Abstract
This study investigates the qualitative analysis of groundwater obtained from the Tonk district of Rajasthan to determine its suitability as potable water. Around 321 ground drinking water samples were collected and analysed for various physico-chemical characteristics including pH, electrical conductivity (EC), total dissolved solid (TDS), HCO3−, F−, Cl−, SO42−, NO3−, Ca2+, Mg2+, total hardness (TH) and total alkalinity (TA) in Tonk district of Rajasthan (India). The groundwater in the region is a little alkaline and hard. The EC during the pre-monsoon period varies from 434 to 7271 µS/cm, while in the post-monsoon period, it ranges from 356 to 8871 µS/cm. TDS of samples ranged between 285 to 5090 mg/L in the pre-monsoon and 247 to 6140 mg/L post-monsoon seasons. Few samples have EC, TDS, and TH levels higher than the permissible limit, restricts its usage as drinking water. The water quality in the study area varies from soft-fresh to hard-brackish in both pre- and post-monsoon seasons. Anion concentrations in analysed samples followed the order: HCO3− > Cl− > SO42− > NO3− > F−. Notably, the Water quality index (WQI) results for all collected samples fall within the range of 4.55–96.83, indicating they are considered safe and suitable for drinking purposes. Water quality data was analysed by correlation study and geochemical processes. A strong correlation between the Water Quality Index (WQI) and fluoride levels is found in both seasons. The geochemical analysis shows that most of the samples are in the rock water interaction zone, with some in the evaporation zone due to the high content of chloride ions. The groundwater chemistry appears to be influenced by rock-water interaction and evaporation sedimentation. The molar ratio of Ca2+/Mg2+ denotes calcite dissolution and silicate weathering revealing enrichment of Ca2+ and HCO3− ions in aquifer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Clean water is the basis of life. Urbanization and industrialization have caused the adulteration of surface water bodies. Dependence on underground water increases as population grows rapidly for the last few decades. According to World Bank and U.N report, water scarcity is affecting more than 40% global population. The proportion of groundwater withdrawal in the overall available freshwater has risen from 12% in 1950 to 25% in 2017 globally. During the same period, the total worldwide ground-water removal has increased from 158 to 959 km3/year. Asia has the highest share of the total quantity withdrawn accounting for two-third of the total quantity withdrawn. According to a report, in 2017, almost two-third of the total estimated groundwater withdrawal was done by nine countries. These countries are China, France, India, Iran, Mexico, Pakistan, Russia, Saudi Arabia and United States of America. Out of the total volume of groundwater withdrawn world-wide, approximately 69% is used for irrigation, 22% for domestic use, and 9% for industrial purposes. Groundwater is mainly used for agriculture purposes across all continents except Europe, where it is primarily extracted for domestic use [1].
The reliance on ground-water in urban India is substantial, accounting for nearly 40% of its total water demand. However, due to excessive usage, rapid decline in ground-water tables is a concerning trend. In most cities it is dropping at a distressing rate of 2–3 m per year. According to the report, India’s position as the largest user of groundwater worldwide, extracting a substantial 251 km3 annually through millions of wells and tube wells, highlights the critical role ground-water plays in meeting the country’s water demands. India’s substantial share of nearly 26% in global groundwater extraction reflects its heavy reliance on this vital resource. India is a developing country with a predominantly agrarian economy and approximately 89% of this groundwater is utilized for irrigation purposes within India [1]. So, sustainable practices and effective management will be crucial to balance this demand and ensure long-term water availability. Per head accessibility of water in India was 1816 m3 in 2001, 1545 m3 in 2011 and presently it is reduced to 1486 m3 in 2021 due to the increase in population. The availability of water to each person may be expected to reduce by 1140 m3 by 2050 [2].
Rajasthan is a dry state with a semiarid region. Aquifers are rapidly depleting in the state to critical condition. By 2030 it is expected that people will be living in water-stressed conditions. The status of groundwater is affected by the geochemistry of the aquifer, the type of rock, weathering process, residence time and physical factors such as evaporation and precipitation [3]. Various reactions, adsorption and desorption take place at the subsurface, pH, TDS, ORP, DO, temperature, anions (SO42−, F−, PO43−, NO3− and silicate) and recharge of water chemistry plays a significant role in leaching of minerals from host rock to groundwater [4,5,6,7]. For assessing groundwater quality, a fuzzy multi-criteria decision-making tool was used in Rajasthan's Bikaner district [8]. Assessing groundwater quality in dry regions of Rajasthan is crucial for both drinking and irrigation [9].
Drinking water quality and aesthetic characteristics were evaluated in the highlands of Ethiopia and advised water source must be treated before supply [10]. The hydrochemistry studies in Ardabil area of Iran determined mixed types of groundwater viz Ca-Mg-HCO3, Na-HCO3, and Na-Cl type. The mineral saturation index and chemical analysis result indicated the predominant hydrogeochemical natural processes, mainly due to weathering, mixing, ion exchange, and anthropogenic activity [11]. The WQI assessment studies by Atta et al., in surrounding of Ismailai canal, Egpyt, evaluated that most observation wells fall within desirable and maximum allowable limits [12]. Using indices like CR, HI, MH, RSC, SAR and Na% to assess groundwater in Saudi Arabia provides a comprehensive understanding of its quality [13]. The geochemical processes and groundwater quality of the Vientiane Plain, Laos is assessed for irrigation and drinking purpose [14].
Groundwater quality of Mahoba, Uttar Pradesh was assessed by using GIS framework and concluded that overall groundwater is safe and potable except at few locations [15]. Basavarajappa et al., analysed the groundwater in pre-cambrian rock of Chitradurga district of Karnataka through Geo informative technique [16].
Rajasthan’s vast expanse in terms of area and arid to semiarid conditions pose unique challenges for water resources. The substantial reliance on groundwater for both drinking and irrigation in Rajasthan underscores its pivotal role in sustaining life and agriculture. The awareness of contamination, as highlighted by the Central Ground Water Board, emphasizes the importance of safeguarding this vital resource [17]. Researchers (Duke University India Initiative) tested 243 groundwater wells in Rajasthan, and found that more than 75% of the wells (which include tubewells and handpumps) in untreated water contain harmful contaminants such as fluoride, nitrate, and uranium. Exceeding both Indian and World Health Organisation (WHO) drinking water standards for contaminants is a serious concern. It emphasizes the urgency of implementing effective measures to address and rectify the groundwater quality issues in Rajasthan [18]. Studies has been done on the quality assessment of drinking water in Rajasthan by using various statistical concepts and applying new tools by hydro-researchers. The use of Generalized Additive Model(GAM) by Pandey et al. to assess the impact of anthropogenic and climatic factors on ground-water quality at 84 stations in arid and semi-arid districts of Rajasthan from 2000 to 2018 provides valuable insights [19]. Additionally, the WQI assessment for drinking and irrigation purposes in Chambal river of Kota (as well in Banasthali, Tonk district, Rajasthan) [20, 21] and neural network modelling for ground-water quality index in Jodhpur demonstrate diverse and advanced approaches to understanding water quality dynamics [22]. Rukshar et al. [23] addressed deteriorating water quality in Jaipur city and through GIS (Geographic Information System) interpolation. Groundwater quality index of 30 samples was evaluated via GIS in Rural Ajmer and Bhilwara district [24].
Gautam et al., assessed the WQI (different method) by collecting few samples in 2016 from different block of Tonk district [25]. Evaluating nitrate levels in groundwater and assessing health risks, as conducted by Saini et al., is crucial for understanding potential hazards in Tonk district [26]. Regular monitoring of water quality parameters is essential for understanding pollution levels, ensuring suitability for human consumption, and mitigating potential environmental threats. Sustainable management practices play a crucial role in preserving these vital resources [27]. Monitoring groundwater quality by the Central Ground Water Authority is crucial, but resource limitations pose challenges. So, more regional studies are required to fill the gap. Therefore, we covered whole district with systematic grid mapping and analyzed the water quality parameters such as pH, Total dissolved solids (TDS), Dissolved oxygen (DO), Electrical conductivity (EC), Oxidation reduction potential (ORP), Temperature, Salinity, Hardness, Alkalinity, anions such as Sulphate (SO42−), Fluoride (F−), Chloride (Cl−), Nitrate (NO3−) and Phosphate (PO43−) in drinking ground water of Tonk district and also tried to find correlation between them in pre- and post-monsoon season.
This study assessed groundwater by collecting 321 water samples across the district during both pre- and post-monsoon seasons to analyze the seasonal influence on water quality. The study aims to evaluate the WQI of ground drinking water in Tonk district (Rajasthan). This involves assessing its suitability for human consumption through a combination of statistical methods and geochemical analysis.
2 Material and methods
2.1 Study area
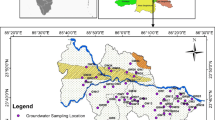
The district situated in the eastern part of Rajasthan, spans between latitudes 25°42′ to 26°34′ and longitudes 75°07′ to 76°19′. Covering a total area of 7194 km2, it comprises seven subdivisions: Deoli, Malpura, Niwai, Peeplu, Toda Raisingh, Tonk and Uniara. The district is home to 1116 villages.
Tonk district experiences a semi-arid climate with hot dry summers and cold winters. The temperature ranges from 5°C to 45ºC. The monsoon season extends from June to mid-September. According to 30 year (1991–2020) statistical data, the district receives an annual average rainfall of 536.96 mm [28]. The water level trend analysis from 2011 to 2020 indicates a decrease of 0.16 m per year in pre-monsoon and 0.04 m per year in post-monsoon seasons. A large number of colonies of the Tonk district receive water supply from the Beesalpur dam therefore a substantial number of water samples were collected from Beesalpur dam supply. A large number of samples were also collected from borewells, open wells and handpumps.
2.1.1 Geology and hydrogeology
The geological composition of Tonk district includes rocks from the Bhilwara Super Group, predominantly mica schist, gneisses, phyllites and quartzites with minor intrusive granite. These durable rocks are covered by recent to sub-recent alluvium, comprising clay, sand and silt [29]. Notably granite and gneisses are visible in Malpura and Toda Raisingh tehsils of Tonk district. Most of the groundwater in the area exists under phreatic conditions. In alluvial regions, it is under water table conditions, while in hard and crystalline rocks, it tends to be under slight pressure. Groundwater movement is influenced by factors such as porosity in the weathered zone, structural features like joints, fissures, fractures, bedding planes, and other structurally weak zones in hard rock. Additionally, the grain size distribution in alluvium plays a significant role in shaping the movement of groundwater in the area. The major hydrogeological formations in the district include quaternary alluvium, phyllites, schists, and granitic gneisses [29]. The two major types of aquifers are hard rock and alluvium (sand) aquifers.
Around 80% area of Tonk is comprised with hard rock aquifer which is suitable for groundwater recharge. The major hard rock formations in the area are mica-schist, phyllites, banded gneissic complex, and quartzites. Mica-schist is indeed a significant and dominant geological formation in the district, playing a key role in shaping the local geology and hydrogeology. In the alluvium, ground water fills the open spaces between particles of sand, gravel and clay kankar. The porosity of these materials allows for the storage and movement of groundwater within the subsurface.
2.2 Sample collection
Groundwater sample were taken by grid mapping (6 × 6 km2) method. Every water sample was collected from drinking sources and centre of the grid for each 36 km2 area. If another source of drinking water was found in same area, then water sample was collected from that source also. Following standard protocol, sample collection was conducted during both pre-monsoon and post-monsoon periods in the study area. Pre-monsoon sampling was carried out from mid of March to mid of June 2021, while post-monsoon sampling has been done from October to December 2021. The information regarding samples was put on record manually as well as a soft copy.
Water samples were collected in polyethene or polypropylene bottles, which were thoroughly cleaned by soaking overnight in soap water on day one, then rinsed with 10% HNO3 on day two, and finally washed with double distilled or ultra-pure water before sampling. At the sampling site, water samples underwent filtration using a 0.45 µm membrane and were subsequently stored for further studies.
COVID -19 has impacted our sampling schedule and analysis procedure, leading to reduced samples collection per day. Setting up a lab near the house campus for experiments is a proactive solution to ensure timely analysis despite limitations. To overcome the challenge of electricity unavailability in village at night, titration and other analyses were conducted during the early hours of the morning.
2.3 Analytical method
Collecting a total of 321groundwater samples from Tonk district, of Rajasthan, 161 samples were obtained during pre-monsoon, and 160 during post monsoon season. The samples were gathered from various drinking sources including hand pumps (34%), bore wells (25–27%), Beesalpur supply (33–35%) and from open wells (6%). Water table depths varied between 20–340 feet in pre-monsoon and 5–340 feet in post-monsoon. The sampling was carried out twice to analyze the impact of seasonal variation in study area.
The water samples collected from the grid points underwent on site examination using a portable multiparameter probe sensor [Eutech PCS-35, ORP and DO meter], measuring pH, temperature, salinity, TDS, EC and ORP (Fig. 1). Standard methods(APHA) were employed for chemical analysis, ensuring a reliable and consistent approach to assess the composition of the collected water samples [30]. Laboratory analysis involved UV visible spectrophotometer (Thermo-Fischer model Aqua Orion 8000) for major anions such as F−, NO3−, PO4− and SO42−. Chloride was determined using argentometric method, while CO3− and HCO3 − were analysed through volumetric analysis. Total hardness and calcium were assessed using the EDTA titrimetric method and magnesium was calculated theoretically. To ensure quality control and precision, all measurement were taken in triplicate and the mean values were reported. Maintaining analytical precision, the error ranged from ± 2 to ± 8%. Three samples with an error exceeding 10% were excluded. Calibration checks of instruments were ensured by running blank and standards at regular intervals, maintaining the quality of analyses.
WQI in this study is determined through the weighted arithmetic index approach, providing a comprehensive assessment of water quality [31].
where, Wn is the unit weight of nth parameter to calculate the water quality index. Rn serves as the quality rating of the parameter. Unit weight (Wn) can be estimated by Eq. (2).
Here k → Proportionality constant, Sn → permissible limit for water quality parameter as per WHO, 2012 and estimated according to Eq. (3).
Quality rating (Qn) was calculated using the Eq. (4):
With the exception of pH and DO, which are regarded as neutral and 14.6 mg/L respectively, Pn represents the actual value of the analyzed water parameter, while Ci represents the ideal concentration of that specific water parameter.
The statistical analysis was carried out by using Pearson correlation matrix and box whisker plot methods. Additionally, hydrochemical processes were elucidated through bivariate plots, shedding light on the dissolution of minerals in the studied context.
3 Results and discussion
The minimum, maximum, standard deviation and average etc. are calculated for the analysed hydrochemical data (Table 1). A low standard deviation implies close proximity to the mean while a high standard deviation suggests a more dispersed dataset. The major water quality parameters like alkalinity, DO, EC, hardness, ORP, pH, salinity, TA, TDS, temperature, TH, F−, Cl−, NO3−, SO42− and PO43− were examined according to the standard protocols [31]. These hydro-chemical parameters could have positive or negative impact on water quality which depends on geochemistry of terrain. To establish the relationship between these hydro-chemical parameters, we conducted statistical analysis and evaluated correlation coefficient. The 33–35% samples have same source of drinking water i.e. supply from Beesalpur dam so the evaluated data is almost similar. The details of the hydrochemical parameters are as follows:
pH: pH is a measure of the hydrogen ion concentration in water. According to WHO/BIS guidelines, the safe pH for drinking water should fall within the range of 6.5–8.5. In the study area, collected water samples show variation from 6.12 to 8.90 during pre-monsoon, averaging at 7.61. In post monsoon, the pH ranges from 5.87 to 8.56 with an average of 7.14. The average value suggests that the majority of water samples in the study area maintain a neutral pH state.
EC/TDS: The electrical conductivity EC) serves as a measure of a substance’s ability to conduct an electric current, with higher EC values indicating greater salt content in groundwater. In the study EC is categorised as type-I if the salt enrichment is low (EC < 1500 µS/cm); type-II if the salt enrichment is medium (EC: 1500–3000 µS/cm); and type-III if the salt enrichment is high (EC > 3000 µS/cm) [32]. During the pre-monsoon season, EC ranges from 434 to 7271 µS cm−1 with a mean of 1390.1 µS cm−1. In post-monsoon season, the range is 356–8871 µS cm−1 with a mean of 1321.2 µS/cm.
Studied samples exhibit positive correlation between TDS and EC which can be mathematically expressed by the empirical relation as follows:
TDS refers to the total concentration of the dissolved inorganic cations, anions and small organic matter in the drinking water. Because the dissolved solids are predominantly ionic species, therefore TDS is strongly correlated with conductivity. TDS values in the collected samples varies from 285–5090 mg/L with a mean value of 980.2 mg/L in pre-monsoon and 247–6140 mg/L with a mean value of 932.1 mg/L in post-monsoon season. 61.25% of pre-monsoon and 50% of post-monsoon water samples have TDS under the accepted limit of 500 mg/L as per the WHO guidelines [33], while 16 samples of pre-monsoon and 14 samples of post-monsoon are found to have TDS above BIS permissible limit of 2000 mg/L [34]). Highest TDS & EC value was found in Mutooka village of Tonk district in both seasons. Minimum amount of TDS 285 mg/L in pre-monsoon and 247 mg/L in post-monsoon was reported in Beesalpur Dam supply at Deoli, Tonk.
ORP and DO: Oxidation and reduction potential (ORP) refers to the capacity of one chemical substance to oxidize or reduce another chemical substance. In pre-monsoon water samples, ORP ranges from − 247 to 185 mV while for post-monsoon it varies from − 101 to 189 mV. Dissolved oxygen (DO) represents the un-combined oxygen in water. In the study area, the average dissolved oxygen is measured at 4.34 during the pre-monsoon season and increases to 4.96 mg/L in post-monsoon season.
Chloride, Fluoride and Nitrate: Chloride is a key contributor to TDS and plays a role in determining salinity of the water. It varies from 59.8 mg/L in village Bhojpura (Uniara) to 1393.2 mg/L in Mutooka village of Tonk district in pre-monsoon. In post-monsoon, lowest chloride level 37.48 mg/L was found at Lamba Haresingh of Malpura Tehsil to maximum 1319.4 mg/L at Mutooka village of Tonk. 82.0% of pre-monsoon and 81.3% of post-monsoon water samples have chloride content less than 250 mg/l as per the BIS desirable limit. 11.80% of pre-monsoon samples and 15.0% of post-monsoon samples have chloride in the range of 250–500 mg/L. Rest 6.2% of pre-monsoon and 3.7% of post-monsoon water samples have chloride between 500 and 1500 mg/L.
The majority of the drinking water samples of the study area have fluoride levels within the permissible limits, but the presence of fluoride in some post-monsoon groundwater samples raises questions about its association with fluoride-containing rocks. In pre-monsoon samples, the amount of fluoride varies from 0.004 to 1.34 mg/L. The minimum concentration of fluoride is found in Bhagwanpura of Malpura tehsil and maximum in Sondiphala of Peeplu tehsil in pre-monsoon. The concentration of fluoride varied between 0.04 and 1.33 mg/L in post-monsoon. The maximum value of fluoride is found at DoongrKalan at Malpura tehsil in post-monsoon. Statistical data obtained for fluoride showed that 88.75% sample in pre-monsoon and 83.75% samples in post-monsoon have amount of fluoride in acceptable limit of 1 mg/L. For 17 pre-monsoon and 27 post-monsoon water samples, fluoride was found to be in the range of 1–1.5 mg/L.
The nitrate concentration variations are notable, ranging from 0.96 to 70.4 mg/L (in Mutooka village) in pre-monsoon, averaging 10.90 mg/L. The minimum concentration 2.77 mg/L of nitrate is found in Aamli in Malpura Tehsil and maximum was found in Payaga village 47.98 mg/L at Uniara tehsil of Tonk district in post-monsoon samples with an average of 18.94 mg/L. 96.0% of pre-monsoon and post-monsoon samples was found to have nitrate content within the permissible limit of 45 mg/L, though 4% samples exceed the threshold limit.
Sulphate and Phosphate: In the groundwater samples collected from the Tonk district, sulphate concentration varies from 2.21 to 291.4 mg/L (in village Mutooka) and 3.51 mg/L (in village Kalmandr in Malpura tehsil) to 162.6 mg/L (in village Alipura in Uniara tehsil) with an average of 42.49 and 38.66 mg/L in pre-monsoon and post-monsoon, respectively. The frequency distribution data reveals that 96.25% of pre-monsoon water samples have sulphate within the acceptable limit of 200 mg/L. In all the post-monsoon samples, concentration of sulphate was within the limits.
The study’s phosphate findings suggest predominantly low concentrations for most of the samples, but elevated levels in certain locations point to nearby anthropogenic activities, like use of fertilizers in agriculture and decomposition of organic matter. The phosphate levels in drinking water samples show a range of 0.001–0.24 mg/L in pre-monsoon and 0.003–0.31 mg/L in post-monsoon seasons. The averages are 0.05 mg/L and 0.11 mg/L, respectively. The minimum phosphate concentration was found in Arniya bassi in pre-monsoon and Lamba Haresingh, in Malpura tehsil in post-monsoon. Mutooka (Tonk tehsil) and Jaharli (Malpura tehsil) shows maximum phosphate concentrations in pre-monsoon and post-monsoon, respectively. From the frequency distribution data of phosphate, it is observed that 98.76% samples lie in the range of 0.05–0.1 mg/L in pre-monsoon. In post-monsoon, 36.81% samples have phosphate in the range of 0.01to 0.1, 51.53% samples have 0.1–0.16 mg/L and 11.6% samples have phosphate concentration above 0.16 mg/L.
Total Alkalinity and Hardness: The prevalence of calcium and bicarbonate ions in 98% of global ground water samples, influencing hardness and alkalinity, points to the impact of limestone weathering in the catchment areas of underground water-beds. Bicarbonate is a dominant anion of the region and it is found to be in the range of 110–1220 mg/L in pre-monsoon and 70–1585 mg/L in post-monsoon water samples. Mean value of 322.7 mg/L in pre-monsoon 264.09 mg/L in post-monsoon was obtained for bicarbonate. Most of the samples have high content of bicarbonate results into high alkalinity. Bicarbonate is commonly produced through the weathering reactions of carbonate and silicate minerals [3].
The total hardness level in pre-monsoon samples vary between 105 and 2910 mg/L and in post-monsoon samples, it varies from 100 to 3270 mg/L. The maximum hardness 2910 mg/L and 3270 mg/L was observed in the samples collected from Tonk tehsil (Mutooka) in pre- and post-monsoon seasons, respectively. The elevated concentration of Calcium and Magnesium were identified in Mutooka village of Tonk district, both in pre-monsoon and post-monsoon samples. Carbonate concentration in Tonk is almost negligible.
The order of anions’ concentration in the water samples is as follows:
HCO3− > Cl− > SO42− > NO3− > F−
Calcium and Magnesium: Ca2+ and Mg2+ ions are often present in water due to the dissipation of minerals like dolomite and calcite exist in the rocks. The Ca2+ is a major cation, ranged from 28 to 686 mg/L with average of 95.07 mg/L in pre-monsoon samples. In post monsoon, it varies from 28 to 926.0 mg/L with an average of 83.78 mg/L. The less value of calcium content in the average post monsoon samples may be assigned to the dilution by rainfall. The Mg2+ during pre-monsoon varied from 4.87 to 290.75 mg/L with an average of 20.26 mg/L and in post monsoon it ranges from 3.65 to 242.0 mg/L, with an average of 24.7 mg/L.
Using a combination of box plots, binary plots and correlation matrices to describe study findings allows for a thorough explanation of data, distribution, relationships between variables and the correlation structure.
3.1 Box plot of data
The use of box- plots for pH, TDS, SO42−, Cl−, HCO3− and TH in both pre- and post-monsoon periods reveals scattered distribution indicating variability in the parameters (Fig. 2). The median is a midpoint of data, which divide box in two parts. The ends in the box-plot are called upper and lower quartiles. The observation of positive skewness in TDS, SO42−, Cl−, HCO3−and TH, where the median is below the mean, suggests that these parameters have a tail of higher values. On the other hand, the negative skewness in pH, with the median above the mean, indicates a tail of lower pH values. The longer upper whisker for all parameters except pH in the box plots suggest that there is greater variability in the values of both parameters. The district seasonal changes in the box plots for pH, SO42− and HCO3− highlight significant variation between the pre- and post-monsoon periods. Conversely, the box plots for TDS, TH and Cl− showing only slight variation suggest relatively stable concentration across two seasons. The observed variation in water parameters, along with the 19% above normal rainfall (664.5 mm), during 2021, suggests a link to the dissolution of minerals after rainfall [35]. The fluctuation in the depth of water level in Tonk, ranging from 2.11 to 36.45 m bgl in pre-monsoon and 0.42–34.15 m bgl in post-monsoon 2021 provides additional evidence of the impact of rainfall on water levels and mineral dissolution in the specified period [29].
3.2 Hydrogeochemical process
The Gibbs diagram is typically used to represent chemical compositions of water and identify the water type. It depicts three separate fields: precipitation zone, evaporation zone and rock-water interaction zone [36]. These zones help in understanding the geochemical process influencing water composition and provide insights into the hydrogeochemical evolution of water in a particular environment. The Gibbs diagram is plotted for approx. hundred samples. The presence of samples in the rock-water interaction and evaporation zone, indicates the influence of these process on the water chemistry as shown in Gibbs ratio anion I plot (Fig. 3). It strongly suggests a significant interaction between groundwater and rock minerals, specifically through weathering process. Temperature(water) in Tonk varies from 25.6 to 36.7 °C in PRM and from 22.9 to 31.5 °C during POM. It experiences a minimum temperature of 5 °C and a maximum temperature of 22 °C during winter. The higher temperature during summer, ranging from 30 and 45 °C, can contribute to increased evaporation [29]. The evaporation process in Tonk could be influenced by both temperature during summer and the presence of a high concentration of chloride [37]. High TDS and elevated Cl- content in samples can indeed contribute to increased salinity [9, 38]. The average concentration of chloride in pre- and post-monsoon is 179.43 and 172.7 mg/L respectively.
Ground water is mainly affected by silicate weathering and, to lesser extent, carbonate dissolution. Dominance of bicarbonate is often attributed to the formation of carbonic acid in soil. Drever [39] describes the reaction of CO2 in unsaturated zone with rainwater results in the production of bicarbonate. Reaction are as follows:
The bivariate plot of Ca2+and HCO3− (Fig. 4a) revealing enrichment of bicarbonate with calcium in the pre-monsoon period suggests a potential source of these ions, possibly linked to silicate weathering. On the other hand the post-monsoon samples showing dissolution of silicate and calcite indicate changes in geochemical processes [40]. Most of the samples lie on or below 1:1 line. Samples above the line have low calcium content which may be attributed to cation exchange and silicate weathering (Fig. 4b). The weathering of calcium rocks and Ca-rich silicates consistently enrich the Ca2+ and HCO3− in aquifer.
Molar ratio of Ca2+/Mg2+ denotes dolomite or calcite dissolution and silicate weathering. The Ca and Mg molar ratio lies on median line indicates dolomite dissolution (Fig. 4b). From the data analysis of samples, the mCa/Mg ratio is greater than 1 with an average of 4.05 in PRM and 3.06 mmol/L in POM. The majority of samples lying below the median line in bivariate plot of Ca2+ and HCO3−, especially with a 1:1 ratio, suggest a higher Ca2+ content in both seasons. This pattern is indicative of calcite dissolution on the study area. The presence of samples with mCa/Mg > 2, especially in the silicate weathering region indicates a dominance of silicate weathering process. High Mg2+ content in some samples during both seasons further supports the influence of silicate weathering on groundwater chemistry.
TDS and TH are crucial parameters for assessing water quality. The majority of samples as ranging from soft fresh to hard brackish water in both pre- and post-monsoon seasons indicates variation in mineral content (Fig. 5).
3.3 Water quality index
The Water Quality Index (WQI) is a valuable tool as it condenses multiple water quality criteria into a single numerical value, providing a convenient way to assess overall water quality. WQI study offers a comprehensive picture of surface and ground water quality allows for evaluation of water suitability for various domestic pur poses. The concentrations of 11 parameters such as pH, EC, TH, TA, TDS, Ca2+, Mg2+, Cl−, SO42−, NO3−, and F− were used to compute water quality indices (WQI) for the samples. Overall WQI value indicating 70% of tested samples as of exceptional quality and 30% as of good quality for drinking purposes is a positive finding. The WQI value and water type of the pre- and post-monsoon season are presented in Table 2 and Fig. 6. The WQI ranges from 4.55 to 82.33 and 6.39 to 96.83 for pre- and post-monsoon seasons, respectively. During post-monsoon the WQI is affected by dissolution of Cl−, NO3−, SO42−, Ca2+, Mg2+ and HCO3− ions. Presence of a high concentration of F− influencing the WQI in both PRM and POM seasons highlights the importance of specific ion in overall water quality.
3.4 Correlation analysis of physico-chemical parameters in Tonk
Correlation analysis is a statistical tool for measuring the strength and direction of a linear relationship between two variables. Correlation coefficient values close to + 1 or − 1 indicate that the variables x and y are likely to have a linear connection. The correlation has been extensively employed in studies related to water quality and physicochemical characteristics. Incorporating the WQI into the correlation matrices for variables such as pH, EC, Salinity, ORP, DO, TDS, TA, TH, F−, Cl−, NO3−, SO42−, PO4 3−, Ca2+, Mg2+ and bicarbonate etc. is summarized in Tables 3 and 4 for pre and post monsoon season allows not only to assess the relationship between individual water parameters but also their collective contribution to the water quality. Strong (r = > 0.9) to good (r = < 0.9 to > 0.5) correlations between various physicochemical parameters such as: (i)TDS/ EC with Cl−, NO3−, SO42−, TH,Ca,Mg and HCO3− (ii) Total hardness with TDS, Cl−, Ca, Mg, NO3−, SO42− (iii) Calcium with TDS, TH, Cl−, NO3−, SO42− (iv) Magnesium with TDS, Ca, TH, Cl−, NO3−, SO42−(v) Bicarbonate with TDS, Ca, Mg, TH, Cl−, NO3−, SO42− have been observed. These observed patterns in both PRM and POM seasons provide valuable insights into the interconnected nature of these parameters. The moderate to strong correlation of SO42− (r = 0.4–0.65) with other parameters in both pre- and post-monsoon suggests physicochemical reactions including oxidation–reduction and ion exchange in the groundwater aquifer system [41]. This correlation implies that these ions likely originated from a common source, indicating a shared geochemical history.
The strong correlation between the WQI and fluoride in both pre- (r = 0.90) and post-monsoon(r = 0.85) suggests a consistent relationship. Additionally, the moderate correlation of WQI with parameters such as TDS, Cl−, NO3−, SO42−, TH, Ca, Mg and HCO3− in post-monsoon indicates a link attributed to rock water interaction following rainfall. This suggests that the WQI is directly or indirectly associated with these parameters, emphasizing the impact of post-monsoon conditions on water quality.
4 Conclusion
Geostatistical and hydrochemical processes have effectively assessed the groundwater quality in the study area. The findings along with Water Quality Index indicate that the majority of the area has good to exceptional quality water suitable for various uses. The strong correlation between WQI and fluoride in both seasons further supports the reliability of evaluation. Additionally, the water quality characteristic for most of the samples is soft fresh to hard brackish water for both the seasons.
Ca2+ is the predominant cation in the groundwater study, and chloride is the most abundant anion after bicarbonate. Ninety six percent of groundwater samples in the study region had nitrate concentrations below acceptable limits reflects a positive aspect for water quality. The majority of samples exhibit TDS levels below the permissible limit set by the BIS(34) in both pre- and post-monsoon seasons. However around ten and nine percent samples have TDS above the permissible limit of 2000 mg/L in pre- and post-monsoon respectively, warrants attention. The wide range of concentration in the primary components indicates the involvement of multiple hydrochemical processes influencing water quality. The average molar ratio of Ca2+/Mg2+ ions at 4.05 in pre-monsoon and 3.06 mmol/L in post-monsoon, suggests variation in the dominance of these ions across season. Bicarbonate emerges as the dominant ion followed by chloride and others highlighting the complex interplay of hydrochemical factors in shaping the groundwater composition.
The order of anions in the area based on concentration is as follows: HCO3− > Cl− > SO42− > NO3− > F−
The Gibbs plot analysis reveals that most of the samples fall in the rock water interaction zone with some in the evaporation zone due to elevated Cl− ion content, suggests a significant influence of geology on the water composition. Beesalpur dam providing a significant portion (33%) of the drinking water in Tonk while other area relying on underground water reserves, rain water harvesting is crucial for sustainable water management. Different hydrochemical measures have been taken to determine groundwater suitability for drinking purpose. While most analysed water is deemed safe for drinking and household use, locations with high TDS, Cl−, Ca2+ and hardness levels may require treatment before use. The unusual concentration of the ions in groundwater possibly influenced by anthropogenic contributions, agricultural activities and industrial pollution emphasize the impact of human activities on water quality. Water quality index is soft to moderate in both seasons indicate a level of water quality monitoring and management.
The findings of this study hold valuable insights for identifying the principal causes of pollution at various locations and the measures to mitigate the source of contamination.
Data availability
The data set generated during current study are available from corresponding author on reasonable request.
Code availability
Not applicable.
References
UNESCO. Grounwater making the invisible visible. 2022. https://unesdoc.unesco.org/ark:/48223/pf0000380721/PDF/380721eng.pdf.multi.
Shakti M of J. Annual Report. New Delhi; 2020.
Chitsazan M, Aghazadeh N, Mirzaee Y, Golestan Y, Mosavi S. Hydrochemical characteristics and quality assessment of urban groundwater in Urmia City, NW Iran. Water Supply. 2017;17(5):1410–25.
Zhu C, Schwartz FW. Hydrogeochemical processes and controls on water quality and water management. Elements. 2011;7(3):169–74.
Chen-Wuing Liu CJ. Characterization of groundwater quality in Kinmen Island using multivariate analysis and geochemical modelling. Hydrol Process. 2008;22(3):376–83.
Rajmohan N, Elango L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the palar and cheyyar river basins, Southern India. Environ Geol. 2004;46:47–61.
Rajesh R, Brindha K, Murugan R, et al. Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environ Earth Sci. 2011. https://doi.org/10.1007/s12665-011-1368-2.
Srinivas R, Bhakar P, Singh AP. Groundwater quality assessment in some selected area of Rajasthan, India using fuzzy multi-criteria decision making tool. Aquatic Procedia. 2015. https://doi.org/10.1016/j.aqpro.2015.02.129.
Chaudhary V, Satheeshkumar S. Assessment of groundwater quality for drinking and irrigation purposes in arid areas of Rajasthan, India. Appl Water Sci. 2018. https://doi.org/10.1007/s13201-018-0865-9.
Addisie MB. Evaluating drinking water quality using water quality parameters and esthetic attributes. Air, Soil Water Res. 2022;15:1–8.
Aghazadeh N, Chitsazan M, Golestan Y. Hydrochemistry and quality assessment of groundwater in the Ardabil area, Iran. Appl Water Sci. 2017;7:3599–616.
Atta HS, Omar MA-S, Tawfik AM. Water quality index for assessment of drinking groundwater purpose case study: area surrounding Ismailia Canal, Egypt. Egypt J Eng Appl Sci. 2022. https://doi.org/10.1186/s44147-022-00138-9.
Fallatah O, Khattab MR. Evaluation of groundwater quality and suitability for irrigation purposes and human consumption in Saudi Arabia. Water. 2023;15(13):2352.
Brindha K, Pavelic P, Sotoukee T, et al. Geochemical characteristics and groundwater quality in the Vientiane plain, Laos. Expo Health. 2017;9:89–104.
Ram A, Tiwari SK, Pandey HK, et al. Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl Water Sci. 2021. https://doi.org/10.1007/s13201-021-01376-7.
Basavarajappa HT, Manjunatha MC. Groundwater quality analysis in precambrian rocks of Chitradurga district, Karnataka, India using geo-informatics techdnique. Aquatic Procedia. 2015;4:1354–65.
CGWB D of water resources. Ground water Year Book 2020–21. Jaipur, INDIA; 2021. http://cgwb.gov.in/Regions/WR/Reports/GWYearBook2020–21-Rajasthan.pdf.
Coyte RM, Singh A, Furst KE, Mitch WA, Vengosh A. Co-occurrence of geogenic and anthropogenic contaminants in groundwater from Rajasthan, India. Sci Total Environ. 2019;20(688):1216–27.
Pandey S, Mohapatra G, Arora R. Groundwater quality, human health risks and major driving factors in arid and semi-arid regions of Rajasthan, India. J Clean Prod. 2023;15(427):139149.
Sisodiya KS, Mathur AK. An Assessment of Water Quality Indices of Chambal River in Kota Cityfor Drinking and Irrigation Purposes. Weentech Conference Proceedings. 2021;121–36.
Sharma KCAM. Assessment of groundwater quality for drinking and irrigation purposes in Banasthali village, district Tonk, Rajasthan. Nat Environ Pollut Technol. 2013;12:679–84.
Sinha KK, Gupta MK, Banerjee MK, Meraj G, Singh SK, Kanga S, Farooq M, Kumar PS. Neural network-based modeling of water quality in Jodhpur, India. Hydrology. 2022. https://doi.org/10.3390/hydrology9050092.
Vyas AD, Bhatnagar N. Assessing the deteriorating water quality in wards of Jaipur city through GIS interpolation. AQUA Water Infrastruct Ecosyst Soc. 2023;72(4):425–37.
Dubey SK, Pathak SK, Sharma D, Babel MS, Kumar M. Assessment of groundwater quality index using geographic information system in parts of rural Ajmer and Bhilwara districts of Rajasthan, India. Water Conserv Sci Eng. 2021;6:221–32.
Gautam R, Sivaranjani S, Rakshit A. Ways of assessment of water quality index in TONK district of Rajasthan, India. Pollut Res EM Int. 2018;37(3):669–77.
Saini A, Kanwar P, Garg J. Potential health risk assessment of nitrate in groundwater of Tonk district in Rajasthan, north western India. Int J Environ Anal Chem. 2024. https://doi.org/10.1080/03067319.2023.2299947.
Shittu OB, Olaitan JO, Amusa TS. Physicochemical and bacteriological analyses of water used for drinking and swimming purposes in Abeokuta, Nigeria. Afr J Biomed Res. 2008;11:285–90.
CGWB, Tonk G of I. National Aquifer Mapping and Management Plan of Tonk District-2021–22. Jaipur, INDIA; 2022. http://cgwb.gov.in/AQM/NAQUIM_REPORT/Rajasthan/Tonk.pdf.
CGWB, Tonk G of I. Ground Water Information Tonk district. Tonk, RAjasthan; 2013. https://cgwb.gov.in/District_Profile/Rajasthan/Tonk.pdf.
APHA. Standards methods for examination of water and wastewater (22nd ed.). Washington, D.C; 2012.
Brown RM, McCleland NI, Deininger RA, Tozer R. A water quality index: do we dare? Water Sewage Works. 1970;117(10):339–43.
Sarath Prasanth SV, Magesh NS, Jitheshlal KVCN. Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha district, Kerala. India Appl Water Sci. 2012;2(3):165–75.
WHO. Guidelines for drinking water quality. Geneva: World Health Organization; 2011.
BIS. Indian Standards Drinking Water Specifications. Second revision IS 10500:2012,eau of Indian Standards, New Delhi. Vol. 2. New Delhi; 2012. http://cgwb.gov.in/Documents/WQ-standards.pdf.
Centre IM. Monsoon Report. Jaipur; 2021.
Gibbs RJ. Mechanisms controlling world water chemistry. Science. 1970;170(3962):1088–90.
Sahu S, Gogoi U, Nayak NC. Groundwater solute chemistry, hydrogeochemical processes and fluoride contamination in phreatic aquifer of Odisha, India. Geosci Front. 2021;12(3):101093.
Howladar MF, Al Numanbakth MA, Faruque MO. An application of Water quality index (WQI) and multivariate statistics to evaluate the water quality around Maddhapara granite mining industrial area, Dinajpur, Bangladesh. Environ Syst Res. 2018. https://doi.org/10.1186/s40068-017-0090-9.
Drever JI. The geochemistry of natural waters. Englewood Cliffs: Prentice Hall; 1988.
Montcoudiol N, Molson J, Lemieux J. Groundwater geochemistry of the Outaouais region (Québec, Canada): a regional-scale study. Hydrogeology. 2015;23(2):377–96.
Batabyal AK, Chakraborty S. Hydrogeochemistry and water quality index in the assessment of groundwater quality for drinking uses. Water Environ Res. 2015. https://doi.org/10.2175/106143015X14212658613956.
Acknowledgements
Board Research in Nuclear Sciences (BRNS), Department of Atomic Energy, supported this investigation. The authors thanks to Raj Rishi College, Alwar (Rajasthan) for their grant access to the project.
Funding
The study was conducted with financial support from the Raj Rishi College, Alwar.
Author information
Authors and Affiliations
Contributions
Avinash Kumar Bairwa: Collection interpretation of data & framing basic draft of the manuscript. Ashok Kumar Gupta: prepared graphs, tables and concept designs. Vivek Mishra: revised and edited the manuscript. Kalpana Gupta: Supervising and drafting the manuscript for intellectual content; have given final approval.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors consented to publish this research article.
Competing interests
The authors declare that they have no competing interests and non-financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bairwa, A.K., Gupta, A.K., Mishra, V. et al. Physico-chemical and correlation study of ground drinking water of Tonk district of Rajasthan (India). Discov Water 4, 23 (2024). https://doi.org/10.1007/s43832-024-00077-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-024-00077-x