Abstract
The present study synthesized magnetite-pectin-chitosan (MPC), which is a nanocomposite adsorbent through partial reduction co-precipitation method. Its structure and morphology were characterized by Scanning Electron Spectroscopy (SEM), X-ray diffraction (XRD), Fourier Transform Infrared Spectrometry (FT-IR) and Vibrating Sample Magnetometry (VSM). One studied the adsorption of Lanthanum and Cerium ions onto MPC in a batch system as a function of initial pH of solution, contact time, initial concentration and temperature. When the initial pH of the solution increased, the La(III)/Ce(III) ions augmented as well. The optimum pH for La/Ce adsorption was 5 and the maximum adsorption achieved within 30 min. The thermodynamics parameters such as enthalpy (\(\Delta {\text{H}}^\circ\)), entropy (\(\Delta {\text{S}}^\circ\)) and Gibbs free energy changes (\(\Delta {\text{G}}^\circ\)) were calculated with the results suggesting that the adsorption was exothermic and spontaneous. To determine the adsorption characteristics, models of Langmuir, Dubinin-Radushkevich, Freundlich, Temkin and Flory–Huggins adsorption isotherms were performed. Langmuir isotherm model enabled the data of adsorption to be described very well. The adsorption capacity was seen to be 8.17 mg/g and 9.72 mg/g for lanthanum and cerium, respectively. The adsorption kinetics of MPC indicated that the adsorption process fit the Pseudosecond-order (PSO) model. Based on these data, it is concluded that short operating time, eco-friendly technology, and easy to apply MPC can be used for La(III)/Ce(III) ions removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare Earths Elements (REEs) such as lanthanum and cerium are employed in many highly advanced optical and electrical materials. Lanthanum has been of special interest thanks to its high/progressive technological characteristics in catalysts, special alloys and organic synthesis [1]. Cerium is used as pigment in applications of flat screen monitors, lights lamps, synthesis of catalysts and biomedicine [2, 3].
With the demand for such elements increasing, various processes of mining, ores and extraction generate wastes of lanthanides which have been discharged into environment. Moreover, one of fission products generated by irradiated nuclear fuel is a group of lanthanide/lanthanoid elements. Natural environment and humans are exposed to the threat of such impurities. Therefore, it is vital to perform their rapid and efficient removal from aquatic environments by means of advanced technology [4]. Numerous studies have been conducted to remove and separate lanthanum and cerium ions from aqueous phases [5, 6]. A number of methods have been used for extracting lanthanides from aqueous phases, such as chemical precipitation, ion-exchange, electrolysis, adsorption and solvent extraction [7]. Of these techniques, adsorption is the most effective, inexpensive and easy to apply with short operating time and eco-friendly technology. The method is used to uptake heavy radioactive metal ions from waste solutions [8,9,10].
Adsorption is the process by which/whereby distinctive biomass exhibits the ability to fix some metal ions from aqueous solutions. Accordingly, magnetic adsorbents, especially of vegetable origin have become very popular for eliminating impurities in aqueous solutions owing to their desirable properties such as low cost, ease of preparation, fast sorbing ability, high sorption capacity and facility in separation without significant secondary waste [11]. It is therefore important to assess vegetative wastes present in nature for the synthesis of the new magnetic adsorbent in terms of potential development of a low-cost, effective and environmentally friendly method [12].
Of the natural materials likely to be used to create nanocomposite materials, pectin is the most popular in particular. Pectin is plant cell wall whose natural heteropolysaccharides are mainly composed of α-1–4 D-galacturonic acid units, which could or could not be methyl-esterified, and possess neutral sugars branching that favor functional moieties [13]. Biocompatible and bioactive, Pectin contains the hydroxyl group as well to enhance its chemical properties thus enabling the COOH group to bind Fe3O4 via the covalent bond [14,15,16].
A natural biodegradable polysaccharide extracted from marine natural sources, Chitosan is also widely employed in biomedical and pharmaceutical processes such as tissue engineering and wound healing. Included in different adsorbents, chitosan as a derivative obtained by N-deacetylation of chitin is a naturally abundant and versatile biopolymer with an immense role in wastewater treatment [17]. Chitosan has excellent absorbent qualities, ease of availability and economic benefits. However, its practical application involves a few challenges, such as its non-selective character, lack of mechanical strength, limited solubility in acidic media and low surface area. The literature has examined many modification methods to enhance the physicochemical characteristics of chitosan [18]. One of the new approaches used to overcome its disadvantages and improve physical and chemical properties is its employment as a composite material in combination with a mineral as cross-linkers [19]. The application of cross-linkers is one of the most common procedures to commonly frequently the stability of chitosan in an acidic environment as well as its mechanical strength [20, 21].
Biopolymer-based magnetic hybrid materials have been extensively studied in recent years. Studies on the drug delivery are limited [14], removal of some heavy metals [22], radionuclides [11] from aqueous solution, and sensor for triglycerides in serum [23]. Our previous study investigated [11] adsorption performance of magnetic-pectin nanobiocomposite material for Sr(II) ion removal from aqueous solutions.
The current study focused on the synthesis and characterization of magnetite-pectin (Fe3O4-pectin) nanocomposite adsorbent using co-precipitation method and functionalization with chitosan to eliminate Lanthanum and Cerium from aqueous phases. The Fe3O4-pectin-Chitosan adsorbent was synthesized and then systematically characterized by numerous different analyses.
The objective of the work was to evaluate the capacity of the Fe3O4-pectin-Chitosan nanocomposite adsorbent to remove lanthanum and cerium ions. The effect of initial solution pH, initial concentration of La(III)/Ce(III) ions, contact time and temperature were studied to eventually perform the research on kinetics, thermodynamic parameters and isotherms related to the process.
2 Material and methods
2.1 Reagents and materials
The chemicals used were lanthanum(III) nitrate [La(NO3)3.6H2O] and cerium(III) nitrate [Ce(NO3)3.6H2O] acquired from Merck. The stock of a 500 mg/L lanthanum and cerium solution was prepared by dissolving an appropriate amount of metal salt in deionized water and acidified by concentrated nitric acid (HNO3) solution to prevent hydrolysis. HNO3 and ammonia (NH3) solutions were added dropwise to adjust the pH of the solutions. Iron (III) chloride [FeCl3.6H2O], sodium sulfite (Na2SO3), hydrochloric acid (HCl), ethanol all from Merck, pectin and chitosan from Aldrich were used for the synthesis of Magnetite-Pectin-Chitosan (MPC). All the chemicals used were used at analytical reagent grade without further purification.
2.2 Magnetite-Pectin-Chitosan synthesis
In the study, one employed the co-precipitation method to synthesize Magnetite-Pectin-Chitosan (MPC). Figure 1 shows the steps related to the MPC sample preparation. It was prepared in 35 mL FeCl3.6H2O HCl solution with the consequent dropwise addition of 25 mL pectin solution into it. The resulting solution was stirred in a magnetic stirrer until it was homogeneous. 25 mL of NaSO3 was placed in the Fe3O4 solution with a slow addition of 14 mL of NH3. The mixture was stirred on a magnetic stirrer for 24 h. The precipitate was then washed in double distilled water until pH was neutral, filtered through Whatman 42 grade filter paper and dried in a vacuum medium at 60 °C. The sample (Fe3O4-pectin) was functionalized with chitosan. Later, 0.05 g chitosan, 5 mL deionized water and 15 mL ethanol were added to the Fe3O4-pectin, the mixture of which was dried under an infrared lamp.
2.3 Instrumental conditions
The MPC was characterized by various methods to indicate their structure: scanning electron microscope (SEM, Coxem EM-30), Fourier Transform Infrared Spectroscopy (FT-IR, Perkin Elmer Spectrum Two), X-Ray Diffraction (XRD, Thermo Scientific Arl K-Alpha) and Vibrating Sample Magnetometry (VSM, VSM550-100, Dexing Magnet Technical Company). The lanthanum and cerium concentrations in aqueous phases were measured by Perkin Elmer Optima 2000 DV Inductively Coupled Plasma Optima Emission Spectrometer (ICP-OES).
Mixture of the solutions was carried out in a thermostated shaker water bath device (GFL-1083 model). pH adjustment of aqueous phases was performed by an 8521 model Hanna Instrument pH meter.
2.4 Adsorption experiments
The adsorption of La(III)/Ce(III) on MPC nanocomposite from aqueous phases was studied by batch method to achieve the effect of various parameters such as pH (2–6), contact time (30–120 min), concentration (25–100 mg/L) and temperature (25–50 °C). 0.05 g MPC was put into Erlenmeyer with 15 mL of known La(III)/Ce(III) concentrations and the sample then adjusted to the desirable pH. Following the adsorption, the MPC sample was isolated by a magnet and the residual solution filtered through filter paper to determine the concentrations of La(III)/Ce(III) in the solutions using the ICP-OES instrument. The adsorption efficiency (%) and adsorption capacity (mg/g) were calculated using Eqs. 1 and 2, respectively.
where Ci and Ce are the initial concentration and the equilibrium concentration of metal ions (mg/L) respectively, W the mass adsorbent (g) and V the volume of the aqueous solution (mL). All the trials were conducted in duplicate with experimental errors within the range of ± 3%.
3 Results
3.1 Characterization of MPC
The pore surface and morphology of (a) chitosan (b) magnetite-pectin and (c) magnetite-pectin-chitosan nanocomposite materials at different magnifications were examined by SEM images illustrated in Fig. 2. The red arrows present on the micrograph (c) showed that magnetite pectin chitosan (MPC) was embedded with the chitosan surface thus exhibiting the rough and porous structure of the MPC texture as well.
Figure 3a, b exhibit the FT-IR spectra of MPC and chitosan, respectively. Those of chitosan (Fig. 3a) before and after uptake show the adsorption bands at 3367 cm−1 and 3288 cm−1 related to the overlapping of O–H and N–H stretching vibrations. The moderate peak at 2875 cm−1 is characteristic of the C-H bond. The one at 1652 cm−1 is assigned to C=O stretching vibrations of amide I. The bands at 1374 cm−1 and 1326 cm−1 exhibit symmetrical deformation attributable to the La/Ce adsorption. Those at 1066 cm−1 and 1028 cm−1 are accounted for by the stretching vibration of C-O bands in the ring of C–OH, C–O–C and CH2–OH. The one at 892 cm−1 exhibited C–H bending of the plane of the ring of monosaccharides [24].
The vibrational band at 3340 cm−1 corresponds to the hydroxyl–OH stretching vibration of MPC as in Fig. 3b [25, 26].
The smaller broad shoulder around 2900 cm−1 shows a decreased signal intensity for –OH groups in the COOH carboxyl groups of pectin connected to dimers by hydrogen bonds.
It follows from the MPC load that the peak at 1592 cm−1 shows both symmetrical and asymmetrical COO- stresses to be observed. Two peaks 1374 cm−1 and 1316 cm−1 correspond to the symmetrical deformation, which could be caused by the La(III)/Ce(III) adsorption. The bands at 1023 cm−1 were due to the vibration connected with the saccharide ring [27]. The structure of Fe3O4 has been proven by Fe–O stretching vibration at 545 cm−1.
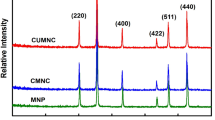
Figure 4 shows the XRD patterns of MPC and chitosan. The diffraction patterns have well-defined peaks suggesting that MPC and chitosan are crystalline. The pattern diffraction of MPC shows 6 peaks at 2\(\uptheta\)° values of 30.1°, 35.5°, 43.5°, 53.8°, 57.1°, and 63.0° which are marked 220, 311, 400, 422, 511 and 440 indices, respectively. The above consequences are in accordance with Fe3O4 crystal at cubic spinel phase of magnetite and also with the literature (JCPDS card no 19-0529) [28, 29].
As the pattern shows, modification of magnetite particles by pectin has not changed the crystal structure. However, the modification of magnetite-pectin by chitosan modifies the structure of magnetite-pectin. The very weak peak of MPC detected at the value of 20° is associated with the amorphous nature of chitosan [30, 31].
The magnetic property of magnetite-pectin nanocomposite was characterized by a vibrating sample magnetometer at room temperature. Considering the VSM data in Fig. 5, the value of 50 emu/g suggests that the samples rise to maximum magnetization very quickly, thus exhibiting superparamagnetic properties of magnetite pectin nanocomposite.
3.2 Effect of pH
pH is one of the most important parameters in controlling the adsorption process, influencing the charge of the adsorbent, degree of ionization and speciation of metal in solution [32, 33]. The effect of initial pH values on the adsorption of La(III) and Ce(III) ions onto MPC is presented in Fig. 6. The adsorption percentage efficiency of La(III) and Ce(III) ions increased from pH 2 to pH 4. The weak adsorption at pH 2 could be explained by the H+ ions at high concentrations preferentially adsorbed by La(III) and Ce(III) ions. Adsorption efficiency at pH 6 was ignored associated with precipitation in the solution, which indicates the hydrolysis of metal ions. The Eh–pH diagram of the La-Ce-H2O system in Fig. 7 was constructed by means of HSC.6 software. At pH 6, La(OH)3 and Ce(OH)3 species are present in the solution. Further adsorptions of La(III) and Ce(III) ions are performed by the MPC experiment at pH 5.
3.3 Effect of concentration
Adsorption of the La(III) and Ce(III) ions on MPC adsorbent as a function of metal concentration was studied in the range from 25 to 100 mg/L. Adsorption efficiencies (%) and adsorption capacities of La(III) and Ce(III) ions are presented in Fig. 8. Increasing La(III) and Ce(III) concentration show decreasing effect on the adsorption of metal ion, which may have been due to the increase in the number of ions which compete for available binding sites on the adsorbent surface at high concentrations. The concentration of metal ions at 25 mg/L is supposed to have the highest adsorption percentage of 68% and 82% for La(III) and Ce(III), respectively. On the other hand, the adsorption capacities of La(III) and Ce(III) by MPC increased with initial metal concentration and reached an equilibrium, which can be explained by the fact that mass transfer driving at the solid–liquid interface enhances as the initial concentration increases [34].
3.4 Effect of contact time
The effect of contact time on the adsorption of metal ions by MPC was examined in the range from 25 to 120 min using 25 mg/L initial concentration of metal ions at pH 5 and 0.05 g amount of the adsorbent. Figure 9 exhibits the adsorption efficiency (%) and capacity as the function of contact time. The adsorption efficiency (%) and capacity augment with the shaking time increasing and reaching an equilibrium at around 98% adsorption. The study consequently found that the adsorption of La(III) and Ce(III) was rapid [35]. The high percentage of adsorption may have been caused by active sites on the adsorbent being filled as the shaking time was increased. Further studies used 30 min as the shaking time in the adsorption.
3.5 Effect of temperature
Temperature is an important parameter in the adsorption process. In order to explain the thermodynamic behaviour of La/Ce ions in their adsorption onto MPC adsorbent, the experiments were performed at 25, 30, 40 and 50 °C. The initial concentration of metal ions was 25 mg/L, the shaking time fixed at 30 min and the amount of nanocomposite 0.05 g. Figure 10 indicates that increase at the temperature does not affect La(III)/Ce(III) ion adsorption.
3.6 Thermodynamic studies
To understand the thermodynamic properties of MPC adsorption, changes in Gibbs free energy (ΔG°), entropy (ΔS°) and enthalpy (ΔH°) as functions of temperature were calculated by Eq. 3 and Eq. 4, respectively.
and
where ΔH° and ΔS° were calculated using the slope and intercepts of the plots of lnKd vs. 1/T, which is shown for lanthanum and cerium adsorption in Fig. 11. Table 1 illustrates the thermodynamic values of ΔH°, ΔS° and ΔG°.
The negative values of enthalpy (ΔH°) suggested the exothermic nature of La(III)/Ce(III) ions adsorption. When the value ΔH° is lower than 40 kJ/mol, the type of adsorption can be described as a physical process. Those of ΔH° are − 70.6 kJ/mol and − 64.3 kJ/mol for La(III) and Ce(III) ions adsorption on MPC, respectively. The consequences indicate that the adsorption was physical, thus involving the attraction of the weak forces [36, 37]. The negative value ΔS° for La(III)/Ce(III) showed the decreased randomness at the solid/solution interface. The negative values ΔG° exhibit the spontaneous adsorption process. Those of ΔG° increased from − 17.371 to − 13.085 kJ/mol and − 21.339 to − 17.880 kJ/mol for La(III) and Ce(III) respectively. The temperatures increased from 298 to 322 K, all of which demonstrate that the favorable behavior of the adsorption process occur at low temperature [38].
3.7 Adsorption isotherms
Adsorption isotherms are useful to describe the mechanism of the adsorption process, the one on the surface, diffusion mechanism, and chemistry reaction. The work at hand shows the adsorption data to be subjected to different adsorption isotherms, namely those of Langmuir, Freundlich, Dubinin-Radushkevich (D-R), Temkin, and Flory–Huggins. Various parameters of adsorption isotherms for La(III)/Ce(III) ions on MPC are presented in Table 2. Adsorption experiments with 25 mg/L for La (III)/Ce(III) solutions and 0.05 g MPC were made to calculate isotherms under the circumstances of 25 °C, 15 mL of aqueous phase, initial pH 5 and in 30 min.
3.8 Langmuir isotherms
Langmuir isotherm predicts the formation of a monolayer on the adsorbent assuming homogeneous distribution of the adsorbed La(III)/Ce(III) ions are on the MPC surface [39]. The linear forms of the Langmuir isotherm model are defined by Eq. 5.
where Ce is the equilibrium concentration of ions, qe the quantity of adsorbed ions under equilibrium conditions and Q0 and bL the Langmuir constants related to adsorption capacity and adsorption energy, calculated from the slope and interception of linear plots of Ce/qe versus Ce, respectively.
One of the essential characteristics of the Langmuir equation can be defined by a dimensionless constant known as the equilibrium parameter or separation factor, RL [40] which is described by Eq. 6.
where C0 is the highest concentration of Lanthanum/Cerium (mg/L) and The value RL the type of isotherm to be irreversible ( RL = 0), favorable ( 0 < RL < 1), or unfavorable ( RL > 1).
3.9 Freundlich isotherm
Freundlich isotherm depends on the assumption that the active adsorption sites are dispensed exponentially concerning based on the heat of adsorption, which can be used as the model to describe reversible and non-ideal adsorption [41]. Freundlich isotherm model is given by Eq. 7.
where KF is the adsorption capacity and nF a dimensionless constant related to adsorption intensity.
3.10 Dubinin–Radushkevich isotherm
The model of Dubinin and Radushkevich isotherm describes that the adsorption of the adsorbent surface occurs in the volume of pores and micropores where the adsorbed particles lose energy [42]. The linearized model of the Dubinin–Radushkevich isotherm is illustrated by Eq. 8.
where Cads (mmol/g) is the amount of the solute adsorbed per its unit weight, Xm (mmol/g or mg/g the adsorption capacity, β (mole/K)2 the constant related to energy and \(\epsilon\) the Polanyi potential which can be computed by Eq. 9.
3.11 Temkin isotherm
In the Temkin isotherm model, the effects of indirect interactions between adsorbate/adsorbent on the adsorption process were considered [43]. All the molecules sorb heat in the layer decreased linearly due to sorbate/sorbent interactions [44]. The Temkin isotherm linear model may be expressed by Eq. 10.
where bTe is the constant of Temkin related to adsorption heat (J/mol), aTe Temkin isotherm constant (L/mg), R the gas constant and T the absolute temperature (K).
Constants bTe and aTe were calculated from the intercept and slope of the straight line of the plot of qe versus Ce.
3.12 Flory–Huggins isotherm
The Flory–Huggins isotherm was applied to find the degree of surface coverage of the sorbate on sorbent [45, 46]. The linearized form of the Flory–Huggins isotherm can be expressed by Eq. 11.
where \(\Theta\) is the degree of surface coverage, KFH the Flory–Huggins model equilibrium constant and nFH the Flory–Huggins model exponent. Θ is calculated by Eq. 12.
The linearized form of the model can be presented by Eq. 13.
The constant of isotherm was extrapolated from plots of plot of \({\text{log}}\frac{\Theta }{{{\text{C}}}_{{\text{i}}}}\) versus, \({\text{log}}\left(1-\Theta \right)\) and values of KFH and nFH calculated from the slope. Intercept of the plot are shown in Table 2.
According to the correlation coefficients (R2) for the four above-mentioned isotherm models, the Langmuir isotherm was found to be the most useful model to describe the La(III)/Ce(III) adsorption onto MPC.
The maximum adsorption capacity (Q0) and the constant of adsorption equilibrium (bL) of MPC from linear regression curves were calculated to be 8.17 mg/g, 0.20 L/mg, and 9.72 mg/g, 0.61 L/mg for La(III) and Ce(III), respectively. The results thus suggest a homogenous surface and monolayer coverage of the adsorbent. Some similar consequences of lanthanum adsorption onto graphene-based magnetic nanocomposite were reported by Oral et al. [37] with maximum adsorption parameters (Q0 = 49.75 mg/g, constant of the adsorption equilibrium (bL = 0.2545 mg/g) and correlation coefficient (R2 = 0.99). Values RL related to MPC adsorbent are 0.05 and 0.02 for La(III) and Ce(III), respectively. They are in the range from 0 to 1, showing that the adsorption of La(III)/Ce(III) ions onto MPC is favorable under experimental conditions. Table 3 presents comparison data of the adsorption capacities of adsorbents for La(III) and Ce(III) with those of different adsorbents reported in the literature. MPC showed a potential for removal of La(III) and Ce(III) ions from aqueous solutions. Chitosan-functionalized Magnetite-Pectin composite could be used for continuous wastewater treatment in respect that it can be recovered and reused many times. The main advantages of biopolymers are their substantially low cost, availability of the biomaterials and economic feasibility. As well as their magnetic property, pectin and chitosan adsorbents have advantages of environment-friendly and non-toxic characteristics and economic feasibility.
3.13 Adsorption kinetics of La (III) and Ce (III)
The adsorption kinetic shows the evolution of the adsorption capacity over time. To examine the potential rate-controlling step (e.g. chemical reaction, diffusion control and mass transfer), several kinetic models were used and the experimental data tested.
3.14 Pseudo first order model (PFO)
Lagergren model describes the adsorption of solute onto adsorbent following the first order mechanism (Eq. 14).
where qe is the amount of metal ion adsorbed onto the adsorbent at equilibrium (mg/g), qt the amount of metal ion adsorbed at various times, t the time of adsorption duration and k1 the rate constant of the Eq. (1/min) [52].
Slopes and intercepts of plots of log(qe – qt) vs. time were obtained for magnetite-pectin-chitosan (MPC) concerning La (III) and Ce(III) to determine the first-order rate constant k1 at different contact time (plots not shown) but the correlation coefficients for the first-order kinetic model were relatively low, indicating that the adsorption of La (III) and Ce(III) ions onto MPC was not of first-order reaction. Results of the model are shown in Table 4.
3.15 Pseudo-second-order model
Pseudo-second-order model assumes that the rate of adsorption of solute is proportional to the available sites on the adsorbent. The reaction rate is dependent on the amount of solute on the surface of the adsorbent-the driving force (qe − qt) is proportional to the number of active sites available on the adsorbent [53]. The pseudo-second-order model can be represented in the following form by Eq. 15.
where k2 (g/mol min) is the second-order rate constant. The linear plot of t/qt as a function of t provided not only the rate constant k2, but an independent evaluation of qe as well. The pseudo-second-order rate constant k2 was determined by the slopes and intercepts of plots of t/qt vs. t [54]. As Fig. 12 exhibits, the correlation coefficients related to the pseudo-second-order kinetic model for both ions were above 0.999. The data allows for perfect fit in this model for La(III) and Ce(III) adsorption onto MPC. Therefore, the rate-limiting step may be chemical sorption or chemisorption through sharing or exchanging electrons between sorbent and adsorbate [35, 55].
3.16 Intra-particle diffusion model
The kinetics of adsorption of La(III) and Ce(III) on/onto the MPC was also assessed by the Morris–Weber model, which has been widely applied as a kinetic model to examine the rate limiting step during adsorption. The adsorption of solute in a solution involves mass transfer of adsorbate (film diffusion), surface diffusion, and pore diffusion. Film diffusion is an independent step while surface and pore diffusion may occur simultaneously/concurrently. Intra-particle diffusion model is studied considering Weber and Morris’s model (Eq. 16) [56].
where qt is the concentration of the adsorbed ion (mg/g) at time t, Kp the rate constant for the intraparticle transport (mg g−1 min−0.5) and C boundary layer thickness. The value C determines the boundary layer effect-higher values. The model allows a graphic plot for qt versus t0.5 to predict the sorption mechanism. The plot of qt vs.\(\sqrt{{\text{t}}}\), gives a linear function. If intraparticle diffusion is the mechanism to underlie the adsorption process, the plot qt against/ versus t0.5 will be linear, and if the plot passes through the origin, the rate-limiting process will be solely due to intraparticle diffusion. Otherwise, some additional process is involved as well as intraparticle diffusion [57].
The intraparticle diffusion model plot has a low regression coefficient (0.692 and 0.687) for La (III) and Ce (III), respectively. Both intercepts are not equal to zero, due to which intraparticle diffusion is likely to have been the only rate-limiting step; hence, kinetics was simultaneously regulated by both liquid film and intraparticle diffusion. It can be inferred from Fig. 13 that intraparticle diffusion and film diffusion are both the rate-limiting steps for La (III) and Ce (III) diffusion onto MPC.
4 Conclusion
The present study synthesized magnetite-pectin-chitosan nanocomposite by means of the partial reduction co-precipitation method to remove lanthanum and cerium from aqueous solutions. The nanocomposite were characterized successfully by SEM, FT-IR, XRD, VSM analyses. The observed saturation magnetization value of the MPC nanocomposite was found to be 50 emu/g.
The parameters to affect the adsorption were examined by batch method. The optimum conditions were inspected in terms of solution pH, contact time, initial La(III) and Ce(III) ion concentrations, contact time and temperature values, all of which are the parameters to affect the adsorption efficiency.
The optimum adsorption of lanthanum and cerium was observed at initial pH 5 and adsorption equilibrium reached at 30 min. The negative values of enthalpy and Gibbs free energy change show that the adsorption process is exothermic and spontaneous. The adsorption data has been subjected to different adsorption isotherms, such as those of Langmuir, Freundlich, Dubinin-Radushkevich (D-R), Temkin, and Flory–Huggins. The adsorption equilibrium fits the Langmuir adsorption model well. Considering such isotherm-related studies, the maximum La(III) and Ce(III) uptake capacity of magnetite pectin chitosan is 8.17 mg/g and 9.72 mg/g, respectively. Kinetic studies have determined that adsorption follows PSO kinetic model.
According to the results, MPC can be employed to remove lanthanum and cerium ions at low concentrations from aqueous phases. The synthesized magnetic nanocomposite sorbent is recommended to be used as the effective material at low concentrations for removing lanthanum and cerium ions from aqueous solutions. Evidence of the study demonstrated that MPC adsorbent can be a low-cost, effective, easily separated and environmentally friendly with a high adsorption yield as well.
It could finally be concluded that the synthesis method proposed in the study does not use any toxic chemicals and the production scale-up can be easily performed with low costly materials. Further research can be carried out to investigate different chemicals and prepare magnetic nanocomposites for a variety of applications with different elements.
Data availability
Supporting information is available in the additional files and further supporting data is available from the corresponding author.
References
Torab-Mostaedi M. Biosorpcija lantana i cerijuma iz vodenih rastvora pomoću kore mandarine (Citrus reticulata): ravnotežna, kinetička i termodinamička ispitivanja. Chem Ind Chem Eng Q. 2013;19(1):79–88. https://doi.org/10.2298/CICEQ120128043T.
Van TL, Le NTN. Spectrophotometric determination of cerium using azocalixarene derivative in geological samples. Int J Chem Eng Appl. 2011;2(6):381–4. https://doi.org/10.7763/ijcea.2011.v2.138.
Alguacil FJ, García-Díaz I, Baquero EE, Largo OR, López FA. On the adsorption of cerium(III) using multiwalled carbon nanotubes. Metals (Basel). 2020;10:1–10. https://doi.org/10.3390/met10081057.
Wu Y, Pang H, Liu Y, Wang X, Yu S, Fu D, et al. Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut. 2019;246:608–20. https://doi.org/10.1016/j.envpol.2018.12.076.
Yarahmadi A, Khani MH, Nasiri Zarandi M, Amini Y, Yadollahi A. Ce(III) and La(III) ions adsorption using Amberlite XAD-7 resin impregnated with DEHPA extractant: response surface methodology, isotherm and kinetic study. Sci Rep. 2023;13(9959):1–19. https://doi.org/10.1038/s41598-023-37136-7.
Gabor AE, Davidescu CM, Negrea A, Ciopec M, Butnariu M, Ianasi C, et al. Lanthanum separation from aqueous solutions using magnesium silicate functionalized with tetrabutylammonium dihydrogen phosphate. J Chem Eng Data. 2016;61:535–42. https://doi.org/10.1021/acs.jced.5b00687.
Crini G, Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett. 2019;17(1):145–55. https://doi.org/10.1007/s10311-018-0785-9.
Liosis C, Papadopoulou A, Karvelas E, et al. Heavy metal adsorption using magnetic nanoparticles for water purification: a critical review. Materials (Basel). 2021;14(24):7500. https://doi.org/10.3390/ma14247500.
Singh BK, Um W. Application of clay materials for sorption of radionuclides from waste solutions. Minerals. 2023. https://doi.org/10.3390/min13020239.
Al-Ghouti MA, Da’ana DA. Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater. 2020;393: 122383. https://doi.org/10.1016/j.jhazmat.2020.122383.
Yusan S, Aytas S, Sert S, Akpolat O, Elmastas GO. Development of environment-friendly magnetic nanobiocomposites and full factorial design (FFD) analysis for strontium removal from aqueous solutions. J Radioanal Nucl Chem. 2023;332:591–606. https://doi.org/10.1007/s10967-023-08803-w.
Şenol ZM, Gül ÜD, Şimşek S. Bioremoval of Safranin O dye by the identified lichen species called Evernia prunastri biomass; biosorption optimization, isotherms, kinetics, and thermodynamics. Biomass Convers Biorefinery. 2022;12(9):4127–37. https://doi.org/10.1007/s13399-020-01216-9.
Lara-Espinoza C, Carvajal-Millán E, Balandrán-Quintana R, López-Franco Y, Rascón-Chu A. Pectin and pectin-based composite materials: beyond food texture. Molecules. 2018;23(4):942. https://doi.org/10.3390/molecules23040942.
Lemos TSA, de Souza JF, Fajardo AR. Magnetic microspheres based on pectin coated by chitosan towards smart drug release. Carbohydr Polym. 2021. https://doi.org/10.1016/j.carbpol.2021.118013.
Birch NP, Schiffman JD. Characterization of self-assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir. 2014;30(12):3441–7. https://doi.org/10.1021/la500491c.
Liu JF, Zhao ZS, Bin JG. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ Sci Technol. 2008;42(18):6949–54. https://doi.org/10.1021/es800924c.
Bhatt P, Joshi S, Urper Bayram GM, Khati P, Simsek H. Developments and application of chitosan-based adsorbents for wastewater treatments. Environ Res. 2023;226: 115530.
Jawad AH, Abdulhameed AS, Kashi E, Yaseen ZM, ALOthman ZA, Khan MR. Cross-linked chitosan-glyoxal/kaolin clay composite: parametric optimization for color removal and COD reduction of remazol brilliant blue R dye. J Polym Environ. 2022;30:164–78. https://doi.org/10.1007/s10924-021-02188-1.
Şen NE, Şenol ZM. Effective removal of Allura red food dye from water using cross-linked chitosan-diatomite composite beads. Int J Biol Macromol. 2023;253: 126632. https://doi.org/10.1016/j.ijbiomac.2023.126632.
Şenol ZM, Elma E, El Messaoudi N, Mehmeti V. Performance of cross-linked chitosan-zeolite composite adsorbent for removal of Pb2+ ions from aqueous solutions: experimental and Monte Carlo simulations studies. J Mol Liq. 2023;391: 123310. https://doi.org/10.1016/j.molliq.2023.123310.
Ganesh SS, Anushikaa R, Swetha Victoria VS, Lavanya K, Shanmugavadivu A, Selvamurugan N. Recent advancements in electrospun chitin and chitosan nanofibers for bone tissue engineering applications. J Funct Biomater. 2023. https://doi.org/10.3390/jfb14050288.
Maleki ST, Beigi P, Babamoradi M. Synthesis of pectin hydrogel /Fe3O4/Bentonite and its use for the adsorption of Pb(II), Cu(II), and Cd(II) heavy metals from aqueous solutions. Mater Sci Eng B. 2023;298: 116899. https://doi.org/10.1016/j.mseb.2023.116899.
Di Tocco A, Robledo SN, Osuna Y, Sandoval-Cortez J, Granero AM, Vettorazzi NR, et al. Development of an electrochemical biosensor for the determination of triglycerides in serum samples based on a lipase/magnetite-chitosan/copper oxide nanoparticles/multiwalled carbon nanotubes/pectin composite. Talanta. 2018;190:30–7. https://doi.org/10.1016/j.talanta.2018.07.028.
Shehap AM, Nasr RA, Mahfouz MA, Ismail AM. Preparation and characterizations of high doping chitosan/MMT nanocomposites films for removing iron from ground water. J Environ Chem Eng. 2019;9(1): 104700. https://doi.org/10.1016/j.jece.2020.104700.
Naushad M, Ahamad T, Sharma G, et al. Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem Eng J. 2016;300:306–16. https://doi.org/10.1016/j.cej.2016.04.084.
Prill B, Yusan S. Synthesis and characterization of magnetic nanoparticles functionalized with different starch types. Part Sci Technol. 2022;40(5):521–30. https://doi.org/10.1080/02726351.2021.1967536.
Gong JL, Wang XY, Zeng GM, et al. Copper (II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chem Eng J. 2012;185–186:100–7. https://doi.org/10.1016/j.cej.2012.01.050.
Azari A, Gharibi H, Kakavandi B, et al. Magnetic adsorption separation process: an alternative method of mercury extracting from aqueous solution using modified chitosan coated Fe3O4 nanocomposites. J Chem Technol Biotechnol. 2017;92(1):188–200. https://doi.org/10.1002/jctb.4990.
Yusan S, Korzhynbayeva K, Aytas S, et al. Preparation and investigation of structural properties of magnetic diatomite nanocomposites formed with different iron content. J Alloys Compd. 2014;608:8–13. https://doi.org/10.1016/j.jallcom.2014.04.064.
Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Dev Ther. 2016;10:483–507. https://doi.org/10.2147/DDDT.S99651.
Rahman NA, Hanifah SA, Mobarak NN, et al. Synthesis and characterizations of o-nitrochitosan based biopolymer electrolyte for electrochemical devices. PLoS ONE. 2019;14(2): e0212066. https://doi.org/10.1371/journal.pone.0212066.
Aksu Z, Işoǧlu IA. Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005;40(2):3031–44. https://doi.org/10.1016/j.procbio.2005.02.004.
Kütahyali C, Şert S, Çetinkaya B, et al. Factors affecting lanthanum and cerium biosorption on Pinus brutia leaf powder. Sep Sci Technol. 2010;45(10):1456–62. https://doi.org/10.1080/01496391003674266.
Akbas YA, Yusan S, Sert S, Aytas S. Sorption of Ce(III) on magnetic/olive pomace nanocomposite: isotherm, kinetic and thermodynamic studies. Environ Sci Pollut Res. 2021;28(40):56782–94. https://doi.org/10.1007/s11356-021-14662-3.
Yusan S, Gok C, Erenturk S, Aytas S. Adsorptive removal of thorium (IV) using calcined and flux calcined diatomite from Turkey: evaluation of equilibrium, kinetic and thermodynamic data. Appl Clay Sci. 2012;67–68:106–16. https://doi.org/10.1016/j.clay.2012.05.012.
Liu Y, Liu YJ. Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol. 2008;61(3):229–42. https://doi.org/10.1016/j.seppur.2007.10.002.
Oral AE, Aytas S, Yusan S, et al. Preparation and characterization of a graphene-based magnetic nanocomposite for the adsorption of lanthanum ions from aqueous solution. Anal Lett. 2020;53(11):1812–33. https://doi.org/10.1080/00032719.2020.1719128.
Gok C, Aytas S. Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater. 2009;168(1):369–75. https://doi.org/10.1016/j.jhazmat.2009.02.063.
Aytas S, Yusan S, Sert S, Gok C. Preparation and characterization of magnetic graphene oxide nanocomposite (GO-Fe3O4) for removal of strontium and cesium from aqueous solutions. Charact Appl Nanomater. 2021;4(1):26. https://doi.org/10.24294/can.v4i1.1291.
Torab-Mostaedi M, Asadollahzadeh M, Hemmati A, Khosravi A. Biosorption of lanthanum and cerium from aqueous solutions by grapefruit peel: equilibrium, kinetic and thermodynamic studies. Res Chem Intermed. 2015;41(2):559–73. https://doi.org/10.1007/s11164-013-1210-4.
Yusan S, Bampaiti A, Erenturk S, et al. Sorption of Th(IV) onto ZnO nanoparticles and diatomite-supported ZnO nanocomposite: kinetics, mechanism and activation parameters. Radiochim Acta. 2016;104(9):635–47. https://doi.org/10.1515/ract-2016-2581.
Dubinin MM. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev. 1969;60(2):235–41.
Foo KY, Hameed BH. Insights into the modeling of adsorption isotherm systems. Chem Eng J. 2010;156(1):2–10. https://doi.org/10.1016/j.cej.2009.09.013.
Hosseini M, Mertens SFL, Ghorbani M, Arshadi MR. Asymmetrical Schiff bases as inhibitors of mild steel corrosion in sulphuric acid media. Mater Chem Phys. 2003;78(3):800–8. https://doi.org/10.1016/S0254-0584(02)00390-5.
Flory PJ. Themodynamics of high polymer solutions. J Chem Phys. 1942;10(1):51–61. https://doi.org/10.1063/1.1723621.
Huggins ML. Some properties of solutions of long-chain compounds. J Phys Chem. 1942;46(1):151–8. https://doi.org/10.1021/j150415a018.
Gabor A, Davidescu CM, Negrea A, et al. Optimizing the lanthanum adsorption process onto chemically modified biomaterials using factorial and response surface design. J Environ Manage. 2017;204:839–44. https://doi.org/10.1016/j.jenvman.2017.01.046.
Farahmand E. Adsorption of cerium (IV) from aqueous solutions using activated carbon developed from rice straw. Open J Geol. 2016;06(03):189–200. https://doi.org/10.4236/ojg.2016.63017.
Keshtkar AR, Moosavian MA, Sohbatzadeh H, Mofras M. La(III) and Ce(III) biosorption on sulfur functionalized marine brown algae Cystoseira indica by xanthation method: response surface methodology, isotherm and kinetic study. Groundw Sustain Dev. 2019;8(3):144–55. https://doi.org/10.1016/j.gsd.2018.10.005.
Xu S, Zhang S, Chen K, et al. Biosorption of La3+ and Ce3+ by Agrobacterium sp. HN1. J Rare Earths. 2011;29(3):265–70. https://doi.org/10.1016/S1002-0721(10)60443-7.
Sert Ş, Kütahyali C, Inan S, et al. Biosorption of lanthanum and cerium from aqueous solutions by Platanus orientalis leaf powder. Hydrometallurgy. 2008;90(1):13–8. https://doi.org/10.1016/j.hydromet.2007.09.006.
Hameed BH, Salman JM, Ahmad AL. Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J Hazard Mater. 2009;163(1):121–6.
William Kajjumba G, Emik S, Öngen A, Kurtulus Özcan H, Aydın S. Modelling of adsorption kinetic processes—errors, theory and application. Adv Sorption Process Appl. 2019. https://doi.org/10.5772/intechopen.80495.
Ijagbemi CO, Baek MH, Kim DS. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater. 2009;166(1):538–46. https://doi.org/10.1016/j.jhazmat.2008.11.085.
Namasivayam C, Sureshkumar MV. Removal of chromium(VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Bioresour Technol. 2008;99(7):2218–25. https://doi.org/10.1016/j.biortech.2007.05.023.
Weber WJJ, Morris JC. Kinetics of adsorption on carbon from solution. J Sanit Eng Div. 1963;89:31–59.
Hasani N, Selimi T, Mele A, Thaçi V, Halili J, Berisha A, et al. Theoretical, equilibrium, kinetics and thermodynamic investigations of methylene blue adsorption onto lignite coal. Molecules. 2022;27(6):1856. https://doi.org/10.3390/molecules27061856.
Acknowledgements
Abdoul-Rachid Chaibou Yacouba gratefully acknowledge Türkiye Scholarship Program.
Funding
This research was in part funded by Türkiye Scholarship Program.
Author information
Authors and Affiliations
Contributions
ARCY: Investigation, methodology, validation and writing; AEO: Data curation, validation; SS: Review and editing; IGKa: Data curation, validation and editing; IN: editing; SY: methodology, supervision, review and editing; SA: methodology, supervision, review, editing and validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to reposition figures and tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaibou Yacouba, AR., Oral, A.E., Sert, S. et al. Removal of lanthanum and cerium from aqueous solution using chitosan-functionalized magnetite-pectin. Discov Water 4, 1 (2024). https://doi.org/10.1007/s43832-023-00054-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00054-w