Abstract
One approach to addressing energy security issues is to produce renewable and sustainable bioenergy using abundant waste resources through anaerobic co-digestion (AcoD). However, the lignocellulosic nature of these biomass resources makes them recalcitrant, and pretreatment is required to make them more amenable to conversion. Iron oxide nanoparticles (ION) have been shown to increase methane yield significantly when added to biomass resources. This study aimed to investigate the effect of ION application on Sorghum stover (SS) and Winery solid waste (WSW) under mesophilic conditions. Hydrothermal synthesis was used to obtain Fe3O4 nanoparticles. Biomethane potential (BMP) tests were carried out in semi-continuous batch reactors with and without ION singly and combined SS: WSW (1:1) during a 30-day retention period. The results showed that the ION application on WSW delivered a higher biogas yield (380 mL), indicating an increase of 162% in biogas production compared to the sample without ION (145 mL). In addition, CH4 generation went from 30 to 114 mLCH4, indicating a 280% increase. However, adding ION to SS inhibited CH4 production. The study found that ION addition significantly improved biogas yield, especially with WSW, where the increase was more than triple, of interest to bioenergy and waste management practitioners.
Graphic Abstract

Highlights
-
ION application significantly improved biogas yield, especially with WSW.
-
WSW methane yield tripled (30–114 mL) with ION addition.
-
Adding ION to SS at an equal ratio inhibited CH4 production and is not advised.
-
AcoD of substrates produced more biogas than mono-AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world faces various challenges, such as environmental pollution caused by fossil fuel energy combustion, increased organic waste generation because of population growth, and rising global energy demand. About 88% of the global energy supply comes from fossil fuels [1,2,3]. The combustion of fossil fuels is wreaking havoc on the earth’s environment and, if not checked, will result in an untenable climate change crisis. For example, due to climate change, Australia recently experienced an unprecedented large-scale forest fire in September 2019, burning 6.3 million hectares [4]. The heat wave across Europe and America in the summer of 2022 set new records in the earth’s history [5].
Furthermore, many countries are dealing with waste management, waste reduction, waste prevention and waste recycling, which have become legislative and environmental issues. Traditional disposal methods (incineration, waste dumping and landfilling) are offensive because they cause pollution and greenhouse gas emissions (GHG). In addition, these methods represent a lost opportunity, as very little waste can be reused, recycled, or extracted from materials. These materials represent suitable biomass for anaerobic digestion [1, 6]. Over the years, researchers have studied ways to improve biogas production from biomass materials through different substrates, such as animal manure [7, 8], agricultural residues [9], Sorghum stover [10], and winery solid wastes [11].
Sorghum stover (SS) is the agricultural residue that remains after the harvest of sorghum grain. It is a lignocellulosic material rich in cellulose, hemicellulose, and lignin. Sorghum stover has been identified as a promising feedstock for biogas production due to its high cellulose content, which can be converted to methane via anaerobic digestion. Several studies have investigated sorghum stover as a substrate for biogas production and reported high biogas yields when combined with other organic wastes [10].
Winery solid wastes (WSW) are generated during the production of wine and consist of grape marc, grape stalks, and grape seeds. These wastes, including cellulose, hemicellulose, and lignin, are rich in organic matter. They have been identified as potential feedstocks for biogas production, and several studies have investigated their use as substrates for anaerobic digestion [12, 13]. However, winery solid wastes are often difficult to digest due to their high lignin content, which can inhibit the activity of methanogenic bacteria [14].
Iron oxide nanoparticles (ION) are a type of magnetic nanoparticle that has received increasing attention in recent years due to their potential applications in environmental remediation and biotechnology. In the context of anaerobic digestion, iron oxide nanoparticles have been shown to improve biogas yields by increasing the availability of nutrients and enhancing the activity of methanogenic bacteria. However, using iron oxide nanoparticles in anaerobic digestion is still a relatively new field of research, and further studies are needed to fully understand their potential benefits and drawbacks.
Each of the above individual components, SS and WSW, has been extensively studied in the context of anaerobic digestion. However, to the best of our knowledge, no study has combined these substrates that are locally available in South Africa together with ION to valorise the wastes for biogas production. Only recently, has interest been shown in using iron oxide nanoparticles to improve biogas and methane yield. Further research is needed to investigate the potential benefits and drawbacks of combining these components in the proposed study.
2 Experimental
2.1 Substrates and inoculum preparation
Sorghum Stover was purchased from ARC-Grains, Pretoria, South Africa. The substrate was semi-dry and weighed 5 kg upon collection. It was dried in an oven at 60 °C for at least 4 h. This step was repeated until the substrate became completely dry. The sorghum stover was milled in a food processor (POLYMIX®Lab Mill, Model PXMFC 90 D 230 V/EU, Bench & Holm, Denmark) set at 6000 × rpm. The obtained substrate was then stored in a sampling bag at room temperature prior to use. Fresh winery waste was collected from a winery farm in the Agricultural Research Council (ARC) premises, Stellenbosch, South Africa. It was dried and ground into powder using a hammer mill (HAM mill SER No. 400, Scientific®, SA) equipped with a 2 mm sieve mesh [15]. Fresh zebra manure was also collected from Vredenberg Farm and Game Reserve in Stellenbosch. South Africa and kept in a foam bag with ice cubes during transportation to the laboratory and later stored in a refrigerator at 4 °C before preparation.
The Zebra manure, which served as inoculum in the current study, was prepared according to the protocol [11] used. This mixture was prepared by mixing approximately 200 g of zebra dung with 1 L of sterilised distilled water in a blender for 10 min. The blended mixture was then transferred into a 3 L deflated plastic bottle, flashed with nitrogen, sealed, and kept at room temperature for 10 × days. For the acclimatisation of the inoculum to the substrates, the produced seed inoculum (3 L) was transferred to a 5 L plastic container in which 5 g of each of the milled substrates was added. This mixture was kept in a water bath at 37 ℃ for 20 days. Afterwards, the inoculum was sieved to remove large particles (> 2 mm) and stored in plastic containers at – 4 ℃ for later use in all required experiments.
2.2 Synthesis of iron oxide nanoparticles
Iron oxide (Fe3O4) nanoparticles are obtained by hydrothermal synthesis. The following components were mixed in an autoclave: 1.98 g iron II chloride, 8.08 g iron III nitrate (anhydrous), 0.7 g sodium dodecyl sulphate, 100 mL water, and 10 mL ammonia. The aqueous solution was then heated in a water bath at 60 °C for 4 h. In addition, the obtained solution was centrifuged at 4000 × rpm for 5 min. This step was repeated until the solution became clear. The resulting precipitate was added into a crucible and dried overnight at 60 °C. Thereafter, it was reduced to a powder form by means of a mortar and pestle.
2.3 Characterisation of substrates
The total solids and volatile solids, ash and moisture content of the substrate were analysed according to the standard methods [16]. The carbon, nitrogen, oxygen, calcium, and phosphorus percentages were also determined by Inductively coupled plasma atomic emission spectrometry (ICP-AES) using Thermo ICap 6200 ICP-AES and the elemental analysis was carried out using Elementar Vario EL Cube elemental analyser. Samples were prepared before using the microwave digester for ICP analysis. According to the method of Mariotti and Tomé [17], the protein content is determined by applying a correction factor of 6.25 to the measured nitrogen content.
2.4 Biomethane potential procedure
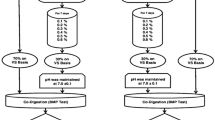
Analytical BMP for this system was done in duplicates. Seven experiments were carried out in fourteen 500 mL Schott bottles with two connected screw caps (model GL 45 from Sigma-Aldrich) immersed in a water bath with an integrated temperature control system (model TR5 and serial number F7571-0717 set at 37 ± 0.5 °C. This gave a total of 2 × 7 = 14 experimental runs as the scope of the present study. 100 ppm dose of ION was selected for inoculation because it gave the best result after initial trial experiments with various doses (20, 40, 60, and 100 ppm). All the steps followed during the BMP tests, including the inoculation, are shown in Table 1 below.
The biogas volume produced was collected and measured using a water column by the water displacement method. All BMP assays were carried out at a mesophilic batch digester set at 37 ± 5 ℃ under anaerobic conditions and achieved by flushing the headspace of the digester with nitrogen (Nitrogen Baseline 5.0, UN No. 1066, Afrox gas, Epping, South Africa) for 5 min before adding the samples as well as the inoculum. The associated CO2 was scrubbed out using the scrubbing solution of three NaOH pellets in a 2 L distilled water and phenol indicator. The aim is to improve the quality of the biogas produced. A Geotech 5000 Biogas Analyzer was used to determine the biogas composition, including the methane content.
3 Results and discussion
3.1 Synthesis of iron oxide nanoparticles
The prepared iron oxide nanoparticle sample’s crystal structure, phase and size are characterised by Transmission electron microscopy (TEM) and X-ray diffraction (XRD). Firstly, the prepared ION sample was subjected to a TEM, and the results are shown in Fig. 1 below, enlarged by × 2 (Fig. 1A) and × 3 (Fig. 1B), respectively. The scale on the TEM image was done using JAVA Image J software to determine nanoparticle shape, size and distribution. The average grain size of the Iron oxide (II) and (III) nanoparticle mixture is 30 nm. Nanoparticles comprise fine sub-crystalline magnetite [18]. The result of the size is consistent with the results obtained by previous work [19] that uses the same method. The result of the shape is one of the different characteristics Fe3O4 nanoparticles have. According to Wang et al. [20], various morphologies of Fe3O4 nanoparticles can be obtained depending on the conditions and parameters of the Iron oxide synthesis method. The synergistic effect between the substrate microorganism and iron nanoparticles may explain the role of nanoparticles in this present study.
Secondly, the ION sample was then subjected to XRD. XRD patterns of the reference synthesised ION (Fe3O4, JCPDS magnetite, Maghemite-Q) shown in Fig. 2 were compared with XRD in Fig. 3 to determine the properties of the prepared sample. The results showed that the positions of the sample diffraction peaks are consistent with the solid blue M bars of Fe3O4 at 24 degrees, 35 degrees, 42 degrees and 51 degrees (Fig. 2). Since the position of the reference sticks matches the data, it confirms the nature of the sample as Fe3O4 material.
However, the intensity is different from the peaks of Fe3O4, whose d value of lattice is 2.61, as shown in Fig. 3. The XRD pattern of the nanomaterial observed in Fig. 3 produces sharp Bragg’s peaks. It indicates that the sample is crystalline, as expected [21]. Figure 3 shows a diffraction peak broadening, which may indicate a smaller crystallite size in the nanocrystalline synthesised ION oxide.
Different instrument configurations may explain it. Minor reference peaks not observed can indicate the lack of purity by the Fe3O4 nanoparticles. It is also important to emphasise the difficulties Fe2O3 and Fe3O4 nanoparticles (Fig. 2) are distinguished by their similar crystal structure [22]. The Fe2O3 pattern is slightly more intense because Fe2O3 diffracts X-rays more efficiently.
3.2 Characterisation of Winery solid waste (WSW) and Sorghum stover (SS)
Chemical composition and biodegradability are essential for biogas and methane production [23]. The characteristics of WSW and SS are indicated in Table 2 below. The volatile solids content of WSW and SS of the two substrates is very similar at 83.86% and 84.09%, respectively. However, the total solids in WSW (95.92%) is higher than that of SS (61.38%). The SS moisture content (38.62%) is much higher than that of WSW (1.15%). Water content affects the production of biogas during anaerobic digestion. The higher the moisture content, the higher the biogas production [24]. Regarding total nitrogen, the WSW with 1.76% is twice that of SS (0.81%). The high volatile solid content indicates that the raw materials have high biodegradability and, therefore, have good potential for biogas production.
WSW’s carbon and nitrogen content was 50.40% and 1.76%, respectively, and SS’s carbon and nitrogen content was 37.77% and 0.81%, respectively. Therefore, within the acceptable range, WSW’s carbon/nitrogen ratio (C/N) can be calculated as 28.63. For high methane production, a C/N ratio between 20:1 and 30:1 is considered appropriate [25]. However, the C/N ratio of SS was found to be 46.63, which is way out of the acceptable range mainly due to its lignocellulosic nature with minimal nitrogen content (0.81%), as shown in Table 2. Hence, there is a need for co-digestion of the substrates. The C/N ratio is usually determined based on the type of material: wet weight, percentage of carbon, percentage of nitrogen, and percentage of moisture. Generally, anaerobic microbes utilise carbon 25–30 times faster than nitrogen. Thus, for efficient biogas production, the C/N ratio in the feedstocks should be maintained at 20–30:1 [25]. In the co-digestion process, a high C/N ratio substrate can balance the ratio of the other to an appropriate range. The protein content of WSW and SS are also 11% and 5%, respectively, like the results obtained by Lohani [26]. A protein-rich substrate typically produces relatively high biogas production and is energy-rich. However, the ammonia contained in protein is toxic to methanogens at high concentrations, thereby inhibiting the production of biogas. Hence, a digester often requires enough protein to provide sufficient nutrients without inhibiting methanogens [23, 27]. The Fe content of SS was found to be very high (636 mg/kg) as compared to WSW, with only 28.05 mg/kg. High Fe content stimulates anaerobic digestion [28] and acts as an external electron acceptor during anaerobic respiration, leading to the degradation of organic matter [29]. For example, Acidiphilium cryptum and Shewanella saccharophila oxidise glucose entirely to CO2 and acetate in the presence of iron (Fe).
Trace metals are essential in anaerobic digestion because they stimulate methanogenic activity. Some metals (Iron, cobalt, nickel, etc.) represent nutrients for methanogens [27]. The phosphorus (0.16%), potassium (1.77%) and calcium (0.06%) in WSW are similar to the findings of Castro Sousa et al. [30], but their calcium content is higher. Likewise, SS’s phosphorus, potassium, and calcium content are 0.08%, 2.76% and 0.41%, respectively, consistent with the findings of Pontieri et al. [31].
3.3 Biomethane potential tests
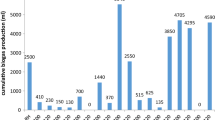
The cumulative amount of biogas obtained after 30 days from the BMP test of the substrates is shown in Table 3, while the daily profiles are represented in Fig. 4. Mostly, biogas production started on Day 2 of the AD period. For the co-digestion of WSW + SS + ION, biogas production became stable on Day 20 and WSW + ION on Day 24. The SS mono-AD without ION biogas production only started on Day 3, became stable on the 18th day, and finally ceased production on Day 20. The amount of biogas the control (inoculum) produced was lower than that of other substrates. It can be said that no significant inhibition of methanogens was observed in any of the substrates tested, although the accompanying methane production for SS was on the contrary. Also, biogas production from ION-added biomass started earlier than non-ION biomass except for SS. A limit press measurement might clarify this and come within the fast increment of biogas generation [32]. The mono-AD of SS delivered 239 mL of biogas, while the mono-AD of WSW delivered 145 mL as well in the present study.
Cumulative biogas yield from different BMP tests: Control, Zebra dung inoculum only; WSW, winery solid waste; SS, Sorghum Stover; WSW + SS, co-digestion of WSW and SS at 50/50 ratio; WSW + ION, Winery solid waste with Iron oxide nanoparticles, SS + ION, Sorghum Stover with Iron oxide nanoparticles, WSW + SS + ION, co-digestion substrates with Iron oxide nanoparticles
The co-digestion of WSW + SS without ION gave 320 mL of biogas. In comparison, WSW + SS with ION gave 365 mL, which is only second to mono-AD of WSW, delivering the highest biogas production of 380 mL observed in this study. In general, the co-digestion of substrates delivered more biogas than mono-AD of individual substrates except for mono-AD of WSW, which separately gave a high biogas yield. The co-digestion of WSW + SS without ION yielded more biogas (an increase of 81 mL) than for SS alone, with an increase of 175 mL for WSW alone. These represent a 34% and 120% increase in biogas production compared to mono-AD of each substrate alone. Similar increases were observed in methane content (Table 3).
Our results confirm the findings of Mata-Alvarez et al. [33] that co-digestion produces more methane than adding the methane produced in both single digestions. The SS + ION delivered 186 mL of biogas compared with 239 mL of SS mono-AD. There is a 22% decrease, which speaks to the nanoparticles’ hindrance of microscopic organisms. According to Faisal et al. [34], the transformation handled by the interaction of nanoparticles is influenced by inorganic contaminants obtained from inorganic fabric and atomic estimate. Nanomaterials may, in this manner, not have a response with SS microorganisms sort of. The WSW + ION delivered 380 mL of biogas, which is 235 mL more than WSW alone and indicates a 162% increment in biogas production. The WSW + SS + ION delivered 365 mL of biogas, indicating an increment of 14%. This affirms the attestation of Casals et al. [32] that ION could be a reasonable added substance to boost biogas yield. According to Casals et al. [32], when 100 ppm of 7 nm iron oxide NPs (Fe3O4) were added to the digester containing sludge (inoculum) from a wastewater treatment plant, the biogas production increased by 180%, which is slightly higher than 162% biogas increase obtained in the present study. This may be due to their use of wastewater sludge, a less complex organic matter than SS and WSW in biodegradability rate. However, the authors failed to determine the composition of the biogas produced at different times. They also reported a total increase in CH4 production of 234%. A similar study by Zhang et al. [35] used 100 mg/L of ION, which showed 109.8 ml CH4/g VSadded methane with a yield increase of 58.7% CH4 compared with the control. The above authors reported on a less complex organic matter than the ones reported in the present study, such as SS and WSW.
For the quality of the biogas, methane content is considered the most crucial objective. Figure 5 shows the cumulative methane production by the substrates with and without adding ION. The WSW methane generation went from 30 mLCH4 to 114 mLCH4 after adding ION, resulting in an increase of 280% for 30 days retention time (Fig. 5A). As measured by the Biogas 5000 analyser, the methane generation rate went up from 21% without ION to 30% when ION was added. In the case of SS, methane generation went from 48 mLCH4 to 76 mLCH4 over 30 days, leading to a methane increment of 58% (Fig. 5B). The SS had 20% methane content at the beginning and went up to 41% after adding ION to the substrate. The co-digestion substrate, WSW + SS, went from 128 mLCH4 by volume to 172 mLCH4 after the addition of ION, representing an increase of 34% (Fig. 5C). The methane generation rate during co-digestion of the substrates went from 40% without ION to 47% after adding ION.
BMP tests indicating cumulative methane production of different substrates with and without the addition of ION: Control (Inoculum); WSW, winery solid waste; SS, Sorghum Stover; WSW + SS, co-digestion of WSW and SS at 50/50 ratio; WSW + ION, Winery solid waste with Iron oxide nanoparticles, SS + ION, Sorghum Stover with Iron oxide nanoparticles, WSW + SS + ION, co-digestion substrates with Iron oxide nanoparticles
In another study by Zhang et al. [35], the authors investigated the effects of Iron oxide nanoparticles on the efficiency of anaerobic digestion of waste-activated sludge. Their results showed that adding Iron oxide nanoparticles enhanced the biogas yield and methane production rate. The authors suggested that Iron oxide nanoparticles could be used as a potential additive to improve the performance of anaerobic digestion systems.
Adding Fe3 + in anaerobic digesters can affect methane production [36]. When Fe3 + is added to the digester, it can undergo reduction to Fe2 + (ferrous iron) through microbial-mediated redox reactions. The outcome of Fe3 + in the anaerobic digester depends on the specific conditions and microbial interactions. They could enhance the syntrophic interactions between different microbial species, facilitating the degradation of complex organic matter and the subsequent methane production. Certain microorganisms can utilise Fe3 + as an electron acceptor, an alternative to methanogenesis. This process is known as iron reduction or dissimilatory iron reduction. Fe3 + acts as an electron acceptor for microorganisms that can respire using ferric iron, breaking down organic compounds and generating energy.
According to Zhu et al. [37], looking at the mechanism of action of ION in a digester containing waste-activated sludge and food waste, they found that it triggered direct interspecific electron transfer (DIET) between bacteria and methanogens. At an equal ratio between the substrates, the hydrogenotrophic methanogens were dominant, and due to an unfavourable environment, bacteria had no contact with Fe3O4 particles. So, it became difficult for ION to trigger DIET and enhance digester performance. The mechanism of action involves microbial communities capable of reducing Fe3 + using organic matter or other electron donors in the biodigester. These microorganisms transfer electrons to Fe3 + , reducing it to Fe2 + while simultaneously oxidising the organic matter. This process can compete with methanogenic pathways for electron availability. However, it is important to note that excessive Fe3 + addition might inhibit methanogenesis as it provides an alternative electron acceptor. This diversion of electrons away from methanogenesis can limit methane production [37].
In the present study, while WSW + SS + ION co-digestion is a good choice due to its high methane production of 172 mLCH4/VSadded, there is no optimism for using SS + ION due to its low methane yield of 76 mLCH4/VSadded. It could be postulated that the ION addition did not play a significant role in SS’s hydrolysis and acidogenesis processes, leading to the slow breakdown of complex organic compounds in the lignocellulolytic biomass. Further pretreatment may be necessary to break down the SS whenever the addition of ION is intended. Acidogenesis is the initial anaerobic digestion phase, where acidogenic bacteria break down complex organic matter into simpler organic compounds such as volatile fatty acids (VFAs). During acidogenesis, the primary focus is hydrolysis and fermentation processes, where complex organic materials are converted into VFAs, alcohols, and other intermediate products. The microbial consortia involved in acidogenesis are not usually directly affected by adding Fe3 + . Hence, Fe3 + addition is more commonly associated with the subsequent methanogenesis step, where methane production occurs. As mentioned earlier, Fe3 + can serve as an electron acceptor for certain microorganisms, and its reduction to Fe2 + through dissimilatory iron reduction can compete with methanogenesis by diverting electrons away from the methane-producing pathways.
In summary, there was an increase of 51% in biogas generation and 124% in methane generation with the addition of ION. This fits with the explanation that the non-toxic Fe3+ ions in oxide nanoparticles upgraded the methane generation by making a different electron transport system that progresses the methane and hydrogen rate and fortifies bacterial development, especially the methanogens [34].
Notwithstanding, the present study did not significantly improve the Hydraulic retention time (HRT) or Solid retention time (SRT). The cumulative methane production of WSW + SS + ION delayed about ten days compared with WSW + SS, which started on Day 2 (Fig. 5). The cumulative methane production of WSW + SS + ION began to increase on the 11th day, while the cumulative methane production of WSW + SS reached the highest on Day 20 and remained stable. This means that while WSW + SS could finish AD within Day 20, WSW + SS + ION must digest for at least 30 days. From the increasing tendency of WSW + SS + ION, its AD may need more than 30 days to complete. Therefore, it is appropriate that SS + ION be given more digestion time due to its lignocellulosic nature, with lignin playing a significant role in this delay. Another critical factor for consideration is the concentration of ION to be added to the substrates. In a recent study by Al-Bkoor-Alrawashdeh et al. [38], the effect of ION on the anaerobic co-digestion (AD) of olive mill wastewater and chicken manure was investigated. They found that supplementing AD with ION at a concentration of 20–30 mg/g VS induced a favourable impact on methane yield (1.3–4.2%) in mesophilic conditions. However, adding ION at a concentration of > 30 mg/g VS inhibited both biogas and methane generation as well as the hydrolysis stage [38]. In summary, the higher the concentration of ION, the lower the methane generation. The present study used ION in an equal ratio (1:1) with the SS, which could have delayed the onset of methanogenesis.
4 Conclusion
The chemical and physical properties of WSW and SS indicate that they have a high total solid content, high volatile solid content, and protein content and are suitable substrates to produce biogas. Iron oxide analysis showed that the nanoparticles were a mixture of Iron oxides (II) and (III) with an average particle size of 30 nm. The mixture contains Fe2+ and Fe3+ ions that are nutritious to methanogenic bacteria. During 30 days of BMP analysis at the optimal temperature of 37 °C ± 0.5, SS and WSW showed excellent results of anaerobic digestion amplification by simply adding ION. The results showed that biogas production from mono-AD of the substrates increased by 51% in yield and 124% in methane generation by adding ION to the digestion mixture. The addition of nanoparticles even tripled the methane production of the WSW from 30 to 114 mLCH4 over 30 days. This confirms that ION is more compatible with WSW than SS.
Furthermore, we can recommend the adoption of ION by the winery industry in valorising this type of waste going forward. On the contrary, we would not recommend using ION with SS at an equal ratio as its addition reduced methane yield, probably due to inorganic SS contaminants that delay the AD process. A low concentration of ION is necessary during AD of SS. Compared with the separate digestion of WSW and SS, co-digestion of the substrates produces more biogas amounts, as clearly shown in the present study.
Data availability
Data will be made available on request.
References
Pullen T. Anaerobic digestion-making biogas-making Energy: the earthscan expert guide. New York: Routledge; 2015. https://doi.org/10.4324/9781315770772.
Achinas S, Achinas V, Euverink GJW. A technological overview of biogas production from biowaste. Engineering. 2017. https://doi.org/10.1016/J.ENG.2017.03.002.
Gaulin N, Le Billon P. Climate change and fossil fuel production cuts: assessing global supply-side constraints and policy implications. Climate Policy. 2020;20:888–901. https://doi.org/10.1080/14693062.2020.1725409.
Ryan J. Australian fires: Everything we know about the crisis and how you can help - CNET. CNET. 2020. https://www.cnet.com/science/australian-fires-everything-we-know-about-the-crisis-and-how-you-can-help/. Accessed 2 Apr 2023.
Prodhomme C, Materia S, Ardilouze C, White RH, Batté L, Guemas V, Fragkoulidis G, García-Serrano J. Seasonal prediction of European summer heatwaves. Clim Dyn. 2022;58:2149–66. https://doi.org/10.1007/S00382-021-05828-3.
Wang J, Chen X, Zhang S, Wang Y. XS Analysis of raw materials and products characteristics from composting and anaerobic digestion in rural areas. J Clean Prod. 2022;338: 130455.
Recebli Z, Selimli S, Ozkaymak M, Gonc O. Biogas production from animal manure. J Eng Sci Technol. 2015;10(6):722–9.
Kozłowski K, Dach J, Lewicki A, Malińska K, Carmo do IE, Czekała W. Potential of biogas production from animal manure in Poland. Arch Environ Prot. 2019;45(3):59–68.
Parralejo AI, Royano L, González J, González JF. Small scale biogas production with animal excrement and agricultural residues. Ind Crops Prod. 2019;1(131):307–14.
Gnanambal VS, Swaminathan K. Biogas production from renewable lignocellulosic biomass. Int J Environ. 2015;4(2):341–7.
Khumalo SC, Oyekola OO, Okudoh VI. Evaluating input parameter effects on the overall anaerobic co-digestion performance of abattoir and winery solid wastes. Bioresour Technol Rep. 2021;13: 100635. https://doi.org/10.1016/j.biteb.2021.100635.
Da Ros D, Cavinato C, Cecchi F, Bolzonella C. Anaerobic co-digestion of winery waste and waste activated sludge: assessment of process feasibility. Water Sci Technol. 2014;69:1461–8. https://doi.org/10.2166/wst.2013.692.
Bharathiraja B, Iyyappan J, Jayamuthunagai J, Kumar RP, Sirohi R, Gnansounou E, Pandey A. Critical review on bioconversion of winery wastes into value-added products. Ind Crops Prod. 2020;15(158): 112954.
Bucić-Kojić A, Tišma M, Šelo G, Grgić J, Perković G, Planinić M. Winery Production residues as feedstocks within the biorefinery concept. Eng Power Bull Croat Acad Eng. 2022;17(1):11–7.
Mkruqulwa U, Okudoh V, Oyekola O. Optimising methane production from co-digestion of cassava biomass and winery solid waste using response surface methodology. Waste Biomass Valorisation. 2020;11:4799–808. https://doi.org/10.1007/s12649-019-00801-y.
American Public Health Association (APHA). Standard methods for the examination of water and wastewater. 21st ed. Washington: American Water Works Association and Water Environment Federation; 2017.
Mariotti F, Tomé D, Mirand PP. Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit Rev Food Sci Nutr. 2008;48(2):177–84. https://doi.org/10.1080/10408390701279749.
Morsi M, Abd EM. Effect of iron doped hydroxyapatite nanoparticles on the structural, morphological, mechanical, and magnetic properties of polylactic acid polymer. J Mater Res. 2019;34(15):2618–29.
Hedayati K, Goodarzi M, Ghanbari D. Hydrothermal synthesis of Fe3O4 nanoparticles and flame resistance magnetic poly styrene nanocomposite. J Nanostructures. 2017;7(2):167–73.
Wang LK, Shammas NK, Hung Y-T. Advanced biological treatment processes. Totowa: Humana Press; 2009. https://doi.org/10.1007/978-1-60327-170-7.
Speakman SA. Introduction to X-ray powder diffraction data analysis. 2021. http://prism.mit.edu/xray/introduction-to-xrpd-data-analysis.pdf. Accessed 12 Feb 2021.
Iida H, Takayanagi K, Nakanishi T, Osaka T. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J Colloid Interface Sci. 2007;314(1):274–80. https://doi.org/10.1016/j.jcis.2007.05.047.
Hagos K, Zong J, Li D, Liu C, Lu X. Anaerobic co-digestion process for biogas production: progress, challenges and perspectives. Renew Sustain Energy Rev. 2017;1(76):1485–96. https://doi.org/10.1016/j.rser.2016.11.184.
Alnakeeb AN, Najim K, Ahmed A. Anaerobic digestion of tomato wastes from groceries leftovers: effect of moisture content. Int J Curr Eng Technol. 2017;7(4):1468–70.
Lohani SP, Havukainen J. Anaerobic digestion: factors affecting anaerobic digestion process. Waste Bioremediation. 2018. https://doi.org/10.1007/978-981-10-7413-4_18.
Harinarayana G, Melkania NP, Reddy B, Gupta SK, Rai KN, Kumar PS. Forage potential of sorghum and pearl millet. 2023. http://oar.icrisat.org/4394/. Accessed 4 Apr 2023.
Rabii A, Aldin S, Dahman Y. A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies. 2019;12(6):1106. https://doi.org/10.3390/en12061106.
Mudhoo A. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. J Environ Sci Health Part A. 2013;10:1383–98. https://doi.org/10.1007/s13762-012-0167-y.
Lovley DR, Coates JD. Novel forms of anaerobic respiration of environmental relevance. Curr Opin Microbiol. 2000;3(3):252–6.
Castro Sousa E, Maria A, Uchôa-Thomaz A, Osvaldo J, Carioca B, Maia De Morais S, De Lima A, Martins CG, Alexandrino CD, Augusto P, Ferreira T, Livya A, Rodrigues M, Rodrigues SP, Do J, Silva N, Rodrigues LL. Chemical composition, and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci Technol. 2014;34:135–42.
Pontieri P, Troisi J, Di Fiore R, Di Maro A, Bean SR, Tuinstra MR, Boffa A, Del Giudice A, Pizzolante G, Alifano P, Del Giudice L. Mineral contents in grains of seven food-grade sorghum hybrids grown in a Mediterranean environment. Search Informit Org. 2014;8:1550–9.
Casals E, Barrena R, García A, González E, Delgado L, Busquets-Fité M, Font X, Arbiol J, Glatzel P, Kvashnina K, Sánchez A, Puntes V. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small. 2014;10:2801–8. https://doi.org/10.1002/smll.201303703.
Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, Astals S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sustain Energy Rev. 2014;36:412–27. https://doi.org/10.1016/j.rser.2014.04.039.
Faisal S, Yusuf Hafeez F, Zafar Y, Majeed S, Leng X, Zhao S, et al. A review on nanoparticles as boon for biogas producers—nano fuels and biosensing monitoring. Appl Sci. 2018;9(1):59.
Zhang Z, Guo L, Wang Y, Zhao Y, She Z, Gao M, et al. Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: impact on the biogas production and the substrate metabolism. Renew Energy. 2020;1(146):2724–35.
Fetra J. Andriamanohiarisoamanana, Ikko Ihara, Gen Yoshida, Kazutaka Umetsu, tackling antibiotic inhibition in anaerobic digestion: the roles of Fe3+ and Fe3O4 on process performance and volatile fatty acids utilisation pattern. Bioresour Technol Rep. 2020;11: 100460. https://doi.org/10.1016/j.biteb.2020.100460.
Zhu R, He L, Li Q, Huang T, Gao M, Jiang Q, Liu J, Cai A, Shi D, Gu L, He Q. Mechanism study of improving anaerobic co-digestion performance of waste activated sludge and food waste by Fe3O4. J Environ Manage. 2021;15(300): 113745. https://doi.org/10.1016/j.jenvman.2021.113745.
Al Bkoor Alrawashdeh K, Al-Zboon KK, Rabadi SA, Gul E, AL-Samrraie SA, Ali R, Al-Tabbal JA. Impact of Iron oxide nanoparticles on sustainable production of biogas through anaerobic co-digestion of chicken waste and wastewater. Front Chem Eng. 2022;4: 974546. https://doi.org/10.3389/fceng.2022.974546.
Acknowledgements
The financial assistance of the African Union (Mwalilu Nyerere Scholarship), as well as the National Research Foundation (NRF) of South Africa (Grant No. UID 99393), towards this research, is acknowledged. Our deepest gratitude goes to Cape Flats Wastewater Treatment Works for providing the digested sludge; Deborah Erasmus of the Horticulture Department for providing laboratory assistance; and to the entire staff of the Functional Material Unit, Department of Chemical Engineering at Cape Peninsula University of Technology, for their willingness to assist.
Author information
Authors and Affiliations
Contributions
CGO: methodology, formal analysis, investigation, writing—original draft. MRC: methodology, writing—review and editing, supervision. VIO: conceptualization, resources, methodology, formal analysis, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ossinga, C.G., Okudoh, V.I. & Chowdhury, M.R. Sorghum stover and winery solid wastes co-digestion: application of iron oxide nanoparticles for biogas yield optimisation. Discov Water 3, 21 (2023). https://doi.org/10.1007/s43832-023-00047-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43832-023-00047-9