Abstract
The mitigation of hazardous effects of chemical dyes on the environment and living organisms, greatly demands an efficient and effective management of dye-laden wastewater. One such solution gaining prominence is the utilization of metal-infused nanofibers-based dye removal techniques, which are simple, effective, and environmentally friendly. In this report, we present the fabrication of mechanically stable hydrophobic nanofibers infused with metal blends, fabricated through the utilization of electrospinning techniques. The successful fabrication of these mechanically stable hydrophobic nanofibers is evidenced through contact angle measurements, tensile tests, and FESEM analysis. While polystyrene-based nanofibrous mats were anticipated to be effective, nanofibrous mats infused with Cu-Fe and Cu-Ni metal blends exhibit exceptional efficacy in degrading dyes. The size and morphology of nanofibers depend on polymer concentration, with the average diameter increasing from 13 to 20%. At a 20% polystyrene concentration, only nanometer-scale fibers of polystyrene polymers were fabricated, while both Cu-Fe and Cu-Ni metal blend-infused fibers were synthesized in micrometers. Fibers infused with Cu-Fe and Cu-Ni metal blend at a 17% polymer concentration displayed nano-scale diameters, confirmed by FESEM characterizations. The heat-based technique is identified as an accessible and cost-effective approach for industries reliant on color-based processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the twenty-first century, contamination of natural resources has become a significant concern. Scientists are actively working to develop effective and affordable cleaning methods to address this problem [1,2,3,4,5]. Many organic, inorganic, and dye-related compounds are discharged into the environment by different types of industries. As the demand for colorful products increased, the release of dye-related pollutants increased. Globally, approximately 10,000 different types of dyes are produced, accounting for the manufacturing of 700,000 tons of dyes [6]. Dye-rich wastewater discharged by numerous textile industries is harmful to the living as well as non-living [7]. These dyes, when dissolved in water, hinder sunlight penetration and reduce photochemical process. This leads to a decrease in dissolved oxygen (DO) levels in water and increased biological oxygen demands as well as chemical oxygen demand (COD), all of which have negative effects on the environment and human health [8]. The effluents from textile and paper industries pose serious problems for aquatic and terrestrial life due to the coloring, toxic, mutagenic, carcinogenic, and teratogenic nature of these color dyes [9, 10]. Numerous methods have been developed to remove biological, chemical, and physical contaminants [11,12,13,14,15]. Latest technologies and designs including physical, chemical, and biological have been investigated from time to time to eliminate dyes from water [16]. Among different dye removal techniques, adsorption-based techniques are widely used to eliminate the contaminants discharged by textile, color, food, printing, and other industries [17]. Polymer-based nanofibers do not accumulate chemicals and are proven to be affordable, highly efficient, durable, and sustainable due to their large surface area-to-volume ratio [18]. Excellent chemical, mechanical, piezoelectric, and biological properties of nanofibers make them unique for different applications like textile, biomedical, nano-sensors, water cleaning, oil separation, air filtration, energy harvesting, etc [19,20,21,22].
Electrospinning involves high electrostatic force to stretch the polymer jet in the fibers fabrication process [23, 24]. Electrospun nanofibers offer advantages such as rapid and easy production, morphological variations, versatility, etc. All these make them suitable for various applications, including filtration, separation, sensing, energy harvesting, tissue engineering, etc. [21, 25,26,27,28,29]. They are particularly useful for large-scale applications due to the ease of industrial production [30, 31]. There are some issues such as size, quality, and morphology, but these can be controlled and resolved by choosing optimum polymer concentration and solvent to produce smooth, rough, cactus, hollow, porous, wrinkled, grooved and crimped structured nanofibers [21, 25, 32]. Polystyrene nanofibers, with their super-hydrophobic and oleophilic properties, are suitable for water-based applications [33, 34]. The use of metal-based nanoparticles has shown promise in pollutant removal [35,36,37,38]. The combination of metals and metal oxides, have gained great attention by their probable potential use in the decontamination process [39,40,41,42].
Blending metals with other materials enhances stability and catalytic activity. Ongoing research in this field has led to significant advancements in efficient cleaning technologies for protecting the environment. Metal combinations have demonstrated strong oxidation and degradation capabilities against organic dyes like methylene blue (MB) and methylene orange (MO) [43]. Nanofibers infused with copper synthesized by electrospinning techniques are found to have remarkable results against dye-rich wastewater treatment [44]. Although polystyrene possesses unique dye-degrading properties, the combination of electrospun nanofibers with metals like Cu-Fe and Cu-Ni presents a promising avenue for applications in filtration, sensing, wastewater treatment, etc. [45,46,47,48,49,50,51]. Copper and nickel have long been recognized for their cleaning and antimicrobial properties. Particularly copper can form coordination complexes with ligands in oxidation states of + 1 and + 2 [52, 53]. Metal complexes have been frequently synthesized for various applications [54,55,56,57,58]. Cu and Ni nanoparticles exhibited excellent biological and chemical dye degradation properties against MB and MO [59, 60]. However, designing a multifunctional filter to cope with problems such as particulate matter, oily wastewater, and microorganisms is a highly challenging task [61, 62]. Cu-Fe and Cu-Ni dual metals are an ideal choice due to their enhanced efficiency instead of a single one for breaking chemical dye bonds[63], making them suitable for decontaminating toxic organic matters [64], heavy metals [65], and other contaminants available in the water [66]. The incorporation of copper with iron and nickel into nanofibers enhances their efficiency. Several dye-based industries utilize heat for several applications. Nanofibers and their applications for heat-based dye degradation approaches may also be found to be more suitable and economical for dye-based industries due to the availability of heat generation structures [67].
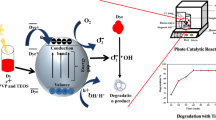
Dyes can be degraded by piezoelectric, photoelectric, and thermoelectric catalysis methods. Out of these, thermoelectric catalysis requires fewer operational conditions which are easily available in color-based industries. The thermal energy greater than the band gap of the catalyst produces a large number of free electrons in the conduction band. Organic dyes such as MB and MO have interesting characteristics in the range of 50–80 ℃ [68]. The large surface of nanofibers provides an easy and fast way to degrade dyes by adsorbing them [69]. The metal catalysts were found effective in accelerating the degradation of dyes into harmless byproducts [59, 60, 70, 71]. The free electrons and holes produced from the catalyst by heat excitation come into contact with nearby oxygen and water molecules to generate hydrogen peroxide, OH and O2.− free radicals, which are supposed to break down dye molecules through several steps into simpler molecules such as water, carbon dioxide, ammonium, sulfates, etc. [72].
The objective of this study is to design an efficient and environmentally friendly technique for dye degradation by synthesizing nanofibers infused with metal blend. The electrospinning process is employed to fabricate the nanofibers of Cu-Fe and Cu-Ni metal blends. The size and morphology of nanofibers are highly dependent on polymer concentration and the average diameter of fibers increased with increasing concentration from 13 to 20% [73, 74]. Mechanical analysis and contact angle measurements have been employed to assess the strength and water-loving nature of the nanofibrous mats. The study aims to showcase an enhancement in the efficiency and rate of dye removal by utilizing various metal blends, with a specific focus on MB and MO dyes under elevated temperature conditions [75].
2 Material and methods
2.1 Materials
Polystyrene (Pallets, Average MW 280000 g/mole, Melt Index 2.8–3.8 g/10 min), Ferric (III) chloride anhydrous (Merk Life Science, Assay ≥ 98%), Cupric acetate monohydrate and Nickel acetate tetrahydrate (Molychem, Min. Assay 98%), N, N-dimethyl formaldehyde (SDFCL, Min. Assay 99.0%), Acetone (Avarice Laboratories, Min. Assay 99.0%).
2.2 Methods
2.2.1 Preparation of polystyrene-based electrospinning solution
The polystyrene pellets were dissolved in a DMF & Acetone mixture (80:20) at different concentrations ranging from 13%, 17%, and 20% (w/v). The solute and solvent were mixed until a homogeneous solution was achieved. A continuous stirring for 5 to 6 h results in a clear solution.
2.2.2 Preparation of Cu-Fe metal blend-based electrospinning solution
A solution was prepared by dissolving 1.5 mol of Cu(CH3COO)2·H2O and 0.15 mol of anhydrous FeCl3 in 3 ml DMF. The resulting metal solution was then mixed, and the combined solution labeled as A. Subsequently, 1 ml of the metal blend solution was added to each beaker containing polystyrene solutions with varying concentrations of 13%, 17%, and 20% (w/v) [73]. The resulting solutions were labeled as B, C, and D, respectively. Each of these solutions (B, C, and D) underwent continuous stirring for 5–6 h at 60 ℃ on a hot plate to obtain the final electrospinning solutions, as depicted in Fig. 1.
2.2.3 Preparation of Cu-Ni metal blend-based electrospinning solution
In a separate container, 2 ml of DMF was used to dissolve 1.5 mol of Cu(CH3COO)2·H2O and 0.15 mol of C4H14NiO8. The solution was thoroughly mixed, resulting in the formation of the metal blend solution designated as E. In two additional containers, polystyrene at concentrations of 17% and 20% (w/v) was taken, and 1 ml of the metal blend solution was added. These resulting solutions of polystyrene and metal blend were labeled as F and G, respectively. The final solutions were allowed to swirl for 5–6 h to obtain the resultant electrospun solutions, as illustrated in Fig. 2.
2.2.4 Electrospinning process for nanofibers
An electrospinning setup, including a high-voltage source, drum collector, syringe pump, computer with software, and a controlled synthesis chamber, was used to fabricate nanofibers. Adjustment to parameters such as high voltage, electrode distance, needle spacing, and feed rate were made to produce bead-free nanofibers. A conducting sheet was placed on a grounded collector at some distance from the needle tip and the voltage range was adjusted to operate during the process. The solution was injected with a suitable feed rate to get bead free fibers. To ensure safety, the impact of air current on the system was minimized by operating the entire setup in a closed environment.
To initiate the electrospinning process, the electrospinning solution was loaded in a syringe, holding a metallic needle at the end. The needle tip was kept at a distance of 40 cm from the collector. The conducting sheet of aluminum was wrapped around the collector plate to collect the deposited fibers. The metallic needle was brought into contact with the high-voltage source 17 kV, while the collector was electrically grounded [76]. The electrospun solution was supplied at a feed rate of 0.8 ml/hr [77]. The metal blend polymer solution elongates to deposit fibers on the collecting sheet [28]. The same electrospinning process was repeated to get a thick sheet of fibers. The solvent nature and concentration variations of the polymer greatly affect the size and morphology of nanofibers. Table 1 acquaint about effect of concentration variations and solvent on the diameter of bead-free fibers fabricated with only polystyrene and polystyrene along with a metal blend.
3 Results and discussion
3.1 Morphological characterization
The morphology of the synthesized fibers was examined through optical microscope and FESEM (Fig. 3). The efficiency of nanofibers is significantly influenced by their diameter and surface nature. Parameters such as concentration, viscosity, and the presence of conducting materials played a substantial role in shaping the synthesis parameters and morphology of the fibers. The FESEM images illustrate the concentration variations of polymers significantly influenced the size of fibers. The bead-free fibers were fabricated by varying the ratio of the mixed solvent mixture. The electrospinning process was applied to produce fibers at 20% polystyrene concentration. The fibers were observed to be bead-free and the diameters were found in the range of nanometers.[S1].
3.1.1 Cu-Fe metal blend nanofibers
The fibers fabricated with polystyrene concentration at 13% and Cu-Fe metal blend were observed structural irregularities and large diameters on optical microscope [S2]. The fibers fabricated with polystyrene concentration at 20% with DMF and acetone (80:20) have revealed continuous and bead-free morphology. The fibers were observed in the micrometer range when characterized by FESEM at 30 µm, 20 µm, and 10 µm scales. However, a close examination at 1 µm exposed rough surface morphology.[S3] FESEM images revealed the fibers in the nanometer range with polystyrene concentration at 17% with a mixture of DMF and acetone (80:20). The magnified images confirmed the uniform and bead-free nanofibers with diameters ranging between 497 and 966 nm [77]. The presence of infused metals was confirmed with the help of EDAX images [S5].
3.1.2 Cu-Ni metal blend nanofibers
The bead free fibers were fabricated with diameters ranging from micrometers to nanometers when polystyrene concentration at 20% including Cu-Ni metal blend. The continuous fibers were confirmed by FESEM images in the micrometer range [S4]. The nanofibers were also observed devoid of beads when electrospun at 17% polystyrene and metal blend solution in a mixture of DMF and acetone (80:20). The fibers were found within the nanometer range. The infused metals were confirmed by EDAX graphs [S6]. Cu-Fe, and Cu-Ni metal blend nanofibrous mats images are presented in Fig. 3a-d.
3.2 Hydrophobicity of Cu-Fe and Cu-Ni metal blend nanofibrous mats
To assess the wetting characteristics of metal blend-infused nanofibers, static contact angle analysis was employed [78,79,80]. A couple of nanofibers of each type were fixed and pressed on a small piece of aluminum sheet to make mat-like designs. Deionized water (DI water) was utilized to assess the hydrophobic characteristics of the nanofibrous mats [81,82,83]. Rapid observations were conducted by capturing 10 consecutive images of a 5 µl droplet on the surface of the nanofibrous mats. All the images of DI water were captured using a fixed camera and digitally processed. The results consistently revealed a contact angle close to 92 degrees for Cu-Fe and nearly 94 degrees for Cu-Ni metal blend-infused nanofibrous mats, as illustrated in Fig. 4. All contact angle values were found to be slightly higher than 90 degrees, indicating that the nanofibrous mats exhibit a marginal hydrophobic characteristic towards DI water. This signified that the cohesive forces were slightly stronger than the adhesive forces and nanofibers have little affinity for fats and oils. Moreover, the contact angle remained relatively stable across 10 successive observations, indicting that the water droplets did not penetrate the surface of the nanofibrous mats [S7,S8].
3.3 Mechanical properties of nanofibrous mats
The mechanical properties of the developed nanofibrous mats were evaluated through tensile testing using a UTM machine. Specimens, measuring 5 mm in width, 20 mm in length, and 1 mm in thickness, were obtained from each nanofibrous mat. The testing was conducted at room temperature, applying stretching forces at a rate of 0.2 mm/min with a constant rate of strain and load. Under the applied stress, the nanofibrous mats underwent stretching and initiated necking down, attributed to the presence of ductile metals in the mats. The stress–strain curves were plotted to analyze the properties of the nanofibrous mats. The tensile test assessed the force sustainability of the nanofibrous mats up to 63.5% strain. The properties of both types of nanofibrous mats were significantly influenced by the presence of metals, a correlation confirmed by EDAX data. Cu-Fe and Cu-Ni metal blend-infused nanofibrous mats demonstrated a maximum strength of up to 14.1 MPa, establishing their suitability for wastewater treatment. The high strain values were attributed to the presence of copper metal in both types of mats. Figure 5 illustrates the closely matched results in the stress–strain curve for both types of mats.
4 Discussion
4.1 Enhancement in dye degradation efficiency
Polystyrene-based nanofibers exhibit inherent efficacy for dye degradation; however, the incorporation of a metal blend into polystyrene yields significant enhancements. While the heat-based degradation technique is straightforward, its efficiency in dye degradation is closely tied to factors such as concentration, contact time, and adsorbent dosages [84].
The enhancement in dye degradation efficiency due to the infusion of metal blend in polystyrene was analyzed on nanofibrous mats. Two different sets of nanofibrous mats of polystyrene, Cu-Fe, and Cu-Ni metal blend-infused nanofibers were prepared. The dye solutions were employed, containing 50 µl of MB in 5 ml of RO water, labeled as X, and pure MO dye labeled as Y. A 50 µl droplet of each solution X and Y was placed on the electrospun nanofibrous mats. Subsequently, these mats were heated to 80 ℃ on a hot plate to initiate the process of moisture evaporation and mass loss [85]. The heating process was maintained at a constant temperature, and observations were recorded over 50 min at regular 10-min intervals. Deformation in shape and color fading of MB and MO drops indicated the effectiveness of the infused metal blend in the polystyrene. The water content in the dye evaporated within a few minutes of heating, leading to a gradual fading of the dye solution color. After some time, a saturation point was reached, showing no significant change in the color of the dye solutions. Pure polystyrene nanofibrous mats exhibited inferior performance and a lower degradation rate against color dyes compared to metal blend-infused nanofibrous mats. No further changes in the degradation efficiency and rate were observed for MB and MO dyes on each nanofibrous mat after 50 min of processing. The degradation efficiency of Cu-Fe metal blend-infused nanofibrous mats was notably high due to the presence of ferric and copper metal blends. As nickel is an active metal, Cu-Ni metal blend nanofibrous mats were anticipated to degrade color dyes at a much faster rate compared to others. The degradation efficiency was observed to be higher for Cu-Fe metal blend-infused nanofibrous mats. Different visual images were collected during the dye degradation process. Tables 2 and 3 demonstrate the enhanced dye degradation efficiency of nanofibers with the insertion of a metal blend in polystyrene.
5 Conclusion
In conclusion, this study outlines the fabrication of mechanically stable hydrophobic nanofibers infused with metal blends. We optimized the polymer concentration to achieve bead-free fibers in the nanometer range. Higher concentrations and viscosities led to reduced polymer conductivity due to chain entanglements. Cu-Fe, Cu-Ni metal blend-infused, and polystyrene-based nanofibers exhibited bead-free surfaces at a 20% polystyrene concentration. Specifically, at a 20% concentration, only polystyrene polymers were fabricated in the nanometer range, while both metal blend-infused fibers were synthesized in micrometers. Cu-Fe and Cu-Ni metal blend-infused fibers formed at a 17% polymer concentration showed nano-scale diameters, confirmed by FESEM characterizations. The elongated polystyrene monomer chains increased solution viscosity, and the inclusion of acetone minimized nanofiber adhesion to the collector sheet. Contact angle measurements revealed that Cu-Fe and Cu-Ni blend-infused nanofibers maintained a mild hydrophobic nature over an extended period, and the stress–strain curve confirmed their strength for wastewater treatment. Cu-Fe, Cu-Ni metal blend-infused, and polystyrene-based nanofibers demonstrated efficacy in degrading both types of dyes. Our results highlighted the superior performance of Cu-Fe metal blend-infused nanofibers compared to Cu-Ni metal blend-infused and only polystyrene-based nanofibers.
This study underscores the promising attributes and potential applications of Cu-Fe and Cu-Ni metal blend-infused nanofibers in environmental cleanup. The technique offers a potentially economical and industrially suitable approach for color and textile industries to degrade their color dyes, reducing reliance on external resources.
Data availability
The data set generated is already presented in the manuscript.
References
Henkel M. 21st century homestead: sustainable agriculture II: farming and natural resources. Morrisville: Lulu Com; 2015.
Brooks BW, Sabo-Attwood T, Choi K, Kim S, Kostal J, LaLone CA, Langan LM, Margiotta-Casaluci L, You J, Zhang X. Toxicology advances for 21st century chemical pollution. One Earth. 2020;2(4):312–6.
Schwitzguebel JP, Kumpiene J, Comino E, Vanek T. From green to clean: a promising and sustainable approach towards environmental remediation and human health for the 21st century. Agrochimica. 2009;53(4):1–29.
Varsha M, Kumar PS, Rathi BS. A review on recent trends in the removal of emerging contaminants from aquatic environment using low-cost adsorbents. Chemosphere. 2022;287:132270.
Lim AP, Aris AZ. A review on economically adsorbents on heavy metals removal in water and wastewater. Rev Environ Sci Biotechnol. 2014;13:163–81.
Moussavi G, Mahmoudi M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J Hazard Mater. 2009;168(2–3):806–12.
Solayman HM, Hossen MA, Abd Aziz A, Yahya NY, Hon LK, Ching SL, Monir MU, Zoh KD. Performance evaluation of dye wastewater treatment technologies: a review. J Environ Chem Eng. 2023;11:109610.
Ahmad A, Mohd-Setapar SH, Chuong CS, Khatoon A, Wani WA, Kumar R, Rafatullah M. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 2015;5(39):30801–18.
Mcyotto F, Wei Q, Macharia DK, Huang M, Shen C, Chow CW. Effect of dye structure on color removal efficiency by coagulation. Chem Eng J. 2021;405:126674.
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov. 2019;3(2):275–90.
Hasan HA, Muhammad MH. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J Water Process Eng. 2020;33:101035.
Srinivasan R. Advances in application of natural clay and its composites in removal of biological, organic, and inorganic contaminants from drinking water. Adv Mater Sci Eng. 2011.
Fu F, Cheng Z, Lu J. Synthesis and use of bimetals and bimetal oxides in contaminants removal from water: a review. RSC Adv. 2015;5(104):85395–409.
Gole A, John D, Krishnamoorthy K, Wagh NS, Lakkakula J, Khan MS, Odeibat HAM, Tarique M, Islam MR. Role of phytonanotechnology in the removal of water contamination. J Nanomater. 2022;2022:1–19.
Guchi E. Review on slow sand filtration in removing microbial contamination and particles from drinking water. Am J Food Nutr. 2015;3(2):47–55.
Nachiyar CV, Rakshi AD, Sandhya S, Jebasta NBD, Nellore J. Developments in treatment technologies of dye-containing effluent: a review. Case Stud Chem Environ Eng. 2023;7:100339.
Alfei S, Grasso F, Orlandi V, Russo E, Boggia R, Zuccari G. Cationic polystyrene-based hydrogels as efficient adsorbents to remove methyl orange and fluorescein dye pollutants from industrial wastewater. Int J Mol Sci. 2023;24(3):2948.
Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS. Electrospinning of nanofibers. J Appl Polym Sci. 2005;96(2):557–69.
Zaarour B, Zhu L, Jin X. Direct generation of electrospun branched nanofibers for energy harvesting. Polym Adv Technol. 2020;31(11):2659–66.
Zaarour B, Liu W. Recent advances of textile sorbents for oil spills cleanup: a review. J Ind Text. 2023;53:15280837231186652.
Zaarour B, Zhu L, Huang C, Jin X. Enhanced piezoelectric properties of randomly oriented and aligned electrospun PVDF fibers by regulating the surface morphology. J Appl Polym Sci. 2019;136(6):47049.
Zaarour B, Tina H, Zhu L, Jin X. Branched nanofibers with tiny diameters for air filtration via one-step electrospinning. J Ind Text. 2022;51(1_suppl):1105S-1117S.
Zaarour B, Zhu L, Huang C, Jin X. Controlling the secondary surface morphology of electrospun PVDF nanofibers by regulating the solvent and relative humidity Nanoscale. Res Lett. 2018;13(1):1–11.
Zaarour B, Alhinnawi MF. A comprehensive review on branched nanofibers: preparations, strategies, and applications. J Ind Text. 2022;51(1_suppl):1S-35S.
Nam C, Lee S, Ryu M, Lee J, Lee H. Electrospun nanofiber filters for highly efficient PM 2.5 capture. Korean J Chem Eng. 2019;36:1565–74.
Halicka K, Cabaj J. Electrospun nanofibers for sensing and biosensing applications—A review. Int J Mol Sci. 2021;22(12):6357.
Zaarour B, Zhu L, Huang C, Jin X. A mini review on the generation of crimped ultrathin fibers via electrospinning: materials, strategies, and applications. Polym Adv Technol. 2020;31(7):1449–62.
Zaarour B, Zhu L, Jin X. A review on the secondary surface morphology of electrospun nanofibers: formation mechanisms, characterizations, and applications. Chem Sel. 2020;5(4):1335–48.
Zaarour B, Zhu L, Huang C, Jin X, Alghafari H, Fang J, Lin T. A review on piezoelectric fibers and nanowires for energy harvesting. J Ind Text. 2021;51(2):297–340.
Ramakrishna S, Fujihara K, Teo WE, Yong T, Ma Z, Ramaseshan R. Electrospun nanofibers: solving global issues. Mater Today. 2006;9(3):40–50.
Schreuder-Gibson HL, Gibson P. Applications of electrospun nanofibers in current and future materials. Washington: American Chemical Society; 2006.
Nady N, Rehim MHA, Badawy AA. Dye removal membrane from electrospun nanofibers of blended polybutylene succinate and sulphonated expanded polystyrene waste. Sci Rep. 2023;13(1):15455.
Eang C, Opaprakasit P. Electrospun nanofibers with super hydrophobicity derived from degradable polylactide for oil/water separation applications. J Polym Environ. 2020;28(5):1484–91.
Gao J, Li B, Wang L, Huang X, Xue H. Flexible membranes with a hierarchical nanofiber/microsphere structure for oil adsorption and oil/water separation. J Ind Eng Chem. 2018;68:416–24.
Zhang L, Fang M. Nanomaterials in pollution trace detection and environmental improvement. Nano Today. 2010;5(2):128–42.
Hussain I, Singh NB, Singh A, Singh H, Singh SC. Green synthesis of nanoparticles and its potential application. Biotech Lett. 2016;38:545–60.
Smith SC, Rodrigues DF. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon. 2015;91:122–43.
Sahoo TR, Prelot B. Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology. In: Nanomaterials for the detection and removal of wastewater pollutants. Amsterdam: Elsevier; 2020. p. 161–222.
Gautam S, Agrawal H, Thakur M, Akbari A, Sharda H, Kaur R, Amini M. Metal oxides and metal organic frameworks for the photocatalytic degradation: a review. J Environ Chem Eng. 2020;8(3):103726.
Khulbe KC, Matsuura T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl Water Sci. 2018;8:1–30.
Bodzek M. Nanoparticles for water disinfection by photocatalysis: a review. Arch Environ Prot. 2022;48(1):3–17.
Prakash J, Krishna SBN, Kumar P, Kumar V, Ghosh KS, Swart HC, Bellucci S, Cho J. Recent advances on metal oxide based nano-photocatalysts as potential antibacterial and antiviral agents. Catalysts. 2022;12(9):1047.
Singh J, Dhaliwal AS. Synthesis of rGO/AgNPs adsorbent for the effective removal of two basic dyes: kinetics, isotherms and thermodynamic studies. Int J Environ Sci Technol. 2022;20:11483–11500.
Wei L, Song Y, Liu P, Kang X. Polystyrene nanofibers capped with copper nanoparticles for selective extraction of glutathione prior to its determination by HPLC. MicrochimicaActa. 2018;185:1–8.
Konieczny J, Rdzawski Z. Antibacterial properties of copper and its alloys. Arch Mater Sci Eng. 2012;56(2):53–60.
Saqib S, Munis MFH, Zaman W, Ullah F, Shah SN, Ayaz A, Farooq M, Bahadur S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc Res Tech. 2019;82(4):415–20.
Radwan HA, Ismail RA, Abdelaal SA, Al Jahdaly BA, Almahri A, Ahmed MK, Shoueir K. Electrospun polycaprolactone nanofibrous webs containing Cu–Magnetite/Graphene oxide for cell viability, antibacterial performance, and dye de-colorization from aqueous solutions. Arab J Sci Eng. 2021;47:303–318.
Savva I, Marinica O, Papatryfonos CA, Vekas L, Krasia-Christoforou T. Evaluation of electrospun polymer–Fe 3 O 4 nanocomposite mats in malachite green adsorption. RSC Adv. 2015;5(21):16484–96.
Yildiz A, Bayramol DV, Atav R, Ağirgan AÖ, Kurç MA, Ergünay U, Mayer C, Hadimani RL. Synthesis and characterization of Fe3O4@ Cs@ Ag nanocomposite and its use in the production of magnetic and antibacterial nanofibrous membranes. Appl Surf Sci. 2020;521:146332.
Merzougui C, Miao F, Liao Z, Wang L, Wei Y, Huang D. Electrospun nanofibers with antibacterial properties for wound dressings. J Biomater Sci Polym Ed. 2022;33(16):2165–83.
Saharan P, Chaudhary GR, Mehta SK, Umar A. Removal of water contaminants by iron oxide nanomaterials. J Nanosci Nanotechnol. 2014;14(1):627–43.
Jadhav MS, Kulkarni S, Raikar P, Barretto DA, Vootla SK, Raikar US. Green biosynthesis of CuO & Ag–CuO nanoparticles from Malusdomestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. New J Chem. 2018;42(1):204–13.
Henzler K, Heilemann A, Kneer J, Guttmann P, Jia H, Bartsch E, Lu Y, Palzer S. Investigation of reactions between trace gases and functional CuO nanospheres and octahedrons using NEXAFS-TXM imaging. Sci Rep. 2015;5(1):17729.
Padil VVT, Černík M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomed. 2013;889–898.
Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–1.
Zhang DY, Nie Y, Sang H, Suo JJ, Li ZJ, Gu W, Tian JL, Liu X, Yan SP. Three structurally related Copper complexes with two isomers: DNA/BSA binding ability, DNA cleavage activity and excellent cytotoxicity. Inorg Chim Acta. 2017;457:7–18.
Arfat YA, Ejaz M, Jacob H, Ahmed J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohyd Polym. 2017;157:65–71.
Gammage MD, Stauffer S, Henkelman G, Becker MF, Keto JW, Kovar D. Ethylene binding to Au/Cu alloy nanoparticles. Surf Sci. 2016;653:66–70.
Chaudhary J, Tailor G, Yadav BL, Michael O. Synthesis and biological function of Nickel and copper nanoparticles. Heliyon. 2019;5(6):e01878.
Chitra K, Manikandan A, Moortheswaran S, Reena K, Antony SA. Zingiber officinale extracted green synthesis of copper nanoparticles: structural, orphological and antibacterial studies. Adv Sci, Eng Med. 2015;7(8):710–6.
Xia M, Xiong Z, Yao Z, Wu Y, Cheng Q, Xu J, Liu K, Wang D. A novel gradient structured nanofiber and silver nanowire composite membrane for multifunctional air Filters, oil water Separation, and health monitoring flexible wearable devices. J Colloid Interface Sci. 2023;630:484–93.
Zhu L, Zaarour B, Jin X. Unexpectedly high oil cleanup capacity of electrospun poly (vinylidene fluoride) fiber webs induced by spindle porous bowl like beads. Soft Mater. 2019;17(4):410–7.
Sun W, Xiao L, Wu X. Facile synthesis of NiO nanocubes for hotocatalysts and supercapacitor electrodes. J Alloy Compd. 2019;772:465–71.
Lofrano G, Libralato G, Adinolfi R, Siciliano A, Iannece P, Guida M, Giugni M, Ghirardini AV, Carotenuto M. Photocatalytic degradation of the antibiotic chloramphenicol and effluent toxicity effects. Ecotoxicol Environ Saf. 2016;123:65–71.
Soltani-nezhad F, Saljooqi A, Shamspur T, Mostafavi A. Photocatalytic degradation of imidacloprid using GO/Fe3O4/TiO2-NiO under visible radiation: ptimization by response level method. Polyhedron. 2019;165:188–96.
Aboelfetoh EF, Aboubaraka AE, Ebeid EZM. Synergistic effect of iron and copper oxides in the removal of organic dyes through thermal induced catalytic degradation process. J Clust Sci. 2023;34:2521–2535.
Cheng L, Liu G, Jin W. Recent advances in facilitated transport membranes for olefin/paraffin separation. Discov Chem Eng. 2021;1:1–11.
Fan L, Su X, Zhu H, Liu H, Lou S, Shi Y, Yan S. Degradation of methylene blue by hot electrons transfer in SnSe. Adv Mater Interfaces. 2023;10(11):2202207.
El-Sharkawy EA, Soliman AY, Al-Amer KM. Comparative study for the removal of methylene blue via adsorption and photocatalytic degradation. J Colloid Interface Sci. 2007;310(2):498–508.
Zulfi A, Fauzi A, Edikresnha D, Munir MM. Synthesis of high-impact polystyrene fibers using electrospinning. IOP Conf Ser: Mater Sci Eng. 2017;202(1):012010.
Yang C, Dong W, Cui G, Zhao Y, Shi X, Xia X, Tang B, Wang W. Highly efficient photocatalytic degradation of methylene blue by P2ABSA-modified TiO 2 nanocomposite due to the photosensitization synergetic effect of TiO 2 and P2ABSA. RSC Adv. 2017;7(38):23699–708.
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann JM. Photocatalytic degradation pathway of methylene blue in water. Appl Catal B. 2001;31(2):145–57.
Veleirinho B, Rei MF, Lopes-DA-Silva JA. Solvent and concentration effects on the properties of electrospun poly (ethylene terephthalate) nanofiber mats. J Polym Sci, Part B: Polym Phys. 2008;46(5):460–71.
Liu W, Huang C, Jin X. Electrospinning of grooved polystyrene fibers: effect of solvent systems. Nanos Res Lett. 2015;10:1–10.
Shuchi SB, Suhan MBK, Humayun SB, Haque ME, Islam MS. Heat-activated potassium persulfate treatment of Sudan Black B dye: degradation kinetic and thermodynamic studies. J Water Process Eng. 2021;39:101690.
Hossain MT, Shahid MA, Ali A. Development of nanofibrous membrane from recycled polyethene terephthalate bottle by electrospinning. OpenNano. 2022;8:100089.
Alam MR, Shahid MA, Alimuzzaman S, Hasan MM, Hoque ME. Electrospun bio-nano hybrid scaffold from collagen, Nigella sativa, and chitosan for skin tissue engineering application. J Bioact Compat Polym. 2023;38(3):234–51.
Yue X, Li Z, Zhang T, Yang D, Qiu F. Design and fabrication of superwettingfiber-based membranes for oil/water separation applications. Chem Eng J. 2019;364:292–309.
Zhao T, Jiang L. Contact angle measurement of natural materials. Colloids Surf, B. 2018;161:324–30.
Zaarour B, Zhu L, Jin X. Direct fabrication of electrospun branched nanofibers with tiny diameters for oil absorption. J Dispersion Sci Technol. 2021;42(14):2085–91.
Yuan Y, Choi SO, Kim J. Analysis of contact area between water and irregular fibrous surface for prediction of wettability. RSC Adv. 2016;6(77):73313–22.
Kulkarni PS, Patel SU, Chase GG. Layered hydrophilic/hydrophobic fiber media for water-in-oil coalescence. Sep Purif Technol. 2012;85:157–64.
Ellison AH, Zisman WA. Wettability studies on nylon, polyethylene terephthalate and polystyrene. J Phys Chem. 1954;58(6):503–6.
Rahman MW, Nipa ST, Rima SZ, Hasan MM, Saha R, Halim MA, Ali Y, Deb A. Pseudo-stem banana fiber as a potential low-cost adsorbent to remove methylene blue from synthetic wastewater. Appl Water Sci. 2022;12(10):242.
Nacowong P, Saikrasun S. Thermo-oxidative and weathering degradation affecting coloration performance of lac dye. Fash Text. 2016;3:1–16.
Acknowledgements
The authors express sincere gratitude to C4DFED, IIT Mandi, for providing access to the Electrospin instrument facilities essential for fabricating the desired nanofibers. Additionally, characterization facilities including FESEM, EDAX, and contact angle measurements were made available by C4DFED, IIT Mandi, in support of this study.
Funding
The work has not received a specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
VKS: conceptualization, synthesis, analysis, design, writing—original draft, PK: synthesis, design, writing—original draft, SK: writing—original draft, analysis, MY: analysis, BA: analysis, design.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval is not required as the data presented here is based on an experimental study.
Consent fot publication
All Authors of this draft express their consent to publish this experimental research.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, V.K., Kumar, P., Kumar, S. et al. Cu-Fe and Cu-Ni metal blend-infused polystyrene-based electrospun nanofibers for dye degradation. Discov Chem Eng 4, 5 (2024). https://doi.org/10.1007/s43938-024-00042-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-024-00042-z