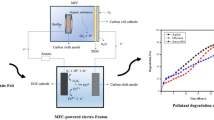
Abstract
Excessive, unregulated usage and reckless disposal of antibiotics have led to the formation of antibiotic resistance in bacteria. Additionally, untreated or partially treated pharmaceutical effluents are discharged into water bodies. With the increasing prevalence of antibiotic resistance across the globe, previously curable diseases are becoming challenging to handle due to the advent of superbugs. It is crucial to ensure complete treatment and removal of antibiotics and pharmaceuticals before discharging them into water bodies. Conventional treatment plants are not specialized in removing such complex, emerging contaminants. The advanced oxidation process is an emerging and promising treatment strategy for the abatement of emerging contaminants such as pharmaceutical compounds. This study explores an electrochemical advanced oxidation process, viz., electro-peroxone for treating ciprofloxacin, a model antibiotic drug. The electro-peroxone system houses electrodes of graphite felt that helps form hydrogen peroxide, an oxidant, in situ. The viability of the carbon-based graphite felt system in reactive oxygen species generation and ciprofloxacin degradation is investigated. A comparison of the electro-peroxone with two benchmark processes, namely, electrolysis and ozonation, is also carried out. Electro-peroxone and ozonation are both quite promising in removing the model contaminant. The in situ generation of H2O2 and •OH is estimated. This is a one-of-a-kind study involving graphite felt as both anode and cathode and achieves an in situ generation of H2O2 of 47 mg/L in 120 min and •OH of 140 µM within 60 min of electro-peroxone. Besides, the efficacy of the system in contaminant degradation is examined at voltammetric and galvanostatic modes of operation. Ozonation and electro-peroxone processes achieved an efficiency between 97% and complete removal of ciprofloxacin in less than an hour. This novel system generates several times higher hydrogen peroxide than the existing graphite electrode system, making it more efficient in radical generation and pollutant abatement. This graphite felt-based electro-peroxone system, on further optimization and up-scaling, can be a promising strategy for abating pharmaceutical compounds and effluents.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, pharmaceutically active compounds in water are becoming a tremendous global health concern [1]. The water bodies get contaminated due to the inefficacy of existing conventional treatment plants in removing pharmaceutical compounds [2]. Pharmaceutical compounds are emerging contaminants that can cause severe health hazards even at very low concentrations (ng/L to µg/L) [3]. One of the most critical and prevalent classes of pharmaceuticals is the antibiotic drugs that might originate from various sources such as pharmaceutical industry effluents, improper disposal of hospital waste into receiving bodies, unmetabolized antibiotics from human and animal excreta and flushing down of unused drugs, and eventually end up in water bodies [4]. Malpractices such as over-the-counter sales of antibiotics and lack of awareness resulting in the consumption of an incomplete course of antibiotics are two critical contributors to the development of antibiotic resistance in microorganisms. Subsequent discharge or land application of such inadequately and inappropriately treated wastewater or sludge results in surface and groundwater contamination. Antimicrobial resistance (AMR) can result in antimicrobials turning ineffective, leading to thousands of deaths due to previously curable infections [4]. Therefore, immediate research leading to technology development is the need of the hour.
In waters associated with a hospital in Ujjain, seven antibiotics were selected (namely, amoxicillin, ceftriaxone, amikacin, ofloxacin, ciprofloxacin, norfloxacin and levofloxacin) and checked for their presence. Ciprofloxacin had the highest residual concentration out of the seven in the hospital wastewater [5]. Similarly, researchers [6] analyzed pharmaceuticals in the effluent from a common effluent treatment plant (CETP) near Hyderabad, India, which serves about 90 bulk drug manufacturers. Several pharmaceutically active compounds were detected in river water near the outfall of CETP, Hyderabad. The concentration ranges of antibiotics are provided within brackets in milligrams per liter: Ciprofloxacin (28–31), Losartan (2.4–2.5), Enrofloxacin (0.78–0.90), Norfloxacin (0.39–0.42), Ofloxacin (0.15–0.16) [6]. The data indicates that conventional wastewater treatment processes are intended to remove traditional and well-established waste or contaminants with regulatory limits and are unable to meet the increasing global demand for clean water.
It is also very evident from the above data that the concentration of ciprofloxacin is exceptionally high. Further, a risk assessment of antibiotic residues in different waters in India showed the highest risk for ciprofloxacin [7]. There is multiple evidence for ciprofloxacin contamination and that they have significant health impacts on humans and other living beings, necessitating its removal from water systems.
Research efforts to keep water bodies from antibiotic contaminants are crucial to prevent avoidable deaths. Several works have been carried out on removing antibiotics and AMR, including membrane separation, adsorption, chlorination, conventional oxidation, and ozonation. Physical techniques such as membrane separation and adsorption have significant limitations because they only result in a mass transfer of the contaminants under consideration. There is no significant degradation of the pollutants observed in such physical techniques. Many of them also suffer from high operational costs.
On the other hand, chemical methods such as chlorination involve chlorine reacting with organic contaminants to form intermediate compounds, which are then expected to break down into less harmful compounds. However, during the course of chlorination, there is the generation of toxic disinfection byproducts (DBPs), such as trihalomethanes (THMs) and haloacetic acids (HAAs), that can be detrimental to human and animal health [8]. Other treatment methods, such as ozonation and advanced oxidation processes (AOPs), can also be effective for ciprofloxacin degradation and may be worth considering. On the flip side, Ozonation has a limitation of being a selective oxidant, resulting in incomplete mineralization of compounds. Insufficient mineralization results from selective oxidants such as ozone, typically reacting with electron-rich organic moieties [9]. Although the rate and efficiency of the degradation process depend on several factors, including the initial concentration of contaminant, solution pH, ozone concentration, and the contact time between ozone and the pollutant, typically, ozonation has a drawback of requiring longer contact times to achieve the desired level of removal. However, this may only sometimes be the case and would depend on the nature of the contaminant.
Therefore, the formation of toxic disinfection by-products, intermediates and incomplete mineralization are critical limitations of the above chemical methods. Consequently, it is essential to carefully assess the benefits and the potential risks associated with each treatment technology before choosing an appropriate treatment method. Advanced oxidation processes are promising in degrading antibiotics and related pharmaceutical drugs [10]. Despite their merits, most AOPs have significant drawbacks, such as a narrow working pH range and the need for frequent chemical addition, sludge removal and post-treatment steps, making it challenging to deploy them at scale.
This study focuses on removing a model antibiotic drug, ciprofloxacin, using electro-peroxone, a promising and contemporary electrochemical advanced oxidation process. The critical properties of ciprofloxacin are presented in Table 1. The electro-peroxone process involves reducing oxygen electrochemically to form H2O2 and further exploits the synergy of electrochemical oxidation and ozonation to generate •OH to remove persistent organic contaminants efficiently.
The current study involves graphite felt, a carbon-based material used as an anode and cathode in this system. The feasibility of graphite felt in driving the two-electron reduction of oxygen to form H2O2 is investigated. Further, the ability of graphite felt to replace expensive anodes such as Platinum is also explored to achieve economic feasibility. To the best of my knowledge, this is one-of-a-kind study wherein graphite felt is deployed as both the anode and cathode in an electro-peroxone system for efficienctly removing pharmaceuticals from water in an eco-friendly manner. The in-situ H2O2 and •OH generation in the graphite felt-based electro-peroxone system is quantified. Furthermore, the system's efficacy in achieving ciprofloxacin degradation is also examined at voltammetric and galvanostatic conditions. Overall, a sustainable and energy-efficient graphite felt system for generating high concentrations of ROS with minimal electric potential and excellent pollution abatement is investigated herein.
2 Materials and methods
2.1 Chemicals and materials
The electrode material, graphite felt, was sourced from Sainergy Fuel Cell Private Limited. Ciprofloxacin with a purity greater than 99% was acquired from Sigma Aldrich and was used to prepare realistic concentrations of ciprofloxacin (10 mg/L) for degradation experiments. Acetonitrile of HPLC grade, Titanium (IV) oxysulfate-sulfuric acid solution (∼27–31%) and the rest of the chemicals were procured from Sigma Aldrich and other chemical suppliers.
2.2 Experimental setup
The electro-peroxone experimental setup consists of an electrochemical reaction vessel housing the working and counter electrodes, as seen in Fig. 1. In this case, both are made of graphite felt. It also provides oxygen and ozone supply via bubble diffusers and a facility for continuous stirring to keep the reaction liquid homogeneous. An oxygen concentrator (Devilbiss, USA) produced oxygen from ambient air, and an ozone generator generated ozone from oxygen. Both oxygen and ozone were supplied into the reactor through two separate lines. Finally, the excess unreacted ozone was sent to an ozone destructor for safe disposal. The entire setup was placed inside a fume hood to ensure safety. Ciprofloxacin is the model pharmaceutical contaminant of concern, and sodium sulfate was used as an electrolyte for the electrochemical system.
2.3 Analytical quantification of reactive oxygen species—H2O2 and •OH
In situ, H2O2 generation was quantified using a colorimetric method, as described in [11]. A colored pertitanic complex was formed and was analyzed using a UV–Visible spectrophotometer (Shimadzu) at a wavelength of 410 nm. Hydroxyl radical quantification was performed by trapping the short-lived radicals using a trapping agent, dimethyl sulfoxide (DMSO). This resulted in the formation of formaldehyde, which was derivatized using dinitrophenylhydrazine (DNPH) at specific conditions, as mentioned in ref. [12]. This process resulted in hydrazone formation that was further analyzed in high-performance liquid chromatography (HPLC) (Dionex) and was detected using the built-in UV detector at a wavelength of 355 nm. Further details of the procedure can be found in ref. [12].
2.4 Degradation experiments
The degradation experiments involved the study of Ciprofloxacin (as a model pharmaceutical contaminant) and its removal during the electro-peroxone process. These experiments were carried out both in voltammetric and galvanostatic conditions. In addition, a comparative study of ciprofloxacin degradation was carried out across electro-peroxone, electrolysis and ozonation. Ciprofloxacin was quantified using HPLC (Dionex). It was detected by the UV-detector at a wavelength of 280 nm [13] owing to its strong absorbance peak in the UV region of the electromagnetic spectrum. Here, the mobile phase comprised acetonitrile and 10 mM phosphate buffer in the volume ratio 20:80 [14]. A constant mobile phase flow rate of 1.5 mL/min was maintained for the quantification. A reverse phase C-18 HPLC column (4.6 × 150 mm; 5 μm) was used for the above quantification.
3 Results and discussion
The quantification of in situ generated reactive oxygen species such as H2O2 and •OH and ciprofloxacin degradation experiments are presented in this section. The quantification of in situ-generated hydrogen peroxide conducted under an electrolysis mode of operation (in the absence of ozone), wherein only oxygen and electric potential are supplied. The quantification was done using a colorimetric method of determination of peroxide concentration wherein 2 mL of aqueous sample drawn out from time to time was mixed with 1 mL of titanium (IV) oxysulfate reagent to form a pertitanic complex. Further, the resulting pertitanic acid complex's absorbance was quantified at a wavelength of 410 nm (UV–Visible Spectrophotometer, Shimadzu) (Eisenberg 1943). The influence of electric potential on hydrogen peroxide generation is presented in Fig. 2. An increase in the applied electric potential resulted in increased peroxide generation. An electric potential of 0.5 V resulted in a maximum of around 20 mg/L, 0.6 V and 0.7 V resulted in a maximum of about 37 and 47 mg/L. This indicated that the peroxide generation depends on the quantum of applied electric potential. Li et al. [15] investigated the application of graphite felt as the working and counter electrodes for methylene blue degradation via the electro-Fenton process. The in-situ generation of H2O2 reported was around 10–12 mg/L at their optimum conditions of an electric potential of 7 V and a solution pH of 2.5 [15]. On the other hand, an electric potential as low as 0.7 V resulted in an in situ H2O2 generation of 47 mg/L. This observation may be attributed to using a higher electrolyte concentration and an oxygen concentrator (to obtain around 93% oxygen) instead of air as a source of oxygen from fish aerators.
Further, quantification of •OH was performed under the electro-peroxone mode using the dimethyl sulfoxide (DMSO)-based HPLC method mentioned in Sect. 2.3. Here, DMSO reacts with a hydroxyl radical to form a methyl radical. Quantification of the methyl radical is achieved by estimating its subsequent oxidation product, formaldehyde. Further, formaldehyde is assessed by derivatization with 2,4-dinitrophenylhydrazine and subsequent analysis of the hydrazone by HPLC–UV/Visible detection [16].
In the electro-peroxone mode, 10% ozone and 1.5 V electric potential were supplied to quantify radical generation. This resulted in a •OH generation of around 140 µM within 60 min of electro-peroxone, as seen in Fig. 3a. Compton et al. [16, 17] investigated using activated carbon impregnated with 5% MnO2 to generate radicals in the presence of H2O2. This system resulted in the generation of 140 μM hydroxyl radicals [16, 17]. This is comparable to the current study wherein a simple, unmodified graphite felt electrode can generate as high as 140 μM hydroxyl radicals in the presence of O2 and O3.
Degradation experiments were also carried out to understand the efficacy of the graphite felt electrode system in removing ciprofloxacin. Degradation in the electrolysis mode was negligible. On the other hand, the electro-peroxone mode at an applied potential of 1.5 V and input ozone of around 10% resulted in 98% and 99.5% ciprofloxacin removal within 30 and 60 min of reaction, respectively.
Thereafter, the three processes, electrolysis and ozonation, were compared across an applied potential of 0.7 V and 30% O3, respectively, and further with electro-peroxone across various applied potentials, as seen in Fig. 4. This experiment was performed in potentiostatic mode. Electrolysis at 0.7 V resulted in only an 8% reduction after 150 min of reaction. All the other conditions of ozonation and electro-peroxone, as seen in Fig. 4 showed outstanding treatment promise. They all resulted in around 97 to 99% ciprofloxacin removal. From this experiment, we understand that if a sufficient ozone dosage is given, ciprofloxacin may be removed with just ozonation and without needing electro-peroxone.
Next, the galvanostatic mode of operation of electro-peroxone involved an ozone input of 40% and an applied electric potential of 5 and 50 mA, as seen in Fig. 5. In the initial 10 min of reaction, the rates of ciprofloxacin removal had variations. However, it resulted in complete removal across all the conditions within 15 min of reaction time.
All the above experiments show that ciprofloxacin is degraded faster as the ozone dosage increases. Ozonation and electro-peroxone processes are efficient in the removal of ciprofloxacin. On the other hand, electrolysis is unable to degrade the contaminant completely. Electrolysis involves driving a non-spontaneous reaction via the application of an electric potential. However, the inability of electrolysis to complete the removal of ciprofloxacin is possibly due to the generation of H2O2 during electrolysis, which has a lower oxidation strength compared to O3 and •OH. The mechanism of ciprofloxacin degradation is detailed below and illustrated in Fig. 6. The graphite felt cathode catalyzes the two-electron reduction of oxygen (from the air pumped into the system) in the presence of an applied electric potential and leads to the in situ generation of H2O2 in bulk solution. Carbon Materials Catalysts (CMCs) generally prefer the Oxygen Reduction Reaction (ORR) due to inherent features like defects and curvature, with O2 adsorption followed by an H-abstraction leading to an OOH intermediate. Subsequently, the OOH intermediate combines with a bulk solution proton, forming H2O2 [18]. As found in this investigation, graphite promotes the explicitly cathodic two-electron reduction of oxygen, resulting in in-situ H2O2 generation. Further, electro-peroxone combines ozone and in-situ generated H2O2 to produce non-selective •OH, aiming to mineralize organic contaminants. Both O3 and •OH contribute to the mineralization of ciprofloxacin by converting it into harmless inorganic products such as CO2 and H2O, as seen in Fig. 6.
Overall, ozonation and graphite-based electro-peroxone systems show great promise as innovative treatment systems for improving the quality of water resources and sustainably protecting public health.
4 Conclusions
This study demonstrates the efficacy of ozonation and electro-peroxone processes in ciprofloxacin degradation. This study has proven the promise that the dual graphite felt electrode system holds in treating antibiotics. Electro-peroxone with a simple and cost-effective electrode material, namely, graphite felt, generated 47 mg/L H2O2 at 0.7 V and 140 µM •OH at 1.5 V. Ozonation and electro-peroxone processes were able to achieve anywhere between 97% and complete removal under various conditions. The current graphite felt-based electro-peroxone system can generate higher hydrogen peroxide than some of the existing graphite electrode systems with one-tenth the electric potential deeming the system several times more efficient than the current conventional system. The mechanism behind ciprofloxacin removal has also been proposed. This system is more than an order of magnitude more efficient in reactive oxygen species generation than the existing graphite-felt-based electro-peroxone systems. Moreover, it has great potential in removing antibiotics, and abatement of antimicrobial resistance associated with antimicrobial drugs.
Data availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
Tijani JO, Fatoba OO, Petrik LF. A review of pharmaceuticals and endocrine-disrupting compounds: Sources, effects, removal, and detections. Water Air Soil Pollut. 2013. https://doi.org/10.1007/s11270-013-1770-3.
Nippes RP, Macruz PD, da Silva GN, Neves Olsen Scaliante MH. A critical review on the environmental presence of pharmaceutical drugs tested for the covid-19 treatment. Process Safety Environ Protection. 2021;152:568–82. https://doi.org/10.1016/j.psep.2021.06.040.
Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F. Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. N Biotechnol. 2015;32:147–56. https://doi.org/10.1016/j.nbt.2014.01.001.
Sivagami K, Vignesh VJ, Srinivasan R, Divyapriya G, Nambi IM. Antibiotic usage, residues and resistance genes from food animals to human and environment: an Indian scenario. Environ Chem Eng J. 2018. https://doi.org/10.1016/j.jece.2018.02.029.
Diwan V, Tamhankar AJ, Khandal RK, Sen S, Aggarwal M, Marothi Y, Iyer RV, Sundblad-Tonderski K, Stålsby-Lundborg C. Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain India. BMC Public Health. 2010;10:414. https://doi.org/10.1186/1471-2458-10-414.
Larsson DGJ, de Pedro C, Paxeus N. Effluent from drug manufacturers contains extremely high levels of pharmaceuticals. J Hazard Mater. 2007;148:751–5. https://doi.org/10.1016/j.jhazmat.2007.07.008.
Mutiyar PK, Mittal AK. Occurrences and the fate of selected human antibiotics in influents and effluents of the sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environ Monit Assess. 2014;186:541–57. https://doi.org/10.1007/s10661-013-3398-6.
Kim J, Chung Y, Shin D, Kim M, Lee Y, Lim Y, Lee D. Chlorination by-products in surface water treatment process. Desalination. 2003;151:1–9. https://doi.org/10.1016/S0011-9164(02)00967-0.
Lee Y, von Gunten U. Oxidative transformation of micropollutants during municipal wastewater treatment: comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010;44:555–66. https://doi.org/10.1016/j.watres.2009.11.045.
Elmolla ES, Chaudhuri M. Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination. 2010;256:43–7. https://doi.org/10.1016/j.desal.2010.02.019.
Srinivasan R, Nambi IM. An electro-peroxone-based multi-pronged strategy for the treatment of ibuprofen and an emerging pharmaceutical wastewater using a novel graphene-coated nickel foam electrode. Chem Eng J. 2022;450: 137618. https://doi.org/10.1016/j.cej.2022.137618.
Divyapriya G, Nambi IM, Senthilnathan J. An innate quinone functionalized electrochemically exfoliated graphene/Fe3O4 composite electrode for the continuous generation of reactive oxygen species. Chem Eng J. 2017;316:964–77. https://doi.org/10.1016/j.cej.2017.01.074.
Divyapriya G, Srinivasan R, Mohanalakshmi J, Nambi IM. Development of a hybrid bifunctional rotating drum electrode system for the enhanced oxidation of ciprofloxacin: an integrated photoelectrocatalysis and photo-electro-Fenton processes. J Water Process Engi. 2022;49: 102967. https://doi.org/10.1016/j.jwpe.2022.102967.
Divyapriya G, Srinivasan R, Nambi IM, Senthilnathan J. Highly active and stable ferrocene functionalized graphene encapsulated carbon felt array—a novel rotating disc electrode for electro-Fenton oxidation of pharmaceutical compounds. Electrochim Acta. 2018;283:858–70. https://doi.org/10.1016/j.electacta.2018.06.186.
Li J, Song D, Du K, Wang Z, Zhao C. Performance of graphite felt as a cathode and anode in the electro-Fenton process. RSC Adv. 2019;9:38345–54. https://doi.org/10.1039/c9ra07525a.
Tai C, Peng JF, Liu JF, Jiang GB, Zou H. Determination of hydroxyl radicals in advanced oxidation processes with dimethyl sulfoxide trapping and liquid chromatography. Anal Chim Acta. 2004;527:73–80. https://doi.org/10.1016/j.aca.2004.08.019.
Compton P, Dehkordi NR, Knapp M, Fernandez LA, Alshawabkeh AN, Larese-Casanova P. Heterogeneous fenton-like catalysis of electrogenerated H2O2 for dissolved RDX removal, frontiers in chemical. Engineering. 2022;4:1–15. https://doi.org/10.3389/fceng.2022.864816.
Chai GL, Hou Z, Ikeda T, Terakura K. Two-electron oxygen reduction on carbon materials catalysts: mechanisms and active sites. J Phys Chem C. 2017;121:14524–33. https://doi.org/10.1021/acs.jpcc.7b04959.
Acknowledgements
We thank the Indian Institute of Technology for providing the facility to perform a portion of this work.
Author information
Authors and Affiliations
Contributions
R.S. did the conceptualisation, implementation, experimentation, analyses, interpretation and writing and reviewing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srinivasan, R. Sustainable degradation of ciprofloxacin in water by the electro-peroxone process via a graphite felt electrode system. Discov Water 4, 4 (2024). https://doi.org/10.1007/s43832-024-00057-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-024-00057-1