the activity of a Cu/ZnO/Al2O3 catalyst owing to the electronic interactions of Ru with Cu over this bimetallic catalyst [71]. Panaritis et al. [72] studied the reaction of CO2 hydrogenation over Ru-Fe bimetallic nanoparticles supported on samarium-doped ceria. It was shown that the addition of a low amount of Ru equal to less than 1 wt.% to Fe led to high stability and CO yield at high temperatures without deactivation. Similar results have been demonstrated for Rh-based catalysts [73, 74], as recently reviewed in Ref. [75]. Although supported rhodium nanoparticles are widely studied for methane production, Wang et al. [76] demonstrated that by selecting molecular sieve crystals as supports and consequently encapsulating Rh nanoparticles, the catalytic selectivity can be adjusted to steer the reaction products towards CO rather than methane. More specifically, their study indicated that core–shell enveloped rhodium nanoparticles at pure Silica (Rh@S-1) showed the highest CO selectivity indicating a strong association among the nanoporosity and CO selectivity, suggesting that S-1 enables the decrease of further hydrogen migration and promotes the rapid CO desorption to prevent excessive hydrogenation. Furthermore, Büchel et al. [53] explored K promotion on a Rh/K/Al2O3 catalyst, and concluded that the catalyst demonstrated minimal CO interaction, suggesting that CO is not retained but is released prior to its reduction to elemental carbon. Iridium was also used as an active phase with variable Ir oxidation states [77], in which partially oxidized Ir species attained complete CO2 conversion to CO, whereas Ir0 favored the formation of CH4. Additionally, while In2O3-supported catalysts are known to inhibit CO yield, a study on bimetallic In-Pd/SiO2 catalysts demonstrated 100% CO selectivity, attributed to weaker CO adsorption on the bimetallic system, suppressing CO2 methanation [78].
Single-atom catalysts (SAC) are a relatively recent promising category for catalytic materials in order to achieve high productivity and investigate the atomic-level reaction mechanism. In recent years, some SACs based on noble metals have been studied for the rWGS reaction. Notably, the product distribution was observed to be largely different from the respective nanoparticle-based catalysts, as shown in indicative works [60, 69, 70, 73, 79,80,81]. However, one of the major issues regarding their use for the rWGS reaction is their largely low thermal stability, which eventually leads to agglomeration at the elevated temperatures required for the rWGS reaction.
2.3.2 Non-noble metal-based catalysts
Despite the higher reaction rates that can be exhibited by noble metal catalysts, their high price and limited availability certainly hinder their implementation at larger scales. In this regard, significant efforts have been put forward on the design of cost-effective and equally active/selective catalysts based on earth abundant transition metals (TMs) as very promising alternatives [15, 39, 82]. Among others, the most used TMs employed for the rWGS reaction are Cu and Fe, either as monometallic phases or promoted entities, whereas the other two active 3d-TMs, Co and Ni, are known to preferentially generate hydrocarbons and methane under CO2/H2 mixtures, respectively [6]. Preliminarily, in a study examining various metals (Ni, Cu, Ag, Rh, Ru, Pt, Pd and Au) supported on ZrO2 [83] demonstrated that Ni and Ru were selective towards CH4. The catalysts based on the remaining metals produced a mixture of CH4, and CO. Similarly, in another study examining M-CeO2 catalysts (M: Ni, Cu, Co, Fe, Mn), Ni and Co showed the highest CO2 conversion values, accompanied by high methane selectivity. On the other hand, Cu, Fe and Mn were practically completely selective towards carbon monoxide production [84]. Apart from the typical metal-based catalysts, transition metal carbides (TMCs) and metal organic frameworks (MOFs) have recently gained particular attention as alternative materials with improved catalytic performance. In the sections below, the most commonly employed non-noble metal catalysts for the rWGS reaction are separately elaborated.
2.3.2.1 Cu-based catalysts
Copper is the most studied active metallic phase for the specific reaction, since it has been proven as active metal for the forward WGS reaction and is largely known for its high CO selectivity [85]. Ternary catalysts of Cu/ZnO supported on Al2O3 are among the widely explored copper-based catalysts for low-temperature rWGS at ambient pressure [32, 71, 86]. An inverse-type Cr2O3/Cu catalyst was designed by Shen et al. [87], which was found to be more active than noble metal catalysts for the rWGS reaction. A Cu-Al spinel catalyst showed very good activity and negligible deactivation after a 40-h stability test [88] and Cu/Al2O3 was efficient in the high-temperature rWGS reaction [37]. Elsewhere, the high activity of a Cu/CeO2 hollow sphere catalyst was attributed to the high oxygen vacancy density, whereas inferior performance was attained by other Cu/CeO2 catalysts characterized by a lower density of vacancies [89, 90]. In a similar manner, the key role of preparation procedure and the synergy between Cu nanoparticles and oxygen vacancies contained in the CeO2 support has been well documented [90,91,92].
However, the major disadvantage of Cu-based catalysts is the low thermal stability of copper which leads to rapid deactivation, especially at high temperatures, as well as re-oxidation above ca. 600 °C. Therefore, most of the Cu-based catalysts are accompanied by an appropriate promoter, predominantly Fe and K. Chen et al. [93] demonstrated that the addition of Fe to Cu/SiO2 and Cu/Al2O3 inhibits Cu sintering and improves stability even above 600 °C. Elsewhere, the addition of high-density Fe oxide clusters proved to be effective textural promoters that enhanced the stability in the high-temperature regime [94]. Also, in another study, Cu3Zr and CuZr3 clusters were suggested as good candidates for CO2 activation and dissociation through the use of DFT calculations, as small barrier heights were observed for these clusters [95]. The CuFe synergy was further explored by Navarro et al. [96], who demonstrated that bimetallic CuFe2O4 spinels favor CO production, due to their abundance on oxygen vacancies, facilitated by the positioning of Fe3+ ions in the tetrahedral sites. Moreover, other alkali metals like K, Na or Cs have been used as promoters, facilitating the CO2 adsorption. Indicatively, potassium increased the surface dispersion of Cu and created new adsorption sites, which led to higher CO2 conversion values compared to K-free Cu/SiO2 catalysts [97]. However, an optimum K loading of 1.9 wt.% was shown, since the addition of higher amounts of potassium significantly suppressed the catalytic performance due to the K-induced coverage of Cu active sites. A similar behavior has been observed for Cs-doped Fe-Cu/Al2O3 catalysts [98]. Cesium addition exerted a strong promoting effect especially at medium temperatures (400–500 °C), evidenced by the higher CO2 conversion values in comparison to the un-promoted catalyst. More importantly, a 50-h stability test demonstrated that the composite catalyst Cs-Fe-Cu/Al2O3 exhibited exceptional long-term behavior, since no coke formation was detected. Also, in our previous work [99] we demonstrated that the addition of Cs into a Cu/CeO2 system is inhibitory towards CO2 conversion, although the alkali promoter effectively favors CO selectivity (Fig. 6) via increased basicity and stabilization of partially reduced copper species.
Effect of Cs loading and surface basicity on CO selectivity over Cs-doped Cu/CeO2 catalysts. Reproduced with permission from [99]
2.3.2.2 Fe-based catalysts
Being the cheapest transition metal, iron can be a potential replacement of noble metals in the reaction of rWGS, along with its sufficient thermal stability. Although there are only a few examples of iron-based rWGS catalysts reported so far due to the fact that the literature on Fe-based catalysts is dominated by its utilization as a Fischer–Tropsch catalyst, the performance of some recently reported catalysts for CO2 hydrogenation to CO was remarkable in terms of both activity and stability [100]. Fishman et al. [101] investigated the catalytic activity of ultrathin FexOy nanomaterials in the rWGS reaction and demonstrated that the nanowire catalyst was highly active and selective towards CO at all temperatures examined.
However, iron is mostly used as a promoter in bimetallic catalytic systems, rather than being the standalone active metallic phase for the rWGS reaction. Indicatively, a bimetallic Fe-Mo catalyst has shown increased stability and complete CO selectivity due to the small particle size with higher Fe dispersion from the formation of a Fe2(MoO4)3 phase [102]. Loiland et al. [103] demonstrated that the addition of potassium to Fe/Al2O3 led to a three-fold increase in the reaction rate. At temperatures above 600 °C, activity of Cu or Ru can be increased by the addition of Fe, owing to their improved thermal stability and oxygen mobility [72, 93, 98]. Also, the bimetallic promoting effect was demonstrated in the study of Yang et al. [104], in which an improved catalytic activity was shown for Fe–Ni/CeO2-Al2O3 and Fe-Cu/CeO2-Al2O3 in comparison to Fe/CeO2-Al2O3, especially at temperatures lower than 600 °C. It was suggested from XPS analysis that Ni and Cu incorporation into the Fe-based sample induced relatively strong interactions between these metals and Fe, in part due to the slight decrease in Fe electron density. The double promotion with Fe and K also improved CO selectivity in carbon-templated BaFe-hexaluminate catalysts [105], whereas a Fe-Ru bimetallic composite could effectively diminish CH4 formation and offer increased stability with the addition of very small amounts of sulfur species [106]. Addition of Fe to Ni was also shown to lead to the formation of Ni-FeOx interfaces, which selectively catalyze CO formation thus suppressing methane formation (Fig. 7), since the strength of the metal-carbonyl interaction was decreased, a fact that facilitated the desorption of CO [107].
Effect of Ni and Fe content on conversions of CO2 (a) and H2 (b), yields of CH4 (c) and CO (d), selectivity of CH4 (e) and CO (f) over zirconia-based catalysts in the reaction of CO2 hydrogenation. Reproduced with permission from [107]
2.3.3 Transition metal carbide catalysts (TMCs)
While copper is predominantly used as the non-noble metal phase for the rWGS reaction, other non-noble metal-based structures have attracted scientific attention as catalytic materials and their performance in CO2 conversion was recently reviewed [108]. Specifically, transition metal carbide catalysts (TMCs) are often referred to as “low-cost precious metals” or “Pt-like” materials [109], due to the combination of more reasonable price with similar catalytic behavior to that of noble metals [110].
The most commonly used metal carbide is molybdenum carbide, Mo2C, due to its excellent ability towards both H2 dissociation and C-O bond cleavage [111]. It was also shown that Mo2C was more active and highly selective to CO compared to other carbides [112]. Elsewhere, it was shown that the CO yield was higher for Mo2C compared to PtCo/CeO2 and PdNi/CeO2 [113]. Additionally, various studies reported enhanced CO2 activation specifically by β-Mo2C, onto which CO2 is bounded in a bent configuration, which facilitates the cleavage of the C-O bond. Subsequently, the dissociated CO desorbs to produce gas-phase CO, while O interacts with Mo2C and forms oxy-carbide moieties (Mo2C-O), which are re-reduced by dissociated hydrogen ad-atoms [113]. More importantly, the combination of various transition metals, such as Co [113] and Cu [114, 115] with Mo2C, can lead to synergistic interactions, resulting in enhanced catalytic activity and stability. In view of above aspects, it has been shown that copper supported on β-Mo2C offered high CO yield values and reaction rates compared to commercial Cu/ZnO/Al2O3 [115]. More importantly, thermal stability was enhanced because of the homogeneous Cu dispersion achieved over the Mo2C support which prevented particle sintering, in comparison with the observed Cu agglomeration on the commercial Zn-Al support.
As for other carbide catalysts, Rodriguez et al. [116] reported on the deposition of small particles of Au, Cu, and Ni on TiC, where it was revealed that the metal coverage on the carbide surface exerted a strong influence on the catalytic activity, i.e., it was significantly higher for smaller metal coverages. Moreover, a vanadium carbide (VC) catalyst with high carbon vacancies demonstrated high conversion and selectivity [117]. The addition of alkali promoters to TMCs strengthens CO2 adsorption and increases selectivity towards CO. Specifically, K-WC/γ-Al2O3 exhibited high CO yield values compared to un-promoted samples [118]. Also, Na and K could act as structural promoters for WC that alter the local geometry and crystallographic orientation of the active sites, affecting their availability for reactant adsorption and activation [119].
2.4 Metal organic frameworks (MOFs) catalysts
Apart from the typical metal/metal oxide and metal carbide catalytic systems, catalysts based on a metal organic framework (MOF) have been attracting more and more interest as alternative materials with improved catalytic performance. These complex compounds constitute a novel class of highly porous crystalline materials built from organic linkers and metal cluster-based secondary building units. Owing to their structural diversity, compositional tunability, and high porosity, MOFs have provided a versatile platform to design solid catalysts with uniform active sites for many reactions such as the CO2 hydrogenation to CO [120]. This rather complex but advanced architecture may potentially result in both remarkable catalytic activity and CO selectivity and the concept of inserting metallic nanoparticles into microporous MOFs will possibly revolutionize future industrial applications.
Specifically, in the recent report by Li et al. [121], CO2 hydrogenation was explored over various transition metal catalysts (M/ZIF-8-C; M: Ni, Fe, Co and Cu) supported on zeolitic imidazolate framework-derived carbon support enriched with pyridinic N sites. The main advantage of using these materials was their dual functionality, since the metal/metal carbide phase facilitates H2 dissociation and pyridinic N is active for CO2 adsorption. On a series of hierarchical FeNiZn-MIL-88B-on-MOF-5 with different morphologies, the octapod catalyst showed both high CO2 conversion and CO selectivity, mainly attributed to the structural superiority of the catalyst, as depicted in Fig. 8 [122]. Elsewhere, the microporous nature of the MOF known as UiO-66 assisted the adsorption of Pt nanoparticles, which in turn enhanced the interaction between Pt and MOF, favoring the formation of isolated and well-dispersed Pt active sites and eventually producing CO [123]. In a similar manner, various multifunctional compositions, such as core–shell Pt/Au@Pd@1Co@MOF [124], Au@Pd@MOF-74 [125], Pt–Pd@UiO-67 nanocages [126], HZSM-5@ZrO2–In2O3@UiO-66 [127] exhibited high rWGS activity and selectivity, ascribed mainly to their advanced nano-architecture in conjunction to the key role of active metal entities. For instance, Fang et al. [127] developed an HZSM-5@ZrO2–In2O3 core–shell catalyst by decomposing the In(NO3)3/HZSM-5@UIO-66 precursor. Their enhanced performance was attributed to the In-Zr interface and the increased number of oxygen vacancies which improved CO2 adsorption, while the special pore structure of UIO-66 enabled HZSM-5@ZrO2–In2O3 offered more active sites.
CO2 conversion (a) and CO selectivity (b) as a function of temperature for different FeNiZn MOFs morphologies (flowers, octapods and cubes) and FeNiZnOx. Reproduced with permission from [122]
In light of the catalytic evaluation studies presented above, a comparison of the most active and selective materials for the rWGS reaction is summarized in Table 1. The comparison is carried out among representative materials based on both noble and non-noble metals under ambient pressure and at reaction conditions that are mostly comparable in terms of the H2/CO2 ratio and weight hourly space velocity (WHSV). More specifically, according to the reaction thermodynamics [128], CO2 conversion is enhanced under elevated H2:CO2 values, while CO selectivity remains unaffected. Consequently and to showcase the affinity towards CO generation instead of the competing reaction of CO2 methanation, Table 1 involves a list of the most active rWGS catalysts at H2:CO2 = 4 and relatively low reaction temperature. This will allow the reader to acquire an understanding about the most promising materials for rWGS reaction, formulating the research rationale and design principles for highly active and selective catalysts.
Collectively, the evaluation of state-of-the-art catalysts in the rWGS process reveals distinct behaviors between noble and non-noble metal-based catalysts. Noble metal catalysts, typically supported on metal oxides like alumina, zirconia, ceria or titania, achieve equilibrium values of CO2 conversion even at lower temperatures. This can be attributed to the synergistic effect between the support and metal, which influences key activity descriptors, such as metal dispersion and basicity. Among the various supports, reducible metal oxides, such as CeO2, exhibit advanced catalytic performance for the rWGS reaction. Several studies demonstrate that the significant improvement of the performance registered for those supports is associated to their direct contribution to the reaction, either through the creation of oxygen vacancies that favor CO2 activation or their strong interaction (of either electronic or geometric nature) with the active metal phase which finally determines chemisorption and catalytic properties. Nevertheless, it was proved that the catalyst's deactivation due to coke deposition was mainly attributed on the support rather than the metal, namely ceria, since its participation to the reaction led to its gradual coverage by the deposited carbon [129]. To address this phenomenon, a combination of two different oxides, such as ceria and zirconia, has proven to enhance catalyst’s stability [134]. Moreover, a significant improvement, in terms of activity and stability, can be obtained through the use of bimetallic phases or alkali promoters, which could be used to fine-tune the local surface chemistry and metal-support or metal–metal interactions towards a pronounced rWGS performance [50, 98, 104, 131].
On the other hand, the scarcity and increased market price of noble metals have directed the focus on non-noble metal catalysts, which even though present lower resistance to deactivation, they are more feasible for scale up applications. Transition metals, particularly Cu, have gathered significant attention, though their tendency to sinter at high temperatures can be a drawback, underscoring the need for thermally stable supports, such as ceria. In this regard, Cu/CeO2 systems are widely explored due to their affordability and the strong metal-support interactions between those counterparts. Notably, different ceria synthesis protocols significantly affect the rWGS performance, through mainly the modifications induced on the structural/textural properties and metal-support interactions [27,28,29, 90, 91, 132, 135].
Moreover, bimetallic catalysts can notably modify the catalytic behavior from single-metal systems by leveraging the synergistic effects between different metals. For instance, doping Ru or In with transition metals like Cu or Ni redirects their tendency to produce methane towards CO formation [71, 132, 134, 136]. In the context of NiFe and FeCu systems, metal addition has shown to be pivotal; Fe addition in FeCu systems not only enhances copper stability due to its high melting point, but also introduces more active sites to the reaction [98]. Similarly, NiFe catalysts demonstrate different behavior from their monometallic counterparts, mainly due to the Ni-FeOx interaction which also improves the catalytic stability and performance.
Apart from metal doping, alkali doping has also been beneficial in promoting catalytic behavior, due to alkali metals inherent characteristics. More specifically, alkali metals tend to enhance electrostatic interactions, increasing the dipole–dipole interaction during CO2 activation, thus favoring catalytic activity. Furthermore, alkali doping can promote the dispersion of active sites [98], however small amounts are required in order not to block them [50]. As mentioned, metal dispersion and more specifically metal particle size are also highly influential to products' selectivity. Even though many different parameters of the preparation procedure affect metal dispersion, metal-support interactions or their combinations, it has been highlighted that particle sizes in the range of 1–20 nm favor CO selectivity [135, 137]. In view of these aspects, non-precious metal catalysts, such as Cs-promoted Fe-Cu/Al2O3 or nanostructured Cu/CeO2 systems, exhibit excellent CO2 hydrogenation performance towards CO production (ca. 40–50% CO yield at 400–500 °C, Table 1), approaching or even surpassing the corresponding performance of noble metals (Table 1).
Figure 9 provides a more thorough comparison among the most active catalysts included in Table 1, in terms of CO formation rate (rCO, μmol g−1 s−1) at 400 °C. In accordance to aforementioned aspects, it is evident that the appropriate catalyst formulation comprising of cost-effective and earth-abundant non-noble metals can lead to highly active and selective catalysts for rWGS.
Relative comparison of the most active catalysts for the rWGS reaction. Reaction conditions and corresponding references are provided in Table 1
In addition to the intrinsic catalytic attributes, reaction conditions also impact product selectivity. This effect is not solely due to thermodynamic constraints, as previously mentioned in relation to temperature, pressure, and reactant ratios, but also because of macroscopic limitations, such as space velocity and mass/heat transport. More specifically, it was highlighted that lower contact time facilitates CH4 formation, especially at the temperature window where methanation competes with the rWGS [98]. Consequently, a meticulous selection of both the operating conditions as well as the metal composition of the catalyst is crucial. The ability to fine-tune these catalysts allows for steering product selectivity towards more beneficial yields while maintaining CO2 conversion values close to equilibrium for extended periods. Additionally, ensuring the stability of the catalysts under these conditions is essential to sustain their performance and economic viability over long-term operation.
3 CO2 hydrogenation to CH4
3.1 Fundamentals and thermodynamics
The hydrogenation of carbon dioxide into methane or CO2 methanation is known as Sabatier reaction, since its discovery by the French chemist Paul Sabatier at the beginning of the twentieth century (Eq. 7). The Sabatier reaction is an eight-electron chemical process that requires the input of a large energy amount in order to overcome the difference in the Gibbs formation energy between the reactant CO2 and product CH4 (− 395 and − 51 kJ/mol, respectively).
In general, CO2 methanation is regarded as the equivalent of two processes, the endothermic reverse water–gas shift reaction (Eq. 1) and the highly exothermic methanation of CO (Eq. 2). In other words, methane is more often than not produced by the intermediate step of CO formation, albeit the reaction mechanism is still not fully elucidated and is catalyst-dependent to a certain extent. Also, considering Le Chatelier’s principle for chemical equilibrium, the reaction of CO2 methanation is thermodynamically favored at high pressures and low temperatures, due to its mole-reducing and exothermic nature, respectively. Nonetheless, a low reaction temperature inevitably hinders the reaction kinetics and thus the use of an appropriate catalyst is practically a prerequisite. At the same time, COx hydrogenation reactions are very exothermic, therefore the maximum conversion of carbon oxides is expected to decrease when operating at higher reaction temperatures.
The effluent composition can be influenced by several factors, mainly the reaction parameters (temperature, pressure, H2:CO:CO2 ratio) and reactor configuration [138]. Additionally, the employed catalyst influences the reaction kinetics, conversion and product distribution [139]. Also, at the reactor outlet, besides methane, the gas mixture includes typically unconverted CO2 and H2, large amounts of H2O, and CO formed by rWGS. A typical operation window for the industrial production of methane from CO2 hydrogenation is 250–500 °C and 10–50 bar [139, 140] and can proceed in a single or multiple reactors in series [141].
As stated above, CO2 methanation is associated with a reduction in gaseous moles, thus an increase in the inlet pressure is expected to shift the equilibrium towards products and increase the yield of CH4 due to molar contraction, thereby increasing methane yield. This is clearly evidenced by the thermodynamic analysis carried out and reflected in the plots of the Gibbs free energy of the reaction at variable pressures (Fig. 10a). Interestingly, the highly negative ΔG values at lower temperatures can theoretically lead to complete CO2 conversion at T < 250 °C, regardless of the reaction pressure (Fig. 10b) whereas the formation of CO is negligible at T < 550 °C, as can be seen in the equilibrium composition plots (dry basis) in Fig. 10c. Although higher (C2+) hydrocarbons can be also theoretically produced as carbonaceous by-products, the associated yields are practically negligible, especially when operating under low to moderate reaction pressures (< 15 bar). Also, the beneficial effect of H2 excess in the feed towards increased CO2-to-CH4 conversion is evidenced in Fig. 10d, considering that the stoichiometric ratio of hydrogen and carbon dioxide according to Eq. 7 is equal to H2:CO2 = 4. Nonetheless, at the operation window where CO2 methanation is thermodynamically favored, significant kinetic limitations exist and thus the design of a highly active catalyst at low reaction temperature is of key importance to attain overall cost-effectiveness from a process engineering perspective. Also, given the intermittent nature of RES-derived H2 in a real-scale process, it is imperative that an appropriate catalyst can also function satisfactorily under variable H2 feed concentrations, providing adequate stability and conversion/selectivity performance with minimum changes in the quality of the final product.
3.2 Mechanistic and kinetic considerations
3.2.1 Proposed mechanisms
Although methanation of carbon dioxide is a relatively old reaction and much effort has been devoted to mechanistic studies, as of yet no definitive answer with regards to the underlying mechanism exists in the literature. As is the case for the rWGS reaction, the reaction mechanism of Sabatier reaction is largely dependent on the employed catalytic system, as well as operating conditions. In this sense, two types of mechanisms have been proposed for the reaction of CO2 methanation. Briefly, the first mechanism is named “associative mechanism” and states that gaseous CO2 is adsorbed associatively and along with the adsorbed hydrogen ad-atoms form oxygenate intermediate species which are eventually hydrogenated to produce gas-phase CH4. The other mechanism, known as “dissociative mechanism” involves firstly the activation of the CO2 molecule via its dissociative adsorption to carbonyl and oxygen surface species, which is followed by the carbonyl hydrogenation to CH4. A vast number of studies is now available regarding the examination of the CO2 methanation mechanism, available in various review papers where detailed information can be found for the interested reader [19, 142, 143], whereas a comparison of the reaction mechanism and kinetics over the more widely used Ni and Ru-based catalysts is presented in [144]. However, since detailed mechanistic analysis of the reaction is beyond the scope of the present review, only the general steps of each proposed mechanism are shown herein (Scheme 2).
Elementary reactions for the main proposed mechanisms for CO2 methanation, adapted from [19]
A number of techniques have been used to confirm the associative or dissociative pathway for CO2 methanation, mostly over nickel-based catalysts. These methods mostly refer to adsorption/desorption analyses of surface species formed upon CO2 adsorption in the absence of H2 and vice versa or with both reactants in the feed, transient response analysis (SSITKA) or in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) experiments [142]. In most cases where the CO-dissociation pathway is proposed, the formation of other surface species (essentially carbonates or formates) is negligible or not detectable. This does not disregard their formation, as more specifically designed mechanistic studies may detect all surface intermediates under variable conditions, as was recently reviewed in the comprehensive work by Feng et al. [16]. However, formates formation cannot be unequivocally excluded as a general rule and at the same time their presence does not necessitate their role as intermediate species, but rather that they are merely spectator species.
In the formate pathway, formates are the main intermediate species without the involvement of adsorbed carbonyl. According to this mechanism, CO2 is linearly adsorbed as mono- or bi- dentate carbonates or bicarbonates, which then react with dissociated H ad-atoms on the metal to form hydrogenated carbonates. These hydrogenated carbonate species in turn react with H(ad) to form monodentate formates, which eventually transform to formaldehyde-like and/or methoxy species, before being desorbed as CH4. It is also possible that formate formation does not necessitate the occurrence of the reaction, but rather these species are merely spectator species.
3.2.2 Reaction kinetics
Both empirical and mechanistic kinetic models have been developed by conducting experiments under differential conditions over a wide range of temperatures and reactant concentrations. The mathematical form of the kinetic rate expressions is typically determined from the derivation of Langmuir–Hinshelwood/Eiley-Rideal models, based on the reaction mechanism. The derived rate equation is ultimately fitted to the experimental rate data. An overview of the recently reported kinetic expressions can be found in Refs. [12, 19], while in the comprehensive review by Jalama [142] detailed information on kinetic studies categorized with regards to the variation of reaction parameters can be found. Considering the reaction of CO2 methanation on Ru-based catalysts, predominantly power-law empirical equations are available [145, 146], although a mechanistic model is reported in [147]. The overview of kinetic models on Ru-based catalysts by Falbo et al. [145] indicated that CO2 methanation rate is more dependent on the partial pressure of hydrogen rather than carbon dioxide, with values for reaction orders in the range of 0.3–2.5 and 0–1, respectively. Recently, Erdőhelyi reviewed the kinetic data for a variety of Rh-based catalysts employed in CO2 methanation [75].
As for nickel-based catalysts, in the seminal work by Weatherbee and Bartholomew [148], one of the most comprehensive kinetic studies for methanation of CO2 over Ni/SiO2 was proposed. It was revealed that the reaction rate is very sensitive to reactant’s concentration at low partial pressures, whereas reaction rates approach zero at high pressures. Another important study modeled the process through the simultaneous reactions of CO methanation, CH4 steam reforming and water gas-shift reaction [149]. Although the model was developed and validated specifically for steam reforming on Ni-based catalysts, it has also been used to kinetically evaluate CO2 methanation by Schlereth and Hinrichsen [150]. Moreover, a mechanistic kinetic expression for Ni/Al2O3 catalyst based on the Langmuir–Hinshelwood-Hougen-Watson approach has been proposed in [151]. Through the CO-intermediate mechanism, the proposed elementary reactions are the ones shown in Scheme 2, assuming the dissociation of CO* surface species to C* and O* to be the rds. A recent study by Miguel et al. [152] also described the reaction mechanism and kinetics over Ni-based catalysts, with the mechanism assuming H2 and CO2 dissociation followed by hydrogenation of adsorbed CO to formyl species and considering hydroxyls as the most abundant species, exhibiting a good fit to the experimental data. In the work by Ibraeva et al. [153], the CO2 methanation kinetics was studied over a NKM-4A nickel-containing catalyst. The kinetic equation was derived involving a formate intermediate without preliminary dissociation of gas-phase CO2 to CO* and O* (reaction pathway in the third column in Scheme 2) and assuming that the reaction between adsorbed CO2 and hydrogen ad-atoms is the rate-determining step. The reaction mechanism involving the formation of formate intermediate was also proposed, in which the formation of hydrogen carbonate is assumed to be the rate-determining step (reaction pathway in the last column in Scheme 2) [154].
In this point, it ought to be mentioned once more that the reaction mechanism of CO2 methanation reaction is strongly dependent on the employed catalytic system. Therefore, the intrinsic properties of the catalytic materials can have a profound influence on the reaction pathway and consequently, on the catalytic activity and selectivity. In particular, catalytic properties such as particle size and shape, metal dispersion, amount of oxygen vacancies and basic sites can significantly affect the metal-support interface and local geometry of metal particles, thus affecting the adsorption and dissociation of gaseous CO2 and H2 towards the formation of specific key intermediates through a specific reaction route. In the following section, the state-of-the-art catalytic materials for the CO2 methanation reaction and the parameters affecting their intrinsic properties and consequently their catalytic performance are thoroughly described.
3.3 Catalysts for CO2 methanation
3.3.1 Noble metal (NM)-based catalysts
For the reaction of CO2 methanation all metals of groups 8–10 exhibit significant performance when employed as active phases. Among them, the best activity is generally reported for catalysts based on Ru and Rh, mostly supported on metal oxides with high surface areas such as SiO2, TiO2, Al2O3 and CeO2. Other active methanation metallic phases include Pt and Pd, whereas most of the remaining group 8–10 metals are generally less active for methane formation and are mostly selective to CO, as discussed above. Contrary to the order for CO2 conversion in the methanation reaction, CH4 selectivity is largely reported to follow the order: Pd > Pt > Ir > Rh > Ru [19, 155, 156]. Indeed, CH4 selectivity depends on different parameters aside from the employed active phase, such as the reaction conditions and mass/heat transfer phenomena. Indicatively, the methanation performance over typical noble metal-based catalysts of variable weight loadings (0.1–5.0 wt.%) and crystallite sizes (1.3–13.6 nm) supported on titania showed that the catalytic activity and methane selectivity were dependent on the noble metal, structural properties and reaction conditions. Rh/TiO2 catalyst attained the highest activity values, being more than three times higher than the corresponding performance of Pd/TiO2 [157].
Taking also into consideration that noble metal-based methanation systems are active even with a low metal loading (even less than 1 wt.%), a cost-effective operation can be implemented. Regarding the resistance to deactivation, noble metal-based catalysts are characterized by higher thermal stability over a wider temperature range and are more tolerant than nickel to sulfur poisoning, carbon deposition or carbides formation [158, 159]. However, the above are general rules that are valid mostly for monometallic systems, whereas the performance of a multi-component catalyst in the reaction of CO2 methanation is not solely determined by the active metal phase, but is a combination of various descriptors, the most prominent of which are the nature of the supporting material, promotion effects, bimetallic effects, metal-support interactions, metal particle size, as well as operating conditions such as the reactant ratio or the presence of impurities in the feed of a real process [143, 160, 161]. Notably, traces of H2S have been shown to promote CO2 methanation on Ru, Rh, and Pd supported on TiO2 and CeO2, since when the support is exposed to H2S new active sites are formed in the metal-support interface and the catalyst becomes more active. However, in the cases of supported catalysts on ZrO2 and MgO or under higher H2S content the reaction rate is substantially suppressed, [162]. Thus, fundamental studies for the development of low-content, highly active and selective noble metal-based catalysts are of key importance and actually significant progress has been made over the last decade with respect to assessing these parameters, as elaborated below for the most widely employed active noble metals.
3.3.1.1 Ru-based catalysts
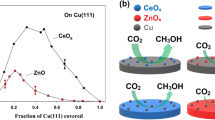
Ruthenium is possibly the most active and selective catalyst for COx methanation reactions, even at low loadings and at low reaction temperature (< 300 °C). Indeed, Ru-based catalysts can attain a methane yield of 99% at atmospheric pressure, being particular active in a temperature range where the reaction thermodynamics is particularly favorable (Fig. 9). Fully reduced metallic Ru is known for its high affinity towards H2 dissociation, which subsequently reacts with adsorbed CO2 on the catalyst surface. Nevertheless, CO2 conversion and CH4 yield over Ru-based catalysts are largely dependent on metal loading, particle size and dispersion, the nature of the support material and metal-support interactions [143]. Notably, the recovery of the initial methanation activity of Ru-based catalysts after accumulation of surface carbonaceous species was demonstrated in various early works employing high-temperature treatment under hydrogen atmosphere [163,164,165].
The influence of the support on Ru-based catalysts was studied using various oxides, including both reducible (CeO2, ZrO2 and TiO2) and non-reducible (Al2O3, SiO2) carriers. Among them, Ru/TiO2 catalyst has been regarded as the most effective and selective [166]. Particularly, the effect of TiO2 crystalline phases (i.e., rutile and anatase titania) on the structure and performance of Ru nanoparticles was explored [167]. In this work, rutile-TiO2 exhibited better Ru dispersion and lower Ru aggregation due to Strong Metal Support Interaction (SMSI) effects via the Ru–O-Ti bond and in turn superior methanation activity and thermal stability in comparison with anatase-TiO2. The structure–activity correlation over Ru/r-TiO2 was corroborated in a follow-up study by varying the pretreatment temperature [168]. It was found that the activity was dependent on the extent of Ru encapsulation by TiOx layers and the amount of surface OH– species on TiOx. Recently, an advanced support consisting of porous hexagonal boron nitride (pBN) material has been employed for the preparation of a catalyst with a very low Ru loading (0.58 wt.%) [169]. By utilizing Density Functional Theory (DFT) simulations, it was claimed that atomic-scale size and low oxidation state of Ru species supported on pBN contributed to a significant enhancement of the methane production rate and a remarkable stability even after 110 h on stream.
In a similar manner, Ru nanoparticles deposited on nanocrystal titania exposing (001) and (101) facets were investigated for CO2 methanation. Ru nanoparticles supported on TiO2 (001) exhibited improved CO2 conversion rate and methanation reactivity, as well as considerable durability in comparison to the TiO2 (101) nanocrystal with the same Ru loading (2.5 wt.% Ru). The dispersion of Ru species and the synergism between Ru and TiO2 were found to be significantly affected by the exposed facets, thus influencing their capability for CO2 activation and determining the CO2 methanation activity [170]. The high catalytic activity of Ru nanoparticles deposited on TiO2 (001) facets, namely 80% CO2 conversion and 100% CH4 selectivity at 350 °C, originates from the nature of (001) facets with high surface energy and oxygen vacancy concentration that could provide more active sites for the CO2activation [170].
Also, the impregnation of Ru with variable loadings on Al2O3 was found to affect the catalytic activity in [171], since although increasing Ru loading from 1 to 5 wt.% did not alter dispersion and particle size, higher contents increased the surface basicity and thus provided enhanced CO2 adsorption capacity, concomitantly increasing CO2 conversion, at the optimum loading of 4 wt.% Ru (Fig. 11). In a separate study over Ru/Al2O3 catalysts with variable Ru loadings, product distribution was highly affected by Ru content [172]. In particular, CO formation was favored over Ru monolayers in the catalyst with 1 wt.% Ru loading, while CH4 formation was favored over nanoclusters being predominant in the catalysts with higher Ru loading. Based on DFT calculations, the authors claimed that the dissociation energy barrier for H2COOH* (the rate-determining step) for the 3D nanoclusters was lower than that for the monolayers. The dependence of product selectivity on Ru loading and particle size in the range of 0.1–5 wt.% was also shown in another work [69], where 3D metal clusters preferably formed on Ru/Al2O3 catalysts with Ru loadings equal and higher than 1 wt.%, resulted in higher CH4 selectivity.
Effect of Ru loading on Ru/Al2O3 catalysts for the reaction of CO2 methanation. Adapted with permission from [171]
A series of Ru catalysts supported on CrOx-modified Al2O3 with various amounts of Cr were prepared through a step impregnation method and used for CO2 methanation, with RuO2/7%CrOx/Al2O3 exhibiting elevated CO2 methanation activity compared to Ru/Al2O3 at low temperatures. The formation of a solid solution structure inhibited the crystallization of RuO2 and the aggregation of Ru during reduction, thus resulting in decreased Ru nanoparticle sizes and enhanced metal-support interactions in the reduced catalysts [173]. The effect of Ru loading (1–5 wt.%) on CeO2 supports has been also studied, with the optimum Ru loading being 2.5 wt.% [174]. Sakpal and Lefferts [175] investigated the CO2 methanation performance over ruthenium catalysts of identical Ru particle size supported on ceria of various shapes (rods, cubes, octahedra) in order to gain insight into the effect of ceria morphology on the catalytic activity. Ru addition promotes the reducibility and increases the oxygen vacancy concentration in comparison to bare ceria, with the rod-shaped catalyst (Ru/CeO2/r) demonstrating abundant oxygen vacancies as compared to cubic and octahedral Ru/Ceria catalysts, thus leading to the optimum CO2 methanation activity (79.3% CO2 conversion and 100% CH4 selectivity at 325 °C). The highest catalytic activity is observed in the catalyst that generates oxygen vacancies most easily, indicating that CO2 activation on oxygen vacancies is the rate determining step. Figure 12 depicts the activity trend at 225 °C, which is well correlated with the vacancy concentration trend obtained from Raman and XPS results, in terms of the I598/I462 and OV/OL ratios, respectively [175].
Correlation between the activity (at 225 °C) and oxygen vacancy concentration of catalysts. Reproduced with permission from [175]
In addition, the size of ruthenium nanoparticles was also found to notably influence CH4 selectivity. In a recent work by Aitbekova et al. [70], it was found that Ru nanoparticles with a size of 2.6 nm pretreated in H2 at 230 °C favored CH4 formation. In contrast, single-site RuOx pretreated subsequently at 230 °C in O2 and H2 both revealed increased CO selectivity (> 90%). This prominent change in the reaction pathway was ascribed to the re-dispersion of Ru nanoparticles into atomically dispersed RuOx species during oxidative pretreatment. These Ru single-site species were associated with weaker CO* adsorption than Ru nanoparticles, thus leading to easier CO desorption in the gas phase. The relationship between the activity in CO2 methanation and variabilities in the Ru particle size including single atoms, nanoclusters (ca. 1 nm) and larger nanoparticles (approximately 4 nm) was systematically investigated over Ru/CeO2 [176], where Ru nanoclusters and large Ru nanoparticles exhibited weaker SMSIs than single Ru atoms, thus facilitating the activation of Ru-CO species, as depicted in Fig. 13. Conversely, single Ru atoms and Ru nanoclusters were not so active towards hydrogen spillover compared to large Ru nanoparticles, thus enhancing water desorption (Fig. 13). The balance between these two factors as well as the optimal activity (TOF of 7.41 × 10−3 s−1 at 190 °C) were achieved by CeO2-supported Ru nanoclusters. In another study, Abe et al. [177] investigated the relationship between the CO2 methanation activity and the mean Ru particle size on TiO2-supported samples. A significant reduction in the onset temperature and concomitantly a significant increase in the methanation activity were observed upon decreasing the particle diameter from 6 to 2.5 nm, whereas both descriptors remained almost constant for a Ru size higher than 6 nm.
Competitive SMSI and H-Spillover effect on the Competing CO Activation and Surface Dehydration for CeO2-Supported Single Ru Atoms, Ru Nanoclusters, and Large Ru Nanoparticles. Reproduced with permission from [176]
Moreover, a Ru/γ-Al2O3 catalyst with an optimal Ru content of 1 wt.%, synthesized by a single step reduction using hydrazine hydrate, showed excellent CO2 conversion and CH4 selectivity of 99% at 325 °C under stoichiometric conditions [178]. It is also worth mentioning that a tandem catalyst (Ru1Ni/CeO2) of two active sites, composed of isolated Ru1 sites and Ni nanoparticles, showed ~ 90% CO2 conversion and ~ 99% CH4 selectivity at 325 °C. In specific, the Ru1 single sites are extremely active for CO2 conversion to CO, while the Ni sites exhibit high efficiency for the sequential CO to CH4 reaction step with the coexistence of these sites significantly promoting the methanation of CO2 [179].
Furthermore, Ru-based catalysts using CeO2, MgO, SiO2 and γ-Al2O3 as the weak basic, medium-strength basic, neutral and acidic support were synthesized through the wet impregnation method. The Ru/CeO2 catalyst exhibits the highest CO2 conversion (86%) and CH4 selectivity (100%), as well as an excellent 30-h stability due to the existence of abundant oxygen vacancies, weak basic sites and good dispersion of Ru species [180].
In another study, the Ru/MnCO3 catalyst exhibited great specific activity, methane selectivity over 99% and long-term anti-CO poisoning stability in CO2 methanation due to the formation of carbonate-modified metal-support interfaces. The Ru species are stabilized by these interfaces, leading to the activation of CO2 and H2 molecules. The Ru-CO* species which are identified as the reaction intermediates and are steadily formed from the dissociation of CO2 exhibit moderate adsorption strength and high reactivity in further hydrogenation to CH4. Moreover, the Ru/MnCO3 carbonates are consumed by hydrogenation towards methane formation and are replenished by exchange with CO2, being in a dynamic equilibrium during the reaction [181]. Also, interstitial carbon doped into RuO2, prepared through a green hydrothermal method, exhibited high activity and selectivity to methane at low temperatures as well as remarkable stability, due to the stabilization of Ru sites in low oxidation states [182].
3.3.1.2 Rh-based catalysts
In addition to Ru, rhodium is a very active and selective metal phase employed in low-temperature CO2 methanation. In accordance with Ru-based catalysts, previous studies on Rh-based catalysts have established the effects of Rh particle size, supporting material and Rh chemical state on CO2 methanation activity, selectivity and reaction mechanism. More importantly, most of the literature studies claim that over Rh-based catalysts the amount of catalytically active sites increased significantly at higher Rh contents, thereby improving overall catalytic activity. In the cases of low Rh loadings, mostly CO formation would be favored, whereas the highly selective formation of methane normally dominates at higher loading values [156, 183].
In specific, the intrinsic activity of Rh/alumina catalysts depended on the Rh particle size, where especially at the low-temperature regime (135–150 °C), larger Rh particles favored CH4 formation due to the higher abundance of lateral faces active for the rate determining step, i.e., the breaking of C-O bond [184]. In contrast, the catalytic performance was independent of the size of Rh nanoparticles between 185 and 200 °C. In a following study, the strong dependence of the reaction kinetics with regards to Rh particle size was also significant up to 165 °C [185]. Smaller Rh clusters possessed fewer active sites and offered stronger adsorption of CO intermediates than larger Rh ones, resulting in lower CO2 reaction order values and higher activation energy values, whereas in contrast smaller particles exhibited higher H2 reaction orders, indicating lower hydrogen coverage. As shown in Fig. 14a, an increase in the specific rate is observed with increasing particle size for up to 7 nm. Also, turnover frequency (TOF) increases by a factor of 10 for particle sizes from 2 to 7 nm (Fig. 14b), with a further increase in particle size up to 19 nm resulting in a threefold rise of TOF. Overall, increasing the Rh particle size from 2 to 7 nm enhanced the surface-normalized CH4 formation rate, while negligible changes were demonstrated for larger particle diameter values. This was also shown in another study over Rh/TiO2, where the turnover frequency was doubled when the crystallite size of Rh was increased from 0.9 to 4.2 nm [185].
Catalytic activity results. Rate of methane formation as a function of Rh particle size and reaction temperature. a Specific rate (\({\text{mol}}_{{\text{CH}}_{4}} {\text{mol}}_{\text{Rh}}^{-1}\) s‒1) and b turnover frequency (\({\text{mol}}_{{\text{CH}}_{4}}\) molRhsurface s‒1). Lines are used as a visual guide. Reproduced with permission from [185]
As shown in Fig. 15 that illustrates the apparent activation energy of the CO2 hydrogenation reaction as a function of Rh particle size, the activation energy of the dissociation of CO(ads) does not vary significantly with particle size, being ~ 15 kcal/mol for all the catalysts studied. Nevertheless, the apparent activation energy of the overall CO2 methanation reaction is constant for catalysts with particle sizes larger than 7 nm (ca. 17 kcal/mol), while increasing sharply for smaller particles, attaining 28.7 kcal/mol for Rh (0.5 wt.%)/TiO2 catalyst of 2 nm particle size [185].
Apparent activation energy for CH4 formation at steady-state conditions compared to the activation energy obtained for CO(ads) dissociation by operando-DRIFTS, as a function of Rh particle size. Reproduced with permission from [185]
Regarding the choice of support, TiO2 is one of the most effective supports for Rh catalysts. Rh/TiO2 catalyst exhibited a methane formation rate that was almost ten times higher than the corresponding values over Rh/Al2O3 and Rh/SiO2. It was reported that the different extent of electronic metal-support interactions could be mainly accounted for the observed differences in activity; as TiO2 is a n-type semiconductor, a much higher interaction is expected in this case, affecting the bonding and reactivity of chemisorbed species [186]. Similarly, Martin et al. [187] explored Rh supported on SiO2, Al2O3 and CeO2 and showed that both Rh/CeO2 and Rh/Al2O3 attained high CH4 yields. Based on a detailed DRIFTS study, the differences in activity were ascribed to the higher degree of electronic interaction of Rh with Al2O3, which could facilitate the adsorption and dissociation of CO2. The high activity over Rh/Al2O3 was associated with the formation of linear Rh-CO and RhOx species upon dissociation of gaseous CO2. Besides, the interaction between Rh and CeO2 was also strong, with Ce3+ ions being active for the activation of CO2 and formate formation at the Ru-CeO2 interface, which were further dissociated to strongly bound CO and OH species [188]. Recently, novel mesoporous Rh nanoparticles were synthesized via a facile wet chemical reduction method and were used as a catalyst for CO2 methanation [189]. The mesoporous Rh catalyst exhibited substantially higher reaction rate than non-porous Rh, attributed to a large number of atomic steps assisting in CO2 adsorption and activation.
Lastly, some studies on bimetallic Rh-based catalysts have been reported. Specifically, Rh addition to Ni supported on a composite zirconia-ceria oxide improved Ni dispersion, resulting in an increase in activity and catalyst lifetime [190]. Also, synergistic interactions were reported for a physical mixture of Ni supported on activated carbon (Ni/C) and a Rh/γ-Al2O3 catalyst compared to the parent materials [191]. The synergy was attributed to the increased H2 adsorption and promotion of carbon hydride formation, as well as the fact that the spillover of hydrogen maintains rhodium particles in a fully reduced state (Rh0), providing high availability of active sites.
3.3.1.3 Pd-based catalysts
Aside from Rh and Ru, Pd is the other mostly used noble metal for CO2 hydrogenation to CH4. Palladium is generally known as an excellent metallic phase for the dissociation and subsequent spillover of gaseous hydrogen, a behavior to which the increased methanation activity of Pd/γ-Al2O3 catalysts was ascribed [58]. Indeed, higher availability of H ad-atoms was essential for obtaining high CH4 yield. This is in accordance with a study examining bifunctional Pd-Mg/SiO2 catalysts, in which the authors showed that Pd-Mg/SiO2 exhibited a relatively high methane yield (~ 57%) at 500 °C, whereas Pd/SiO2 largely produced CO [192]. Notably, the Pd-free sample was completely inactive for the reaction, an interesting result which was ascribed to the absence of the bifunctional mechanism induced by Pd, which provides active H(ad) species in nearby Mg-bound carbonates for the eventual desorption of gas-phase CH4. Elsewhere, in a study examining the impact of different crystallographic orientations and mean coordination numbers on a series of Pd/SiO2 catalysts, it was showcased that under-coordinated sites (i.e., corners, edges and steps) were more active towards methane formation than over-coordinated Pd (100) and Pd (111) facets on shape-controlled Pd nanoparticles of cubic and polyhedral morphology embedded in mesoporous silica [193]. In another work, a MOF-supported Pd sample attained high methanation activity via the synergy between Pd nanoparticles and UiO-66. As expected, Pd promoted the availability of active hydrogen ad-atoms, while at the same time the Zr6O4(OH)4 nods of UiO-66 facilitated CO2 adsorption and activation, with the optimum activity acquired for a loading of 6 wt.% Pd [194]. Upon addition of further amounts of palladium, agglomerated Pd nanoparticles with uneven dispersion were formed, which were responsible for inferior catalytic performance. Vajda and co-workers have investigated the fine-tuning of CO2 methanation performance down at the atomic level [195, 196]. In particular, they theoretically explored, through density functional theory (DFT) calculations, the mechanism of CO2 methanation on the zirconia (Zr12O24)-supported bimetallic tetramer Cu3PdO2, revealing the direct participation of Pd atom in the hydrogenation steps through the lowering of the energy barriers and the key role of the tetramers-zirconia interaction that leads to support redistribution in the clusters [195].
3.3.2 Transition metal-based catalysts
Although Rh-based and other noble metal-based catalysts are very promising for CO2 methanation reaction under different working conditions, they are associated with economic impediments arising from the rather expensive mining costs and general uncertainties regarding their availability. It has been also reported that these catalysts are prone to sintering at elevated reaction temperatures, which is detrimental to their performance [197]. In this regard, particular attention has been recently paid to the rational design and fine-tuning of non-noble metal-based catalysts in order to obtain catalytic systems with comparable or even superior reactivity to that of NMs [27,28,29]. Even as early as the year 1902, Sabatier and Senderens discovered that metallic Ni is able to catalyze the hydrogenation of COx by producing a mixture of CH4 and H2O. In the following decades, the reaction of CO2 methanation was investigated over several catalytic systems based on non-noble VIIIB group metals. Among them only nickel, cobalt and iron were active towards the specific reaction, albeit differences in product distribution were observed. In particular, Ni- and Co-based catalysts produce predominantly CH4 and to a lesser extent CO, while Fe is mostly active for chain growth and the generation of significant amounts of C2+ hydrocarbons, so in general, have been mostly utilized in Fischer–Tropsch and ammonia synthesis reactions [159]. There is a lot of room for progress towards determining the role of active Fe species, as well as understanding phase transformations, structural changes and participation of surface carbon species during CO2 methanation [198, 199]. Supported nickel catalysts thus remain by far the most widely studied metallic phases, even considering noble metal-based catalysts, followed by a few studies concerning Co-based catalysts, as thoroughly elaborated below.
3.3.2.1 Ni-based catalysts
Nickel is the first and by a large margin the most studied active metallic phase for the reaction of CO2 methanation, clearly evidenced by the vast number of scientific papers examining Ni-based catalysts for the methanation reaction of carbon dioxide. In fact, nickel-containing CO2 methanation catalysts dominate the relevant literature and the recent advances solely for Ni-based catalysts in the low-temperature production of CH4 from CO2 hydrogenation have been extensively summarized in dedicated reviews [200,201,202]. In the present survey, the key parameters affecting the activity and selectivity of state-of-the-art Ni-based catalysts are elaborated, in the light of obtaining reliable structure-performance relationships and revealing the most active materials in the field.
One of the most significant factors impacting the low-temperature CO2 methanation activity of Ni-based catalysts is the employed supporting material. Specifically, the physicochemical properties of the support can alter the intrinsic characteristics of Ni entities as well as the extent of metal-support interactions, thereby affecting reactants adsorption and activation. In particular, the dispersion of the Ni species over the catalyst surface is greatly affected by the structure of the support, as high-surface supports accompanied by large pore volumes may offer an abundance of Ni active sites and high dispersion [203, 204]. Nickel oxidation state is also notably affected by the charge transfer between Ni and the support that leads to augmented redox properties; the high electron density of Ni is associated with the higher affinity between Ni and the carbon atom of CO2, thereby allowing the dissociation of the C-O bond [205].
In light of the above, a plethora of materials have been employed for Ni-based catalytic composites, most prominently metal oxides such as CeO2, Al2O3, TiO2, SiO2, ZrO2 or their combinations in multi-oxide supports [206,207,208,209,210,211,212,213]. Ascribed to strongly evidenced metal-support interaction phenomena, the formation of a solid solution is usually favored over oxide-supported Ni catalysts. Furthermore, given that Ni0 is far more active than Ni2+ towards CO2 methanation, nickel-based catalysts need to be reduced (almost always via hydrogenation at relatively elevated temperatures) in order to increase the catalyst surface coverage of metallic nickel nanoparticles. Apparently, metal oxide supports with high surface areas are advantageous in dispersing Ni0 species, whereas the formation of a solid solution is beneficial for forming Ni nanoparticles with ultra-small sizes after reduction [214,215,216]. Apart from conventional metal oxides, other novel supporting materials like zeolites [217,218,219,220], mesoporous materials [221,222,223], graphene oxide [224, 225] and MOFs [226,227,228] have also been employed. Compared with metal oxide supports, these novel materials may potentially offer several advantages such as excellent textural characteristics (i.e., surface area, pore volume and pore diameter) and improved properties with regards to surface nickel dispersion.
Generally, the most prominent disadvantages regarding Ni-based catalysts are their proneness to sintering at high reaction temperature and their poisoning by the formation of mobile nickel sub-carbonyl species at low temperatures [229, 230]. However, these drawbacks can be negated by the addition of a second metallic phase and/or an appropriate promoter into a Ni-based catalytic composite [201, 231,232,233]. Although the additive itself may be inactive, it can significantly promote the catalytic performance of the complex catalyst by regulating Ni dispersion and surface properties, by altering the catalyst structure, electronic properties and/or surface acidity/basicity. The same effect can be demonstrated upon promoting Ni-based catalysts with low amounts of a noble metal like Ru, Pt or Pd. For instance, in bimetallic Ni-Ru catalysts existing in the form of hetero-structures, Ru increases the availability of active sites for CO2 activation as well as enhancing the spillover of hydrogen into adjacent Ni sites [234,235,236]. On the other hand, palladium and platinum mostly modify nickel electronic properties via alloy formation [237,238,239,240]. In all, the synergistic effect between Ni and the second metal entity may greatly enhance reactants dissociation and products desorption and lead to higher CH4 yield values compared to un-promoted samples [107, 241,242,243,244,245,246,247]. For instance, a 3 wt.% Ru-doped 30 wt.% Ni/CexZr1−xO2 catalyst, prepared by the one-pot hydrolysis method, exhibited improved low-temperature catalytic activity, due to high nickel dispersion and large amounts of basic sites induced by Ru promotion [248]. Similarly, the Ni-Ru/CeO2-Al2O3 catalyst of 15 wt.% Ni and 1 wt.% Ru reached equilibrium conversion at 350 °C. The increased specific surface area of the Ni-Ru/CeO2-Al2O3 catalyst, ascribed to the alumina carrier, and the small particle size of nickel resulted in this remarkable activity and stability for CO2 methanation [249]. Taking into account the key role of textural and structural characteristics of the catalyst's counterparts on intrinsic reactivity and metal-support interactions, the synthesis method [223, 250,251,252,253,254,255,256] and pretreatment protocols [211, 252, 257,258,259] can be used as additional modulating tools to influence the catalytic activity of Ni-based catalysts. In particular, Ni dispersion and metal-support interactions, which are closely related to catalyst activity, can be notably influenced by the preparation method, mainly the nickel precursors or the synthesis protocol used. The conventional wet impregnation method remains an economical and efficient method, exploiting the capillary pressure to push the active components into the pore channels of the support. Nonetheless, in recent years, new methods have been explored and developed by employing new technologies, such as microwave assisted, hydrothermal, plasma and urea hydrolysis methods. Regarding the pretreatment procedure, it mainly involves changes in the catalytic activity induced by the different treatment atmosphere (i.e., gas and temperature employed), before actually testing the catalysts under the CO2/H2 mixture. Although the calcination step is necessary for the removal of residual precursor ions, an increased calcination temperature may significantly decrease Ni dispersion and the specific surface area, in turn yielding inferior catalytic activity.
Finally, Ni content (and equivalently the size of Ni particles) has been shown to largely affect the catalytic performance, as in the case of other metal-based CO2 hydrogenation catalysts. Notably, there is no consensus regarding the optimum range for the particle size of Ni, as there exists great ambiguity in the literature, showcased by a variety of contradicting reports demonstrating both improvement and inhibition of the methanation activity upon an increase in Ni loading and in turn the particle size of Ni crystallites. Indicatively, an enhancement in catalytic activity by larger Ni particles was shown in Refs. [26, 260,261,262,263,264], whilst the increase in Ni content led to decreased CH4 yield or turnover frequency values in other works [265,266,267,268,269]. In this perspective, we recently explored the structure-sensitivity of CO2 methanation reaction over nickel catalysts with variable particle sizes supported on CeO2 nanorods [270]. Specifically, an optimum Ni particle size of ca. 20 nm was revealed, offering remarkable CO2 methanation activity [270]. Interestingly, on the ground of a comprehensive structure-sensitivity analysis (Fig. 16), it was disclosed that a compromise between the length of the Ni-CeO2 perimeter and the presence of larger Ni particles is required to obtain the optimum performance.
Structure-sensitivity of CO2 methanation over Ni/CeO2-nanorod with variable Ni particle sizes: effect of Ni particle size on the total nickel-ceria perimeter (a), site time yield normalized by a single Ni crystallite, STYb (b) perimeter-normalized reaction rate, rp (c) and relationship between the mass-normalized reaction rate and Ni-CeO2 perimeter (d). Reproduced with permission from [270]
In most cases, though, a different optimum value for the Ni content/size is proposed, conducive of the fact that this parameter should be assessed in a case-by-case basis. The above differences were generally ascribed to the variabilities in various relevant parameters like hydrogen dissociation affinity and reducibility, Ni-support interface properties, nickel particles local geometry, reaction pathway and carbonyl bonding energy. Under these perspectives, the rational design of highly active and stable Ni-based CO2 hydrogenation catalysts by means of structural and electronic engineering—via advanced synthetic and promotional routes—constitutes one of the main research pillars in heterogeneous catalysis, as reviewed elsewhere [28].
In view of the above aspects, ceria-supported nickel catalysts (Ni/CeO2) with different Ni loadings ranging from 0.1 to 5 wt.%, were examined for CO2 hydrogenation reaction. It was revealed that the catalytic activity and the product selectivity are closely related to the interfacial oxygen vacancies and the structure of Ni species, respectively. As the Ni loading increases, more surface oxygen vacancies located at the nickel-ceria interface are responsible for CO2 adsorption and activation. In the meantime, the increase in Ni particle size and the higher amount of metallic Ni species contribute to the gradual transformation of the product selectivity from CO to CH4. The Ni/CeO2 catalyst of 5 wt.% Ni loading exhibits the optimum CO2 conversion (78.3%) and CH4 selectivity (99.3%) at 290 °C [271]. Elsewhere, with the scope of improving the low-temperature CO2 methanation activity and tolerance to sintering, Ni catalysts supported on ZrO2, CeO2 and Ce-Zr-O solid solutions were prepared through a citrate complexation-impregnation method. The Ni/Ce0.2Zr0.8O2 catalyst exhibited the highest CO2 conversion (71%) and complete methane selectivity at 250 °C, along with the highest stability, mainly due to the high nickel dispersion and small particle size. The enhanced interaction between nickel nanoparticles and the Ce0.2Zr0.8O2 support was accounted for the high sintering resistance of the Ni/Ce0.2Zr0.8O2 catalyst, as the aggregation of nickel species was suppressed by inhibiting their migration to the surface or the flow through the sub-surface lattice [272]. Moreover, Ni catalysts supported on ceria of various nano-morphologies (rods, cubes, octahedra, particles) were examined in CO2 methanation reaction with the catalyst supported on nanoparticles exhibiting the highest catalytic activity (81.1% CO2 conversion and 100% CH4 selectivity at 300 °C) and stability (Fig. 17) [273].
Effect of catalyst preparation method in the pre-exponential factor over the different 5Ni/CeO2 catalysts; reaction conditions: 0.1 g catalyst, WGHSV = 12,000 mL g‒1 h‒1), H2/CO2 = 4, 1 atm. Reproduced with permission from [273]
In particular, during CO2 methanation over these 5Ni/CeO2 catalysts, the formate species were the main reaction intermediates for the 5Ni/NRs and 5Ni/NCs catalysts, whereas the carbonate species were the main intermediates for the 5Ni/NPs and 5Ni/NOs ones, thus leading to a faster methane formation over the latter catalysts. The different Ni coordination environments on the various ceria crystal facets could be accounted for the high activities of the 5Ni/NPs and 5Ni/NOs catalysts. In general, the hydrogenation of CO2 to carbonate species dominated the Ni-based catalysts supported on CeO2 exposing (111) crystal facets, whereas the reductive dissociation of CO2 to CO was slightly more favorable over the Ni-based catalysts supported on CeO2 exposing (100) and (110) crystal facets [273].
In the same manner, ceria nanocubes of various sizes (30.7‒41.3 nm), exposing (100) crystal facets, were hydrothermally synthesized by modifying the concentration of NaOH (6 M, 9 M, 12 M) and explored for the CO2 methanation over Ni/CeO2 catalysts. The Ni/CeO2-6M had the smallest ceria size, the highest oxygen vacancy concentration and the largest Ni/support interfacial area and exhibited the best catalytic performance, i.e. 58% CO2 conversion and 97.5% CH4 selectivity at 300 °C [274]. A correlation was found between the oxygen vacancy concentration derived by the Raman (ID/IF2g) and XPS [Ce3+/(Ce3+ + Ce4+)] ratios with CO2 conversion rate, as shown in Fig. 18 [274].
The structure-effect relationships between CO2 reaction rate at 548 K and concentration of oxygen vacancies. Reaction conditions: T reduced = 723 K, P = 0.1 MPa, WHSV = 36 L g‒1 h‒1. Reproduced with permission from [274]
Moreover, as depicted in Fig. 19a, a linear correlation between CO2 reaction rate and the amount of effective basic sites for CO2 adsorption was found, indicating that the oxygen vacancies related to the nickel-ceria interface as the effective basic sites play a profound role in CO2 activation, which is the rate-determining step for CO2 methanation. Figure 19b illustrates the relationship between the CO2 methanation activity (in terms of apparent activation energy) and the ceria particle size. A gradual increase in the apparent activation energy was observed with increasing CeO2 particle size, following the order: Ni/CeO2-12M (120.0 kJ/mol) > Ni/CeO2-9M (112.9 kJ/mol) > Ni/CeO2-6M (104.7 kJ/mol), indicating that Ni/CeO2-6M with the effective interfacial sites decreases the apparent activation energy of CO2 methanation and increases the catalytic activity [274].
Plot of CO2 consumption rate at 548 K versus effective basic sites of Ni/CeO2 catalysts in CO2 methanation (a) and the apparent activation energy in CO2 methanation over the Ni/CeO2 catalysts as a function of CeO2 nanocubes size (b). Reaction conditions: P = 0.1 MPa, Treaction = 523 K, WHSV = 36 L g‒1 h‒1. Reproduced with permission from [274]
Ni supported on ceria-zirconia nanopolyhedra showed excellent catalytic activity, achieving 85.6% CO2 conversion and 100% selectivity towards CH4 at 325 °C due to their higher Ni dispersion, higher pore volume, reducibility and moderate basicity, along with their abundance in adsorbed surface oxygen and oxygen vacancies, as compared to the corresponding materials in the form of nanocubes and nanospheres. Additionally, the existence of the more reactive (100) and (110) ceria facets in the polyhedral catalyst was another contributing factor to its superior catalytic performance [275]. A remarkable low-temperature CO2 methanation activity (75.5% CO2 conversion and 99.9% CH4 selectivity at 250 °C) was observed with the introduction of Eu3+ cations to Ni/CeO2, as they promoted the nickel-ceria interaction through the formation of abundant Ni–O–Ce interfacial sites. The Ni/CeEu catalyst with a Ce:Eu mole ratio of 9:1 exhibited higher Ni dispersion and more interfacial sites for the bidentate carbonates adsorption, thus resulting in enhanced low-temperature CO2 methanation performance [276]. Tsiotsias et al. [277] prepared a series of Ni-based catalysts supported on Pr-doped CeO2 of various porosities and nanostructures through the sol–gel method. The Ni/Ce0.9Pr0.1O2-δ-MPC catalyst, in which the support was synthesized by the modified Pechini method, displayed 77% CO2 conversion and 99% methane selectivity at 350 °C. The modified Pechini synthesis through the use of ethylene glycol in the absence of water resulted in a metal oxide support of larger pore size and volume, an indispensable factor for the deposition of medium-sized Ni nanoparticles confined into the nanoporous structure [277].
In order for the complex relationship between Frustrated Lewis Pair (FLP) structures and CO2 methanation performance to be investigated, Xie et al. [278] constructed different distributions of FLP structures over nickel-ceria catalysts of various morphologies (nanorods, nanocubes and nanooctahedra). Among the investigated catalytic materials, Ni/CeO2 nanorods (Ni/CeO2-R) were the most prone to FLP fabrication due to the nature of ceria (110) facet and the steric hindrance between the hydroxyl species (OH) and the oxygen vacancies (OV). The Ni/CeO2-R FLPs-enriched catalyst exhibited higher CO2 conversion (84.2%) and CH4 selectivity (97.8%) at a very low temperature (225 °C), as compared to the other catalysts of fewer FLPs. According to the experimental results, both the emerged CO* route and formate pathway were accounted for the low-temperature methanation performance over the FLP-enriched Ni/CeO2-R catalyst. The formate route was positively correlated with the concentration of FLPs-activated HCO3* species. In addition, instead of the traditional CO pathway (activated by Ni0), the emerged CO* route (promoted by FLPs) was involved in the low-temperature CO2 methanation process (below 225 °C) [278].
A series of inverse CeO2–Cr2O3/Ni model catalysts with controlled CeO2–Ni and Cr2O3–Ni interfacial structures were synthesized by the ion-exchange method and the role of interface was investigated in CO2 methanation performance. The CeO2–Cr2O3/Ni catalyst showed 92% CO2 conversion and full methane selectivity at 310 °C. It was revealed that the catalyst with only CeO2–Ni interfaces followed the formate pathway while the Cr2O3-Ni interface which was produced with the introduction of Cr2O3, modified the nearby CeO2-Ni interface through electron transfer, thus bringing the additional CO reaction pathway on the catalyst [279]. Figure 20 shows the relationship between the CO2 methanation rate and the weak basic site content at 200–230 °C. The linear correlation between these parameters revealed the significant role of the weak basic sites in the activation of CO2 at low temperatures [279].
Relationship between the reaction rate and weak basic site content. Reproduced with permission from [279]
In another study by Wang et al. [280], ZrO2-x-supported Ni nanocatalysts with a distinctive interface structure (Ni–O–Zr3+-Vö) were synthesized based on NiZrAl-LDHs precursors. The optimum Ni/ZrO2-x-S2 (15 wt.% Zr, 50 wt.% Ni) catalyst exhibited high CO2 conversion (~ 72%) and full CH4 selectivity at a temperature as low as 230 °C. It was revealed that the main active centers were the interfacial sites with the Zr3+-Vö sites promoting the CO2 adsorptive activation and H2 dissociation taking place at the metallic Ni sites, followed by hydrogen spillover onto the interfacial sites for intermediate hydrogenation to methane. Both the formate and CO pathways were involved in CO2 methanation in the presence of the Ni/ZrO2-x-S2 catalyst. In order for a structure–property relationship to be established, the intrinsic reaction rate was plotted as a function of surface Zr3+ species ratio (Fig. 21a) and surface oxygen vacancy ratio (Fig. 21b), respectively, resulting in a linear correlation [280]. Moreover, it is worth mentioning that sub-nanometer copper clusters on zirconia supports were found to be highly active for CO2 methanation with the catalyst prepared by supersonic cluster beam deposition (Cu12/NS ZrOx) becoming active at ~ 175 °C and attaining a rate of methane formation of 0.05 molecules atom−1 s−1 at 375 °C [196]. A highly stable Ni/ZrO2 catalyst dually promoted by La and Ce was synthesized through the impregnation method, exhibiting robust durability over 200 h at 600 °C for CO2 methanation. It was shown that the dispersion and sintering resistance of nickel were highly promoted by the formation of a La2O3-CeO2 solid solution [281].
Reaction rate as a function of surface Zr3+/(Zr3+ + Zr4+) ratio (a) and surface oxygen vacancy ratio (b). Reproduced with permission from [280]
Moreover, Zafeiratos et al., [282] investigated the CO2 methanation performance of Ni-doped CeO2 nanoparticles which exhibited an extremely high Ni mass-specific activity and CH4 selectivity. The NiCeOx nanoparticles of 1.3 wt.% Ni loading showed maximum CO2 conversion (ca. 70%) and CH4 selectivity (ca. 98%) at 400 °C. Operando characterization results revealed that the CO2 methanation performance was closely related to the ionic Νi and Ce3+ surface sites, whereas the formation of metallic nickel did not promote the reaction considerably. In addition, the stability of interstitial ionic nickel sites on CeO2 surfaces was corroborated by theoretical calculations which highlighted the role of Ce–O frustrated Lewis pair (FLP), Ni–O classical Lewis pair (CLP) and Ni-Ce pair sites to the H2 and CO2 activation. It is worth mentioning that the performance of Ni-doped ceria nanoparticles is comparable with that of ceria-supported nickel catalysts containing 5 times higher Ni loading (~ 10 wt.% or even more). As a consequence, the nickel mass-specific CO2 conversion rate (5.6 molCO2 h‒1 gNi‒1) and CH4 yield (1700 μmolCH4 h‒1 gNi‒1) were among the highest reported for Ni/CeO2 catalysts [282]. The Ni/CeO2-EDA catalyst, whose preparation was assisted by ethylenediamine (EDA), showed enhanced CO2 conversion (84.2%) and CH4 selectivity (97.8%) at 225 °C, without any decrease in CO2 conversion even after 70 h. Apparently, nickel dispersion is facilitated by the EDA, thus providing more active sites for hydrogen dissociation [283].
Nickel-based catalysts supported on ZrO2-Al2O3 composite support with different Al/Zr molar ratios (Ni/Zr-Al-X) were synthesized through a co-precipitation method by doping the nickel-zirconia catalyst with different amounts of Al and were evaluated in CO2 methanation reaction [284]. The incorporation of a suitable aluminum amount into the support increased the metal Ni dispersion and promoted H2 activation and dissociation, thus significantly improving the CO2 methanation activity at low-temperatures (< 300 °C). Nevertheless, the addition of an excessive Al amount hinders the formation of metal nickel, thus reducing the number of active metal sites and weakening the catalyst's performance. So, the Ni/Zr-Al-0.1 catalyst (molar ratio of Al/Zr = 0.1) is the optimum catalyst in the low-temperature range, achieving 84.4% CO2 conversion and 99.4% CH4 selectivity at 280 °C [284].
On the basis of a dynamic analysis of intermediates and oxygen vacancies correlations, a series of Ni-based CeO2 catalysts was prepared through impregnation and electrospinning methods and studied in CO2 methanation. The NiNPs@CeO2NF (Ni nanoparticles encapsulated in ceria nanofibers) catalyst of 10 wt.% Ni loading, prepared by the co-electrospinning method exhibited superior catalytic performance with 83.7% CO2 conversion and 98.2% selectivity to methane at 325 °C, as well as excellent stability at 400 °C [285]. It was revealed that NiNPs@CeO2NF could form abundant active oxygen vacancies for the activation and subsequent hydrogenation of CO2. The hydrogenation of CO2 followed the formate pathway over the catalysts, with the monodentate and bridging bidentate formate being the key intermediates. As depicted in Fig. 22, the nanofibrous samples show higher ID/IF2g ratios, indicating their ability to facilely generate oxygen vacancies, which are favorable for the cleavage of the C–O bond and CO2 activation [285].
Ratio of the intensity of defect peak and F2g peak derived by Raman results for each catalyst. Figure created by data taken with permission from [285]
Zhen et al. [286] employed the double solvent method (DSM) and multiple impregnation method (IM) for the development of Ni nanoparticles (NPs) encapsulated in a highly ordered metal–organic framework (MIL-101) as catalysts for CO2 methanation. The 20Ni@MIL-101 catalyst with a Ni loading of 20% exhibited excellent methanation activity and selectivity, achieving complete CO2 conversion and full methane selectivity at 300 °C. The high dispersion (42.3%) of small-sized (2.6 nm) Ni NPs, which were stabilized in the MIL-101 frameworks, while exposing the (111) nickel facet is accounted for the superior CO2 methanation performance [286].
In another study by Kawi and co-workers [287], Ni and Ni-Mg phyllosilicate mesoporous SBA-15 catalysts were synthesized through ammonia evaporation (AE) method. The catalysts originated from the phyllosilicate structure showed superior CO2 methanation in comparison to the ones prepared through the wetness impregnation (WI) method, owing to the weak basic sites provided by surface hydroxyl groups and the enhanced metal-support interaction. In addition, Mg incorporation with an optimum loading of 5 wt.% into the phyllosilicate structure increases the medium basic sites, which are able to promote the formation of monodentate formates and improve the CO2 methanation activity at lower temperatures. Moreover, the strong metal-support interaction originated from the phyllosilicate structure and the confinement effect of the SBA-15 support can suppress metal sintering, thus leading to good stability. The Ni-Mg/SBA-15-AE catalyst (10 wt.% and 5 wt.% Ni and Mg loading, respectively) showed 73% CO2 conversion and 99% CH4 selectivity at 350 °C. It is worth mentioning that a correlation between TOF of CO2 conversion and the concentration of basic sites was observed, as shown in Fig. 23. In particular, an increase in TOF values is observed with increasing total basicity, indicating the key role of abundant basic sites in high CO2 adsorption capacity [287].
The plot of TOF of CO2 conversion (black solid line based on TEM and red dash line based on N2O pulse titration) as a function of total basicity of Ni-based SBA-15 catalysts. Reproduced with permission from [287]
The role of alkaline earth metals in CO2 methanation was studied over promoted Ni/M0.1Ce0.9Ox catalysts, in which ceria was modified by different alkaline earth metal oxides with a M/Ce molar ratio of 1/9 (M = Mg, Ca, Sr, Ba). The MgO and CaO oxides are dissolved into the ceria lattice, forming solid solutions, while the SrO and BaO oxides are dispersed as carbonates on the ceria surface. The alkaline earth metal oxides improved the intrinsic activity of the supported nickel catalysts, following the order: Ni/Ca0.1Ce0.9Ox > Ni/Sr0.1Ce0.9Ox > Ni/Mg0.1Ce0.9Ox > Ni/Ba0.1Ce0.9Ox > Ni/CeO2. Specifically, the Ni/Ca0.1Ce0.9Ox catalyst exhibited the best CO2 methanation performance with 75% CO2 conversion and 99% CH4 selectivity at 290 °C, mainly attributed to its high Ni dispersion and abundant moderate alkaline sites [288]. In order to elucidate the role of the moderate alkaline sites in CO2 methanation, TOF values at 250 °C were plotted against the amount of moderate alkaline sites over the Ni/M0.1Ce0.9Ox catalysts, as shown in Fig. 24, revealing a linear relationship between these parameters and suggesting the significance of the amount of moderate alkaline sites in CO2 methanation activity [288].
Linear relationship between the TOF values at 250 °C and the amount of surface moderate alkaline sites on the Ni/M0.1Ce0.9Ox catalysts. a Ni/CeO2; b Ni/Mg0.1Ce0.9Ox; c Ni/Ca0.1Ce0.9Ox; d Ni/Sr0.1Ce0.9Ox; e Ni/Ba0.1Ce0.9Ox; f Ni/Ca0.1Ce0.9Ox-IMP. Reproduced with permission from [288]
Furthermore, the alkaline earth metals magnesium (Mg) and calcium (Ca) are considered as promising promoters for CO2 methanation due to their exceptional basic characteristics. Therefore, Ahmad et al. [289] developed nickel-based catalysts promoted with Mg and Ca supported on exfoliated graphitic carbon nitride (eg-C3N4). The Ni-Mg/eg-C3N4 catalyst exhibited superior methanation activity (CO2 conversion of 77% and CH4 selectivity > 99% at 322 °C), mainly ascribed to the creation of a NiO-MgO solid solution, which facilitates metal-support interactions, thus promoting the formation of highly dispersed nickel nanoparticles. Also, Mg incorporation resulted in the generation of Lewis basic sites and in the modification of eg-C3N4 support basicity, therefore increasing the number of effective CO2 adsorption sites.
A novel nickel–yttrium–cerium/diatomite (Ni–YCe/Dia) composite was investigated in CO2 methanation exhibiting 89% CO2 conversion and 99% CH4 selectivity in a 150-h stability test, thereby providing a new perspective on the rational regulation between metal oxides and natural minerals. The diatomite template enhanced the dispersion of the Ni–YCe oxide nanoparticles, thus providing extra hydroxyl groups for the adsorption of CO2, while the citric acid improved Ni species dispersion and favored H2 dissociation. Also, the introduction of yttrium enhanced Ni nanoparticles dispersion and inhibited carbon deposition [290].
Recently, Polychronopoulou and co-workers [291] investigated the impact of various parameters (pore volume, ceria loading, preparation method) on the interfacial phenomena and the CO2 methanation performance of Ni/CeO2/Al2O3 catalysts. The Ni-20Ce/mpAl catalyst (alumina of medium-porosity co-impregnated with nickel and ceria) exhibited excellent performance of 70% CO2 conversion and > 98% CH4 selectivity at 350 °C, mainly ascribed to the presence of oxygen vacancies, the abundance in surface Ce3+ species, its high carbonyl activation capacity and H-spillover capability accompanied by strong metal-support interactions. The optimum catalyst followed a CO-mediated reaction mechanism, as verified by the rapid depletion of adsorbed carbonyls, along with the formation of thermally stable bicarbonates [291]. A nickel-based catalyst supported on CeO2-Al2O3 with a molar ratio of Al/Ce = 0.1 exhibited high CO2 conversion (88.8%) and methane selectivity (99.9%) at 240 °C, and excellent stability for low-temperature CO2 methanation. The addition of Al into the nickel-ceria catalyst preserves a moderate metal-support interaction, thus promoting the formation of active Ni species, while improving Ni dispersion and H2 dissociation [292].
3.3.2.2 Co-based catalysts
It is generally known that Co is the second most active metal phase among the Group VIII metals for CO2 methanation after nickel [293, 294], although only a few Co-based catalysts exhibit both high CH4 yield values and adequate stability [295, 296]. Also, cobalt-based catalysts generally require elevated reaction pressures for high catalytic activity, compared to nickel. As in the case of Ni-based catalysts, various parameters influence the catalytic activity of cobalt-containing catalysts, most notably the morphology and surface orientation [297], catalyst support [298,299,300] and metal particle size [295, 301]. Also, it has been shown that cobalt-based catalysts may require to be subjected to a specific preparation procedure. Among the promising synthesis techniques are solid-state reactions, colloidal chemistry and NH3-temperature programmed reactions, which have shown to improve catalytic activity compared to conventional methods such as wet impregnation [144, 294].
Regarding the CO2 methanation performance of bare Co3O4 oxides, Co3O4(110) nanorods exhibited considerably higher activity and methane selectivity than Co3O4 nanoparticles exposing (111) and (001) facets, revealing the key role of the crystallographic planes exposed to the reaction environment [297]. In this regard, a series of Co nanorods (CoNR) with preferential exposure of (110) surface facets supported on Al2O3, SiO2 and TiO2 were explored for CO2 methanation [300]. It was found that at low temperatures (< 300 °C), the CoNR/TiO2 sample exhibited higher catalytic activity, attributed to the strong interaction between Co3O4 and TiO2, while above 300 °C CoNR/Al2O3 showed enhanced activity, largely due to the increased thermal conductivity of alumina.
Srisawad et al. [302] investigated the effect of cobalt precursor over Co/Al2O3 catalysts. Compared to the catalysts prepared by conventional impregnation using cobalt nitrate, solid-state catalysts exhibited higher methanation activity with comparable product distribution. The solid-state reaction resulted in CoxOy dispersion mostly on the external surface of alumina, ergo the adsorption and subsequent dissociation of gaseous CO2 to CO* and O* ad-species were not diffusion-controlled.
Elsewhere, cobalt was added onto highly-ordered cubic mesoporous silica KIT-6, which exhibited superior catalytic activity and selectivity compared with Co/meso-SiO2 [298]. This was attributed to the higher Co dispersion over the large surface area of KIT-6. Additionally, the ordered mesoporous structure of KIT-6 enables high methane selectivity by facilitating the diffusion and desorption of surface-bound CH4. A screening of Co catalysts supported on various oxides (i.e., TiO2, ZrO2, SiO2, SiC, Al2O3 and activated carbon) was recently conducted [296]. It was disclosed that Co supported on zirconia exhibited the highest CH4 yield and stability after 300 h on stream. The formation of a Co-Zr phase at the Co-ZrO2 interface discerned by the use of TEM analysis was deemed to play a key role in CO2 adsorption. In the follow-up study, an attempt to maximize this active interface was made by decreasing Co particle size [295]. Indeed, the suitable Co-ZrO2 interaction was able to induce more reduced active sites and oxygen vacancies, thus providing a higher ability for CO2 adsorption. Similarly, Díez-Ramírez et al. [299] revealed the significant role of support during the CO2 methanation under hydrogen excess conditions. In specific, methane yield at 300 °C followed the order: Co/CeO2 > Co/ZnO > Co/Gd2O3 ~ Co/ZrO2. The authors postulated that the superior performance of Co/CeO2 may be attributed to its enhanced reducibility. Moreover, Co/CeO2 demonstrated stable conversion/selectivity performance under subsequent reaction cycles, in contrast to Co/ZnO, directly related to modifications induced by the pretreatment in reaction conditions in the elemental chemical states and surface composition of Co/ZnO. Besides the significant role of support, aliovalent doping can be employed to adjust the local surface chemistry and in turn CO2 methanation reactivity [28]. For instance, bimetallic Co-Ti catalyst supported on mesoporous silica MCM-41 exhibited high activity and stability performance for CO2 methanation [303]. It was inferred that the addition of titanium species induced significant modifications in the properties of Co catalysts that are favorable for CO2 methanation, by facilitating the reduction of CoxOy species interacting strongly with the support and preventing the formation of inactive silicate moieties. Recently, a remarkably high methanation activity was reported over Co4N/γ-Al2O3 catalysts [304]. The results revealed that cobalt nitride was a superior active phase for CO2 conversion compared to metallic cobalt; the formation of Co4N led to increased basicity and strong metal-support interactions by varying the metal dispersion and particle size. Moreover, the remarkable resistance to coking and metal sintering of Co4N/γ-Al2O3 was confirmed by a 250-h stability test.
On the basis of aforementioned catalytic studies of CO2 hydrogenation to methane, a comparison of the most active and selective catalysts ‒ in terms of CO2 conversion and CH4 selectivity ‒ is presented in Table 2. As in the case of rWGS reaction, a straightforward comparison is not possible due to the variations in testing conditions. In this regard, only the results acquired at certain reaction conditions, i.e., stoichiometric reactant ratio of H2:CO2 = 4 and atmospheric pressure, are included in Table 2.
On the basis of the state-of-the-art catalysts included in Table 2, the following conclusions can be drawn:
-
(i)
Ru-based catalysts are among the most active and selective noble metals for the CO2 methanation, offering > 85% yield to methane at temperatures as low as 300 °C.
-
(ii)
Bi-metallic compositions combined with reducible supports (e.g., Ru-Ni/CeO2) can further enhance the low temperature methanation performance, resulting in a methane yield of > 90%.
-
(iii)
From non-noble metals, Ni-based catalysts are the most promising, offering comparable or even superior methanation performance to noble metals.
-
(iv)
Through the use of advanced synthesis and promotional routes, extremely active multifunctional Ni-based composites can be obtained, exhibiting a yield to methane > 90% at temperatures lower than 300 °C.
-
(v)
Ceria is the commonly used support due to its excellent redox properties, although other supporting materials such as TiO2, ZrO2, MgO and Al2O3 could offer adequate methanation performance.
-
(vi)
In total, the CO2 methanation performance can be exceptionally enhanced by the appropriate regulation of the surface properties, metal dispersion and coordination environment through the modification of the catalyst structure, electronic properties and the surface acidity/basicity.
In light to the above aspects, Figure 25 provides at a glance a comparison among the most active catalysts of Table 2, on the basis of methane formation rate (rCH4, μmol g−1 s−1) at 250 °C. It is evident that Ru-based catalysts are among the most active noble metal-based catalysts. However, appropriate fine-tuning on non-noble metal-based catalysts can lead to the development of highly active and selective catalytic materials with similar or even superior CO2 methanation performance as compared to noble metals.
Relative comparison of the most active catalysts for CO2 methanation. Reaction conditions and the corresponding references are provided in Table 2
4 Conclusions and outlooks
Essentially, the current challenge for the heterogeneous catalytic CO2 hydrogenation reaction as an important step in the synthesis of added-value products lies in the design of materials that can achieve both high selectivity towards the case-specific product and sufficient reactivity and thermal stability at the medium-to-low temperature range, along with versatility under variable operating conditions, especially hydrogen concentration, intrinsically linked to the intermittency of renewable energy generation when employing electrolytic hydrogen. As established in this review, even though a plethora of materials have already been studied and have attained promising results in terms of catalytic performance, there is still room for improvement, especially considering that many reports are not directly comparable due to differences in the experimental procedure. Furthermore, if the reaction of CO2 hydrogenation is to be implemented at a large scale towards mitigating CO2 captured emissions, a catalyst based on earth-abundant materials is preferable from an environmental and cost standpoint.
In specific, for the production of either CO via the reverse water–gas shift reaction or CH4 via the Sabatier reaction, significant progress has been made recently towards the understanding of the reaction mechanism, with the relationships between active sites nature and the reaction intermediates so far understood via insights gained from molecular dynamic simulations and operando mechanistic works, which is beneficial to the development of state-of-the-art catalysts. In any of the proposed mechanisms, the intermediate species are further hydrogenated and/or decomposed to form the final products. Also, it is known that these intermediate species are preferentially formed on the metal-support interface and can participate in the reaction, since the adsorbed hydrogen atom on the metal nanoparticle can then react with the intermediate entities and produce easily-desorbed CO or CH4 and H2O. Vacancies on the catalyst surface are also generally known to facilitate CO2 adsorption and activation. Therefore, a material with a relatively high concentration of oxygen vacancies, medium reactant adsorption strength and weak metal-intermediate bond strength can prove to be an effective catalyst, in accordance with the Sabatier principle [17]. In this regard, the most commonly used reducible supports are CeO2 and ZnO, largely due to their high oxygen vacancy concentration and high oxygen mobility. These supporting oxides can be very promising as an alternative to Al2O3, a material widely employed so far in commercial catalysts due to its sufficient performance and cost-effectiveness given its abundance.
Based on the available literature, it can be reasonably postulated that the abundance of surface hydroxyl groups acts as a descriptor for the differences in the underlying reaction mechanism reported on different metal oxide supports and can determine the final product. That is, carbon dioxide can be adsorbed onto these sites and form oxy-hydrogenated intermediate species, whereas in the absence of these hydroxyl groups, the redox mechanism takes place for the production of CO. Besides, the products' distribution and in essence CO/CH4 selectivity is strongly influenced by the presence of a second metallic phase. More often than not, this is a direct effect of the change in the local surface chemistry and electronic properties of the primary metal, induced by the promoting metal. Thus, the charge transfer leads to easier CO2 adsorption via the creation of partial positive/negative charges or changes in the strength of the metal-bound carbonyl, given that metal atoms and ions exhibit different values for CO adsorption energy. Also, the promoter leads to the emergence of active site in the form of metal–metal oxide moieties.
In light of the above, several efforts have been made in order to address the existing problems and fine-tune appropriate CO2 hydrogenation catalysts, either by modulating the metal-support interfacial interactions, changing the active metal particle size, adding a second metal in multi-metallic composites, especially by the addition of an alkali promoter for supported catalysts or by doping with additional heteroatoms and, lastly, by tuning the exposed crystal planes for oxide catalysts that have shown facet-dependent catalytic behavior. Additionally, transition metal carbides are increasingly becoming an attractive alternative supporting material category for industrial purposes, due to the combination of high H2 dissociation and C-O bond cleavage affinity along with their potentially similar behavior to reducible oxides.
As for the employed active phase, copper and platinum are so far the most advanced metals studied for the rWGS reaction, although their activity can be enhanced by their employment in bimetallic formulations, such as the co-presence of an alkali metal or a second transition metal. Moreover, catalytic composites that are associated with metallic entities in higher oxidation states tend to largely favor the facile desorption of carbonyl species and generate gas-phase CO. For the methanation reaction, catalysts based on Ni and Ru and supporting metal oxides are given preference due to the remarkably high CO2 conversion and CH4 selectivity of the obtained composites under a variety of conditions. However, Ni-based catalysts greatly suffer from heat resistance (i.e., thermal instability) and sulphur tolerance, which is due to the interaction of nickel metal particles with CO and the formation of mobile nickel sub-carbonyls. In this regard, the fine-tuning of Ni local and interfacial environment by aliovalent doping in conjunction to structural/defect engineering strategies can provide an effective tool in the design of novel and stable catalytic materials with the desired CO2 hydrogenation performance.
Lastly, it must be mentioned that as of yet, the thermocatalytic CO2 hydrogenation process has not reached a commercial stage, since most developed catalytic systems do not demonstrate remarkable activity and stability when scaled up. However, for the preferable solution of non-noble metal-based catalysts, there is still further development to be made in order to achieve high catalytic activity and sufficient stability at lower reaction temperatures (ideally < 300 °C and < 500 °C for the Sabatier and the rWGS reaction, respectively) without significant poisoning and/or deactivation phenomena, a progress which will certainly boost the industrial applicability of the process. Additionally, in the scope of further scaling up the production of value-added products via CO2 hydrogenation, structured catalysts with adequate thermal properties need to be employed for large-scale processes, thus further fine-tuning of catalysts needs to be made in order to be implemented at an even more intensified production scheme.
Data availability
No datasets were generated or analysed during the current study.
References
Intergouvernemental Panel on Climate Change. Climate Change 2021: The Physical Science Basis: Summary for Policymakers: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change.
Yan J, Zhang Z. Carbon capture, utilization and storage (CCUS). Appl Energy. 2019;235:1289–99. https://doi.org/10.1016/j.apenergy.2018.11.019.
Thonemann N, Pizzol M. Consequential life cycle assessment of carbon capture and utilization technologies within the chemical industry. Energy Environ Sci. 2019;12:2253–63. https://doi.org/10.1039/c9ee00914k.
Lopes JVM, Bresciani AE, Carvalho KM, Kulay LA, Alves RMB. Multi-criteria decision approach to select carbon dioxide and hydrogen sources as potential raw materials for the production of chemicals. Renew Sust Energ Rev. 2021;151: 111542. https://doi.org/10.1016/j.rser.2021.111542.
Chauvy R, Meunier N, Thomas D, De Weireld G. Selecting emerging CO2 utilization products for short- to mid-term deployment. Appl Energy. 2019;236:662–80. https://doi.org/10.1016/j.apenergy.2018.11.096.
González-Castaño M, Dorneanu B, Arellano-García H. The reverse water gas shift reaction: a process systems engineering perspective. React Chem Eng. 2021;6:954–76. https://doi.org/10.1039/d0re00478b.
Gao X, Cai P, Wang Z, Lv X, Kawi S. Surface acidity/basicity and oxygen defects of metal oxide: impacts on catalytic performances of CO2 reforming and hydrogenation reactions. Top Catal. 2023;66:299–325. https://doi.org/10.1007/s11244-022-01708-0.
Albeladi N, Alsulami QA, Narasimharao K. Recent progress in nickel and silica containing catalysts for CO2 hydrogenation to CH4. Catalysts. 2023;13:1104. https://doi.org/10.3390/catal13071104.
Xie Y, Wen J, Li Z, Chen J, Zhang Q, Ning P, Hao J. Double-edged sword effect of classical strong metal-support interaction in catalysts for CO2 hydrogenation to CO, methane, and methanol. ACS Mater Lett. 2023;5:2629–47. https://doi.org/10.1021/acsmaterialslett.3c00640.
Panzone C, Philippe R, Chappaz A, Fongarland P, Bengaouer A. Power-to-liquid catalytic CO2 valorization into fuels and chemicals: focus on the Fischer-Tropsch route. J CO2 Util. 2020;38:314–47. https://doi.org/10.1016/j.jcou.2020.02.009.
Wang H, Liu Y, Laaksonen A, Krook-Riekkola A, Yang Z, Lu X, Ji X. Carbon recycling—an immense resource and key to a smart climate engineering: a survey of technologies, cost and impurity impact. Renew Sust Energ Rev. 2020;131: 110010. https://doi.org/10.1016/j.rser.2020.110010.
Ashok J, Falbo L, Das S, Dewangan N, Visconti CG, Kawi S. Catalytic CO2 conversion to added-value energy rich C1 products. An economy based on carbon dioxide and water, Springer International Publishing; 2019, p. 155–210. https://doi.org/10.1007/978-3-030-15868-2_5.
Mathew T, Saju S, Raveendran SN. Survey of Heterogeneous Catalysts for the CO2 Reduction to CO via Reverse Water Gas Shift. Engineering Solutions for CO2 Conversion, Wiley-VCH GmBH; 2021, p. 281–316. https://doi.org/10.1002/9783527346523.ch12.
Zhu M, Ge Q, Zhu X. Catalytic reduction of CO2 to CO via reverse water gas shift reaction: recent advances in the design of active and selective supported metal catalysts. Trans Tianjin Univ. 2020;26:172–87. https://doi.org/10.1007/s12209-020-00246-8.
Chen X, Chen Y, Song C, Ji P, Wang N, Wang W, Cui L. Recent advances in supported metal catalysts and oxide catalysts for the reverse water-gas shift reaction. Front Chem. 2020;8:709. https://doi.org/10.3389/fchem.2020.00709.
Feng K, Wang Y, Guo M, Zhang J, Li Z, Deng T, Zhang Z, Yan B. In-situ/operando techniques to identify active sites for thermochemical conversion of CO2 over heterogeneous catalysts. J Energy Chem. 2021;62:153–71. https://doi.org/10.1016/j.jechem.2021.03.054.
Bahmanpour AM, Signorile M, Kröcher O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction. Appl Catal B: Environ. 2021;295: 120319. https://doi.org/10.1016/j.apcatb.2021.120319.
Podrojková N, Sans V, Oriňak A, Oriňaková R. Recent developments in the modelling of heterogeneous catalysts for CO2 conversion to chemicals. ChemCatChem. 2020;12:1802–25. https://doi.org/10.1002/cctc.201901879.
Jangam A, Das S, Dewangan N, Hongmanorom P, Hui WM, Kawi S. Conversion of CO2 to C1 chemicals: catalyst design, kinetics and mechanism aspects of the reactions. Catal Today. 2020;358:3–29. https://doi.org/10.1016/j.cattod.2019.08.049.
Saeidi S, Najari S, Fazlollahi F, Nikoo MK, Sefidkon F, Klemeš JJ, Baxter LL. Mechanisms and kinetics of CO2 hydrogenation to value-added products: a detailed review on current status and future trends. Renew Sust Energ Rev. 2017;80:1292–311. https://doi.org/10.1016/j.rser.2017.05.204.
Atsbha TA, Yoon T, Seongho P, Lee C-J. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J CO2 Util. 2021;44:101413. https://doi.org/10.1016/j.jcou.2020.101413.
Cheng D, Negreiros FR, Aprà E, Fortunelli A. Computational approaches to the chemical conversion of carbon dioxide. Chemsuschem. 2013;6:944–65. https://doi.org/10.1002/cssc.201200872.
Dietz L, Piccinin S, Maestri M. Mechanistic insights into CO2 activation via reverse water—gas shift on metal surfaces. J Phys Chem C. 2015;119:4959–66. https://doi.org/10.1021/jp512962c.
Nolen MA, Tacey SA, Kwon S, Farberow CA. Theoretical assessments of CO2 activation and hydrogenation pathways on transition-metal surfaces. Appl Surf Sci. 2023;637: 157873. https://doi.org/10.1016/j.apsusc.2023.157873.
Liu X, Sun L, Deng W-Q. Theoretical investigation of CO2 adsorption and dissociation on low index surfaces of transition metals. J Phys Chem C. 2018;122:8306–14. https://doi.org/10.1021/acs.jpcc.7b12660.
Vogt C, Groeneveld E, Kamsma G, Nachtegaal M, Lu L, Kiely CJ, Berben PH, Meirer F, Weckhuysen BM. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat Catal. 2018;1:127–34. https://doi.org/10.1038/s41929-017-0016-y.
Konsolakis M, Lykaki M. Facet-dependent reactivity of ceria nanoparticles exemplified by CeO2-based transition metal catalysts: a critical review. Catalysts. 2021;11:452. https://doi.org/10.3390/catal11040452.
Varvoutis G, Lykaki M, Marnellos GE, Konsolakis M. Recent advances on fine-tuning engineering strategies of CeO2-based nanostructured catalysts exemplified by CO2 hydrogenation processes. Catalysts. 2023;13:275. https://doi.org/10.3390/catal13020275.
Konsolakis M, Lykaki M. Recent advances on the rational design of non-precious metal oxide catalysts exemplified by CuOx/CeO2 binary system: implications of size, shape and electronic effects on intrinsic reactivity and metal-support interactions. Catalysts. 2020;10:160. https://doi.org/10.3390/catal10020160.
Ernst K-H, Campbell CT, Moretti G. Kinetics of the reverse water-gas shift reaction over Cu(110). J Catal. 1992;134:66–74. https://doi.org/10.1016/0021-9517(92)90210-9.
Nakamura J, Rodriguez JA, Campbell CT. Does CO2 dissociatively adsorb on Cu surfaces? J Phys: Condens Mater. 1989;1:SB149–60. https://doi.org/10.1088/0953-8984/1/SB/026.
Ginés MJL, Marchi AJ, Apesteguía CR. Kinetic study of the reverse water-gas shift reaction over CuO/ZnO/Al2O3. Appl Catal A: Gen. 1997;154:155–71. https://doi.org/10.1016/S0926-860X(96)00369-9.
Chen CS, Wu JH, Lai TW. Carbon dioxide hydrogenation on Cu nanoparticles. J Phys Chem C. 2010;114:15021–8. https://doi.org/10.1021/jp104890c.
Osaki T, Narita N, Horiuchi T, Sugiyama T, Masuda H, Suzuki K. Kinetics of reverse water gas shift (RWGS) reaction on metal disulfide catalysts. J Mol Catal A Chem. 1997;125:63–71. https://doi.org/10.1016/S1381-1169(97)00080-0.
Fornero EL, Chiavassa DL, Bonivardi AL, Baltanás MA. Transient analysis of the reverse water gas shift reaction on Cu/ZrO2 and Ga2O3/Cu/ZrO2 catalysts. J CO2 Util. 2017;22:289–98. https://doi.org/10.1016/j.jcou.2017.06.002.
Kim SS, Lee HH, Hong SC. A study on the effect of support’s reducibility on the reverse water-gas shift reaction over Pt catalysts. Appl Catal A: Gen. 2012;423–424:100–7. https://doi.org/10.1016/j.apcata.2012.02.021.
Choi S, Sang B-I, Hong J, Yoon KJ, Son J-W, Lee J-H, Kim B-K, Kim H. Catalytic behavior of metal catalysts in high-temperature RWGS reaction: In-situ FT-IR experiments and first-principles calculations. Sci Rep. 2017;7:41207. https://doi.org/10.1038/srep41207.
Bao J, Yang G, Yoneyama Y, Tsubaki N. Significant advances in C1 catalysis: highly efficient catalysts and catalytic reactions. ACS Catal. 2019;9:3026–53. https://doi.org/10.1021/acscatal.8b03924.
Su X, Yang X, Zhao B, Huang Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: recent advances and the future directions. J Energy Chem. 2017;26:854–67. https://doi.org/10.1016/j.jechem.2017.07.006.
Wang Y, Winter LR, Chen JG, Yan B. CO2 hydrogenation over heterogeneous catalysts at atmospheric pressure: from electronic properties to product selectivity. Green Chem. 2021;23:249–67. https://doi.org/10.1039/d0gc03506h.
Yaccato K, Carhart R, Hagemeyer A, Lesik A, Strasser P, Volpe AF Jr, Turner H, Weinberg H, Grasselli RK, Brooks C. Competitive CO and CO2 methanation over supported noble metal catalysts in high throughput scanning mass spectrometer. Appl Catal A: Gen. 2005;296:30–48. https://doi.org/10.1016/j.apcata.2005.07.052.
Kattel S, Liu P, Chen JG. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J Am Chem Soc. 2017;139:9739–54. https://doi.org/10.1021/jacs.7b05362.
Porosoff MD, Chen JG. Trends in the catalytic reduction of CO2 by hydrogen over supported monometallic and bimetallic catalysts. J Catal. 2013;301:30–7. https://doi.org/10.1016/j.jcat.2013.01.022.
Kattel S, Yu W, Yang X, Yan B, Huang Y, Wan W, Liu P, Chen JG. CO2 hydrogenation over oxide-supported PtCo catalysts: the role of the oxide support in determining the product selectivity. Angew Chem Int Ed. 2016;55:7968–73. https://doi.org/10.1002/ange.201601661.
Kattel S, Yan B, Chen JG, Liu P. CO2 hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: importance of synergy between Pt and oxide support. J Catal. 2016;343:115–26. https://doi.org/10.1016/j.jcat.2015.12.019.
Kim SS, Park KH, Hong SC. A study of the selectivity of the reverse water-gas-shift reaction over Pt/TiO2 catalysts. Fuel Process Technol. 2013;108:47–54. https://doi.org/10.1016/j.fuproc.2012.04.003.
Lee SM, Eom H, Kim SS. A study on the effect of CeO2 addition to a Pt/TiO2 catalyst on the reverse water gas shift reaction. Environ Technol. 2021;42:182–92. https://doi.org/10.1080/09593330.2019.1625954.
Liang B, Duan H, Su X, Chen X, Huang Y, Chen X, Delgado JJ, Zhang T. Promoting role of potassium in the reverse water gas shift reaction on Pt/mullite catalyst. Catal Today. 2017;281:319–26. https://doi.org/10.1016/j.cattod.2016.02.051.
Yang X, Su X, Chen X, Duan H, Liang B, Liu Q, Liu X, Ren Y, Huang Y, Zhang T. Promotion effects of potassium on the activity and selectivity of Pt/zeolite catalysts for reverse water gas shift reaction. Appl Catal B: Environ. 2017;216:95–105. https://doi.org/10.1016/j.apcatb.2017.05.067.
Seuser G, Martinelli M, Garcia ES, Upton GF, Ayala M, Villarreal J, Rajabi Z, Cronauer DC, Kropf AJ, Jacobs G. Reverse water-gas shift: Na doping of m-ZrO2 supported Pt for selectivity control. Appl Catal A: Gen. 2023;650: 119000. https://doi.org/10.1016/j.apcata.2022.119000.
Yu W, Porosoff MD, Chen JG. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem Rev. 2012;112:5780–817. https://doi.org/10.1021/cr300096b.
Wang H, Bootharaju MS, Kim JH, Wang Y, Wang K, Zhao M, Zhang R, Xu J, Hyeon T, Wang X, Song S, Zhang H. Synergistic interactions of neighboring platinum and iron atoms enhance reverse water-gas shift reaction performance. J Am Chem Soc. 2023;145:2264–70. https://doi.org/10.1021/jacs.2c10435.
Büchel R, Baiker A, Pratsinis SE. Effect of Ba and K addition and controlled spatial deposition of Rh in Rh/Al2O3 catalysts for CO2 hydrogenation. Appl Catal A: Gen. 2014;477:93–101. https://doi.org/10.1016/j.apcata.2014.03.010.
Chukwu E, Molina L, Rapp C, Morales L, Jin Z, Karakalos S, Wang H, Lee S, Zachman MJ, Yang M. Crowded supported metal atoms on catalytically active supports may compromise intrinsic activity: a case study of dual-site Pt/α-MoC catalysts. Appl Catal B: Environ. 2023;329: 122532. https://doi.org/10.1016/j.apcatb.2023.122532.
Karelovic A, Ruiz P. Improving the hydrogenation function of Pd/γ-Al2O3 catalyst by Rh/γ-Al2O3 addition in CO2 methanation at low temperature. ACS Catal. 2013;3:2799–812. https://doi.org/10.1021/cs400576w.
Erdöhelyi A, Pásztor M, Solymosi F. Catalytic hydrogenation of CO2 over supported palladium. J Catal. 1986;98:166–77. https://doi.org/10.1016/0021-9517(86)90306-4.
Qian C, Sun W, Hung DLH, Qiu C, Makaremi M, Hari Kumar SG, Wan L, Ghoussoub M, Wood TE, Xia M, Tountas AA, Li YF, Wang L, Dong Y, Gourevich I, Singh CV, Ozin GA. Catalytic CO2 reduction by palladium-decorated silicon–hydride nanosheets. Nat Catal. 2019;2:46–54. https://doi.org/10.1038/s41929-018-0199-x.
Wang X, Shi H, Kwak JH, Szanyi J. Mechanism of CO2 hydrogenation on Pd/Al2O3 catalysts: kinetics and transient DRIFTS-MS studies. ACS Catal. 2015;5:6337–49. https://doi.org/10.1021/acscatal.5b01464.
Wang X, Shi H, Szanyi J. Controlling selectivities in CO2 reduction through mechanistic understanding. Nat Commun. 2017;8:513. https://doi.org/10.1038/s41467-017-00558-9.
Kwak JH, Kovarik L, Szanyi J. Heterogeneous catalysis on atomically dispersed supported metals: CO2 reduction on multifunctional Pd catalysts. ACS Catal. 2013;3:2094–100. https://doi.org/10.1021/cs4001392.
Aguirre A, Collins SE. Selective detection of reaction intermediates using concentration- modulation excitation DRIFT spectroscopy. Catal Today. 2013;205:34–40. https://doi.org/10.1016/j.cattod.2012.08.020.
Lebarbier V, Dagle R, Datye A, Wang Y. The effect of PdZn particle size on reverse-water-gas-shift reaction. Appl Catal A: Gen. 2010;379:3–6. https://doi.org/10.1016/j.apcata.2010.02.008.
Xu H, Li Y, Luo X, Xu Z, Ge J. Monodispersed gold nanoparticles supported on a zirconium-based porous metal-organic framework and their high catalytic ability for the reverse water-gas shift reaction. Chem Commun. 2017;53:7953–6. https://doi.org/10.1039/c7cc02130e.
Rezvani A, Abdel-Mageed AM, Ishida T, Murayama T, Parlinska-Wojtan M, Behm RJ. CO2 reduction to methanol on Au/CeO2 catalysts: mechanistic insights from activation/deactivation and SSITKA measurements. ACS Catal. 2020;10:3580–94. https://doi.org/10.1021/acscatal.9b04655.
Bobadilla LF, Santos JL, Ivanova S, Odriozola JA, Urakawa A. Unravelling the role of oxygen vacancies in the mechanism of the reverse water-gas shift reaction by operando DRIFTS and ultraviolet-visible spectroscopy. ACS Catal. 2018;8:7455–67. https://doi.org/10.1021/acscatal.8b02121.
Carrasquillo-Flores R, Ro I, Kumbhalkar MD, Burt S, Carrero CA, Alba-Rubio AC, Miller JT, Hermans I, Huber GW, Dumesic JA. Reverse water-gas shift on interfacial sites formed by deposition of oxidized molybdenum moieties onto gold nanoparticles. J Am Chem Soc. 2015;137:10317–25. https://doi.org/10.1021/jacs.5b05945.
Kyriakou V, Vourros A, Garagounis I, Carabineiro SAC, Maldonado-Hódar FJ, Marnellos GE, Konsolakis M. Highly active and stable TiO2-supported Au nanoparticles for CO2 reduction. Catal Commun. 2017;98:52–6. https://doi.org/10.1016/j.catcom.2017.05.003.
Pacchioni G. Electronic interactions and charge transfers of metal atoms and clusters on oxide surfaces. Phys Chem Chem Phys. 2013;15:1737–57. https://doi.org/10.1039/c2cp43731g.
Kwak JH, Kovarik L, Szanyi J. CO2 reduction on supported Ru/Al2O3 catalysts: cluster size dependence of product selectivity. ACS Catal. 2013;3:2449–55. https://doi.org/10.1021/cs400381f.
Aitbekova A, Wu L, Wrasman CJ, Boubnov A, Hoffman AS, Goodman ED, Bare SR, Cargnello M. Low-temperature restructuring of CeO2-supported Ru nanoparticles determines selectivity in CO2 catalytic reduction. J Am Chem Soc. 2018;140:13736–45. https://doi.org/10.1021/jacs.8b07615.
Zhuang Y, Currie R, McAuley KB, Simakov DSA. Highly-selective CO2 conversion via reverse water gas shift reaction over the 0.5wt% Ru-promoted Cu/ZnO/Al2O3 catalyst. Appl Catal A: Gen. 2019;575:74–86. https://doi.org/10.1016/j.apcata.2019.02.016.
Panaritis C, Edake M, Couillard M, Einakchi R, Baranova EA. Insight towards the role of ceria-based supports for reverse water gas shift reaction over RuFe nanoparticles. J CO2 Util. 2018;26:350–8. https://doi.org/10.1016/j.jcou.2018.05.024.
Matsubu JC, Yang VN, Christopher P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J Am Chem Soc. 2015;137:3076–84. https://doi.org/10.1021/ja5128133.
Bando KK, Soga K, Kunimori K, Arakawa H. Effect of Li additive on CO2 hydrogenation reactivity of zeolite supported Rh catalysts. Appl Catal A: Gen. 1998;175:67–81. https://doi.org/10.1016/S0926-860X(98)00202-6.
Erdőhelyi A. Hydrogenation of carbon dioxide on supported Rh catalysts. Catalysts. 2020;10:155. https://doi.org/10.3390/catal10020155.
Wang C, Guan E, Wang L, Chu X, Wu Z, Zhang J, Yang Z, Jiang Y, Zhang L, Meng X, Gates BC, Xiao F-S. Product Selectivity controlled by nanoporous environments in zeolite crystals enveloping rhodium nanoparticle catalysts for CO2 hydrogenation. J Am Chem Soc. 2019;141:8482–8. https://doi.org/10.1021/jacs.9b01555.
Li S, Xu Y, Chen Y, Li W, Lin L, Li M, Deng Y, Wang X, Ge B, Yang C, Yao S, Xie J, Li Y, Liu X, Ma D. Tuning the selectivity of catalytic carbon dioxide hydrogenation over iridium/cerium oxide catalysts with a strong metal-support interaction. Angew Chem Int Ed. 2017;56:10761–5. https://doi.org/10.1002/ange.201705002.
Ye J, Ge Q, Liu C. Effect of PdIn bimetallic particle formation on CO2 reduction over the Pd-In/SiO2 catalyst. Chem Eng Sci. 2015;135:193–201. https://doi.org/10.1016/j.ces.2015.04.034.
Wang Y, Arandiyan H, Scott J, Aguey-Zinsou K-F, Amal R. Single atom and nanoclustered Pt catalysts for selective CO2 reduction. ACS Appl Energy Mater. 2018;1:6781–9. https://doi.org/10.1021/acsaem.8b00817.
Matsubu JC, Zhang S, DeRita L, Marinkovic NS, Chen JG, Graham GW, Pan X, Christopher P. Adsorbate-mediated strong metal-support interactions in oxide-supported Rh catalysts. Nat Chem. 2017;9:120–7. https://doi.org/10.1038/NCHEM.2607.
Xing Y, Ouyang M, Zhang L, Yang M, Wu X, Ran R, Weng D, Kang F, Si Z. Single atomic Pt on SrTiO3 catalyst in reverse water gas shift reactions. Catalysts. 2021;11:738. https://doi.org/10.3390/catal11060738.
Ebrahimi P, Kumar A, Khraisheh M. A review of CeO2 supported catalysts for CO2 reduction to CO through the reverse water gas shift reaction. Catalysts. 2022;12:1101. https://doi.org/10.3390/catal12101101.
Wambach J, Baiker A, Wokaun A. CO2 hydrogenation over metal/zirconia catalysts. Phys Chem Chem Phys. 1999;1:5071–80. https://doi.org/10.1039/A904923A.
Dai B, Zhou G, Ge S, Xie H, Jiao Z, Zhang G, Xiong K. CO2 reverse water-gas shift reaction on mesoporous M-CeO2 catalysts. Can J Chem Eng. 2017;95:634–42. https://doi.org/10.1002/cjce.22730.
Konsolakis M. The role of Copper-Ceria interactions in catalysis science: recent theoretical and experimental advances. Appl Catal B: Environ. 2016;198:49–66. https://doi.org/10.1016/j.apcatb.2016.05.037.
Nakamura I, Fujitani T, Uchijima T, Nakamura J. The synthesis of methanol and the reverse water-gas shift reaction over Zn-deposited Cu(100) and Cu(110) surfaces: comparison with Zn/Cu(111). Surf Sci. 1998;400:387–400. https://doi.org/10.1016/S0039-6028(97)00899-6.
Shen Y, Xiao Z, Liu J, Wang Z. Facile preparation of inverse nanoporous Cr2O3/Cu catalysts for reverse water-gas shift reaction. ChemCatChem. 2019;11:5439–43. https://doi.org/10.1002/cctc.201901259.
Bahmanpour AM, Héroguel F, Kılıç M, Baranowski CJ, Artiglia L, Röthlisberger U, Luterbacher JS, Kröcher O. Cu-Al spinel as a highly active and stable catalyst for the reverse water gas shift reaction. ACS Catal. 2019;9:6243–51. https://doi.org/10.1021/acscatal.9b01822.
Zhang Y, Liang L, Chen Z, Wen J, Zhong W, Zou S, Fu M, Chen L, Ye D. Highly efficient Cu/CeO2-hollow nanospheres catalyst for the reverse water-gas shift reaction: investigation on the role of oxygen vacancies through in situ UV-Raman and DRIFTS. Appl Surf Sci. 2020;516: 146035. https://doi.org/10.1016/j.apsusc.2020.146035.
Zhou G, Xie F, Deng L, Zhang G, Xie H. Supported mesoporous Cu/CeO2-δ catalyst for CO2 reverse water–gas shift reaction to syngas. Int J Hydrogen Energy. 2020;45:11380–93. https://doi.org/10.1016/j.ijhydene.2020.02.058.
Ebrahimi P, Kumar A, Khraisheh M. Combustion synthesis of copper ceria solid solution for CO2 conversion to CO via reverse water gas shift reaction. Int J Hydrogen Energy. 2022;47:41259–67. https://doi.org/10.1016/j.ijhydene.2021.12.142.
Yang S-C, Pang SH, Sulmonetti TP, Su W-N, Lee J-F, Hwang B-J, Jones CW. Synergy between ceria oxygen vacancies and Cu nanoparticles facilitates the catalytic conversion of CO2 to CO under mild conditions. ACS Catal. 2018;8:12056–66. https://doi.org/10.1021/acscatal.8b04219.
Chen C-S, Cheng W-H, Lin S-S. Study of iron-promoted Cu/SiO2 catalyst on high temperature reverse water gas shift reaction. Appl Catal A: Gen. 2004;257:97–106. https://doi.org/10.1016/S0926-860X(03)00637-9.
Lin L, Yao S, Rui N, Han L, Zhang F, Gerlak CA, Liu Z, Cen J, Song L, Senanayake SD, Xin HL, Chen JG, Rodriguez JA. Conversion of CO2 on a highly active and stable Cu/FeOX/CeO2 catalyst: tuning catalytic performance by oxide-oxide interactions. Catal Sci Technol. 2019;9:3735–42. https://doi.org/10.1039/c9cy00722a.
Megha A, Mondal K, Ghanty TK, Banerjee A. Adsorption and activation of CO2 on small-sized Cu-Zr bimetallic clusters. J Phys Chem A. 2021;125:2558–72. https://doi.org/10.1021/acs.jpca.1c00751.
Navarro JC, Hurtado C, Gonzalez-Castaño M, Bobadilla LF, Ivanova S, Cumbrera FL, Centeno MA, Odriozola JA. Spinel ferrite catalysts for CO2 reduction via reverse water gas shift reaction. J CO2 Util. 2023;68:102356. https://doi.org/10.1016/j.jcou.2022.102356.
Chen C-S, Cheng W-H, Lin S-S. Study of reverse water gas shift reaction by TPD, TPR and CO2 hydrogenation over potassium-promoted Cu/SiO2 catalyst. Appl Catal A: Gen. 2003;238:55–67. https://doi.org/10.1016/S0926-860X(02)00221-1.
Pastor-Pérez L, Baibars F, Le Sache E, Arellano-García H, Gu S, Reina TR. CO2 valorisation via reverse water-gas shift reaction using advanced Cs doped Fe-Cu/Al2O3 catalysts. J CO2 Util. 2017;21:423–8. https://doi.org/10.1016/j.jcou.2017.08.009.
Varvoutis G, Lykaki M, Papista E, Carabineiro SAC, Psarras AC, Marnellos GE, Konsolakis M. Effect of alkali (Cs) doping on the surface chemistry and CO2 hydrogenation performance of CuO/CeO2 catalysts. J CO2 Util. 2021;44:101408. https://doi.org/10.1016/j.jcou.2020.101408.
De S, Dokania A, Ramirez A, Gascon J. Advances in the design of heterogeneous catalysts and thermocatalytic processes for CO2 utilization. ACS Catal. 2020;10:14147–85. https://doi.org/10.1021/acscatal.0c04273.
Fishman ZS, He Y, Yang KR, Lounsbury AW, Zhu J, Tran TM, Zimmerman JB, Batista VS, Pfefferle LD. Hard templating ultrathin polycrystalline hematite nanosheets: effect of nano-dimension on CO2 to CO conversion via the reverse water-gas shift reaction. Nanoscale. 2017;9:12984–95. https://doi.org/10.1039/c7nr03522e.
Kharaji AG, Shariati A, Takassi MA. A novel γ-alumina supported Fe-Mo bimetallic catalyst for reverse water gas shift reaction. Chin J Chem Eng. 2013;21:1007–14. https://doi.org/10.1016/S1004-9541(13)60573-X.
Loiland JA, Wulfers MJ, Marinkovic NS, Lobo RF. Fe/γ-Al2O3 and Fe-K/γ-Al2O3 as reverse water-gas shift catalysts. Catal Sci Technol. 2016;6:5267–79. https://doi.org/10.1039/c5cy02111a.
Yang L, Pastor-Pérez L, Villora-Pico JJ, Gu S, Sepúlveda-Escribano A, Reina TR. CO2 valorisation via reverse water-gas shift reaction using promoted Fe/CeO2-Al2O3 catalysts: showcasing the potential of advanced catalysts to explore new processes design. Appl Catal A: Gen. 2020;593: 117442. https://doi.org/10.1016/j.apcata.2020.117442.
Utsis N, Landau MV, Erenburg A, Nehemya RV, Herskowitz M. Performance of reverse water gas shift on coprecipitated and C-templated BaFe-hexaaluminate: the effect of Fe loading, texture, and promotion with K. ChemCatChem. 2018;10:3795–805. https://doi.org/10.1002/cctc.201800709.
Hakeem AA, Rajendran J, Kapteijn F, Makkee M. Sulfur as a selectivity modifier in a highly active Rh/Fe2O3/ZrO2 catalyst for water-gas shift. ChemCatChem. 2014;6:2240–3. https://doi.org/10.1002/cctc.201402161.
Yan B, Zhao B, Kattel S, Wu Q, Yao S, Su D, Chen JG. Tuning CO2 hydrogenation selectivity via metal-oxide interfacial sites. J Catal. 2019;374:60–71. https://doi.org/10.1016/j.jcat.2019.04.036.
Zhang Q, Pastor-Pérez L, Gu S, Reina TR. Transition metal carbides (TMCs) catalysts for gas phase CO2 upgrading reactions: a comprehensive overview. Catalysts. 2020;10:955. https://doi.org/10.3390/catal10090955.
Levy RB, Boudart M. Platinum-like behavior of tungsten carbide in surface catalysis. Science. 1973;181:547–9. https://doi.org/10.1126/science.181.4099.547.
Gao J, Wu Y, Jia C, Zhong Z, Gao F, Yang Y, Liu B. Controllable synthesis of α-MoC1-x and β-Mo2C nanowires for highly selective CO2 reduction to CO. Catal Commun. 2016;84:147–50. https://doi.org/10.1016/j.catcom.2016.06.026.
Liu P, Rodriguez JA. Water-gas-shift reaction on molybdenum carbide surfaces: essential role of the oxycarbide. J Phys Chem B. 2006;110:19418–25. https://doi.org/10.1021/jp0621629.
Porosoff MD, Kattel S, Li W, Liu P, Chen JG. Identifying trends and descriptors for selective CO2 conversion to CO over transition metal carbides. Chem Commun. 2015;51:6988–91. https://doi.org/10.1039/c5cc01545f.
Porosoff MD, Yang X, Boscoboinik JA, Chen JG. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO. Angew Chem Int Ed. 2014;53:6705–9. https://doi.org/10.1002/anie.201404109.
Zhang Q, Pastor-Pérez L, Jin W, Gu S, Reina TR. Understanding the promoter effect of Cu and Cs over highly effective β-Mo2C catalysts for the reverse water-gas shift reaction. Appl Catal B: Environ. 2019;244:889–98. https://doi.org/10.1016/j.apcatb.2018.12.023.
Zhang X, Zhu X, Lin L, Yao S, Zhang M, Liu X, Wang X, Li Y-W, Shi C, Ma D. Highly dispersed copper over β-Mo2C as an efficient and stable catalyst for the reverse water gas shift (RWGS) reaction. ACS Catal. 2017;7:912–8. https://doi.org/10.1021/acscatal.6b02991.
Rodriguez JA, Evans J, Feria L, Vidal AB, Liu P, Nakamura K, Illas F. CO2 hydrogenation on Au/TiC, Cu/TiC, and Ni/TiC catalysts: production of CO, methanol, and methane. J Catal. 2013;307:162–9. https://doi.org/10.1016/j.jcat.2013.07.023.
Pajares A, Prats H, Romero A, Viñes F, de la Piscina PR, Sayós R, Homs N, Illas F. Critical effect of carbon vacancies on the reverse water gas shift reaction over vanadium carbide catalysts. Appl Catal B: Environ. 2020;267: 118719. https://doi.org/10.1016/j.apcatb.2020.118719.
Morse JR, Juneau M, Baldwin JW, Porosoff MD, Willauer HD. Alkali promoted tungsten carbide as a selective catalyst for the reverse water gas shift reaction. J CO2 Util. 2020;35:38–46. https://doi.org/10.1016/j.jcou.2019.08.024.
Koverga AA, Flórez E, Dorkis L, Rodriguez JA. CO, CO2, and H2 interactions with (0001) and (001) tungsten carbide surfaces: importance of carbon and metal sites. J Phys Chem C. 2019;123:8871–83. https://doi.org/10.1021/acs.jpcc.8b11840.
Khan A, Verpoort F, Asiri AM, Hoque ME, Bilgrami AL, Azam M, Naidu KCB, editors. Metal-organic frameworks for chemical reactions: from organic transformations to energy applications. Elsevier; 2021. https://doi.org/10.1016/C2019-0-04610-9.
Li Y, Cai X, Chen S, Zhang H, Zhang KHL, Hong J, Chen B, Kuo D-H, Wang W. Highly dispersed metal carbide on ZIF-derived pyridinic-N-doped carbon for CO2 enrichment and selective hydrogenation. Chemsuschem. 2018;11:1040–7. https://doi.org/10.1002/cssc.201800016.
Zhang Q, Yang Z, Chen B, Liang X. Phase-competition-driven formation of hierarchical FeNiZn-MIL-88B-on-MOF-5 octapods displaying high selectivity for the RWGS reaction. Chem Commun. 2019;55:8450–3. https://doi.org/10.1039/c9cc03716k.
Zheng Z, Xu H, Xu Z, Ge J. A monodispersed spherical Zr-based metal-organic framework catalyst, Pt/Au@Pd@UIO-66, Comprising an Au@Pd core-shell encapsulated in a UIO-66 center and its highly selective CO2 hydrogenation to produce CO. Small. 2018;14:1702812. https://doi.org/10.1002/smll.201702812.
Zhao X, Xu H, Wang X, Zheng Z, Xu Z, Ge J. Monodisperse metal-organic framework nanospheres with encapsulated core-shell nanoparticles Pt/Au@Pd@{Co2(oba)4(3-bpdh)2}4H2O for the highly selective conversion of CO2 to CO. ACS Appl Mater Interfaces. 2018;10:15096–103. https://doi.org/10.1021/acsami.8b03561.
Han Y, Xu H, Su Y, Xu Z, Wang K, Wang W. Noble metal (Pt, Au@Pd) nanoparticles supported on metal organic framework (MOF-74) nanoshuttles as high-selectivity CO2 conversion catalysts. J Catal. 2019;370:70–8. https://doi.org/10.1016/j.jcat.2018.12.005.
Zhang H, Xu H, Li Y, Su Y. Octahedral core–shell bimetallic catalysts M@UIO-67 (M = Pt–Pd nanoparticles, Pt–Pd nanocages): metallic nanocages that enhanced CO2 conversion. Appl Mater Today. 2020;19: 100609. https://doi.org/10.1016/j.apmt.2020.100609.
Fang H, Zhao G, Cheng D, Liu J, Lan D, Jiang Q, Liu X, Ge J, Xu Z, Xu H. MOF-derived bimetallic core-shell catalyst HZSM-5@ZrO2-In2O3: high CO2 conversion in reverse water gas shift reaction. Mater Chem Front. 2022;6:2826–34. https://doi.org/10.1039/d2qm00307d.
Ahmad K, Upadhyayula S. Greenhouse gas CO2 hydrogenation to fuels: a thermodynamic analysis. Environ Prog Sustain Energy. 2019;38:98–111. https://doi.org/10.1002/ep.13028.
Goguet A, Meunier F, Breen JP, Burch R, Petch MI, Faur GA. Study of the origin of the deactivation of a Pt/CeO2 catalyst during reverse water gas shift (RWGS) reaction. J Catal. 2004;226:382–92. https://doi.org/10.1016/j.jcat.2004.06.011.
Sikora E, Prekob Á, Halasi G, Vanyorek L, Pekker P, Kristály F, Varga T, Kiss J, Kónya Z, Viskolcz B. Development and application of carbon-layer-stabilized, nitrogen-doped, bamboo-like carbon nanotube catalysts in CO2 hydrogenation. ChemistryOpen. 2018;7:789–96. https://doi.org/10.1002/open.201800162.
Liu Y, Li L, Zhang R, Guo Y, Wang H, Ge Q, Zhu X. Synergetic enhancement of activity and selectivity for reverse water gas shift reaction on Pt-Re/SiO2 catalysts. J CO2 Util. 2022;63:102128. https://doi.org/10.1016/j.jcou.2022.102128.
Li M, My Pham TH, Ko Y, Zhao K, Zhong L, Luo W, Züttel A. Support-dependent cu-in bimetallic catalysts for tailoring the activity of reverse water gas shift reaction. ACS Sustain Chem Eng. 2022;10:1524–35. https://doi.org/10.1021/acssuschemeng.1c06935.
Nityashree N, Price CAH, Pastor-Perez L, Manohara GV, Garcia S, Maroto-Valer MM, Reina TR. Carbon stabilised saponite supported transition metal-alloy catalysts for chemical CO2 utilisation via reverse water-gas shift reaction. Appl Catal B: Environ. 2020;261: 118241. https://doi.org/10.1016/j.apcatb.2019.118241.
Le Saché E, Pastor-Pérez L, Haycock BJ, Villora-Picó JJ, Sepúlveda-Escribano A, Reina TR. Switchable catalysts for chemical CO2 recycling: a step forward in the methanation and reverse water-gas shift reactions. ACS Sustainable Chem Eng. 2020;8:4614–22. https://doi.org/10.1021/acssuschemeng.0c00551.
Iqbal MW, Yu Y, Simakov DSA. Enhancing the surface area stability of the cerium oxide reverse water gas shift nanocatalyst via reverse microemulsion synthesis. Catal Today. 2023;407:230–43. https://doi.org/10.1016/j.cattod.2021.11.029.
Guo J, Wang Z, Li J, Wang Z. In-Ni intermetallic compounds derived from layered double hydroxides as efficient catalysts toward the reverse water gas shift reaction. ACS Catal. 2022;12:4026–36. https://doi.org/10.1021/acscatal.2c00671.
Zakharova A, Iqbal MW, Madadian E, Simakov DSA. Reverse microemulsion-synthesized high-surface-area Cu/γ-Al2O3 catalyst for CO2 conversion via reverse water gas shift. ACS Appl Mater Interfaces. 2022;14:22082–94. https://doi.org/10.1021/acsami.2c01959.
Gao J, Wang Y, Ping Y, Hu D, Xu G, Gu F, Su F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012;2:2358–68. https://doi.org/10.1039/c2ra00632d.
Lehner M, Tichler R, Steinmüller H, Koppe M. Springer briefs in energy, power-to-gas: technology and business models. 1st ed. Heidelberg, New York, Dordrecht, London: Springer Cham; 2014.
Götz M, Lefebvre J, Mörs F, McDaniel Koch A, Graf F, Bajohr S, Reimert R, Kolb T. Renewable Power-to-Gas: a technological and economic review. Renew Energy. 2016;85:1371–90. https://doi.org/10.1016/j.renene.2015.07.066.
Romeo LM, Bailera M. Design configurations to achieve an effective CO2 use and mitigation through power to gas. J CO2 Util. 2020;39:101174. https://doi.org/10.1016/j.jcou.2020.101174.
Jalama K. Carbon dioxide hydrogenation over nickel-, ruthenium-, and copper-based catalysts: review of kinetics and mechanism. Catal Rev Sci Eng. 2017;59:95–164. https://doi.org/10.1080/01614940.2017.1316172.
Ashok J, Pati S, Hongmanorom P, Tianxi Z, Junmei C, Kawi S. A review of recent catalyst advances in CO2 methanation processes. Catal Today. 2020;356:471–89. https://doi.org/10.1016/j.cattod.2020.07.023.
Aziz MAA, Jalil AA, Triwahyono S, Ahmad A. CO2 methanation over heterogeneous catalysts: recent progress and future prospects. Green Chem. 2015;17:2647–63. https://doi.org/10.1039/c5gc00119f.
Falbo L, Martinelli M, Visconti CG, Lietti L, Bassano C, Deiana P. Kinetics of CO2 methanation on a Ru-based catalyst at process conditions relevant for Power-to-Gas applications. Appl Catal B: Environ. 2018;225:354–63. https://doi.org/10.1016/j.apcatb.2017.11.066.
Lunde PJ, Kester FL. Rates of methane formation from carbon dioxide and hydrogen over a ruthenium catalyst. J Catal. 1973;30:423–9. https://doi.org/10.1016/0021-9517(73)90159-0.
Wang X, Hong Y, Shi H, Szanyi J. Kinetic modeling and transient DRIFTS–MS studies of CO2 methanation over Ru/Al2O3 catalysts. J Catal. 2016;343:185–95. https://doi.org/10.1016/j.jcat.2016.02.001.
Weatherbee GD, Bartholomew CH. Hydrogenation of CO2 on group VIII Metals II. Kinetics and mechanism of CO2 hydrogenation on nickel. J Catal. 1982;77:460–72. https://doi.org/10.1016/0021-9517(82)90186-5.
Xu J, Froment GF. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989;35:88–96. https://doi.org/10.1002/aic.690350109.
Schlereth D, Hinrichsen O. A fixed-bed reactor modeling study on the methanation of CO2. Chem Eng Res Des. 2014;92:702–12. https://doi.org/10.1016/j.cherd.2013.11.014.
Lim JY, McGregor J, Sederman AJ, Dennis JS. Kinetic studies of CO2 methanation over a Ni/γ-Al2O3 catalyst using a batch reactor. Chem Eng Sci. 2016;141:28–45. https://doi.org/10.1016/j.ces.2015.10.026.
Miguel CV, Mendes A, Madeira LM. Intrinsic kinetics of CO2 methanation over an industrial nickel-based catalyst. J CO2 Util. 2018;25:128–36. https://doi.org/10.1016/j.jcou.2018.03.011.
Ibraeva ZA, Nekrasov NV, Gudkov BS, Yakerson VI, Beisembaeva ZT, Golosman EZ, Kiperman SL. Kinetics of methanation of carbon dioxide on a nickel catalyst. Theor Exp Chem. 1991;26:584–8. https://doi.org/10.1007/BF00531916.
Yu Y, Chan YM, Bian Z, Song F, Wang J, Zhong Q, Kawi S. Enhanced performance and selectivity of CO2 methanation over g-C3N4 assisted synthesis of Ni–CeO2 catalyst: kinetics and DRIFTS studies. Int J Hydrogen Energy. 2018;43:15191–204. https://doi.org/10.1016/j.ijhydene.2018.06.090.
Ravanchi MT, Sahebdelfar S. Catalytic conversions of CO2 to help mitigate climate change: recent process developments. Process Saf Environ Prot. 2021;145:172–94. https://doi.org/10.1016/j.psep.2020.08.003.
Younas M, Loong Kong L, Bashir MJK, Nadeem H, Shehzad A, Sethupathi S. Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels. 2016;30:8815–31. https://doi.org/10.1021/acs.energyfuels.6b01723.
Panagiotopoulou P. Hydrogenation of CO2 over supported noble metal catalysts. Appl Catal A: Gen. 2017;542:63–70. https://doi.org/10.1016/j.apcata.2017.05.026.
Bartholomew CH. Mechanisms of catalyst deactivation. Appl Catal A: Gen. 2001;212:17–60. https://doi.org/10.1016/S0926-860X(00)00843-7.
Mills GA, Steffgen FW. Catalytic methanation. Catal Rev Sci Eng. 1974;8:159–210. https://doi.org/10.1080/01614947408071860.
Bacariza MC, Spataru D, Karam L, Lopes JM, Henriques C. Promising catalytic systems for CO2 hydrogenation into CH4: a review of recent studies. Processes. 2020;8:1646. https://doi.org/10.3390/pr8121646.
Lee WJ, Li C, Prajitno H, Yoo J, Patel J, Yang Y, Lim S. Recent trend in thermal catalytic low temperature CO2 methanation: a critical review. Catal Today. 2021;368:2–19. https://doi.org/10.1016/j.cattod.2020.02.017.
Szailer T, Novák É, Oszkó A, Erdőhelyi A. Effect of H2S on the hydrogenation of carbon dioxide over supported Rh catalysts. Top Catal. 2007;46:79–86. https://doi.org/10.1007/s11244-007-0317-5.
Dalla Betta RA, Shelef M. Heterogeneous methanation: In situ infrared spectroscopic study of RuAl2O3 during the hydrogenation of CO. J Catal. 1977;48:111–9. https://doi.org/10.1016/0021-9517(77)90082-3.
Ekerdt JG, Bell AT. Synthesis of hydrocarbons from CO and H2 over silica-supported Ru: reaction rate measurements and infrared spectra of adsorbed species. J Catal. 1979;58:170–87. https://doi.org/10.1016/0021-9517(79)90255-0.
Mukkavilli S, Wittmann C, Tavlarides LL. Carbon deactivation of Fischer-Tropsch ruthenium catalyst. Ind Eng Chem Process Des Dev. 1986;25:487–94. https://doi.org/10.1021/i200033a023.
Wang W, Wang S, Ma X, Gong J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem Soc Rev. 2011;40:3703–27. https://doi.org/10.1039/c1cs15008a.
Lin Q, Liu XY, Jiang Y, Wang Y, Huang Y, Zhang T. Crystal phase effects on the structure and performance of ruthenium nanoparticles for CO2 hydrogenation. Catal Sci Technol. 2014;4:2058–63. https://doi.org/10.1039/c4cy00030g.
Xu J, Su X, Duan H, Hou B, Lin Q, Liu X, Pan X, Pei G, Geng H, Huang Y, Zhang T. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J Catal. 2016;333:227–37. https://doi.org/10.1016/j.jcat.2015.10.025.
Fan M, Jimenez JD, Shirodkar SN, Wu J, Chen S, Song L, Royko MM, Zhang J, Guo H, Cui J, Zuo K, Wang W, Zhang C, Yuan F, Vajtai R, Qian J, Yang J, Yakobson BI, Tour JM, Lauterbach J, Sun D, Ajayan PM. Atomic Ru immobilized on porous h-BN through simple vacuum filtration for highly active and selective CO2 methanation. ACS Catal. 2019;9:10077–86. https://doi.org/10.1021/acscatal.9b02197.
Chai S, Men Y, Wang J, Liu S, Song Q, An W, Kolb G. Boosting CO2 methanation activity on Ru/TiO2 catalysts by exposing (001) facets of anatase TiO2. J CO2 Util. 2019;33:242–52. https://doi.org/10.1016/j.jcou.2019.05.031.
Quindimil A, De-La-Torre U, Pereda-Ayo B, Davó-Quiñonero A, Bailón-García E, Lozano-Castelló D, González-Marcos JA, Bueno-López A, González-Velasco JR. Effect of metal loading on the CO2 methanation: a comparison between alumina supported Ni and Ru catalysts. Catal Today. 2020;356:419–32. https://doi.org/10.1016/j.cattod.2019.06.027.
Yan Y, Wang Q, Jiang C, Yao Y, Lu D, Zheng J, Dai Y, Wang H, Yang Y. Ru/Al2O3 catalyzed CO2 hydrogenation: oxygen-exchange on metal-support interfaces. J Catal. 2018;367:194–205. https://doi.org/10.1016/j.jcat.2018.08.026.
Zhang Z, Deng S, Fang X, Xu J, Xu X, Wang X. CrOx-promoted Ru/Al2O3 for CO2 methanation: formation of surface Cr-doped RuO2 solid solution plays key roles. Fuel. 2023;339: 127414. https://doi.org/10.1016/j.fuel.2023.127414.
López-Rodríguez S, Davó-Quiñonero A, Bailón-García E, Lozano-Castelló D, Bueno-López A. Effect of Ru loading on Ru/CeO2 catalysts for CO2 methanation. Mol Catal. 2021;515: 111911. https://doi.org/10.1016/j.mcat.2021.111911.
Sakpal T, Lefferts L. Structure-dependent activity of CeO2 supported Ru catalysts for CO2 methanation. J Catal. 2018;367:171–80. https://doi.org/10.1016/j.jcat.2018.08.027.
Guo Y, Mei S, Yuan K, Wang D-J, Liu H-C, Yan C-H, Zhang Y-W. Low-temperature CO2 methanation over CeO2-supported Ru single atoms, nanoclusters, and nanoparticles competitively tuned by strong metal-support interactions and H-spillover effect. ACS Catal. 2018;8:6203–15. https://doi.org/10.1021/acscatal.7b04469.
Abe T, Tanizawa M, Watanabe K, Taguchi A. CO2 methanation property of Ru nanoparticle-loaded TiO2 prepared by a polygonal barrel-sputtering method. Energy Environ Sci. 2009;2:315–21. https://doi.org/10.1039/b817740f.
Jabotra G, Yadav PK, Kumar S, Sharma S. CO2/CO methanation over Ru and Ni supported γ-Al2O3: a study on the effect of the stoichiometry of reactant gases. Mol Catal. 2023;547: 113365. https://doi.org/10.1016/j.mcat.2023.113365.
Zhang T, Zheng P, Gu F, Xu W, Chen W, Zhu T, Han Y-F, Xu G, Zhong Z, Su F. The dual-active-site tandem catalyst containing Ru single atoms and Ni nanoparticles boosts CO2 methanation. Appl Catal B: Environ. 2023;323: 122190. https://doi.org/10.1016/j.apcatb.2022.122190.
Wang C, Lu Y, Zhang Y, Fu H, Sun S, Li F, Duan Z, Liu Z, Wu C, Wang Y, Sun H, Yan Z. Ru-based catalysts for efficient CO2 methanation: synergistic catalysis between oxygen vacancies and basic sites. Nano Res. 2023;16:12153–64. https://doi.org/10.1007/s12274-023-5592-3.
Wang Q, Gao Y, Tumurbaatar C, Bold T, Wei F, Dai Y, Yang Y. Tuned selectivity and enhanced activity of CO2 methanation over Ru catalysts by modified metal-carbonate interfaces. J Energy Chem. 2022;64:38–46. https://doi.org/10.1016/j.jechem.2021.04.039.
Tébar-Soler C, Martin-Diaconescu V, Simonelli L, Missyul A, Perez-Dieste V, Villar-García IJ, Brubach J-B, Roy P, Haro ML, Calvino JJ, Concepción P, Corma A. Low-oxidation-state Ru sites stabilized in carbon-doped RuO2 with low-temperature CO2 activation to yield methane. Nat Mater. 2023;22:762–8. https://doi.org/10.1038/s41563-023-01540-1.
Rönsch S, Schneider J, Matthischke S, Schlüter M, Götz M, Lefebvre J, Prabhakaran P, Bajohr S. Review on methanation—from fundamentals to current projects. Fuel. 2016;166:276–96. https://doi.org/10.1016/j.fuel.2015.10.111.
Karelovic A, Ruiz P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: effect of the metal particle size on catalytic performances and reaction mechanism. Appl Catal B: Environ. 2012;113–114:237–49. https://doi.org/10.1016/j.apcatb.2011.11.043.
Karelovic A, Ruiz P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J Catal. 2013;301:141–53. https://doi.org/10.1016/j.jcat.2013.02.009.
Solymosi F, Erdöhelyi A, Bánsági T. Methanation of CO2 on supported rhodium catalyst. J Catal. 1981;68:371–82. https://doi.org/10.1016/0021-9517(81)90106-8.
Martin NM, Velin P, Skoglundh M, Bauer M, Carlsson P-A. Catalytic hydrogenation of CO2 to methane over supported Pd, Rh and Ni catalysts. Catal Sci Technol. 2017;7:1086–94. https://doi.org/10.1039/c6cy02536f.
Martin NM, Hemmingsson F, Schaefer A, Ek M, Merte LR, Hejral U, Gustafson J, Skoglundh M, Dippel A-C, Gutowski O, Bauer M, Carlsson P-A. Structure-function relationship for CO2 methanation over ceria supported Rh and Ni catalysts under atmospheric pressure conditions. Catal Sci Technol. 2019;9:1644–53. https://doi.org/10.1039/c8cy02097c.
Arandiyan H, Kani K, Wang Y, Jiang B, Kim J, Yoshino M, Rezaei M, Rowan AE, Dai H, Yamauchi Y. Highly selective reduction of carbon dioxide to methane on novel mesoporous Rh catalysts. ACS Appl Mater Interfaces. 2018;10:24963–8. https://doi.org/10.1021/acsami.8b06977.
Ocampo F, Louis B, Kiwi-Minsker L, Roger A-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1-xO2 catalysts for carbon dioxide methanation. Appl Catal A: Gen. 2011;392:36–44. https://doi.org/10.1016/j.apcata.2010.10.025.
Swalus C, Jacquemin M, Poleunis C, Bertrand P, Ruiz P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl Catal B: Environ. 2012;125:41–50. https://doi.org/10.1016/j.apcatb.2012.05.019.
Park J-N, McFarland EW. A highly dispersed Pd-Mg/SiO2 catalyst active for methanation of CO2. J Catal. 2009;266:92–7. https://doi.org/10.1016/j.jcat.2009.05.018.
Martins J, Batail N, Silva S, Rafik-Clement S, Karelovic A, Debecker DP, Chaumonnot A, Uzio D. CO2 hydrogenation with shape-controlled Pd nanoparticles embedded in mesoporous silica: elucidating stability and selectivity issues. Catal Commun. 2015;58:11–5. https://doi.org/10.1016/j.catcom.2014.08.027.
Jiang H, Gao Q, Wang S, Chen Y, Zhang M. The synergistic effect of Pd NPs and UiO-66 for enhanced activity of carbon dioxide methanation. J CO2 Util. 2019;31:167–72. https://doi.org/10.1016/j.jcou.2019.03.011.
Mravak A, Vajda S, Bonačić-Koutecký V. Mechanism of catalytic CO2 hydrogenation to methane and methanol using a bimetallic Cu3Pd cluster at a zirconia support. J Phys Chem C. 2022;126:18306–12. https://doi.org/10.1021/acs.jpcc.2c04921.
Halder A, Lenardi C, Timoshenko J, Mravak A, Yang B, Kolipaka LK, Piazzoni C, Seifert S, Bonačić-Koutecký V, Frenkel AI, Milani P, Vajda S. CO2 methanation on Cu-cluster decorated zirconia supports with different morphology: a combined experimental in situ GIXANES/GISAXS, ex situ XPS and theoretical DFT study. ACS Catal. 2021;11:6210–24. https://doi.org/10.1021/acscatal.0c05029.
Galadima A, Muraza O. Catalytic thermal conversion of CO2 into fuels: perspective and challenges. Renew Sustain Energy Rev. 2019;115: 109333. https://doi.org/10.1016/j.rser.2019.109333.
Kirchner J, Baysal Z, Kureti S. Activity and structural changes of Fe-based catalysts during CO2 hydrogenation towards CH4—a mini review. ChemCatChem. 2020;12:981–8. https://doi.org/10.1002/cctc.201901956.
Franken T, Heel A. Are Fe based catalysts an upcoming alternative to Ni in CO2 methanation at elevated pressure? J CO2 Util. 2020;39:101175. https://doi.org/10.1016/j.jcou.2020.101175.
Lv C, Xu L, Chen M, Cui Y, Wen X, Li Y, Wu C, Yang B, Miao Z, Hu X, Shou Q. Recent progresses in constructing the highly efficient Ni based catalysts with advanced low-temperature activity toward CO2 methanation. Front Chem. 2020;8:269. https://doi.org/10.3389/fchem.2020.00269.
Tsiotsias AI, Charisiou ND, Yentekakis IV, Goula MA. Bimetallic Ni-based catalysts for CO2 methanation: a review. Nanomaterials. 2021;11:28. https://doi.org/10.3390/nano11010028.
Shen L, Xu J, Zhu M, Han Y-F. Essential role of the support for nickel-based CO2 methanation catalysts. ACS Catal. 2020;10:14581–91. https://doi.org/10.1021/acscatal.0c03471.
Bacariza MC, Graça I, Bebiano SS, Lopes JM, Henriques C. Micro- and mesoporous supports for CO2 methanation catalysts: a comparison between SBA-15, MCM-41 and USY zeolite. Chem Eng Sci. 2018;175:72–83. https://doi.org/10.1016/j.ces.2017.09.027.
Bian Z, Kawi S. Highly carbon-resistant Ni-Co/SiO2 catalysts derived from phyllosilicates for dry reforming of methane. J CO2 Util. 2017;18:345–52. https://doi.org/10.1016/j.jcou.2016.12.014.
Liu W, Li L, Zhang X, Wang Z, Wang X, Peng H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J CO2 Util. 2018;27:297–307. https://doi.org/10.1016/j.jcou.2018.08.003.
Muroyama H, Tsuda Y, Asakoshi T, Masitah H, Okanishi T, Matsui T, Eguchi K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J Catal. 2016;343:178–84. https://doi.org/10.1016/j.jcat.2016.07.018.
Abate S, Mebrahtu C, Giglio E, Deorsola F, Bensaid S, Perathoner S, Pirone R, Centi G. Catalytic performance of γ-Al2O3-ZrO2-TiO2-CeO2 composite oxide supported ni-based catalysts for CO2 methanation. Ind Eng Chem Res. 2016;55:4451–60. https://doi.org/10.1021/acs.iecr.6b00134.
Italiano C, Llorca J, Pino L, Ferraro M, Antonucci V, Vita A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl Catal B: Environ. 2020;264: 118494. https://doi.org/10.1016/j.apcatb.2019.118494.
Le TA, Kim MS, Lee SH, Kim TW, Park ED. CO and CO2 methanation over supported Ni catalysts. Catal Today. 2017;293–294:89–96. https://doi.org/10.1016/j.cattod.2016.12.036.
Mebrahtu C, Abate S, Perathoner S, Chen S, Centi G. CO2 methanation over Ni catalysts based on ternary and quaternary mixed oxide: a comparison and analysis of the structure-activity relationships. Catal Today. 2018;304:181–9. https://doi.org/10.1016/j.cattod.2017.08.060.
Ocampo F, Louis B, Kiennemann A, Roger AC. CO2 methanation over Ni-Ceria-Zirconia catalysts: effect of preparation and operating conditions. IOP Conf Ser Mater Sci Eng. 2011;19: 012007. https://doi.org/10.1088/1757-899X/19/1/012007.
Ahn JY, Chang SW, Lee SM, Kim SS, Chung WJ, Lee JC, Cho YJ, Shin KS, Moon DH, Nguyen DD. Developing Ni-based honeycomb-type catalysts using different binary oxide-supported species for synergistically enhanced CO2 methanation activity. Fuel. 2019;250:277–84. https://doi.org/10.1016/j.fuel.2019.03.123.
Lee YH, Ahn JY, Nguyen DD, Chang SW, Kim SS, Lee SM. Role of oxide support in Ni based catalysts for CO2 methanation. RSC Adv. 2021;11:17648–57. https://doi.org/10.1039/d1ra02327f.
Aziz MAA, Jalil AA, Triwahyono S, Mukti RR, Taufiq-Yap YH, Sazegar MR. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl Catal B: Environ. 2014;147:359–68. https://doi.org/10.1016/j.apcatb.2013.09.015.
Tao M, Xin Z, Meng X, Bian Z, Lv Y. Highly dispersed nickel within mesochannels of SBA-15 for CO methanation with enhanced activity and excellent thermostability. Fuel. 2017;188:267–76. https://doi.org/10.1016/j.fuel.2016.09.081.
Rossetti I, Biffi C, Bianchi CL, Nichele V, Signoretto M, Menegazzo F, Finocchio E, Ramis G, Di Michele A. Ni/SiO2 and Ni/ZrO2 catalysts for the steam reforming of ethanol. Appl Catal B: Environ. 2012;117–118:384–96. https://doi.org/10.1016/j.apcatb.2012.02.006.
Quindimil A, De-La-Torre U, Pereda-Ayo B, González-Marcos JA, González-Velasco JR. Ni catalysts with La as promoter supported over Y- and BETA- zeolites for CO2 methanation. Appl Catal B: Environ. 2018;238:393–403. https://doi.org/10.1016/j.apcatb.2018.07.034.
Wang X, Zhu L, Liu Y, Wang S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci Total Environ. 2018;625:686–95. https://doi.org/10.1016/j.scitotenv.2017.12.308.
Sholeha NA, Mohamad S, Bahruji H, Prasetyoko D, Widiastuti N, Abdul Fatah NA, Abdul Jalil A, Taufiq-Yap YH. Enhanced CO2 methanation at mild temperature on Ni/zeolite from kaolin: effect of metal-support interface. RSC Adv. 2021;11:16376–87. https://doi.org/10.1039/d1ra01014j.
Czuma N, Zarębska K, Motak M, Gálvez ME, Da Costa P. Ni/zeolite X derived from fly ash as catalysts for CO2 methanation. Fuel. 2020;267: 117139. https://doi.org/10.1016/j.fuel.2020.117139.
Shen W, Momoi H, Komatsubara K, Saito T, Yoshida A, Naito S. Marked role of mesopores for the prevention of sintering and carbon deposition in dry reforming of methane over ordered mesoporous Ni-Mg-Al oxides. Catal Today. 2011;171:150–5. https://doi.org/10.1016/j.cattod.2011.04.003.
Xu L, Wang F, Chen M, Zhang J, Yuan K, Wang L, Wu K, Xu G, Chen W. CO2 methanation over a Ni based ordered mesoporous catalyst for the production of synthetic natural gas. RSC Adv. 2016;6:28489–99. https://doi.org/10.1039/c6ra01139j.
Aljishi A, Veilleux G, Lalinde JAH, Kopyscinski J. The effect of synthesis parameters on ordered mesoporous nickel alumina catalyst for CO2 methanation. Appl Catal A: Gen. 2018;549:263–72. https://doi.org/10.1016/j.apcata.2017.10.012.
He H, Sekoulopoulos S, Zygmunt S. Single-electron activation of CO2 on graphene-supported ZnO nanoclusters: effects of doping in the support. J Phys Chem C. 2016;120:16732–40. https://doi.org/10.1021/acs.jpcc.6b04526.
Ma H, Ma K, Ji J, Tang S, Liu C, Jiang W, Yue H, Liang B. Graphene intercalated Ni-SiO2/GO-Ni-foam catalyst with enhanced reactivity and heat-transfer for CO2 methanation. Chem Eng Sci. 2019;194:10–21. https://doi.org/10.1016/j.ces.2018.05.019.
Zhen W, Li B, Lu G, Ma J. Enhancing catalytic activity and stability for CO2 methanation on Ni@MOF-5 via control of active species dispersion. Chem Commun. 2015;51:1728–31. https://doi.org/10.1039/c4cc08733j.
Lin X, Wang S, Tu W, Hu Z, Ding Z, Hou Y, Xu R, Dai W. MOF-derived hierarchical hollow spheres composed of carbon-confined Ni nanoparticles for efficient CO2 methanation. Catal Sci Technol. 2019;9:731–8. https://doi.org/10.1039/c8cy02329h.
Zeng L, Wang Y, Li Z, Song Y, Zhang J, Wang J, He X, Wang C, Lin W. Highly dispersed Ni catalyst on metal-organic framework-derived porous hydrous zirconia for CO2 methanation. ACS Appl Mater Interfaces. 2020;12:17436–42. https://doi.org/10.1021/acsami.9b23277.
Shen WM, Dumesic JA, Hill CG Jr. Criteria for stable Ni particle size under methanation reaction conditions: nickel transport and particle size growth via nickel carbonyl. J Catal. 1981;68:152–65. https://doi.org/10.1016/0021-9517(81)90048-8.
Agnelli M, Kolb M, Mirodatos C. CO hydrogenation on a nickel catalyst: I. Kinetics and modeling of a low-temperature sintering process. J Catal. 1994;148:9–21. https://doi.org/10.1006/jcat.1994.1180.
Alrafei B, Polaert I, Ledoux A, Azzolina-Jury F. Remarkably stable and efficient Ni and Ni-Co catalysts for CO2 methanation. Catal Today. 2020;346:23–33. https://doi.org/10.1016/j.cattod.2019.03.026.
Xu L, Yang H, Chen M, Wang F, Nie D, Qi L, Lian X, Chen X, Wu M. CO2 methanation over Ca doped ordered mesoporous Ni-Al composite oxide catalysts: the promoting effect of basic modifier. J CO2 Util. 2017;21:200–10. https://doi.org/10.1016/j.jcou.2017.07.014.
Vrijburg WL, Moioli E, Chen W, Zhang M, Terlingen BJP, Zijlstra B, Filot IAW, Züttel A, Pidko EA, Hensen EJM. Efficient base-metal NiMn/TiO2 catalyst for CO2 methanation. ACS Catal. 2019;9:7823–39. https://doi.org/10.1021/acscatal.9b01968.
Polanski J, Lach D, Kapkowski M, Bartczak P, Siudyga T, Smolinski A. Ru and Ni—privileged metal combination for environmental nanocatalysis. Catalysts. 2020;10:992. https://doi.org/10.3390/catal10090992.
Zhen W, Li B, Lu G, Ma J. Enhancing catalytic activity and stability for CO2 methanation on Ni-Ru/γ-Al2O3 via modulating impregnation sequence and controlling surface active species. RSC Adv. 2014;4:16472–9. https://doi.org/10.1039/c3ra47982j.
Lange F, Armbruster U, Martin A. Heterogeneously-catalyzed hydrogenation of carbon dioxide to methane using RuNi bimetallic catalysts. Energy Technol. 2015;3:55–62. https://doi.org/10.1002/ente.201402113.
Kikkawa S, Teramura K, Asakura H, Hosokawa S, Tanaka T. Isolated platinum atoms in Ni/γ-Al2O3 for selective hydrogenation of CO2 toward CH4. J Phys Chem C. 2019;123:23446–54. https://doi.org/10.1021/acs.jpcc.9b03432.
Li Y, Zhang H, Zhang L, Zhang H. Bimetallic Ni–Pd/SBA-15 alloy as an effective catalyst for selective hydrogenation of CO2 to methane. Int J Hydrogen Energy. 2019;44:13354–63. https://doi.org/10.1016/j.ijhydene.2019.03.276.
Kikkawa S, Teramura K, Asakura H, Hosokawa S, Tanaka T. Ni−Pt alloy nanoparticles with isolated Pt atoms and their cooperative neighboring Ni atoms for selective hydrogenation of CO2 toward CH4 evolution: in situ and transient Fourier transform infrared studies. ACS Appl Nano Mater. 2020;3:9633–44. https://doi.org/10.1021/acsanm.0c01570.
Yan C, Wang C-H, Lin M, Bhalothia D, Yang S-S, Fan G-J, Wang J-L, Chan T-S, Wang Y, Tu X, Dai S, Wang K-W, He J-H, Chen T-Y. Local synergetic collaboration between Pd and local tetrahedral symmetric Ni oxide enables ultra-high-performance CO2 thermal methanation. J Mater Chem A. 2020;8:12744–56. https://doi.org/10.1039/d0ta02957b.
Serrer M-A, Gaur A, Jelic J, Weber S, Fritsch C, Clark AH, Saraçi S, Studt F, Grunwaldt JD. Structural dynamics in Ni-Fe catalysts during CO2 methanation—role of iron oxide clusters. Catal Sci Technol. 2020;10:7542–54. https://doi.org/10.1039/d0cy01396j.
Liu X, Wang D, Li Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today. 2012;7:448–66. https://doi.org/10.1016/j.nantod.2012.08.003.
Huynh HL, Zhu J, Zhang G, Shen Y, Tucho WM, Ding Y, Yu Z. Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. J Catal. 2020;392:266–77. https://doi.org/10.1016/j.jcat.2020.10.018.
Huang Z, Yuan Y, Song M, Hao Z, Xiao J, Cai D, Ibrahim AR, Zhan G. CO2 hydrogenation over mesoporous Ni-Pt/SiO2 nanorod catalysts: determining CH4/CO selectivity by surface ratio of Ni/Pt. Chem Eng Sci. 2022;247: 117106. https://doi.org/10.1016/j.ces.2021.117106.
Liang C, Ye Z, Dong D, Zhang S, Liu Q, Chen G, Li C, Wang Y, Hu X. Methanation of CO2: impacts of modifying nickel catalysts with variable-valence additives on reaction mechanism. Fuel. 2019;254: 115654. https://doi.org/10.1016/j.fuel.2019.115654.
Yan Y, Dai Y, He H, Yu Y, Yang Y. A novel W-doped Ni-Mg mixed oxide catalyst for CO2 methanation. Appl Catal B: Environ. 2016;196:108–16. https://doi.org/10.1016/j.apcatb.2016.05.016.
Zhang R, Wei A, Zhu M, Wu X, Wang H, Zhu X, Ge Q. Tuning reverse water gas shift and methanation reactions during CO2 reduction on Ni catalysts via surface modification by MoOx. J CO2 Util. 2021;52:101678. https://doi.org/10.1016/j.jcou.2021.101678.
Shang X, Deng D, Wang X, Xuan W, Zou X, Ding W, Lu X. Enhanced low-temperature activity for CO2 methanation over Ru doped the Ni/CexZr(1–x)O2 catalysts prepared by one-pot hydrolysis method. Int J Hydrogen Energy. 2018;43:7179–89. https://doi.org/10.1016/j.ijhydene.2018.02.059.
Merkouri L-P, Le Saché E, Pastor-Pérez L, Duyar MS, Reina TR. Versatile Ni-Ru catalysts for gas phase CO2 conversion: bringing closer dry reforming, reverse water gas shift and methanation to enable end-products flexibility. Fuel. 2022;315: 123097. https://doi.org/10.1016/j.fuel.2021.123097.
Jomjaree T, Sintuya P, Srifa A, Koo-amornpattana W, Kiatphuengporn S, Assabumrungrat S, Sudoh M, Watanabe R, Fukuhara C, Ratchahat S. Catalytic performance of Ni catalysts supported on CeO2 with different morphologies for low-temperature CO2 methanation. Catal Today. 2021;375:234–44. https://doi.org/10.1016/j.cattod.2020.08.010.
Ratchahat S, Surathitimethakul S, Thamungkit A, Mala P, Sudoh M, Watanabe R, Fukuhara C, Chen SS, Wu KC-W, Charinpanitkul T. Catalytic performance of Ni/CeO2 catalysts prepared from different routes for CO2 methanation. J Taiwan Inst Chem Eng. 2021;121:184–96. https://doi.org/10.1016/j.jtice.2021.04.008.
Bacariza MC, Graça I, Westermann A, Ribeiro MF, Lopes JM, Henriques C. CO2 hydrogenation over Ni-based zeolites: effect of catalysts preparation and pre-reduction conditions on methanation performance. Top Catal. 2016;59:314–25. https://doi.org/10.1007/s11244-015-0435-4.
Shanmugam V, Neuberg S, Zapf R, Pennemann H, Kolb G. Effect of support and chelating ligand on the synthesis of Ni catalysts with high activity and stability for CO2 methanation. Catalysts. 2020;10:493. https://doi.org/10.3390/catal10050493.
Gac W, Zawadzki W, Rotko M, Greluk M, Słowik G, Kolb G. Effects of support composition on the performance of nickel catalysts in CO2 methanation reaction. Catal Today. 2020;357:468–82. https://doi.org/10.1016/j.cattod.2019.07.026.
Wen X, Xu L, Chen M, Shi Y, Lv C, Cui Y, Wu X, Cheng G, Wu C, Miao Z, Wang F, Hu X. Exploring the influence of nickel precursors on constructing efficient Ni-based CO2 methanation catalysts assisted with in-situ technologies. Appl Catal B: Environ. 2021;297: 120486. https://doi.org/10.1016/j.apcatb.2021.120486.
Beierlein D, Häussermann D, Pfeifer M, Schwarz T, Stöwe K, Traa Y, Klemm E. Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure-sensitive? Appl Catal B: Environ. 2019;247:200–19. https://doi.org/10.1016/j.apcatb.2018.12.064.
Chen M, Guo Z, Zheng J, Jing F, Chu W. CO2 selective hydrogenation to synthetic natural gas (SNG) over four nano-sized Ni/ZrO2 samples: ZrO2 crystalline phase & treatment impact. J Energy Chem. 2016;25:1070–7. https://doi.org/10.1016/j.jechem.2016.11.008.
Kokka A, Ramantani T, Petala A, Panagiotopoulou P. Effect of the nature of the support, operating and pretreatment conditions on the catalytic performance of supported Ni catalysts for the selective methanation of CO. Catal Today. 2020;355:832–43. https://doi.org/10.1016/j.cattod.2019.04.015.
Gao X, Ashok J, Kawi S. A review on roles of pretreatment atmospheres for the preparation of efficient Ni-based catalysts. Catal Today. 2022;397–399:581–91. https://doi.org/10.1016/j.cattod.2021.06.009.
Vita A, Italiano C, Pino L, Frontera P, Ferraro M, Antonucci V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl Catal B: Environ. 2018;226:384–95. https://doi.org/10.1016/j.apcatb.2017.12.078.
Marconi E, Tuti S, Luisetto I. Structure-sensitivity of CO2 methanation over nanostructured Ni supported on CeO2 nanorods. Catalysts. 2019;9:375. https://doi.org/10.3390/catal9040375.
Tada S, Nagase H, Fujiwara N, Kikuchi R. What are the best active sites for CO2 methanation over Ni/CeO2? Energy Fuels. 2021;35:5241–51. https://doi.org/10.1021/acs.energyfuels.0c04238.
Lin L, Gerlak CA, Liu C, Llorca J, Yao S, Rui N, Zhang F, Liu Z, Zhang S, Deng K, Murray CB, Rodriguez JA, Senanayake SD. Effect of Ni particle size on the production of renewable methane from CO2 over Ni/CeO2 catalyst. J Energy Chem. 2021;61:602–11. https://doi.org/10.1016/j.jechem.2021.02.021.
Lin J, Ma C, Wang Q, Xu Y, Ma G, Wang J, Wang H, Dong C, Zhang C, Ding M. Enhanced low-temperature performance of CO2 methanation over mesoporous Ni/Al2O3-ZrO2 catalysts. Appl Catal B: Environ. 2019;243:262–72. https://doi.org/10.1016/j.apcatb.2018.10.059.
Hao Z, Shen J, Lin S, Han X, Chang X, Liu J, Li M, Ma X. Decoupling the effect of Ni particle size and surface oxygen deficiencies in CO2 methanation over ceria supported Ni. Appl Catal B: Environ. 2021;286: 119922. https://doi.org/10.1016/j.apcatb.2021.119922.
Wu HC, Chang YC, Wu JH, Lin JH, Lin IK, Chen CS. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: the influence of particle size on selectivity and reaction pathway. Catal Sci Technol. 2015;5:4154–63. https://doi.org/10.1039/c5cy00667h.
Kesavan JK, Luisetto I, Tuti S, Meneghini C, Iucci G, Battocchio C, Mobilio S, Casciardi S, Sisto R. Nickel supported on YSZ: the effect of Ni particle size on the catalytic activity for CO2 methanation. J CO2 Util. 2018;23:200–11. https://doi.org/10.1016/j.jcou.2017.11.015.
Jia X, Zhang X, Rui N, Hu X, Liu C. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl Catal B: Environ. 2019;244:159–69. https://doi.org/10.1016/j.apcatb.2018.11.024.
Garbarino G, Riani P, Magistri L, Busca G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int J Hydrogen Energy. 2014;39:11557–65. https://doi.org/10.1016/j.ijhydene.2014.05.111.
Varvoutis G, Lykaki M, Stefa S, Binas V, Marnellos GE, Konsolakis M. Deciphering the role of Ni particle size and nickel-ceria interfacial perimeter in the low-temperature CO2 methanation reaction over remarkably active Ni/CeO2 nanorods. Appl Catal B: Environ. 2021;297: 120401. https://doi.org/10.1016/j.apcatb.2021.120401.
Zheng H, Liao W, Ding J, Xu F, Jia A, Huang W, Zhang Z. Unveiling the key factors in determining the activity and selectivity of CO2 hydrogenation over Ni/CeO2 catalysts. ACS Catal. 2022;12:15451–62. https://doi.org/10.1021/acscatal.2c04437.
Sun H, Wang H, Liu X, Zhang Z, Zhang S, Wang X, Liu Y. Stable and highly dispersed nickel catalysts on Ce-Zr-O solid solutions for CO2 methanation. ChemistrySelect. 2022;7: e202200113. https://doi.org/10.1002/slct.202200113.
Bian Y, Xu C, Wen X, Xu L, Cui Y, Wang S, Wu C, Qiu J, Cheng G, Chen M. CO2 methanation over the Ni-based catalysts supported on nano-CeO2 with varied morphologies. Fuel. 2023;331: 125755. https://doi.org/10.1016/j.fuel.2022.125755.
Lin S, Li Z, Li M. Tailoring metal-support interactions via tuning CeO2 particle size for enhancing CO2 methanation activity over Ni/CeO2 catalysts. Fuel. 2023;333: 126369. https://doi.org/10.1016/j.fuel.2022.126369.
Ullah N, Tang R, Li Z. Nanostructured ceria-zirconia supported Ni catalysts for high performance CO2 methanation: phase and morphology effect on activity. J Taiwan Inst Chem Eng. 2022;134: 104317. https://doi.org/10.1016/j.jtice.2022.104317.
Zhang Z, Yu Z, Feng K, Yan B. Eu3+ doping-promoted Ni-CeO2 interaction for efficient low-temperature CO2 methanation. Appl Catal B: Environ. 2022;317: 121800. https://doi.org/10.1016/j.apcatb.2022.121800.
Tsiotsias AI, Charisiou ND, Harkou E, Hafeez S, Manos G, Constantinou A, Hussien AGS, Dabbawala AA, Sebastian V, Hinder SJ, Baker MA, Polychronopoulou K, Goula MA. Enhancing CO2 methanation over Ni catalysts supported on sol-gel derived Pr2O3-CeO2: an experimental and theoretical investigation. Appl Catal B: Environ. 2022;318: 121836. https://doi.org/10.1016/j.apcatb.2022.121836.
Xie Y, Chen J, Wu X, Wen J, Zhao R, Li Z, Tian G, Zhang Q, Ning P, Hao J. Frustrated lewis pairs boosting low-temperature CO2 methanation performance over Ni/CeO2 nanocatalysts. ACS Catal. 2022. https://doi.org/10.1021/acscatal.2c02535.
Tian J, Zheng P, Zhang T, Han Z, Xu W, Gu F, Wang F, Zhang Z, Zhong Z, Su F, Xu G. CO2 methanation over Ni nanoparticles inversely loaded with CeO2 and Cr2O3: catalytic functions of metal oxide/Ni interfaces. Appl Catal B: Environ. 2023;339: 123121. https://doi.org/10.1016/j.apcatb.2023.123121.
Wang H, Li Z, Cui G, Wei M. Synergistic catalysis at the Ni/ZrO2-x interface toward low-temperature CO2 methanation. ACS Appl Mater Interfaces. 2023;15:19021–31. https://doi.org/10.1021/acsami.3c01544.
Jiang Y, Sun H, Fang Y, Zhang C, Liu Y. Robust stability of La- and Ce- dual-promoted Ni/ZrO2 catalyst for CO2 methanation. Catal Today. 2024;436: 114752. https://doi.org/10.1016/j.cattod.2024.114752.
Barreau M, Salusso D, Li J, Zhang J, Borfecchia E, Sobczak K, Braglia L, Gallet J-J, Torelli P, Guo H, Lin S, Zafeiratos S. Ionic nickel embedded in ceria with high specific CO2 methanation activity. Angew Chem Int Ed. 2023;62: e202302087. https://doi.org/10.1002/anie.202302087.
Zhao R, Xie Y, Li Z, Weng H, Zhu D, Mao Y, Wang H, Zhang Q. Unveiling the promotion effect of ethylenediamine on preparation of Ni/CeO2 catalyst for low-temperature CO2 methanation. Int J Hydrogen Energy. 2024;51:451–63. https://doi.org/10.1016/j.ijhydene.2023.08.216.
Fu H, Sun S, Lian H. Enhanced low-temperature CO2 methanation over Ni/ZrO2-Al2O3 catalyst: effect of Al addition on catalytic performance and reaction mechanism. J CO2 Util. 2023;69:102415. https://doi.org/10.1016/j.jcou.2023.102415.
Hu F, Ye R, Jin C, Liu D, Chen X, Li C, Lim KH, Song G, Wang T, Feng G, Zhang R, Kawi S. Ni nanoparticles enclosed in highly mesoporous nanofibers with oxygen vacancies for efficient CO2 methanation. Appl Catal B: Environ. 2022;317: 121715. https://doi.org/10.1016/j.apcatb.2022.121715.
Zhen W, Gao F, Tian B, Ding P, Deng Y, Li Z, Gao H, Lu G. Enhancing activity for carbon dioxide methanation by encapsulating (111) facet Ni particle in metal–organic frameworks at low temperature. J Catal. 2017;348:200–11. https://doi.org/10.1016/j.jcat.2017.02.031.
Hongmanorom P, Ashok J, Zhang G, Bian Z, Wai MH, Zeng Y, Xi S, Borgna A, Kawi S. Enhanced performance and selectivity of CO2 methanation over phyllosilicate structure derived Ni-Mg/SBA-15 catalysts. Appl Catal B: Environ. 2021;282: 119564. https://doi.org/10.1016/j.apcatb.2020.119564.
Liu K, Xu X, Xu J, Fang X, Liu L, Wang X. The distributions of alkaline earth metal oxides and their promotional effects on Ni/CeO2 for CO2 methanation. J CO2 Util. 2020;38:113–24. https://doi.org/10.1016/j.jcou.2020.01.016.
Ahmad KN, Samidin S, Rosli MI, Yusop MR, Kassim MB, Ayodele BV, Yarmo MA, Isahak WNRW. Alkaline earth metal modified nickel nanoparticles supported on exfoliated g-C3N4 for atmospheric CO2 methanation. J Environ Chem Eng. 2023;11: 111109. https://doi.org/10.1016/j.jece.2023.111109.
Liu Q, Wang S, Lv S, Han F, Ouyang J. High stability of the Ni-YCe/diatomite catalyst for CO2 methanation: the synergistic coupling of citric acid and Y2O3. ACS Sustain Chem Eng. 2023;11:12946–58. https://doi.org/10.1021/acssuschemeng.3c02287.
Alkhoori AA, Elmutasim O, Dabbawala AA, Vasiliades MA, Petallidou KC, Emwas A-H, Anjum DH, Singh N, Baker MA, Charisiou ND, Goula MA, Efstathiou AM, Polychronopoulou K. Mechanistic features of the CeO2-modified Ni/Al2O3 catalysts for the CO2 methanation reaction: experimental and Ab initio studies. ACS Appl Energy Mater. 2023;6:8550–71. https://doi.org/10.1021/acsaem.3c01437.
Fu H, Lian H. Optimizing low-temperature CO2 methanation with aluminum-doped Ni/CeO2 catalysts: insights into reaction pathway adjustments and strong metal-support interactions. Chem Eng J. 2024;489: 151021. https://doi.org/10.1016/j.cej.2024.151021.
Weatherbee GD, Bartholomew CH. Hydrogenation of CO2 on group VIII metals: IV. Specific activities and selectivities of silica-supported Co, Fe, and Ru. J Catal. 1984;87:352–62. https://doi.org/10.1016/0021-9517(84)90196-9.
Fan T, Liu H, Shao S, Gong Y, Li G, Tang Z. Cobalt catalysts enable selective hydrogenation of CO2 toward diverse products: recent progress and perspective. J Phys Chem Lett. 2021;12:10486–96. https://doi.org/10.1021/acs.jpclett.1c03043.
Li W, Liu Y, Mu M, Ding F, Liu Z, Guo X, Song C. Organic acid-assisted preparation of highly dispersed Co/ZrO2 catalysts with superior activity for CO2 methanation. Appl Catal B: Environ. 2019;254:531–40. https://doi.org/10.1016/j.apcatb.2019.05.028.
Li W, Nie X, Jiang X, Zhang A, Ding F, Liu M, Liu Z, Guo X, Song C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl Catal B: Environ. 2018;220:397–408. https://doi.org/10.1016/j.apcatb.2017.08.048.
Jimenez JD, Wen C, Lauterbach J. Design of highly active cobalt catalysts for CO2 hydrogenation via the tailoring of surface orientation of nanostructures. Catal Sci Technol. 2019;9:1970–8. https://doi.org/10.1039/c9cy00402e.
Zhou G, Wu T, Xie H, Zheng X. Effects of structure on the carbon dioxide methanation performance of Co-based catalysts. Int J Hydrogen Energy. 2013;38:10012–8. https://doi.org/10.1016/j.ijhydene.2013.05.130.
Díez-Ramírez J, Sánchez P, Kyriakou V, Zafeiratos S, Marnellos GE, Konsolakis M, Dorado F. Effect of support nature on the cobalt-catalyzed CO2 hydrogenation. J CO2 Util. 2017;21:562–71. https://doi.org/10.1016/j.jcou.2017.08.019.
Jimenez J, Bird A, Santos Santiago M, Wen C, Lauterbach J. Supported cobalt nanorod catalysts for carbon dioxide hydrogenation. Energy Technol. 2017;5:884–91. https://doi.org/10.1002/ente.201600575.
Jimenez JD, Wen C, Royko MM, Kropf AJ, Segre C, Lauterbach J. Influence of coordination environment of anchored single-site cobalt catalyst on CO2 hydrogenation. ChemCatChem. 2020;12:846–54. https://doi.org/10.1002/cctc.201901676.
Srisawad N, Chaitree W, Mekasuwandumrong O, Shotipruk A, Jongsomjit B, Panpranot J. CO2 hydrogenation over Co/Al2O3 catalysts prepared via a solid-state reaction of fine gibbsite and cobalt precursors. Reac Kinet Mech Cat. 2012;107:179–88. https://doi.org/10.1007/s11144-012-0459-8.
Janlamool J, Praserthdam P, Jongsomjit B. Ti-Si composite oxide-supported cobalt catalysts for CO2 hydrogenation. J Nat Gas Sci Eng. 2011;20:558–64. https://doi.org/10.1016/S1003-9953(10)60213-7.
Razzaq R, Li C, Usman M, Suzuki K, Zhang S. A highly active and stable Co4N/γ-Al2O3 catalyst for CO and CO2 methanation to produce synthetic natural gas (SNG). Chem Eng J. 2015;262:1090–8. https://doi.org/10.1016/j.cej.2014.10.073.
Aziz MAA, Jalil AA, Triwahyono S, Sidik SM. Methanation of carbon dioxide on metal-promoted mesostructured silica nanoparticles. Appl Catal A: Gen. 2014;486:115–22. https://doi.org/10.1016/j.apcata.2014.08.022.
Tada S, Ochieng OJ, Kikuchi R, Haneda T, Kameyama H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int J Hydrogen Energy. 2014;39:10090–100. https://doi.org/10.1016/j.ijhydene.2014.04.133.
Goodarzi F, Kock M, Mielby J, Kegnæs S. CO2 methanation using metals nanoparticles supported on high surface area MgO. J CO2 Util. 2023;69:102396. https://doi.org/10.1016/j.jcou.2023.102396.
Schubert M, Pokhrel S, Thomé A, Zielasek V, Gesing TM, Roessner F, Mädler L, Bäumer M. Highly active Co-Al2O3-based catalysts for CO2 methanation with very low platinum promotion prepared by double flame spray pyrolysis. Catal Sci Technol. 2016;6:7449–60. https://doi.org/10.1039/c6cy01252c.
Tsiotsias AI, Charisiou ND, Italiano C, Ferrante GD, Pino L, Vita A, Sebastian V, Hinder SJ, Baker MA, Sharan A, Singh N, Polychronopoulou K, Goula MA. Ni-noble metal bimetallic catalysts for improved low temperature CO2 methanation. Appl Surf Sci. 2024;646: 158945. https://doi.org/10.1016/j.apsusc.2023.158945.
Tada S, Shimizu T, Kameyama H, Haneda T, Kikuchi R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int J Hydrogen Energy. 2012;37:5527–31. https://doi.org/10.1016/j.ijhydene.2011.12.122.
Liu J, Li C, Wang F, He S, Chen H, Zhao Y, Wei M, Evans DG, Duan X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal Sci Technol. 2013;3:2627–33. https://doi.org/10.1039/c3cy00355h.
Zhao Y, Girelli V, Ersen O, Debecker DP. CO2 methanation with high-loading mesoporous Ni/SiO2 catalysts: toward high specific activity and new mechanistic insights. J Catal. 2023;426:283–93. https://doi.org/10.1016/j.jcat.2023.07.021.
Li B, Wang F, Li K, Ning P, Chen M, Zhang C. CeO2-supported Fe, Co and Ni toward CO2 hydrogenation: tuning catalytic performance via metal-support interaction. J Rare Earths. 2023;41:926–32. https://doi.org/10.1016/j.jre.2023.02.009.
Zou X, Liu J, Li Y, Shen Z, Zhu X, Xia Q, Cao Y, Zhang S, Ge Z, Cui L, Wang Y. Molybdenum-doping promoted surface oxygen vacancy of CeO2 for enhanced low-temperature CO2 methanation over Ni-CeO2 catalysts. Appl Surf Sci. 2024;661: 160087. https://doi.org/10.1016/j.apsusc.2024.160087.
Wang T, Tang R, Li Z. Enhanced CO2 methanation activity over Ni/CeO2 catalyst by adjusting metal-support interactions. Mol Catal. 2024;558: 114034. https://doi.org/10.1016/j.mcat.2024.114034.
Hu F, Jin C, Wu R, Li C, Song G, Gani TZH, Lim KH, Guo W, Wang T, Ding S, Ye R, Lu Z-H, Feng G, Zhang R, Kawi S. Enhancement of hollow Ni/CeO2-Co3O4 for CO2 methanation: from CO2 adsorption and activation by synergistic effects. Chem Eng J. 2023;461: 142108. https://doi.org/10.1016/j.cej.2023.142108.
Fan WK, Tahir M, Alias H, Mohamed AR. Catalytic CO2 hydrogenation to produce methane over NiO/TiO2 composite: effect of TiO2 structure. Int J Hydrogen Energy. 2024;51:462–78. https://doi.org/10.1016/j.ijhydene.2023.06.241.
Gonçalves LPL, Mielby J, Soares OSGP, Sousa JPS, Petrovykh DY, Lebedev OI, Pereira MFR, Kegnæs S, Kolenko YV. In situ investigation of the CO2 methanation on carbon/ceria-supported Ni catalysts using modulation-excitation DRIFTS. Appl Catal B Environ. 2022;312:121376. https://doi.org/10.1016/j.apcatb.2022.121376.
Ratchahat S, Sudoh M, Suzuki Y, Kawasaki W, Watanabe R, Fukuhara C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J CO2 Util. 2018;24:210–9. https://doi.org/10.1016/j.jcou.2018.01.004.
Loder A, Siebenhofer M, Lux S. The reaction kinetics of CO2 methanation on a bifunctional Ni/MgO catalyst. J Ind Eng Chem. 2020;85:196–207. https://doi.org/10.1016/j.jiec.2020.02.001.
Shahul Hamid MY, Triwahyono S, Jalil AA, Che Jusoh NW, Izan SM, Tuan Abdullah TA. Tailoring the properties of metal oxide loaded/KCC-1 toward a different mechanism of CO2 methanation by in situ IR and ESR. Inorg Chem. 2018;57:5859–69. https://doi.org/10.1021/acs.inorgchem.8b00241.
Wang W, Duong-Viet C, Ba H, Baaziz W, Tuci G, Caporali S, Nguyen-Dinh L, Ersen O, Giambastiani G, Pham-Huu C. Nickel nanoparticles decorated nitrogen-doped carbon nanotubes (Ni/N-CNT); a robust catalyst for the efficient and selective CO2 methanation. ACS Appl Energy Mater. 2019;2:1111–20. https://doi.org/10.1021/acsaem.8b01681.
Yin S, Xu C, Yang H, Wu C, Wu M, Xu J, Zhu H, Qiu J, Xu L, Chen M. Facilely preparing highly dispersed Ni-based CO2 methanation catalysts via employing the amino-functionalized KCC-1 support. Fuel. 2024;365: 131162. https://doi.org/10.1016/j.fuel.2024.131162.
Zhang L, Bian L, Zhu Z, Li Z. La-promoted Ni/Mg-Al catalysts with highly enhanced low-temperature CO2 methanation performance. Int J Hydrogen Energy. 2018;43:2197–206. https://doi.org/10.1016/j.ijhydene.2017.12.082.
Ahmad W, Younis MN, Shawabkeh R, Ahmed S. Synthesis of lanthanide series (La, Ce, Pr, Eu & Gd) promoted Ni/γ-Al2O3 catalysts for methanation of CO2 at low temperature under atmospheric pressure. Catal Commun. 2017;100:121–6. https://doi.org/10.1016/j.catcom.2017.06.044.
Abate S, Barbera K, Giglio E, Deorsola F, Bensaid S, Perathoner S, Pirone R, Centi G. Synthesis, characterization, and activity pattern of Ni-Al hydrotalcite catalysts in CO2 methanation. Ind Eng Chem Res. 2016;55:8299–308. https://doi.org/10.1021/acs.iecr.6b01581.
Takano H, Izumiya K, Kumagai N, Hashimoto K. The effect of heat treatment on the performance of the Ni/(Zr-Sm oxide) catalysts for carbon dioxide methanation. Appl Surf Sci. 2011;257:8171–6. https://doi.org/10.1016/j.apsusc.2011.01.141.
Tan J, Wang J, Zhang Z, Ma Z, Wang L, Liu Y. Highly dispersed and stable Ni nanoparticles confined by MgO on ZrO2 for CO2 methanation. Appl Surf Sci. 2019;481:1538–48. https://doi.org/10.1016/j.apsusc.2019.03.217.
Quatorze IF, Gonçalves LPL, Kolenko YV, Soares OSGP, Pereira MFR. CO2 methanation over Ni supported on Carbon–ZrO2: an optimization of the composite composition. Catal Today. 2023;422:114215. https://doi.org/10.1016/j.cattod.2023.114215.
Zhu M, Tian P, Cao X, Chen J, Pu T, Shi B, Xu J, Moon J, Wu Z, Han Y-F. Vacancy engineering of the nickel-based catalysts for enhanced CO2 methanation. Appl Catal B: Environ. 2021;282: 119561. https://doi.org/10.1016/j.apcatb.2020.119561.
Chen J, Shen X, Wang Q, Wang J, Yang D, Bold T, Dai Y, Tang Y, Yang Y. CO2 methanation over γ-Al2O3 nanosheets-stabilized Ni catalysts: effects of MnOx and MoOx additives on catalytic performance and reaction pathway. J CO2 Util. 2022;63:102113. https://doi.org/10.1016/j.jcou.2022.102113.
Chen X, Ullah S, Ye R, Jin C, Hu H, Hu F, Peng Y, Lu Z-H, Feng G, Zhou L, Zhang R. Insights into the Zn promoter for improvement of Ni/SiO2 catalysts prepared by the ammonia evaporation method toward CO2 methanation. Energy Fuels. 2023;37:3865–74. https://doi.org/10.1021/acs.energyfuels.2c03701.
Deng L, Tan Y, Chen C, Li F, Zhang X. NiCo composite catalysts for CO2 methanation: the effect of preparation methods on catalyst structure and activity. New J Chem. 2023;47:8032–41. https://doi.org/10.1039/d3nj00434a.
Zurrer T, Wong K, Horlyck J, Lovell EC, Wright J, Bedford NM, Han Z, Liang K, Scott J, Amal R. Mixed-metal MOF-74 templated catalysts for efficient carbon dioxide capture and methanation. Adv Funct Mater. 2021;31:2007624. https://doi.org/10.1002/adfm.202007624.
Acknowledgements
This research has been co‐financed by the European Union NextGenerationEU under the call RESEARCH—CREATE—INNOVATE 16971 Recovery and Resilience Facility (project code: TAEDK-06169).
Author information
Authors and Affiliations
Contributions
GV, EM, ML and AL contributed to the design and implementation of the research. GV, EM, ML and MK contributed to the analysis of the results and to the writing of the manuscript. MK and GM conceived the original and supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lykaki, M., Mandela, E., Varvoutis, G. et al. State-of-the-art thermocatalytic systems for CH4 and CO production via CO2 hydrogenation: critical comparison, mechanistic considerations and structure-performance insights. Discov Chem Eng 4, 11 (2024). https://doi.org/10.1007/s43938-024-00048-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-024-00048-7