Abstract
Groundwater and surface water quality is of great significance for humanity as they serve as the primary drinking water sources globally. Due to population growth and the need to provide people with necessities that depend on water as an essential resource, these bodies of water are becoming more polluted. The present study involved the collection of groundwater and surface water samples from Kampala and Mbarara districts in Uganda during the dry and wet seasons. Also, concentrations of lead (Pb), manganese (Mn), cadmium (Cd), copper (Cu), and iron (Fe) were analyzed in order to evaluate the toxicity of metals, identify potential sources, and determine the health risk associated with their presence in water. Results showed that metals were observed with higher concentration during the wet season than the dry season. The concentrations of Fe (8.646 ± 0.00 mg/L), Mn (2.691 ± 0.01 mg/L) and Cd (0.090 ± 0.41 mg/L) measured in groundwater were significantly higher than those measured in surface water. However, only Cu was observed with higher concentration (0.322 ± 0.06 mg/L) in surface water during the wet season. Furthermore, the degree of contamination (Cd) and the heavy metal pollution index (HPI) were evaluated for both the wet and dry seasons. Results showed that few samples were found in the category of portable drinking water while majority are within the “poor” and “very poor” classes which require proper treatment before consumption. The oral hazard index (HIoral) results showed that none of the samples are suitable for consumption; therefore, cause potential non-carcinogenic health issues to the consumer (HIoral > 1). Moreover, children are more at risk than adults in the study districts (higher HI values in both seasons). This study recommends frequent monitoring of quality of water and also effluent waste treatment by the major source of pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The United Nations convention regards the right to access clean drinking water as a universal human right [1, 2]. However, for many developing nations in Asia, South America, and Africa, this human right is still merely an aspiration [3]. A number of human activities are related to the pollution of soil, groundwater, and surface water. These activities include mining, agriculture, landfilling, industrialization, urbanization and technological advancement. After a period of heavy rainfall, the pollutants are transported by runoff water into water reservoirs, where they are later consumed by people and animals [4,5,6]. Heavy metals have a high geochemical activity and are readily migrated through sediments, water, and suspended particles [7]. Thus, one of the global environmental problems that is more prevalent is the pollution of soils, sediments, groundwater, and surface water by different heavy metals [8,9,10]. In addition, most heavy metals have considerable biological activity and are resistant to biodegradation, which means they may accumulate in the food cycle and enter the human body [11]. Heavy metals can cause harmful effects such as carcinogenicity, teratogenicity, and mutagenicity when their amount in the human body surpass the regulatory limit [12]. For instance, elevated Pb concentrations have been linked to human cognitive deterioration [13], while high Cd concentrations have been linked to liver and skeletal problems as well as a higher chance of lung cancer [14]. In addition, long-term exposure to these hazardous metals may result in fatal conditions including cancer, Parkinson’s, Alzheimer’s, and multiple sclerosis [15]. Moreover, short-term exposure to these metals can induce physical, muscular, and neurological issues [16, 17]. According to previous studies, fast economic growth has led to an increase in the amount of sewage released by chemical companies and the use of pesticides and fertilizers in agriculture, both of which have raised the level of heavy metals in aquatic ecosystems [18].
Uganda is a low-income nation where a large number of people lack access to clean water supplied by National Water and Sewerage Cooperation (NWSC) [19]. According to data from the Uganda NWSC, 34% of the nation's villages and 26% of Uganda's urban areas lack access to safe drinking water [20]. Thus, this population is dependent on groundwater and surface water, both of which are often contaminated. These sources usually include toxic heavy metals like lead, cadmium, chromium, zinc, and mercury along with dissolved organic and inorganic substances like trichloroethylene, volatile organic compounds, chlorides, fluorides, sulphates, carbonates, sodium, potassium, calcium, and magnesium [21]. Individuals who consume such types of water might experience further health issues [22]. Several studies have been conducted on heavy metals in surface and groundwater in various regions of Uganda. These include assessment of heavy metals in Pager River [23], drinking water [24], copper mine and tailing sites [25], groundwater [26], wastewater channel [27] and River Rwizi [28]. Bakyayita and co-workers [29] reported the toxicity of heavy metals in selected groundwater and surface water sources in Kampala city; however, the seasonal variation of the heavy metals from each location were not discussed. More studies on heavy metal regarding regional water quality is necessary to estimate the level of contamination and develop potential mitigation strategies. This is due to the fact that there is a growing need for water in the districts of Kampala and Mbarara to meet the demands of industry, agriculture, and household water supply.
This study presents the results of an extensive investigation to determine the concentration of heavy metals (Cu, Pb, Fe, Cd, and Mn) in surface and groundwater from the districts of Kampala and Mbarara. The two primary objectives of this study were: (1) Examine the presence of heavy metals in the water sources as well as the seasonal and spatial distribution. (2) Assess the pollution indices and the human health risks that these heavy metals contamination poses to the consumers.
2 Materials and methods
2.1 Study area
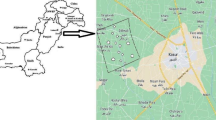
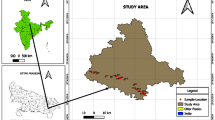
The study focused on Kampala and Mbarara districts which are located in the central and western parts of Uganda respectively with sampling points on groundwater (Fig. 1) and surface water (Fig. 2). The coordinates of the sampling sites mapped in the Figs. 1 and 2 are shown in Table 1. In Kampala district (the nation’s most industrialized commercial district), water samples were collected from 2 protected springs (S1 and S2), 5 shallow wells (S3, S4, S5, S6 and S7) and 2 open springs (S8 and S9). In Mbarara district, wastewater samples were collected from 1 receiving pond (S10), 1 upstream pond (S11) and 1 downstream pond (S12). Moreover, the open ponds were sampled in the Katete sewage pond located in the Mbarara district (Fig. 2). The sample locations were selected based on criteria such as population density, the degree of human activity in the region, and the population's dependency on surface water and groundwater. To understand more fully the seasonal variations in occurrence and distribution of the heavy metals, the study covered three months in a dry season (February, March, and April 2023) as well as three months in a wet season (June, July, and August 2023).
2.2 Sample collection
Water sampling, handling, storage, and chemical analysis were all performed according to international standard methods [30]. One-liter (1 L) polythene containers were used to collect the samples. Sampling containers were thoroughly washed with a 5% solution (v/v) of nitric acid and then rinsed with deionized water 24 h before collecting the samples. Moreover, all the containers were rinsed three times with the sample water before collection at each site. The collected samples were filtered, acidified with nitric acid [31] and stored at 4 °C before analysis.
2.3 Digestion and analysis of heavy metals
Exactly 50 ml aliquot of water sample was transferred into a Teflon vessel and 5 mL of concentrated HNO3 was added. A thermostatic hot plate was then used to gently boil the mixture for about thirty minutes. The digested samples were transferred into a 50 mL standard flask and diluted to volume. An aliquot was taken from the resulting solution and sent for Atomic Absorption Spectroscopy (AAS) analysis. The concentrations of heavy metals in the samples were measured using the calibration curve results of each standard. For the quantitative determination of the heavy metals (Fe, Cd, Pb, Mn andCu), a Thermo Electron Atomic Absorption Spectrometer, model S SERIES system was used. The equipment was calibrated using reference standards of the metals and also blank. All samples were analyzed in the laboratory in triplicate at the Central Instrumentation Research Facility, Covenant University, Otta, Ogun State, Nigeria. The relative standard deviation of the method obtained are: Pb (2.70%), Cd (3.33%), Fe (1.50%), Cu (2.00%), and Mn (1.37%).
2.4 Heavy metal pollution index
Heavy metal pollution index (HPI) was calculated to determine the overall impact of individual metals on water quality. The rating and weight of each metal are used to calculate HPI. According to Rajmohan et al., the rate is an undefined number ranging from 0 to 1, and weight is inversely related to the drinking water regulatory limit of various heavy metal [32]. The maximum admissible concentration of Cu, Fe, Mn, Pb and Cd are 2.00, 1.00, 0.40, 0.01 and 0.005 mg/L respectively [33]. According to Basahi et al., the HPI is classified as: excellent with values ranging from 0 to 25, good (26–50), poor (51–75), very poor (76–100) and unsuitable when HPI value is greater than 100 [34].
2.5 Degree of contamination
The impact of heavy metals on a water body is measured by the degree of contamination (Cd) [34, 35]. The categories of heavy metal pollution based on Cd are as follows: Cd < 1 indicates low pollution, Cd from 1 to 3 indicates moderate pollution, and Cd > 3 indicates high pollution in the water due to trace metals. Cd is calculated using Eqs. (1) and (2).
where Mi and MACi are the measured level and the maximum acceptable concentration (MAC) of the ith metal as recommended by the [33], respectively, and Cfi is the contamination factor.
2.6 Statistical analysis
The average, standard deviation, and Pearson's correlation coefficient were obtained using Minitab® statistical software version 21.4.1. The variance in the trace metals was examined using the ANOVA test. Additionally, Varimax rotation factor analysis was carried out to determine the best fit and explain the correlation among the measured heavy metals.
2.7 Human health risk
Oral injection of contaminated drinking water is the primary means which heavy metals pollution poses health risks to both adults and children. Alternative routes such as cutaneous contacts and aspiration are minor when compared to oral ingestion. The hazard quotient (HQ) and chronic daily intake (CDI, mg/kg/day) were determined in the present study using procedures recommended by the United States Environmental Protection Agency [36]. The approach reported by Rajmohan et al. [37] was also employed to determine the non–carcinogenic health risks (which depend on the hazard index) of each metal. The sum of the HQoral values of each heavy metal was used to evaluate the oral hazard index (HIoral) for individual sample. While samples with values greater than 1 may provide non-carcinogenic health concerns to the consumer, those with HIoral < 1, CDIoral < 1, and HQoral < 1 are safe to drink.
3 Results and discussion
3.1 Heavy metals distribution and assessment of drinking water quality
Table 2 shows the heavy metal concentrations in each water source. During the dry season, site 6 had the highest mean Pb concentration (0.214 mg/L), with concentrations varying from 0.113 to 0.315 mg/L, while site 9 had the lowest average level (0.096 mg/L), with values varying from 0.069 to 0.121 mg/L. Additionally, there is a change in the spatial variations during the wet season, site 10 had the highest average concentration (0.197 mg/L), whereas site 9 was found to still have the lowest mean concentration in the wet season (0.112 mg/L). Results showed that almost all the sampling locations recorded Pb values which exceed the World Health Organization (WHO) maximum allowable concentration of drinking water (0.01 mg/L) [33]. Drinking water contaminated with lead (Pb) may lead to several health issues, including chronic bronchitis, gastrointestinal discomfort, lung cancer, neurological abnormalities, and impaired lung function [38]. Pb concentrations varied significantly (p < 0.05) across the sample locations; however, no significant variations (p > 0.05) were observed in the seasonal patterns of Pb distribution (Table 3). The average levels of Pb recorded in this study for dry and wet seasons (0.152 and 0.166 mg/L respectively) were substantially lower than mean concentrations (7.09 and 7.32 mg/L) of Pb in surface water, Bangladesh [39] but higher than the mean concentrations (0.006 and 0.003 mg/L) of Pb in Aiba Reservoir, Iwo, Nigeria [40].
Site 6 had the highest average concentration of Mn in the dry season (1.225 mg/L), with values varying from 1.115 to 1.335 mg/L. On the other hand, Site 3 had the lowest mean concentration of Mn (0.014 mg/L), with values ranging from below the detection limit (ND) to 0.067 mg/L. Additionally, site 4 recorded the highest average Mn concentration (2.691 mg/L), with concentrations varying from 2.688 to 3.101 mg/L during the wet season. However, Mn concentration was below the detection limit (ND) in site 3 throughout the wet season. The high concentrations in Mn detected in the shallow wells could be related to the increase in the release of manganese (Mn) from soil into groundwater through runoff from landfills or compost (inorganic matter). Results showed that 25% of samples had a mean concentration of Mn which surpassed the WHO allowable limit for drinking water (0.40 mg/L) while 75% were below the limit and are good for consumption. The seasonal patterns in the Mn distribution were significantly different (p < 0.05), although the Mn concentrations across the sample sites did not change significantly (p > 0.05). The average concentrations of Mn in this study for dry and wet season (0.431 and 0.668 mg/L) were higher than the average values (0.22 and 0.13 mg/L) of Mn in surface water, Niger Delta, Nigeria [41] and also average concentration (0.03 mg/L) of Mn in Gomti River, Bangladesh [42].
Moreover, the highest average level of Cd was observed at site 11 in dry season (0.019 mg/L) with values varying from 0.018–0.021 mg/L whereas the lowest average level was detected at site 2 (0.011 mg/L) with values varying from 0.008–0.016 mg/L. During the wet season, there were changes in the spatial distribution of Cd concentrations. At site 2, the mean concentration of Cd was the lowest (0.082 mg/L) and at site 4, the highest (0.090 mg/L). The high concentrations of Cd recorded in almost all the sampling locations could be attributed to run off of phosphate fertilizers into the soil (phosphate contains Cd as contamination) and also combustion emissions. Significant differences in Cd concentrations were observed for both seasonal and spatial variability across the locations (p < 0.05). The average Cd concentrations in this study (0.016 and 0.085 mg/L) were less than those in the Awash River (0.06 and 0.13 mg/L), Ethiopia [43], and dam water (3.76 and 5.12 mg/L) in Nairobi, Kenya [44].
During the dry season, site 10 had the highest mean concentration of Cu (0.146 mg/L), while site 12 had the lowest (0.034 mg/L), with values ranging from 0.003–0.086 mg/L. In addition, site 10 was found to have the highest mean level of Cu (0.322 mg/L) during wet season, with concentrations varying from 0.312 to 0.327 mg/L, while site 4 had the lowest mean concentration (0.235 mg/L). Results showed that all the samples had Cu concentrations below 0.400 mg/L and also are within the recommended limit (2.00 mg/L). Also, Cu concentrations varied significantly (p < 0.05) across the sites for both seasonal and spatial variations. The average concentrations of Cu (0.122 and 0.262 mg/L) observed in this study were lower than that of Cu (0.422 and 1.078 mg/L) in groundwater from OkeOdo, Iwo, Nigeria [45], but substantially higher than the concentration of Cu (7.3 µg/L) in groundwater, Hyderabad City, India [46].
The mean Fe concentration ranged from 0.081–4.45 mg/L from site 7 to site 10, with the latter having the highest average value (2.632 mg/L) in dry season. Furthermore, during the wet season, there was variation in the mean value across the study sites. At site 4, the highest mean value (8.646 mg/L) was recorded, with concentrations varying from 5.237 to 11.770 mg/L, while at site 2, the lowest concentration (0.081 mg/L) was recorded, with concentrations varying from 0.068 to 0.089 mg/L. Results indicated that 25% of samples surpassed the WHO allowable limit for drinking water while the remaining 75% of samples were within the limit (1.0 mg/L). The high value of Fe observed at site 4 during wet season could be related to corrosion of iron or the steel pipes used for the shallow well. The Fe concentrations at each sample location varied significantly (p < 0.05). On the other hand, there was no significant changes (p > 0.05) in the seasonal trends of the Fe distribution (Table 3). The average concentrations (0.865 and 1.223 mg/L) of Fe measured in this study for dry and wet season were significantly lower than the average concentrations (2.574 and 6.09 mg/L) in groundwater [45]. However, Omopariola and Adeniyi [47] reported mean concentrations of Fe in groundwater in Ayedaade, Oyo State, Nigeria, with lower concentration (0.75 mg/L) in dry season and higher (1.95 mg/L) concentration in wet season.
Results showed the following order of the mean metal concentrations (high to low): During the dry season, Fe > Mn > Pb > Cu > Cd. However, during wet season, the following decreasing sequence of heavy metal concentrations was observed: Fe > Mn > Cu > Pb > Cd. The high concentrations of Fe observed in wet season could be related to substantial runoff which had washed the iron-bearing soil particles into water sources. It could also be attributed to corrosion of iron or steel used for the shallow wells.
3.2 Comprehensive evaluation of trace metal pollution
3.2.1 Heavy metal pollution index
Table 4 shows that during the dry season, HPI varied from 14.14 to 220.48 with an average of 89.75, while in wet season, it varied from 9.46 to 638.13 with an average of 127.90. Figure 3a and 4a represent the grouping of the samples during both seasons. In dry season, results showed that 8% of samples fall into “excellent”, 25% fall into “good” while the “unsuitable” class had the highest (34%). The other 33% of samples are categorized into “poor” (8%) and “very poor” (25%). In addition, results also showed that 42% of samples fall into the “poor” category in wet season, however; there was a significant increase in samples with “excellent” category (16%). It could be said that few samples fall in the category of portable drinking water while majority are within the “poor” and “very poor” classes which require proper treatment and management.
3.2.2 Degree of contamination (Cd)
The contamination index (Cd), similar to the HPI, is an essential indicator for evaluating the level of pollution in water sources. It was computed using Eqs. (1) and (2), and the statistical information is shown in Table 4. The Cd concentration in the sample locations varied from -2.45 to 1.59 with an average value of -1.00 during the dry season, whereas it ranged from -2.56 to 12.49 with a mean value of -0.11 during the wet season. Results indicated that in the rainy season, 8% of the samples were classified as high (Cd > 3); however, no sample was recorded with Cd > 3 in dry season (Fig. 3b and 4b). In this study, site 4 (shallow well) was observed to have the highest Cd value (12.49) and this occurred in the wet season. The Cd correlates with the high value of HPI (638.13) observed. This could be due to the high concentration of Fe and Mn measured in wet season at the sampling site.
3.3 Multivariate statistical analysis
3.3.1 Pearson correlation coefficient
In order to investigate the relationships between the variables and to identify the source of the metals found in the particulate matter, the Pearson correlation coefficient is employed [48]. Strong correlation was defined as having a Pearson’s correlation coefficient between 0.9 and 1, while moderate correlation was defined as having a correlation coefficient between 0.9 and 0.5 [49, 50]. This classification was also included in the analysis of this study in order to have a general understanding of the performance of each of the assessed water quality indicators. There was no relationship between the variables Pb, Mn, Cd, Cu, and Fe during the dry season. For each relationship, the correlation coefficient was less than 0.5, and no statistically significant association was identified (Table 5). However, during wet season, Cu had significant positive correlation with Pb (r = 0.579), Fe had significant positive correlation with Cd (r = 0.589) and also strong positive correlation with Mn (r = 0.881). Other relationships found in the correlation matrix were not statistically significant. Moreover, the absence of no significant relationship between variables in the dry season indicates that sources of contamination of water bodies might be from different factors which are not common to all. Therefore, contamination sources could be from sewage discharge, wastewater treatment or surface runoff of agro-chemicals from agricultural practices. The results of this study showed that during the wet season, Fe and Mn exhibited a significant relationship. This could have occurred concurrently due to partial overlaps in the redox processes during reduction of Fe and Mn oxides, and it may be related to the significant concentrations observed at the sources [51].
3.3.2 Factor analysis
The factor analysis result obtained for dry season showed strong positive loading for only Pb in factor 1, negative strong loading in factor 2 and factor 3 for only Cu and Cd respectively (Table 6). It could be said that an increase in concentration of any of the variables will not significantly affect others (no significant correlation between the variables). Moreover, it could be said that the sources for each metal contamination in the water bodies are not similar; therefore, different factors could have contributed to the level of contamination. The statistical analysis indicated significant positive loadings of Mn and Fe in the wet season (Table 7), with factor 1 showing the largest variance (0.38%). The presence of positive loadings may result from a range of geogenic processes and anthropogenic activities including landfill leachate and sewage discharge. Furthermore, factor 2 indicated significant positive loadings for Pb and Cu. The strong correlation means that an increase in concentration of Pb leads to increase in concentration of Cu and this could be related to anthropogenic activities such as industrial processes, runoff of agrochemicals into surface water and over-pumping in shallow wells. However, only Cd showed a strong positive loading in factor 3 with no significant correlation with other variables. This means that an increase in level of Cd does not have any significant change in other variables. A possible explanation for this might be pollution from sources like sewage sludge and landfills in nearby areas of the study locations.
3.3.3 Human health risk assessment
In an effort to quantify the possible health risk associated with adults and children in the study districts consuming metal-polluted surface and groundwater orally, the human health risk assessment (HRA) was conducted. The present study estimated the hazard index (HI), hazard quotient (HQoral), and chronic daily intake (CDI, mg/kg/day) for each metal. Figures 3c and 4c show the proportion of samples that fall below the accepted limit (HQ < 1) for each metal. During dry season, Mn and Fe had HQ < 1 for both adults and children (100% of samples). However, only Fe had HQ value which was within the recommended limit in wet season while Mn had HQ > 1 (children) for 8% of the samples. In addition, Cu and Pb had HQ > 1 (more than 90% of samples) while Cd had 8% of samples with HQ > 1 for children in dry season. Moreover, Cu, Pb and Cd had HQ > 1 (adults and children) for more than 90% of the samples in wet season. The metals exhibit the following decreasing pattern, based on the proportion of samples that surpassed the permissible level: Pb > Cd = Mn = Fe = Cu (adults) and Pb > Cu > Cd > Mn = Fe (children) for dry season. Similarly, in wet season, the metals follow the decreasing trend for wet season: Cu = Cd > Pb > Mn = Fe (adults) and Pb = Cu = Cd > Mn > Fe (children). It could be said that HQoral values of metals which exceeded the recommended limit were mostly recorded during wet season with Pb having the highest percentage.
During the dry season, HIoral calculated for adults in the study districts varied from 2.03 to 3.26 with an average of 2.61 and 3.43 to 5.51 with an average of 4.41 for children. Moreover, there was fluctuation in the HIoral computed in wet season, it varied from 5.11 to 5.66 with a mean value of 5.84 and 8.64 to 11.24 with a mean value of 7.86 for adults and children respectively (Table 4). Results showed that the HIoral values computed for all the sites were more than 1, this suggests that consumers could be exposed to potential non-carcinogenic health risks. Moreover, in the study locations children are significantly more susceptible to non-carcinogenic risks than adults. Similarly, the HIoral results reported in Owan River, Nigeria [52] and Ossiomo River, Nigeria [53] showed that children in the receptor population were predisposed to non-carcinogenic risk (HIoral > 1). Hence, the groundwater and surface water should be managed well by consumers and also treated (when necessary) before consumption.
4 Conclusion
Significant seasonal and spatial variation is observed in the majority of heavy metals assessed. In addition, the quality of water in the study sites with “poor” and “very poor” classes could be improved by proper treatment of wastewater and sewage before discharging to the environment, regulation of extensive application of agrochemicals and routine monitoring of water by protection agencies. According to the HI results, none of the research sites are appropriate for drinking and may provide non-carcinogenic health concerns to consumers (HI > 1). Hence, a mitigation plan should be implemented by the environmental protection agency to ensure that the community at large have access to quality drinking water.
Data availability
All data generated and analysed during this study are included in this article.
References
Gorchev H, Ozolins G. Guidelines for drinking-water quality Geneva. Switzerland: WHO; 2011.
World Health Organization. Safe drinking-water from desalination (No. WHO/HSE/WSH/11.03). Geneva: World Health Organization; 2011.
Goretti E, Pallottini M, Ricciarini MI, Selvaggi R, Cappelletti D. Heavy metals bioaccumulation in selected tissues of red swamp crayfish: an easy tool for monitoring environmental contamination levels. Sci Total Environ. 2016;559:339–46.
Kasozi KI, Namubiru S, Kamugisha R, Eze ED, Tayebwa DS, Ssempijja F, et al. Safety of drinking water from primary water sources and implications for the general public in Uganda. J Environ Public Health. 2019. https://doi.org/10.1155/2019/7813962.
Sardar K, Ali S, Hameed S, Afzal S, Fatima S, Shakoor MB, Tauqeer HM. Heavy metals contamination and what are the impacts on living organisms. Greener J Environ Manage Public Safety. 2013;2(4):172–9.
Mansour SA, Gad MF. Risk assessment of pesticides and heavy metals contaminants in vegetables: a novel bioassay method using Daphnia magna Straus. Food Chem Toxicol. 2010;48(1):377–89.
Zhuang Q, Li G, Liu Z. Distribution, source and pollution level of heavy metals in river sediments from South China. CATENA. 2018;170:386–96. https://doi.org/10.1016/j.catena.2018.06.037.
Karimi A, Naghizadeh A, Biglari H, Peirovi R, Ghasemi A, Zarei A. Assessment of human health risks and pollution index for heavy metals in farmlands irrigated by effluents of stabilization ponds. Environ Sci Pollut Res. 2020;27:10317–27.
Dehghani MH, Jahed GR, Zarei A. Investigation of low-pressure ultraviolet radiation on inactivation of Rhabitidae Nematode from water. Iran J Public Health. 2013;42(3):314.
Kanu I, Achi OK. Industrial effluents and their impact on water quality of receiving rivers in Nigeria. J Appl Technol Environ Sanitation. 2011;1(1):75–86.
Zhang T, Xu W, Lin X, Yan H, Ma M, He Z. Assessment of heavy metals pollution of soybean grains in North Anhui of China. Sci Total Environ. 2019;646:914–22. https://doi.org/10.1016/j.scitotenv.2018.07.335.
Rehman IU, Ishaq M, Ali L, Khan S, Ahmad I, Din IU, et al. Enrichment, spatial distribution of potential ecological and human health risk assessment via toxic metals in soil and surface water ingestion in the vicinity of Sewakht mines, district Chitral, Northern Pakistan. Ecotoxicol Environ Saf. 2018;154:127–36. https://doi.org/10.1016/j.ecoenv.2018.02.033.
Lazo P, Steinnes E, Qarri F, Allajbeu S, Kane S, Stafilov T, et al. Origin and spatial distribution of metals in moss samples in Albania: a hotspot of heavy metals contamination in Europe. Chemosphere. 2018;190:337–49. https://doi.org/10.1016/j.chemosphere.2017.09.132.
Liu R, Bao K, Yao S, Yang F, Wang X. Ecological risk assessment and distribution of potentially harmful trace elements in lake sediments of Songnen Plain, NE China. Ecotoxicol Environ Saf. 2018;163:117–24. https://doi.org/10.1016/j.ecoenv.2018.07.037.
Mishra S, Dwivedi SP, Singh RB. A review on epigenetic effect of heavy metal carcinogens on human health. Open Nutraceutical J. 2010;3(1):188–93.
Ali MM, et al. Heavy metal concentrations in commercially valuable fishes with health hazard inference from Karnaphuli river, Bangladesh. Human and Ecol Risk Assessment Int J. 2019. https://doi.org/10.1080/10807039.2019.1676635.
Saha P, Paul B. Assessment of heavy metal toxicity related with human health risk in the surface water of an industrialized area by a novel technique. Hum Ecol Risk Assess Int J. 2019;25(4):966–87.
Bai J, Zhao Q, Wang W, Wang X, Jia J, Cui B, et al. Arsenic and heavy metals pollution along a salinity gradient in drained coastal wetland soils: depth distributions, sources and toxic risks. Ecol Ind. 2019;96:91–8. https://doi.org/10.1016/j.ecolind.2018.08.026.
Uganda Bureau of Statistics. Uganda bureau of statistics 2020 statistical abstract. Uganda Bureau of Statistics, Kampala. 2020.
National Water and Sewerage Corporation (NWSC). Uganda water management and environment sector performance report. Kampala, Uganda. 2018.
Sharma S, Bhattacharya A. Drinking water contamination and treatment techniques. Appl Water Sci. 2017;7:1043–67. https://doi.org/10.1007/s13201-016-0455-7.
Ngomsik AF, Bee A, Siaugue JM, Talbot D, Cabuil V, Cote G. Co (II) removal by magnetic alginate beads containing Cyanex 272®. J Hazard Mater. 2009;166(2–3):1043–9.
Onen P, Akemkwene R, Nakiguli CK, Nimusiima D, Ruma DH, Khanakwa AV, Angiro C, Bamanya G, Opio B, Gonzaga A, et al. Health risks from intake and contact with toxic metal-contaminated water from Pager river Uganda. J Xenobiot. 2023;13:544–59. https://doi.org/10.3390/jox13040035.
Bamuwamye M, Ogwok P, Tumuhairwe V, Eragu R, Nakisozi H, Ogwang PE. . Human health risk assessment of heavy metals in Kampala (Uganda) drinking water. 2022.
Abraham MR, Susan TB. Water contamination with heavy metals and trace elements from Kilembe copper mine and tailing sites in Western Uganda; implications for domestic water quality. Chemosphere. 2017;169:281–7.
Guma BE, Muwanga A, Owor M. Hydrogeochemical evolution and contamination of groundwater in the Albertine Graben, Uganda. Environ Earth Sci. 2021;80:1–17.
Dietler D, Babu M, Cissé G, Halage AA, Malambala E, Fuhrimann S. Daily variation of heavy metal contamination and its potential sources along the major urban wastewater channel in Kampala, Uganda. Environ Monit Assess. 2019;191:1–13.
Ojok W, John W, Ntambi E. Assessment of seasonal variation in water quality in river Rwizi using multivariate statistical techniques. Uganda: Mbarara Municipality; 2017.
Bakyayita GK, Norrström AC, Kulabako RN. Assessment of levels, speciation, and toxicity of trace metal contaminants in selected shallow groundwater sources, surface runoff, wastewater, and surface water from designated streams in Lake Victoria Basin Uganda. J Environ Public Health. 2019. https://doi.org/10.1155/2019/6734017.
APHA. Standard methods for the examination of water and wastewater. 23rd ed. Washington, DC: American Public Health Association American Water Works Association. Water Environment Federation; 2017.
APHA. Standard methods for examination of water and waste water. 22nd ed. Washington, DC, USA: APHA; 2012.
Rajmohan N, Niyazi BAM, Masoud MHZ. Trace metals pollution, distribution and associated health risks in the arid coastal aquifer, Hada Al-Sham and its vicinities Saudi Arabia. Chemosphere. 2022;297:134246.
WHO. Guidelines for drinking water quality: fourth edition incorporating the first addendum. Geneva: World Health Organization; 2017.
Basahi JM, Masoud MH, Rajmohan N. Effect of flash flood on trace metal pollution in the groundwater-Wadi Baysh Basin, western Saudi Arabia. J Afr Earth Sc. 2018;147:338–51.
Backman B, Bodiš D, Lahermo P, Rapant S, Tarvainen T. Application of a groundwater contamination index in Finland and Slovakia. Environ Geol. 1998;36:55–64.
USEPA. Exposure factors handbook: 2011 Edition (Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F, 2011.
Rajmohan N, Masoud MH, Niyazi BA, Alqarawy AM. Appraisal of trace metals pollution, sources and associated health risks using the geochemical and multivariate statistical approach. Appl Water Sci. 2023;13(5):113.
WHO. Lead in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization, Geneva. WHO/SDE/WSH/03.04/09/Rev/1. 2003.
Proshad R, Islam S, Tusher TR, Zhang D, Khadka S, Gao J, Kundu S. Appraisal of heavy metal toxicity in surface water with human health risk by a novel approach: a study on an urban river in vicinity to industrial areas of Bangladesh. Toxin Rev. 2021;40(4):803–19.
Atobatele OE, Olutona GO. Spatio-seasonal discrimination of water quality and sediment heavy metals distribution in Aiba reservoir, Iwo, Nigeria. Humboldt Kolleg Osogbo-2017. 2017.
Aigberua A, Tarawou T, Abasi C. Spatial and seasonal assessment of heavy metals in surface waters of the Middleton river in the Niger Delta, Nigeria. Int J Nat Res Ecol Manage. 2017;2(5):94–8.
Ahmed AS, Hossain MB, Babu SOF, Rahman MM, Sarker MSI. Human health risk assessment of heavy metals in water from the subtropical river, Gomti, Bangladesh. Environ Nanotechnol Monitor Manag. 2021;15: 100416.
Eliku T, Leta S. Spatial and seasonal variation in physicochemical parameters and heavy metals in Awash River, Ethiopia. Appl Water Sci. 2018;8:1–13.
Ndeda LA, Manohar S. Determination of heavy metals in Nairobi dam water, (Kenya). J Environ Sci Toxicol Food Technol. 2014;8(5):68–73.
Alagbe OA, Olutona GO, Olafisoye ER, Olayiwola KO. Impact of a waste disposal site on groundwater quality (a case study of okeodo refuse dumpsite, Iwo Osun state southwestern, Nigeria). Int J Adv Res. 2019;7(Mar):32–43.
Kamble BS, Saxena PR, Kurakalva RM, Shankar K. Evaluation of seasonal and temporal variations of groundwater quality around Jawaharnagar municipal solid waste dumpsite of Hyderabad city, India. SN Appl Sci. 2020;2:1–22.
Omopariola OA, Adeniyi IF. Statistical investigation of the spatial and seasonal variations of heavy metal contents of groundwater sources in Ayedaade area, Southwestern, Nigeria. J Environ Pollut Hum Health. 2019;7(2):100–6.
Proshad R, Kormoker T, Islam S. Distribution, source identification, ecological and health risks of heavy metals in surface sediments of the Rupsa river. Toxin reviews. 2019. https://doi.org/10.1080/15569543.2018.1564143.
Sojobi AO. Evaluation of groundwater quality in a rural community in North Central of Nigeria. Environ Monit Assess. 2016;188(3):192.
Tirkey P, Bhattacharya T, Chakraborty S, Baraik S. Assessment of groundwater quality and associated health risks: a case study of Ranchi city, Jharkhand, India. Groundw Sustain Dev. 2017;5:85–100.
Ying SC, Schaefer MV, Cock-Esteb A, Li J, Fendorf S. Depth stratification leads to distinct zones of manganese and arsenic contaminated groundwater. Environ Sci Technol. 2017;51(16):8926–32.
Egun NK, Oboh IP. Assessment of water quality for suitability and human health risk: a study of the Owan River, Edo State, Nigeria. Afr J Aquatic Sci. 2023;48(1):19–27. https://doi.org/10.2989/16085914.2022.2156468.
Biose E, Egun NK, Uzoh N, Omoigberale MO. Assessment of water quality and associated human health risk of a tropical freshwater body in Edo state Nigeria. Afr J Aquatic Sci. 2024. https://doi.org/10.2989/16085914.2023.2292125.
Author information
Authors and Affiliations
Contributions
Conceptualization, sample preparation, data collecting and analysis, and paper preparation were done by IOS. Conceptualization, research design, supervision, and paper writing were done by GOO. IGW participated in the project design, supervision, execution, and editing. HO also assisted with the design, execution, and editing of the project. All authors read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanusi, I.O., Olutona, G.O., Wawata, I.G. et al. Heavy metals pollution, distribution and associated human health risks in groundwater and surface water: a case of Kampala and Mbarara districts, Uganda. Discov Water 4, 27 (2024). https://doi.org/10.1007/s43832-024-00087-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-024-00087-9