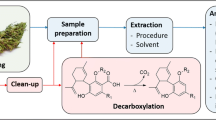
Abstract
Δ9-THC, the psychotropic cannabinoid in Cannabis sativa L., for many years has been the focus of all the pharmacological attention as the main promising principle of the plant. Recently, however, cannabidiol (CBD) has brought a sudden change in the scenario, exponentially increasing the interest in pharmacology as the main non-psychotropic cannabinoid with potential therapeutic, cosmetical and clinical applications. Although the reactivity of CBD and Δ9-THC has been considered, little attention has been paid to the possible photodegradation of these cannabinoids in the vegetal matrix and the data available in the literature are, in some cases, contradictory. The aim of the present work is to provide a characterization of the photochemical behaviour of CBD and Δ9-THC in three cannabis chemotypes, namely I (Δ9-THC 2.50%w/w), II (CBD:Δ9-THC 5.82%w/w:3.19%w/w) and III (CBD 3.02%w/w).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Flowering plants of the Cannabinaceae family can be classified into several taxonomic species, within which Cannabis sativa L. can be further defined according to the content of both tricyclic and bicyclic cannabinoids which determine their use and application [1, 2]. In this context, the psychoactive molecule Δ9-tetrahydrocannabinol (1, Δ9-THC, Fig. 1), is the main compound belonging to the class of tricyclic cannabinoids (which includes all THC derivatives, cannabinol and hexahydrocannabinol isomers), while cannabidiol (2, CBD, Fig. 1) is the main component of the bicyclic group [3].
Cannabis was introduced into Western medicine in 1839 by W. B. O’Shaughnessy [4] and triggered a massive series of pharmacological experiments outlining its therapeutic utility [5,6,7,8]. In recent years CBD dramatically emerged as the most important non-psychotropic cannabinoid with therapeutic benefits [9], due to the strong interaction with the cannabinoid receptor type 1 (CB1, of which CBD is a negative allosteric modulator [5,6,7,8,9,10]) as well as its regulatory role on the action of Δ9-THC [11]. Such properties can be exploited in the treatment of several pathological conditions including Parkinson’s disease [12], epilepsy [13], and to counteract some side effects of common chemotherapeutic regimens [7]. Other investigations pointed out the role of CBD as an agonist towards several receptors, including, among others, the transient receptor potential vanilloid type 1 receptor (TRPV1) and the 5-hydroxytryptamine 1A receptor (5-HT1A) [14].
Recently, CBD has also attracted the attention of the cosmetic industry, mainly due to its anti-inflammatory, analgesic, hydrating, moisturizing, and wrinkle-reducing properties [15]. CBD also acts as a sebostatic agent, inhibiting the proliferation of sebocytes and sebum, making it a promising therapeutic agent for the treatment of acne vulgaris [16].
Because of its many uses and applications, researchers have focused on the thermal and photochemical stability of cannabinoids. The first attempt at photochemical studies on CBD is to be attributed to Loewe in 1950 [16], which was followed by a serial of seminal papers by Shani and Mechoulam [17]. The nature of the products resulting from the UV-induced decomposition of CBD has recently been discussed [6, 18, 19] and its possible photochemical conversion into Δ9-THC is a moot point. Despite what has been observed in solution [17, 19, 20], where Δ9-THC is formed as a minor photodegradation product, most recent investigations firmly exclude such behaviour (at least in the natural matrix [18, 21] since a decrease in the concentration of CBD results in an unchanged concentration of Δ9-THC).
Δ9-THC is reported to break down during storage to a less psychoactive compound, cannabinol (CBN) and the rate of degradation has been found to be accelerated by exposure to light and heat [22, 23]. Although this conversion is thought to be the main pathway through which cannabis reduces its psychotropic activity, the rate at which it occurs in the vegetal matrix remains to be determined, as does whether degradation results in more than one product. Fairbarn et al. conducted experiments on the stability of Δ9-THC solutions, made in different solvents exposed to light [22]. Exposure to light appears to be the key factor in the degradation of Δ9-THC, a hypothesis confirmed by more recent studies [24, 25]; the same behaviour has been observed with cannabis resin and cannabis resin extracts. The effect of both daylight and temperature on the stability of cannabinoids in such matrixes was investigated in detail by Lindholst and coworkers [26].
Further studies on the thermal and photochemical degradation of cannabinoids derivative have been carried out on cannabis resin (i.e., hashish), but not on the inflorescences [27]. Furthermore, photochemical investigations have not been carried out under controlled and reproducible conditions showing few degradation products.
To further investigate the photochemical stability of active ingredients in the vegetal matrix and to shed light on the anecdotical reports of intoxication from the many attempts to convert CBD into THC, and given how little is known about the appropriate storage conditions and degradation patterns of cannabis, this work aims to investigate the degradation rate of different cannabinoids in their native plant matrix. It should be noted that commercially available vegetal material often contains a mixture of decarboxylated and non-decarboxylated cannabinoids, and in the aim of ensuring homogeneity to the samples, we focused on extensively decarboxylated cannabis.
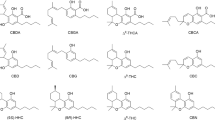
We conducted a systematic characterization of the photochemical behaviour of Δ9-THC (1) and CBD (2) in three different cannabis chemotypes: chemotype I (c-I, Δ9-THC 2.50%w/w), II (c-II, CBD:Δ9-THC 5.82%w/w:3.19%w/w) and III (c-III, CBD 3.02%w/w), by means of an extraction procedure combined to GC–MS analyses. Taking advantage of the interconversion of cannabinoids into each other and the possible photochemical conversion of the main ones into lesser-known but still active derivatives, we compared the products of CBD and Δ9-THC photochemical degradation with our extensive GC–MS library consisting of 22 cannabinoids (including THD and HHC isomers).
2 Results and discussion
The experimental set up used for the irradiation of the Cannabis samples is reported in Supporting Information. Briefly, the irradiation was performed in a Solar Box equipped with a xenon lamp (spectral field from 290 to 800 nm, colour temperature 6000 K) and a power set up at 500 W/m2. The cannabinoids investigated in the present work (shown in Fig. 2) were identified by GC–MS comparison with the cannabinoids from our library. The main GC–MS data obtained, together with the validation of the chromatographic method, have been reported by our group in previous works [19, 20] and are briefly discussed in the Supporting Information for the sake of completeness. The results obtained allowed for the assembling of a library of cannabinoids (Fig. 2) that was employed in the present work. All the molecules found in the examined samples were identified and quantified by means of GC–MS analyses, as reported below in Table 1. We therefore considered any other cannabinoid other than those reported to be absent or present below the Limit of Detection (LODs). Experiments were performed in triplicate, and the values reported are the mean of the results obtained. In all cases, as can be seen from the following figures—including error bars—and tables, the standard deviation is < 20% of the mean value, which is acceptable due to the intrinsic heterogeneity of the plant material and of the intrinsic variability of the experimental set up.
The concentration of cannabinoids in the three herbal products (see Table 1) resulted to be:
-
c-I: Δ9-THC 2.50%w/w, CBN, 0.88%w/w. Weight ratio of the two cannabinoids approximately about 3:1 (Δ9-THC:CBN);
-
c-II: CBD, 5.82%w/w, Δ9-THC 3.19%w/w, CBN 2.34%w/w. Weight ratio of the three cannabinoids approximately about 6:3:2 (CBD:Δ9-THC:CBN);
-
c-III: CBD only, 3.02%w/w.
For all cannabis chemotypes we focused on the photodegradation trends of the cannabinoids that characterize the chemotypes (Δ9-THC and CBN for c-I; CBD, Δ9-THC and CBN for c-II; CBD for c-III); indeed, thanks to their abundance, it is possible to evaluate their behaviour over time and obtain data relating to degradation that are not significantly affected by the different strains. All graphs are constructed by relating the irradiation time to the normalized ratio of the cannabinoid peak area to the internal standard’s peak area.
In addition to the main cannabinoids, the presence of minor amounts of other related compounds, namely CBT, CBL, CBE, CBC, CBG and CBND has been observed by GC–MS analyses. It should be noticed that the presence of other isomers (among the available standards) has been excluded when found at levels below 0.01%w/w in cannabis. An overall decrease in the concentration of all cannabinoids was observed, during irradiation with some slight variations for CBE in c-II when irradiated under a nitrogen atmosphere. However, the trend for these cannabinoids is equivocal as the small amounts give rise to a large variability in the corresponding analytical signals. All the data regarding the photodegradation kinetics and the comparison between the different atmospheres evaluated are presented in Table 2 and commented on the “Discussion”.
2.1 Photodegradation of Δ9−THC in Cannabis sativa (c-I)
When operating in air (i.e., in the presence of 20% oxygen), in 108 h the content of Δ9-THC in the c-I sample decreases from 100% (2.50%w/w in cannabis) to 1.9% (0.05%w/w in cannabis) and for CBN from 100% (0.88%w/w in cannabis) to 19.6% (0.17%w/w in cannabis) (see Fig. 3). The Δ9-THC:CBN ratio changes from 3:1 to about 1:4, indicating either a faster degradation rate for Δ9-THC and/or a low photochemical conversion of Δ9-THC into CBN. The irradiation times are consistent with the other experiments carried out in air..
In the nitrogen atmosphere, Fig. 4, the concentration of Δ9-THC in 480 h decreases from 100% (2.50%w/w in cannabis) to 1.1% (0.03%w/w in cannabis) and that of CBN from 100% (0.88%w/w in cannabis) to 8.8% (0.08%w/w in cannabis). The Δ9-THC:CBN ratio changes from 3:1 to 3:8, which is the most significant change of all the experiments.
2.2 Photodegradation of CBD + Δ9−THC in Cannabis sativa (c-II)
In Fig. 5 the degradation profiles of CBD and Δ9-THC in the c-II sample in air are reported. The concentration of CBD decreases from 100% (5.82%w/w in cannabis) to 0.9% (0.05%w/w in cannabis) in 108 h. There is also a decrease in Δ9-THC from 100% (3.19%w/w in cannabis) to below 0.01%w/w in 96 h. CBN ranges from 100% (2.34%w/w in cannabis) to 0.7% (0.02%w/w in cannabis) in 108 h. Δ9-THC degrades faster than CBD does, while CBN shows a degradation kinetic comparable to that of CBD; it can be observed how the ratio between these cannabinoids changes from 6:3:2 (CBD:Δ9-THC:CBN) at time zero to approximately a CBD:CBN 5:2 mixture (Δ9-THC falls below 0.01%w/w) after 108 h. In this context, the slow conversion of Δ9-THC to CBN cannot be safely excluded [21].
Under nitrogen atmosphere, see Fig. 6, in 480 h a decrease in CBD concentration from 100% (5.82%w/w in cannabis) to 45.4% (2.64%w/w in cannabis) was observed. Δ9-THC decreased from 100% (3.19%w/w in cannabis) to 9.0% (0.29%w/w in cannabis), and CBN from 100% (2.34%w/w in cannabis) to 20.1% (0.47%w/w in cannabis). In this atmosphere, Δ9-THC and CBN showed a similar kinetic, degrading at a faster rate than CBD does; the ratio of the three cannabinoids changed from 6:3:2 to 10:1:2 (CBD:Δ9-THC:CBN). The duration of this test under nitrogen atmosphere is comparable to that of the c-I sample, although in this chemotype the degradation is less accentuated at 480 h when compared to c-I.
Contrary to previous experiments, irradiation of this type of cannabis resulted also in the formation and subsequent degradation of CBND, due to the photodegradation of CBN [28] with a formation peak at 144 h equal to 0.72%w/w. Usually, the degradation of CBND results in the formation of cannabiphenantrene (CBP) [28], but no such behaviour was observed under these conditions.
2.3 Photodegradation of CBD in Cannabis sativa (c-III)
When the degradation procedure was carried out in an oxygenated environment (i.e., air), we observed a decrease in concentration from 100% (quantity normalized to the initial CBD content which corresponds to 3.02%w/w in cannabis) to 1.9% (0.06%w/w in cannabis) of CBD in 108 h. The timing of the experiment is comparable to that of c-II in air: both reached a percentage concentration in cannabis < 0.1%w/w in a time of 108 h (Figs. 7 and 8).
In a nitrogen atmosphere, there was a decrease in concentration from 100% (3.02%w/w in cannabis) to 7.8% (0.24%w/w in cannabis) of CBD in 144 h.
2.4 Discussion
Looking at the first points of the graphs for CBD and Δ9-THC, the photodegradation of these compounds in its early stages has a linear trend and exhibits a first-order kinetics. By interpolating the data we (see S3 in S.I.) were able to calculate the degradation constants, kd, shown in Table 2 which further confirm the higher photodegradation rate of CBD in air compared to nitrogen. The shelf-life (t90%) of the main cannabinoids in the vegetal matrix was calculated with Eq. 2 through the kd in both air and nitrogen atmospheres. The data obtained are reported in Table 2.
As apparent from Table 2, cannabis appears to be a relatively fragile matrix that should be protected from light exposure throughout the production chain, including collection, drying and preparation of the pharmaceutical form.
Comparing the graphs obtained from the c-III irradiation, the experiment carried out in a nitrogen atmosphere achieves an almost complete degradation of CBD (residual amount < 0.5%w/w compared to the initial) in 108 h, which is 85 h more than in air. The difference in degradation rate is due to the different atmospheres in which the experiment is carried out: in the presence of oxygen, some oxidation reactions (which significantly increase the degradation rate of organic compounds) can take place, causing CBD to be consumed much faster than in a situation where photodegradation is carried out in an oxygen-free environment. The same can be observed with c-II..
The behaviour of c-I cannot be directly compared to that of c-III, because of the lack of a common cannabinoid to follow, but it is, however, still possible to evaluate Δ9-THC degradation. Indeed, the discrepancy in irradiation time in different atmospheres in c-I leads to a difference of 42 h between nitrogen and air, which is the shortest discrepancy among the three chemotypes evaluated, highlighting the effect of different atmospheres on cannabis degradation. Although it was not possible to identify the degradation products of the cannabinoids, we performed HPLC-TOF analyses of the extracts that does not show the presence of cannabinoids oligomers in significant amount. In this context, we consider that under the examined conditions and in vegetable matrix, generation of phenoxy radical intermediates [29] from irradiated cannabinoids followed by grafting of the lignocellulosic matrix represents a reasonable degradation path for the examined substrates [30,31,32].
While all three experiments lead to the same conclusion, it is worth noting that the photodegradation of cannabis c-I and c-II in a nitrogen atmosphere appears to have a distinctly different timing compared to the corresponding photodegradation of c-III: c-I and c-II experiments require at least 480 h of irradiation time to be considered complete, with c-I showing a substantial degradation of all cannabinoids present (concentrations dropping to 0.03%w/w for Δ9-THC, and 0.08%w/w for CBN), while c-II still contains a significant amount of cannabinoids (2.64%w/w for CBD, 0.29%w/w for Δ9-THC and 0.47%w/w for CBN); finally, c-III degrade to 0.24%w/w in 144 h.
The Δ9-THC chemotypes of cannabis show a different behaviour under a nitrogen atmosphere compared to the CBD chemotype. In the presence of oxygen, however, the photodegradation rate observed for the three cannabis chemotypes is comparable, as all three have halved their cannabinoid concentration by the 10-h mark or slightly less.
3 Conclusions
In the present manuscript, we investigated the behaviour of different decarboxylated chemotypes of cannabis when exposed to sunlight, to assess the possible photochemical conversion of the present cannabinoids into different compounds and to obtain information on the photodegradation profile of the strands studied, thus providing more data on the optimal storage conditions for cannabis. The presence of all the 22 cannabinoids present in our library was investigated in each sample collected. In addition to the main cannabinoids, small amounts of other related compounds (CBT, CBL, CBE, CBC, CBG and CBND) were observed by GC–MS analyses.
While exposure to light results in a significant degradation of all cannabinoids without favouring the formation of any type of cannabinoid artefacts, the atmosphere used for storage seems to be the key factor when it comes to the survival of the cannabinoids. The presence of oxygen dramatically increases degradation rates due to the oxidation reactions and the formation of non-cannabinoid oxidation products, critically reducing shelf-life. CBD shows a better resistance to light exposure, followed by CBN; out of the three cannabinoids considered in this work, Δ9-THC seems to be the most light-sensitive cannabinoid: evidence can be found mainly in the change of CBD:Δ9-THC:CBN ratio during c-II irradiation. Storing the sample in an inert atmosphere would be a better solution to preserve all the active constituents for a longer period– always bearing in mind that exposure to light will, eventually, lead to the degradation of cannabinoids regardless of the atmosphere.
The experiments carried out on c-III certainly indicate that a photochemical conversion of CBD into the psychoactive Δ9-THC, as sometimes reported in the literature [17, 20], is not feasible and this is crucial to exclude the risk of any accidental contamination by Δ9-THC in CBD-containing cannabis, even if not properly protected from light and atmospheric conditions.
Data availability
Not applicable.
Abbreviations
- CBT:
-
Cannabicitran
- HCDN:
-
Dihydro-cannabinodiol
- CBND:
-
Cannabinodiol
- CBL:
-
Cannabicyclol
- CBD:
-
Cannabidiol
- CBC:
-
Cannabichromene
- Δ8-iso-THC:
-
Δ8-Iso-tetrahydrocannabinol
- iso-CBD:
-
iso-Cannabidiol
- DHD:
-
Dihydro-cannabidiol
- cis-HHC:
-
cis-Hexahydrocannabinol
- trans-HHC:
-
trans-Hexahydrocannabinol
- cis-THD:
-
cis-Tetrahydrocannabidiol
- trans-THD:
-
trans-Tetrahydrocannabidiol
- Δ7-CBD:
-
Δ7-Cannabidiol
- CBS:
-
Cannabielsol
- Δ8-THC:
-
Δ8-Tetrahydrocannabinol
- CBE:
-
Cannabielsoin
- Δ9-THC:
-
Δ9-Tetrahydrocannabinol
- CBQN:
-
Cannabinoquinone
- CBG:
-
Cannabigerol
- CBN:
-
Cannabinol
- CBP:
-
Cannabiphenantrene
References
Hazekamp, A., Fischedick, J. T., Llano, M. D., Lubbe, A., & Ruhaak, R. L. (2010). Chemistry of Cannabis. Comprehensive Natural Products II: Chemistry and Biology, 3, 1033–1084. https://doi.org/10.1016/b978-008045382-8.00091-5
Mechoulam, R. (1896). Cannabinoids as therapeutic agents. CRC Press.
Russo, E. B. (2011). Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, 163(7), 1344–1364. https://doi.org/10.1111/j.1476-5381.2011.01238.x
Mikuriya, T. H. (1969). Marijuana in medicine: Past, present and future. California Medicine, 110(1), 34–40.
Pertwee, R. G. (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-Tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. British Journal of Pharmacology, 153(2), 199–215. https://doi.org/10.1038/sj.bjp.0707442
Bonini, S. A., Premoli, M., Tambaro, S., Kumar, A., Maccarinelli, G., Memo, M., & Mastinu, A. (2018). Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. Journal of Ethnopharmacology, 227, 300–315. https://doi.org/10.1016/j.jep.2018.09.004
Sawtelle, L., & Holle, L. M. (2021). Use of cannabis and cannabinoids in patients with cancer. Annals of Pharmacotherapy, 55(7), 870–890. https://doi.org/10.1177/1060028020965224
Dariš, B., Verboten, M. T., Knez, Ž, & Ferk, P. (2019). Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosnian Journal of Basic Medical Sciences, 19(1), 14–23. https://doi.org/10.17305/BJBMS.2018.3532
Burstein, S. (2015). Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic & Medicinal Chemistry, 23, 1377–1385. https://doi.org/10.1016/j.bmc.2015.01.059
Mackie, K. (2008). Cannabinoid receptors: Where they are and what they do. Journal of Neuroendocrinology, 20, 10–14. https://doi.org/10.1111/j.1365-2826.2008.01671.x
Schubart, C. D., Sommer, I. E. C., van Gastel, W. A., Goetgebuer, R. L., Kahn, R. S., & Boks, M. P. M. (2011). Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophrenia Research, 130(1–3), 216–221. https://doi.org/10.1016/j.schres.2011.04.017
Iuvone, T., Esposito, G., De Filippis, D., Scuderi, C., & Steardo, L. (2009). Cannabidiol: A promising drug for neurodegenerative disorders? CNS Neuroscience & Therapeutics, 15(1), 65–75. https://doi.org/10.1111/j.1755-5949.2008.00065.x
Devinsky, O., Cilio, M. R., Cross, H., Fernandez-Ruiz, J., French, J., Hill, C., Katz, R., Di Marzo, V., Jutras-Aswad, D., Notcutt, W. G., Martinez-Orgado, J., Robson, P. J., Rohrback, B. G., Thiele, E., Whalley, B., & Friedman, D. (2014). Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia, 55(6), 791–802. https://doi.org/10.1111/epi.12631
De Almeida, D. L., & Devi, L. A. (2020). Diversity of molecular targets and signaling pathways for CBD. Pharmacology Research & Perspectives, 8(6), 1–10. https://doi.org/10.1002/prp2.682
Jhawar, N., Schoenberg, E., Wang, J. V., & Saedi, N. (2019). The growing trend of cannabidiol in skincare products. Clinics in Dermatology, 37(3), 279–281. https://doi.org/10.1016/j.clindermatol.2018.11.002
Oláh, A., Tóth, B. I., Borbíró, I., Sugawara, K., Szöllõsi, A. G., Czifra, G., Pál, B., Ambrus, L., Kloepper, J., Camera, E., Ludovici, M., Picardo, M., Voets, T., Zouboulis, C. C., Paus, R., & Bíró, T. (2014). Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. The Journal of Clinical Investigation, 124(9), 3713–3724. https://doi.org/10.1172/JCI64628
Loewe, S. (1950). Active principals of the cannabis and the pharmacology of the cannabinols. Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie, 211(2), 175–193.
Shani, A., & Mechoulam, R. (1971). Photochemical reactions of cannabidiol. Tetrahedron, 27(3), 601–606. https://doi.org/10.1016/s0040-4020(01)90728-8
Lydon, J., & Teramura, A. H. (1987). Photochemical decomposition of cannabidiol in its resin base. Phytochemistry, 26(4), 1216–1217. https://doi.org/10.1016/S0031-9422(00)82388-2
Seccamani, P., Franco, C., Protti, S., Porta, A., Profumo, A., Caprioglio, D., Salamone, S., Mannucci, B., & Merli, D. (2021). Photochemistry of cannabidiol (CBD) revised. A combined preparative and spectrometric investigation. Journal of Natural Products, 84(11), 2858–2865. https://doi.org/10.1021/acs.jnatprod.1c00567
Franco, C., Protti, S., Porta, A., Pollastro, F., Profumo, A., Mannucci, B., & Merli, D. (2022). Stability of cannabidiol (CBD) in solvents and formulations: A GC–MS approach. Results in Chemistry, 4(August), 100465. https://doi.org/10.1016/j.rechem.2022.100465
Grafström, K., Andersson, K., Pettersson, N., Dalgaard, J., & Dunne, S. J. (2019). Effects of long term storage on secondary metabolite profiles of cannabis resin. Forensic Science International, 301, 331–340. https://doi.org/10.1016/j.forsciint.2019.05.035
Fairbairn, J. W., Liebmann, J. A., & Rowan, M. G. (1976). The stability of cannabis and its preparations on storage. Journal of Pharmacy and Pharmacology, 28(1), 1–7. https://doi.org/10.1111/j.2042-7158.1976.tb04014.x
Harvey, D. J. (1990). Stability of cannabinoids in dried samples of cannabis dating from around. Journal of Ethnopharmacology, 28, 117–128. https://doi.org/10.1016/0378-8741(90)90068-5
Trofin, I. G., Vlad, C. C., Dabija, G., & Filipescu, L. (2011). Influence of storage conditions on the chemical potency of herbal cannabis. Revista de Chimie, 62(6), 639–645.
Trofin, I. G., Dabija, G., Vaireanu, D. I., & Filipescu, L. (2012). Long term storage and cannabis oil stability. Revista de Chimie, 63(3), 293–297.
Lindholst, C. (2010). Long term stability of cannabis resin and cannabis extracts. Australian Journal of Forensic Sciences, 42(3), 181–190. https://doi.org/10.1080/00450610903258144
Jaidee, W., Siridechakorn, I., Nessopa, S., Wisuitiprot, V., Chaiwangrach, N., Ingkaninan, K., & Waranuch, N. (2022). Kinetics of CBD, Δ9-THC degradation and cannabinol formation in cannabis resin at various temperature and pH conditions. Cannabis and Cannabinoid Research, 7(4), 537–547. https://doi.org/10.1089/can.2021.0004
Bowd, A., Swann, D. A., & Turnbull, J. H. (1975). Photochemical transformations of cannabinol. Journal of the Chemical Society, Chemical Communications, 310(19), 797–798. https://doi.org/10.1039/C39750000797
Rayne, S., Forest, K., & Friesen, K. J. (2009). Mechanistic aspects regarding the direct aqueous environmental photochemistry of phenol and its simple halogenated derivatives. A review. Environment International, 35, 425–437. https://doi.org/10.1016/j.envint.2008.09.004
Elegir, G., Kindl, A., Sadocco, P., & Orlandi, M. (2008). Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzyme and Microbial Technology, 43, 84–92. https://doi.org/10.1016/j.enzmictec.2007.10.003
Qi, Y., Huang, X., Zhai, H., Shi, M., Zhang, M., Zhang, Y., & Zhao, Y. (2022). Synthesis and photoinitiation properties of lignin model compounds. Progress in Organic Coatings, 173, 107210. https://doi.org/10.1016/j.porgcoat.2022.107210
Wojnàrovits, L., Foldvary, C. M., & Takàcs, E. (2010). Radiation-induced grafting of cellulose for adsorption of hazardous water pollutants: A review. Radiation Physics and Chemistry, 79, 848–862. https://doi.org/10.1016/j.radphyschem.2010.02.006
Acknowledgements
We are grateful to Dr. Barbara Mannucci (Centro Grandi Strumenti, University of Pavia) for GC-MS analyses A.B., A. P., S.P and D. M. acknowledge support from the Ministero dell’Università e della Ricerca (MUR) and the University of Pavia through the program “Dipartimenti di Eccellenza 2023–2027”. Then authors also acknowledge support from the Ministero dell’Università e della Ricerca (MUR) and the University of Pavia through the program “Dipartimenti di Eccellenza 2023–2027. Funded by European Union—NextGenerationEU. I punti di vista e le opinioni espresse sono tuttavia solo quelli degli autori e non riflettono necessariamente quelli dell’Unione europea o della Commissione europea. Né l’Unione europea né la Commissione europea possono essere ritenute responsabili per essi—NOrCa—Not Ordinary Cannabis—Exploring the chemical space around hemp (Cannabis sativa L.) waste and by products from a circular economy perspective PRIN: PROGETTI DI RICERCA DI RILEVANTE INTERESSE NAZIONALE—Bando 2022 PNRR Prot. P2022TXJX8.

Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bini, A., Salerno, S., Protti, S. et al. Photodegradation of cannabidiol (CBD) and Δ9-THC in cannabis plant material. Photochem Photobiol Sci (2024). https://doi.org/10.1007/s43630-024-00589-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43630-024-00589-4