Abstract
This Assessment Update by the Environmental Effects Assessment Panel (EEAP) of the United Nations Environment Programme (UNEP) considers the interactive effects of solar UV radiation, global warming, and other weathering factors on plastics. The Assessment illustrates the significance of solar UV radiation in decreasing the durability of plastic materials, degradation of plastic debris, formation of micro- and nanoplastic particles and accompanying leaching of potential toxic compounds. Micro- and nanoplastics have been found in all ecosystems, the atmosphere, and in humans. While the potential biological risks are not yet well-established, the widespread and increasing occurrence of plastic pollution is reason for continuing research and monitoring. Plastic debris persists after its intended life in soils, water bodies and the atmosphere as well as in living organisms. To counteract accumulation of plastics in the environment, the lifetime of novel plastics or plastic alternatives should better match the functional life of products, with eventual breakdown releasing harmless substances to the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solar ultraviolet radiation (UV; 290–400 nm) is a contributing factor in the environmental fate of toxic chemicals and other contaminants, with potential consequences—both beneficial and detrimental—for the health of humans and the environment. Chemical contaminants that are intentionally or accidentally released into the environment are highly diverse in their chemistry and responses to solar UV radiation. The current assessment focuses on the degradation of plastic debris in the environment, and particularly the effect of solar UV radiation and other environmental factors on the formation of micro- and nanoplastic particles.
The Montreal Protocol on Substances that Deplete the Ozone Layer (hereafter referred to as the “Montreal Protocol”) has been universally ratified by all 198 member states of the United Nations and this treaty has successfully prevented large, global-scale increases in solar UV-B radiation (290–315 nm) at the Earth’s surface [1]. Moreover, because many ozone-depleting substances (ODS) and their replacements are potent greenhouse gases, this treaty, together with its adjustments and Amendments, has significantly reduced global warming. Furthermore, changes in stratospheric ozone also affect climate and vice versa.
Previous assessments by the Environmental Effects Assessment Panel (EEAP) of the United Nations Environment Programme (UNEP) have extensively reported on a range of direct and indirect interactive effects between UV radiation and contaminants and highlighted the important consequences for the environment and human health. Some examples of beneficial and detrimental effects noted were:
-
1.
Solar UV radiation reacts with atmospheric gases including nitrogen oxides (NOx) and volatile organic compounds (VOC) to create photochemical smog, including increased levels of tropospheric ozone and secondary particulate matter, with adverse effects on human health and plants [2].
-
2.
UV-driven photochemical reactions in the atmosphere convert some ozone-depleting substances (ODSs) and substitutes into degradation products such as trifluoroacetic acid (TFA), which, although persistent in the environment, presents de minimis risk to humans and the ecosystem [2].
-
3.
In the troposphere, UV radiation drives the formation of hydroxyl radicals and other reactive oxygen species (ROS) that act as atmospheric cleaning agents [2]. Hydroxyl radicals also play an important role in surface waters in the transformation and breakdown of pollutants e.g. pesticides [3].
-
4.
Widespread use of topical sunscreens, for protection from UV radiation, has resulted in UV-filters entering aquatic ecosystems, with potential harms for aquatic organisms [4].
-
5.
UV radiation can increase the toxicity of oil pollutants to many types of aquatic organisms [4, 5], but also contributes to their degradation and removal from the environment [6].
-
6.
Wildfires have become more frequent and more extreme, at least partially because of climate change. They are becoming a significant source of pollutants, including aerosols, which affect human health and reduce surface UV radiation. Aerosols from wildfires may also cause depletion of stratospheric ozone [7].
Amongst the most ubiquitous of all pollutants is plastic, such that the current era has been referred to by some as the ‘plasticene’ [8]. We define ‘plastics’ as a broad range of synthetic and semi-synthetic organic polymers, which can be moulded into various shapes, as well as elastomers, for example, rubbers and composite materials. Various additives, such as stabilisers, dyes, antistatic agents, flame retardants, and plasticisers are included in the plastics formulation, resulting in substantial variations in the chemical composition, and hence functional and structural properties, of plastics.
World annual plastic production was estimated at 391 million metric tonnes in 2021 [9]. A substantial fraction of plastic waste generated from consumer products (especially packaging which consumes ~ 40% of the resins used in plastic production) ultimately ends up in the environment. Nonetheless, there are substantial uncertainties regarding the actual fate and longevity of plastic debris in the environment. Plastic waste that is exposed to solar UV radiation will photo-oxidise and gradually degrade into microplastic (< 5 mm) and nanoplastic (< 0.1 µm) particles. Such particles are now ubiquitous in the environment and have been discovered in freshwater and marine systems, soils and the atmosphere across diverse geographical regions. Once released into the environment, microplastics are ingested by organisms ranging from microorganisms to humans and have been found in human blood, placental tissue, heart muscle, and urine [10,11,12].
Photo-oxidation and weathering are key steps in UV-induced degradation of plastic debris and determine the useful lifespan of plastic products. The resulting plastic fragments have potential ecological impacts that are closely related to the size of the plastic particles. UV-induced degradation also results in leaching of chemicals from plastic fragments, with further potential ecological impacts. The extent of UV-induced degradation of plastics in the atmosphere and terrestrial and aquatic environments depends on both the amount and spectral composition of solar UV radiation. By preventing significant increases in surface UV radiation, the Montreal Protocol and its Amendments have likely decreased the rate of UV-B-driven photodegradation of plastics, contributed to an increase in the durability of materials and reduced the production and influx of micro- and nanoplastic particles in the environment. This assessment considers the interactive effects of UV radiation and climate change on plastic distribution, weathering, longevity, and ultimately the fate of plastic debris. Findings from this assessment address a number of the United Nations Sustainable Development Goals (SDGs, https://sdgs.un.org/goals) (Table 1).
Recently, at the request of the United Nations Environment Assembly, the Executive Director of UNEP convened an Intergovernmental Negotiating Committee (INC) to develop an international legally binding agreement to end plastic pollution. This initiative will address the following: (a) the global scale of plastics in the environment; (b) an improved understanding of the global impact of plastic pollution, sustainable production, and consumption of plastics; (c) a full life cycle approach; and (d) capacity-building through scientific and technical cooperation. This UNEP EEAP assessment seeks to contribute to this international effort to mitigate the impact of plastic pollution. The current state of knowledge about stratospheric ozone and consequent UV radiation at the Earth’s surface is summarised and findings are used to assess the effects of UV radiation and interacting climate change factors on plastic materials, focussing both on durability of products as well as production and dispersal of micro- and nano-plastic pollutants in the environment.
2 Ultraviolet radiation throughout the twenty-first century
An assessment of the effects of solar UV radiation on plastic pollution requires an understanding of how this part of the solar spectrum has changed over modern times. The EEAP conducts a detailed assessment of the environmental impacts of changes in stratospheric ozone and UV radiation every four years. The most recent Quadrennial Assessment [13] included a projection of the intensity of UV radiation at the Earth’s surface throughout the twenty-first century, which was based on an earlier EEAP assessment [14] and a study by another group [15]. The assessment assumed a realistic scenario of the emission of greenhouse gases (RCP 6.0, see Supplementary Information), time-invariant amounts of atmospheric aerosols, and that there is continued compliance with the Montreal Protocol. This Quadrennial Assessment concluded that erythemal (“sunburning”) UV radiation will decrease by 2–5% at northern and 4–6% at southern mid-latitudes (30°–60°) between 2015 and 2090. Changes for the tropics were projected to be smaller than 1%.
There have been no new projections of changes in UV radiation on a global scale since the most recent EEAP assessment [13]. However, recent studies have identified a number of factors that may affect stratospheric ozone and other factors that may affect ground-level UV radiation throughout the twenty-first century. These include increasing greenhouse gas concentrations; very short-lived substances (ozone-depleting halogen-containing chemicals with a lifetime of less than six months, which are largely produced by natural processes such as emissions from macroalgae (seaweed) and phytoplankton; effects of climate change on cloud cover, aerosols, atmospheric circulation, and surface reflectivity; air pollution and tropospheric aerosols; wildfires; supersonic aircraft; potential nuclear war; potential future climate intervention (geoengineering such as stratospheric aerosol injection); and volcanic eruptions. For details on these effects see the Supplementary Information. Many of these processes do not lead to long-term changes in UV radiation reaching the Earth’s surface in excess of a few percent and would be similar in magnitude to projected trends that have been published during the last ~ 10 years [13, 14, 16]. However, larger changes in UV radiation could be caused by a severe breach in the adherence to the Montreal Protocol, extreme climate events, and severe wildfires, which can lead to large, localised ozone depletion events lasting several months. Likewise, reductions in air pollution can lead to large (> 40%) localised increases in UV radiation. While “colossal” volcanic eruptions that occur on millennial time scales could substantially disturb the ozone layer and Earth’s climate for many years, they are not a result of human activities and are therefore typically not considered in projections of the future climate and atmospheric composition. Likewise, assessments of the effects of nuclear war are only based on scenarios, which will likely differ from the actual situation should such a war occur.
3 UV radiation and plastics: mechanisms of weathering
3.1 UV radiation-driven photo-oxidation and formation of microplastics
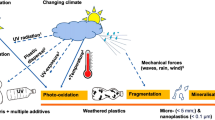
Pathways of UV-induced transformation of plastics have been identified [17]. Direct exposure of plastics to solar UV-B radiation induces free-radical photoreactions resulting in the photo-oxidation of the plastic (Fig. 1). Exposure of a photosensitiser (e.g. dissolved organic matter (DOM)) can also result in degradation of some plastics via the production of hydroxyl radicals and other reactive oxygen species [18]. The consequent deterioration of physical properties, surface erosion and discoloration, are referred to as weathering. Exposure to UV radiation renders common plastics such as polyethylene (PE) [19, 20] and polypropylene (PP) [21,22,23,24] weak and brittle. This makes them more susceptible to fragmentation under environmental mechanical stresses [19, 22, 23, 25, 26] which leads to the release of microplastics and nanoplastics into the environment (Fig. 1). Some fragmentation can also occur due to mechanical forces alone, for instance, during agricultural processes (Sect. 4.2) and in the marine environment [27,28,29,30]. To counter weathering and the deterioration of mechanical properties, the practice of adding UV-protective substances to plastics is widespread, prolonging useful lifetimes of plastic products used outdoors [31].
Conceptual diagram depicting the formation of micro- and nanoplastics under natural conditions. UV radiation and mechanical stress (not shown) drive the weathering and fragmentation of larger plastic waste into smaller fragments and other by-products (e.g. CO2, CH4, and leachates; not shown in the figure). The photodegradation rate depends on properties of the plastic polymer (such as its chemical composition, the presence of specific additives, and the object’s weathering history) and the exposure to UV radiation, including its intensity and spectral composition. Climate change impacts photodegradation, amongst others, by modifying the intensity of UV-B radiation and increasing the ambient temperature
Studies on the spectral dependence of the oxidation process indicate that solar UV-B wavelengths (290–315 nm) are far more effective in oxidising and embrittling common plastics such as PE or PP [32] as compared to UV-A (315–400 nm) or visible (400–700 nm) radiation [33, 34]. Numerous factors, primarily the chemical class of plastics [26], the types of additives incorporated [23, 35], molecular and morphological features such as chain branching, crosslinking, and fractional crystallinity [36], and the thickness of the material [37] affect the rate at which plastics degrade and/or fragment after exposure to solar UV radiation [22, 23] (Fig. 1). Common plastics oxidise more slowly in aqueous environments than in the air, likely primarily due to the lower oxygen availability in water [17]. This, together with limited UV-penetration into the water column, and surface biofouling (the growth of microbial films on surfaces of plastics), allows plastics to persist for extended, but poorly quantified, periods in ocean and freshwater environments.
While the pathways of photo-oxidation of plastics are well understood [17], those of consequent fragmentation are yet to be ascertained. Notwithstanding this knowledge gap, microscale fragments of plastic are ubiquitous across water bodies, air, and soil, as well as in organisms ranging from microorganisms to humans [38]. The contribution of UV radiation to the global load of environmental microplastics cannot be reliably quantified at present due to a lack of studies that have quantitatively assessed photo-oxidation and fragmentation in natural ecosystems [27, 39]. Nevertheless, without the Montreal Protocol the environmental load of microplastics would likely have been higher than it is now, due to long-term exposure of plastics to elevated UV-B radiation.
Photo-oxidation also causes changes in the surface properties of plastics by increasing surface roughness and hydrophilicity [40,41,42]. The hydrophilic functional groups on the surface of microplastic generated by photo-oxidation can interact with organic pollutants via hydrogen bonding, thus having further effects on the composition of the aquatic environment. Furthermore, UV-driven photo-oxidation and fragmentation of plastics can also increase the leaching rates of additives that are present in nearly all commercially used plastics [43, 44]. Additives are added to plastics, to improve functional properties, and are not chemically bound to, but are dissolved in the plastic matrix. Hence, they can easily leach out from the polymer matrix, especially after fragmentation into smaller particles [45]. Specifically, additives with small molecular size such as di(2-ethylhexyl)phthalate and brominated flame retardants diffuse freely [46] and become slowly desorbed into the surrounding medium. Plastics also contain a cocktail of largely unknown residual monomers, solvents, catalysts, impurities, and degradation products [47, 48], all of which can leach out into the environment.
3.2 Ultraviolet radiation and microfibres
Microfibres are the main category of microplastic particles found in both the water column and in organisms living in surface water or marine environments [49,50,51,52]. All fabrics and textiles based on synthetic polymer fibres release microfibres during their manufacture and use, especially during washing and drying cycles [53,54,55,56,57]. Upon exposure to UV radiation, increased numbers of microfibres and microplastics are generated from fabrics containing poly(ethyleneterephthalate) (PET, polyester) [58, 59], polyamide (PA [60]) and PP fibres [61]. For example, weathering of polyester fabrics exposed to high UV radiation for 60 days (60 W/m2) generated 20–40 times more microfibres during laundering compared to control fabrics [58]. UV irradiation also resulted in a four-fold increase in the number of microfibres released from protective facemasks [62]. Similarly, artificial lawns and synthetic turf sports fields, which cover large surfaces exposed to solar radiation worldwide, release large amounts of microplastic fibres which, via run-off, end up in local rivers and the nearshore sea area [63].
As with bulk plastics, long-term exposure of fibres and fabrics to UV radiation reduces their mechanical properties, and thus durability. This has been reported for a variety of fibres and fabrics, including polyvinyl chloride (PVC)-coated PET fabrics [64], poly(3-hydroxybutyrate) electrospun fibres loaded with zinc oxide nanoparticles [65], high-performance fibres used in firefighters' protective clothing [66,67,68], carbon fibre/epoxy composites [69], and poly(lactide acid), and/or poly(hydroxybutyrate) yarns and fabrics [70]. Research is ongoing to reduce the impacts of UV-induced photo-oxidation on fabrics and textiles, particularly by introducing surface modifications. For instance, bio-based finishing of polyester fabrics using chitosan helps to strengthen the bond between fibres and matrix, which reduces the shedding of microfibre fragments during use, and improves durability of the fabric [71]. The use of UV-absorbing stabilisers as coating materials in UV-sensitive fabrics can also improve durability [72, 73]. For instance, thermochromic materials which are often used in certain textiles and packaging, display low UV-stability but their durability significantly improves after incorporating UV-absorbers into these materials [73].
3.3 Outdoor weathering of plastic materials in a changing climate
Deterioration of plastic materials under outdoor conditions is a process driven by the interaction between solar UV radiation, and other weathering factors such as temperature. Given ongoing climate change, this is projected to change in the future (IPCC 2021). Consequently, it is expected that materials will degrade at a less predictable pace, the distribution of degradation products may also change, and harmful substances may leach into the environment at different rates.
Two examples of the importance of weathering by UV radiation and climate factors are from tyres and solar panels. The generation of small rubber particles from tyres is a process affected by exposure to UV radiation as well as heat, eroded tarmac, and impervious road surfaces [74]. It is estimated to annually contribute 6 million metric tonnes of microplastics worldwide [75]. Higher road surface temperatures due to climate change can also accelerate tyre degradation. Microplastics from tyre wear are prominent in urban environments, and in road run-off [74]. An emerging area of concern is that these microplastics can undergo further photothermal-oxidation and their subsequent degradation products may be toxic [76], although this issue remains largely unexplored [77,78,79].
As part of climate change mitigation strategies, installing of solar energy generating capacity is expected to triple between 2022 and 2027 according to the International Energy Agency [80]. Thus, a thorough understanding is required of the impact of UV radiation and climate change on weathering of solar panels, and this applies particularly to plastic components such as the backsheet or outer, protective layer of solar photovoltaic cells. Solar panels retrieved from commercial sites, after operating for up to 28 years in many different climatic zones, and with different materials, displayed variable degrees of weathering of backsheets [81]. Many of the PET backsheets showed microcracking and chalking, surface embrittlement and material erosion, which are generally driven by UV radiation, and which would potentially limit the functional life of the solar panel. Fluorinated plastics such as polyvinylidene difluoride, polytetrafluoroethylene-co-hexafluoropropylene-co-vinylidene fluoride, and polyvinyl fluoride display the least amount of degradation, but still show some surface erosion causing localised regions of embrittled plastics [81]. Careful consideration of the choice of future backsheet materials will be essential to avoid additional release of microplastics into the environment [82], especially given the rapid increase of solar energy harvesting devices and their location in sunny (UV-exposed) locations.
3.4 Ultraviolet radiation-driven mineralisation of plastics
Degradation of plastics occurs through two basic mechanisms: (1) photo-oxidation followed by fragmentation and release of dissolved organic matter (DOM); and (2) mineralisation. Mineralisation refers to the final step in the degradation process where the plastics are being decomposed, usually oxidatively, into inorganic end-products such as water and carbon dioxide. Fragmentation, DOM release and mineralisation processes occur concurrently in the dark, but rates are enhanced, albeit to a variable extent, in plastics exposed to solar UV radiation [83]. Solar UV-facilitated oxidation, and subsequent fragmentation, produces large numbers of nanoscale or very small microscale plastic fragments [20]. Given their high specific area, these microplastics undergo further degradation releasing dissolved organic matter [84, 85]. Some of the evidence for photomineralisation of microplastics is based on accelerated laboratory studies using microplastics suspended in aqueous media and exposed to high-intensity UV radiation. These studies of DOM release and mineralisation have only been conducted under a narrow range of laboratory conditions and there is much uncertainty about mineralisation rates in the natural environment. There is also uncertainty about how polymer additives [86, 87], natural DOM [88], and biotic factors, which are usually excluded in laboratory studies, further modify mineralisation rates. Direct evidence of mineralisation in the natural environment is lacking. However, leaching of DOM from solar UV-exposed plastics under outdoor conditions has been reported, indicating at least the partial conversion of a fraction of the plastics into water-soluble organic compounds [89]. Nevertheless, even under accelerated exposure the process is slow, and it might be speculated to be even slower in natural environments (see also Sect. 4.3).
4 Plastic pollutants in the environment
4.1 Nano- and microplastics in the atmosphere
Both micro- and nanoplastics have been found in the atmosphere (Fig. 2). Recent evidence indicates that microplastics are transported through the atmosphere to remote regions by wind [90], with different shapes (e.g. fibres vs fragments, [91]) being distributed to different degrees. These micro- and nanoplastics are emitted by a variety of different sources, but emission of microplastics from the ocean to the atmosphere has been the focus of recent research [92] (Fig. 2). Microplastics can be ejected from the ocean like sea spray aerosol [93, 94]. Stratospheric ozone depletion changes natural marine aerosol fluxes via the strength and position of the westerly jet in the Southern Ocean [95]. Marine microplastic fluxes may be similarly affected, but this has not yet been examined.
Effects of UV(-B) radiation on plastic litter in various environmental compartments. In the atmosphere, micro- and nanoplastics are exposed to high levels of UV-B radiation; only aerosols and clouds provide a partial UV screen. In aquatic environments, UV-B radiation penetrates only to a limited extent into the water column, leading to a gradient of UV-B varying from high exposure at the water surface to virtually zero exposure deeper in the water column and within sediments. How UV exposure varies with depth depends on various factors, including the mixed layer depth, water transparency, and the presence of surface ice. Biofouling—the growth of a biofilm on the surface of plastic debris—further limits the amount of UV-B radiation plastic fragments are exposed to both by screening radiation and by altering buoyancy (i.e. the position of plastic debris within the water column). In terrestrial environments, plastic can be exposed to high UV-B radiation at the soil surface, but UV is fully screened at depths greater than a few centimetres. Plant canopy cover can reduce further UV radiation at the soil surface. Differences in arrow length and width depict the different availability of UV radiation across the three environments; a decrease in arrow width indicates a decrease in UV-B availability
Experimental data are scarce, but global chemical transport model simulations indicate that microplastics with diameters of 0.5–70 μm can be present at levels of 0.001 μg plastic per m−3 in the atmosphere at remote locations, compared to 0.1 μg m−3 in polluted environments [96]. These levels should be placed in context of the total atmospheric load of particulate matter (PM) with a diameter smaller than 2.5 μm (PM2.5) (e.g. sea salt, mineral dust, organic aerosols, sulphate, soot), which ranges in order of magnitude from 1 μg m−3 in remote locations to 100 μg m−3 in polluted environments [97]. The mass of atmospheric aerosols with diameters less than 70 μm (PM70) is not routinely measured so no data are available. However, their mass should be substantially greater than that of PM2.5. This means that, overall, microplastics are likely to comprise less than 0.1% of the overall mass of atmospheric aerosols. There is no evidence that microplastic aerosols have effects that are disproportionately large compared to other atmospheric aerosols. Consequently, and given technical limitations in quantifying atmospheric plastics, the contribution of microplastics to atmospheric chemistry and physics is currently expected to be negligible.
Few atmospheric observations exist of microplastics smaller than about 10 μm because of technical limitations [98]. Smaller particles may be transported over longer distances and provide more surface area (per unit mass) for chemical and radiative processes. Recent modelling studies have examined whether microplastics in the atmosphere contribute to climate change. Assuming that microplastics are confined to the boundary layer (bottom 2 km of the atmosphere), direct microplastic-radiation interactions may have a weak cooling effect, but this is subject to large uncertainties in concentration and distribution of particles [98]. Microplastics can also act as ice-condensation nuclei (ICN) and/or cloud condensation nuclei (CCN) as they undergo ageing [99]. However, as stated above, their abundance is small compared with other sources of CCN and/or ICN. For example, cloud water collected near Mt. Fuji, Japan, contained only 120 plastic particles L−1, many orders of magnitude below the concentration needed to have an effect on cloud formation [100]. While their CCN and ICN efficiency has not yet been assessed, given their low abundance microplastics are not expected to make a substantial contribution to radiative forcing via indirect microplastic-cloud interactions. However, further quantitative monitoring of microplastics in the atmosphere is required to confirm this interpretation.
Microplastics suspended in the atmosphere are subjected to some of the highest levels of UV radiation in their life cycles, with contributions from direct and diffuse sky radiation, as well as reflections from the surface (Fig. 2). Photons of shorter wavelengths (e.g. UV-B) are more abundant in the atmosphere than in other environments (e.g. aquatic) where microplastics are found. Hence microplastics are likely to degrade faster in the atmosphere by UV-driven processes, although data on this process are currently lacking. UV-B radiation also affects the air surrounding suspended particles, generating highly reactive hydroxyl radicals (OH) that readily oxidise micro- and nanoplastics. Shorter wavelengths, which are most sensitive to changes in stratospheric ozone, have stronger effects on UV-driven weathering of plastic than longer wavelengths [40]. Although knowledge of the spectral dependence of the relevant photo-oxidation reactions is limited, changing UV radiation in the context of stratospheric ozone depletion and recovery, as well as accelerating climate change, will likely affect the lifetime of microplastics in the atmosphere.
4.2 Plastics in terrestrial environments: agroecosystems and the built environment
Plastics are found in terrestrial ecosystems because of their use in agriculture and horticulture, building and construction activities, their disposal in landfills, and as debris generated on land. Agriculture, horticulture, and forestry are major sources of microplastics in terrestrial environments [101]. The use of plastics in these sectors is increasing with global economic development, plastic affordability and in countering the abiotic stresses imposed on crops by climate change [102]. This section addresses the effects of UV radiation on plastics in terrestrial ecosystems with an emphasis on the generation of microplastics in agroecosystems and implications for food security and sustainable agriculture.
The United Nations Food and Agriculture Organisation [102] has estimated that agricultural usage of plastics (mainly PE and PP) is 12.5 million tonnes annually. Plastic materials are widely used as netting, irrigation pipes, seed coatings, greenhouses, growth tunnels, tree guards and shelters, and packaging [103]. Agricultural plastic mulch is considered a key source of small plastics in soils [104]. Mulching involves seasonal covering of soil with lightweight plastic sheets (often PE) and this practice is increasingly used to retain soil moisture and heat, and to prevent soil erosion and weed growth [105]. With increasing frequency of droughts under climate change [106], use of mulching is expected to escalate to control water loss from agricultural soils [107, 108]. The disposal of plastic mulch represents a major challenge and belies its promotion as a sustainable product. If tilled and buried, it becomes a source of microplastics and leached additives in soil ecosystems [109,110,111]. Plastic pollution is not limited to agricultural soils; substantial amounts of microplastics are present in all soils studied, including in industrial and urban environments [112].
It has been estimated that annually some 57,000–390,000 and 39,000–272,000 tonnes of microplastics are added to European and North American farmlands, respectively, making soils a larger reservoir for plastics than the oceans [113]. Use of plastic mulch is associated with soil contamination of between 0.1 and 324.5 kg ha−1 plastics (> 5 mm), and up to 1076 ± 347 microplastic particles per kg soil [104]. It is likely that breakdown of buried plastics is slow; consequently, soils accumulate plastic debris [112, 114]. Another key source of soil plastic pollution is sludge from wastewater treatment plants, which is applied to condition and enrich soils on agricultural land [115]. Sludge can incorporate a variety of chemically different plastic particles [116]. Other sources of plastic in soils include general plastic litter, tyre wear, and atmospheric deposition [117, 118].
Any plastic on the soil surface, such as plastic mulch, will be exposed to solar radiation, oxygen, and ambient temperatures and becomes brittle due to UV radiation-mediated weathering (Figs. 2, 3). This makes these plastics prone to subsequent fragmentation under minimal mechanical stress [23, 25]. Even plastic buried ~ 1 mm deep in soil and exposed to UV radiation in the laboratory can generate microplastics through weathering [119]. Photo-oxidation in combination with fragmentation, will increase the downward migration of plastic fragments in the soil, with particles found on soil surfaces and to depths of ca 100 cm. This poses a risk due to potential contamination of groundwater systems and drinking water [114] (Fig. 2). It is especially likely that smaller (< 1 µm) plastic particles will migrate downwards at a faster rate and contaminate aquifers [120]. However, there are still considerable knowledge gaps in our understanding of the vertical migration of plastics through soils. For example, incorporation of plastics into soil aggregates limits vertical transport, while water infiltration and activities of soil fauna can enhance downward movement. Conversely, the roots of some plants are associated with the upward movement of plastics. Thus, a picture emerges of variable transport kinetics, and this in combination with ploughing may affect exposure to UV radiation, photo-oxidation and further fragmentation of the plastics [121] (Figs. 2, 3).
Plastics in agricultural environments. Plastic mulch is photo-oxidised and fragmented due to exposure to UV radiation and mechanical stressors. This process releases variously sized plastic fragments (grey) and leachates (blue stars). These degradation products are dispersed downwards into the soil, where they are not subjected to further photo-oxidation and thus tend to accumulate. The fate of buried plastics is subject to considerable uncertainties. Potentially, microbial processes can degrade plastics, contributing to its removal. Plastic fragments may also interfere with plant nutrient uptake. Full arrows indicate processes that are well established; dashed arrows show processes whose occurrence is less certain
There is accumulating evidence concerning the broad spectrum of impacts of microplastics on plants, including crops, as well as other soil biota. Most studies are performed using unrealistically high concentrations of micro- and nanoplastics, and it remains to be ascertained which of the identified hazards poses a realistic risk. Nevertheless, there is now evidence that microplastics alter the structure, water-holding capacity, microbial activities, nutrient cycling, and bioavailability of other pollutants in contaminated soil, and exert phytotoxic effects in combination with other pollutants [117, 122]. Effects of microplastics on plant growth and development may relate to the above-mentioned changes in soil conditions (e.g. nutrient cycling and soil structure [122]), or can be due to direct effects of microplastics on plants [117, 123], including interactions with abiotic factors (e.g. UV radiation and drought stress [124]. Uptake of plastics by plants has been demonstrated for smaller micro- and nanoplastics [125]. This process has potential consequences for food chains and food quality including human nutrition [123]. However, it is possible that adherence to roots can also be exploited as part of a bioremediation strategy whereby plants are used to remove plastic particles from contaminated soils [126]. Furthermore, microplastics in the soil may, under some conditions, increase the release of greenhouse gases such as carbon dioxide, methane and nitrogen dioxide [127]. Overall, better-substantiated experimental data are required to fully assess effects of microplastics on plants, crops and soil ecosystems.
4.3 Nano- and microplastics in aquatic environments
Plastics are also ubiquitous in the aquatic environment. For example, a recent survey of 38 lakes and reservoirs across 23 countries found plastic debris > 250 µm in size in all water bodies and sometimes at concentrations substantially higher than in ocean gyresFootnote 1 where plastics are known to accumulate at high concentrations [128, 129]. Most of these plastics are transported by rivers and the atmosphere into the ocean environment [90].
As in terrestrial environments, exposure of plastics to solar UV radiation in the aquatic environment is an important factor leading to fragmentation into microplastics. As described in Sect. 3.4, the exposure of many types of plastics to UV radiation releases DOM and other products, including greenhouse gases (e.g. CO2, CH4) [84]. Release of DOM occurs by photochemical breakdown of microplastics, a process for which UV-B radiation plays a key role in regulating the initial rate [84]. One study estimated overall photodegradation rates of ~ 2% of carbon mass per year for floating plastics in the sub-tropical ocean with DOM release accounting for more than 50% of the carbon loss. However, this estimate cannot be generalised, since it only applies to one size of PE and PP fragments, and relies on rates extrapolated from laboratory exposures [130]. The chemical composition of the plastic, particle size, and previous environmental processing all affect the amount of DOM released, with greater DOM production reported from aged plastics [89]. Additionally, exposure to UV radiation accelerates the degradation rate of the remaining material by biological (microbial) processes, as shown in a study of cellulose diacetate, a common synthetic fabric polymer [131].
The DOM leached from photodegraded plastics comprises tens of thousands of compounds ranging from relatively bioavailable, low molecular weight organics to large, complex, and recalcitrant polymer fragments [132]. While the formation of these breakdown products is part of the process of the ultimate removal of plastics from aquatic ecosystems, DOM may also act as a photosensitiser that further catalyses plastic degradation.
Most of the dissolved organic carbon released during photodegradation is rapidly assimilated by bacteria [89, 133]. Organic carbon derived from plastics may be more accessible to organisms for uptake and metabolism than natural organic carbon and foster more bacterial growth [134]. However, there is concern that some leached substances may be potentially toxic to aquatic organisms. In a recent study of polyethylene bags exposed underwater to combined UV and visible radiation (in a solar simulator), no acute toxicity was reported in zebrafish from photoproducts, although RNA sequencing showed changes in gene expression associated with disrupted neuromuscular processes [135]. The study also showed that the net impacts of released photoproducts likely depend on the leachate composition, as differences were noted in leachate-induced gene expression amongst polyethylene bags depending on the presence of additives such as titanium oxide (TiO2). Additives reduce the photo-degradation of plastics, but this benefit should be balanced against the potential phototoxicity of the additive itself.
Studies on the spectral dependency of plastic degradation in the aquatic environment using natural or ecologically relevant laboratory exposures are still in the early phases. One contributing factor to this difference is that laboratory studies often neglect to account for biofouling that can reduce incident UV irradiation to the underlying plastic [136]. Biofouling can also increase apparent plastic density, thereby allowing fragments to sink deeper in the water column where they are exposed to less UV radiation [136, 137] (Fig. 2). Additional variables that impact plastic photodegradation, but are seldom investigated, are environmental history (i.e. virgin vs aged plastic; [89], and water type (i.e. seawater vs. freshwaters) [138].
Changes in water transparency and mixing depth modify the amount of solar radiation received by plastic debris in the surface mixed layer, affecting both the cumulative exposure of plastics to solar UV radiation as well as the spectrum of the radiation (Fig. 2). Climate change is warming aquatic ecosystems and changing wind patterns, both of which interact to determine mixing depth. This interaction deepens mixing in some areas (mainly marine) and leads to shallower mixing in other water bodies (mainly freshwater) [4]. Exposure of microplastics to UV radiation in the Arctic Ocean will increase in the future because of sea ice loss. This effect outweighs small decreases in UV-B radiation that are projected in response to the anticipated recovery of stratospheric ozone at high latitudes [13]. Both effects are expected with a warming climate [13]. The Arctic cryosphere (sea ice, glaciers, and permafrost) is assumed to function as a temporal sink for microplastics, similar to that for many other pollutants that are transported there either by precipitation from the air or by currents entering the Arctic Ocean from the Pacific or Atlantic Oceans [139, 140]. The decade-long model by Huserbråten and colleagues [141] examined transport of buoyant microplastics into the Arctic Ocean from the major rivers in Northern Europe and Russia. It shows that over time there will be an accumulation of particles in specific regions of the Arctic Ocean. However, the ability to forecast the effects of these microplastics in the Arctic is challenged by limited data [140]. There are no specific studies on photochemical transformations of microplastics in the Arctic. Nevertheless, given the key role of UV-B radiation in forming and degrading microplastics, rates of accumulation of microplastics in the Arctic will depend on many factors including long-term changes in stratospheric ozone, ice cover and other factors as discussed in Sect. 2 and the Supplementary Information.
5 Environmental risks associated with weathered and fragmented plastics
As outlined above, UV radiation-driven weathering affects the physical properties of plastics including strength, brittleness, surface charges and colour, as well as the attached biofilm (biofouling). In turn, this can affect the rates at which organisms ingest microplastics with potential biological impacts [142]. There is a need for comprehensive research into the risks associated with microplastics in the environment. Such research needs to include direct risks associated with exposure to larger plastic debris (e.g. entanglement) and micro- or nanoplastic particles, as well as indirect risks associated with exposure to pollutants adsorbed to plastic particles or leachates containing plastic breakdown products generated during photo-oxidation. The impacts of microplastic pollution on organisms are not central to this assessment, but it is noted that frameworks designed to discern relevant eco-toxicological effects are being developed [143]. Currently, knowledge about the toxicity of microplastics to organisms under environmentally relevant conditions is limited, and so is our understanding of the impact of UV radiation and climate change conditions thereupon [144]. Nevertheless, concerns are justified given the ubiquitous presence of microplastics in the environment and within organisms. In accordance with the precautionary principle, protective action should be taken to reduce the risks of exposure of organisms to plastic degradation products despite scientific uncertainty [145].
5.1 Leachates from weathered plastics
Plastics typically contain multiple types of additives that improve performance and ensure their durability. Some legacy additives are toxic [146, 147], being potential carcinogens or hormone-mimicking endocrine disruptors that cause adverse responses at very low concentrations [148]. The fraction of the additive chemicals in a plastic composition varies from less than 1% by weight (UV stabilisers or biocides in polyolefins) to over 50% by weight (plasticisers used in PVC).
Photo-oxidation and weathering affect the rates at which additives and products of plastic degradation leach into the environment. These effects of UV radiation on leaching are associated with fragmentation caused by weathering, which increases the specific surface area of the fragmented particle. For example, photo-oxidation of polycarbonate can result in the leaching of the endocrine disruptor bisphenol A [44], while photo-oxidation of PE can enhance the leaching of another endocrine disruptor, dibutylphthalate [43]. It is not yet known whether these endocrine disruptors leach to such an extent that they affect biota. Similarly, exposure of common plastics (e.g. polystyrene (PS), low-density PE, and high-density PE) to solar UV radiation resulted in increased leaching of the harmful plasticiser bis(2-ethylhexyl) phthalate [149]. Organisms may be affected by leachates through ingestion of plastics or through contact with leachate present in the environment [150]. In both cases, leachates containing additives can contribute to the potential toxicity of plastics [151]. However, while effects of UV radiation on the generation of leachates have been reported, there is still considerable uncertainty regarding their biological effects.
An important topic of emerging concern is the weathering of tyres (in part due to exposure to UV radiation, see Sect. 3.3), and the consequences thereof for leachate release. Among the hazardous chemicals leaching from weathered tyres are p-phenylenediamines, used as antioxidants. P-phenylenediamines have been found with high frequency bound to particulate matter in air (PM2.5) as well as in urban runoff, roadside soil [152], and urban surface water [79]. These aromatic amines transform to toxic quinone derivatives by reaction with ozone and environmental oxidants [153]. Weathering affects the composition of the leachates released from tyres. Some hazardous chemicals are not found in leachates when tyres are subjected to natural ageing, while the concentration of other leaching substances increases [78]. At present the chemical composition of leachates of weathered tyres and other plastic products is subject to considerable uncertainty [78]. As a consequence, potential environmental effects of leachates from weathered plastics remain largely unknown.
Chemical compounds are also leached from weathered fabrics [59, 60]. Composite fabrics have drawn attention as they tend to release complex chemical molecules into the environment. For instance, polyurethane coated PET-based fabrics release a large number of carbon- and nitrogen-containing substances during photodegradation [59]. Per- and polyfluoroalkyl substances (PFAS) are released from water-repellent PA textile fabrics and microfibres following weathering [60]. In turn, these PFAS are of concern because of their potential toxicity, persistence, and capacity to undergo long-range transport to remote regions [154].
5.2 Changes in the adsorption of pollutants to plastics due to photo-oxidation
Photo-oxidation will cause changes in the surface properties of microplastics by increasing their surface roughness and hydrophilicity (Sect. 3.1) [40,41,42]. These surface changes will affect the adsorption of organic pollutants [41, 155,156,157]. However, it is difficult to predict whether the adsorption of various pollutants onto microplastic will increase or decrease subsequent to photo-oxidation. For metals, such as lead, the situation is more predictable; their adsorption onto photo-oxidised microplastic compared to pristine ones will increase [158]. However, current data are not adequate to assess whether these adsorbed metals are bioavailable and/or cause a toxic effect.
Changes in surface properties of microplastics do not just concern the adsorption of pollutants, but also of natural organic substances. For example, UV-oxidation can enhance adsorption of humic acids in the freshwater environment [159]. As a result, photo-oxidised microplastics can become more dispersible in the water column, and exhibit altered settling rates and settling depth [160]. Hence, in addition to the adsorption of pollutants, photo-oxidation of microplastics may alter the distribution of plastics in the environment. However, overall understanding of the environmental consequences of UV-oxidation of plastics remains incomplete [161].
Photo-oxidation, which can initiate formation and growth of cracks in plastics may also enhance biofilm formation through surface roughening. In turn, algal biofilms increase cracks, pores, surface areas, and further oxidise plastics and microplastics. These biofilm-initiated degradation mechanisms have synergistic interactions with other pollutants [42, 162, 163]. For example, microplastics with biofilms adsorb or release pollutants [162], including antibiotics, with potential consequences for organisms.
6 Nano- and microplastics and human health
Humans are exposed to microplastics through ingestion, inhalation and skin contact. Exposure is primarily through ingestion of drinking water and food including crustaceans and other seafoods, sea salt, honey, beer, and other components of foods [164]. The second route of exposure is through the inhalation of air and dust containing microplastics. Exposure of the skin is considered the least likely exposure route due to the protective barrier of the outermost layer (stratum corneum) of the skin. Studies have shown that microplastics smaller than 150 µm can pass through the gastrointestinal epithelium in mammals [165], and thus the absorption of microplastics in humans is plausible. Smaller microplastics (≤ 10 µm) appear to infiltrate tissues and pass through cellular membranes [165]. Microplastics have been detected in human tissues collected in clinical settings, including placenta, lung, liver, colon, sputum, and bronchoalveolar lavage fluid. They have also been detected in blood, breast milk, saliva, urine, and faeces (including the first stool produced by a newborn) [165, 166].
Concerns about the potential risk of microplastics and their leachates to human health have focussed mainly on toxicity to the lungs, gastrointestinal tract, and liver; putative mechanisms include oxidative stress at the cellular level and inflammatory reactions at the tissue level [144, 167]. However, the majority of studies on the health impacts of plastics are limited by small sample sizes and may suffer from cross-contamination of samples during collection and processing. In addition, knowledge about the toxicity of microplastics under environmentally relevant conditions, with realistic exposure levels, is limited [144]. Potential mechanisms of toxicity, and the role of microplastics as potential carriers of chemical contaminants and pathogens remain to be determined.
One potential leachate from degrading plastic is PFAS (Sect. 5.1). While many studies have investigated potential health effects of PFAS exposure [168], there is ongoing research to clarify links with health outcomes. Human exposure to PFAS from plastics relative to other sources has not been fully quantified but is likely to be small.
The ecological, social and economic impacts of macro, micro- and nanoplastic pollution are inequitably experienced, often primarily affecting communities that are marginalised and vulnerable [169]. Inequitable impacts of plastic pollution are apparent at all stages of the plastic life cycle as follows [170]:
-
Higher abundance of plastic litter due to poor waste management practices threatens the health of economically disadvantaged communities and damages their natural environments;
-
Harm to people and ecosystems located near plastic production facilities from contamination of air, water and soil by plastic borne chemicals and microplastics;
-
Inequities arising from the availability of higher grades of plastic resins and relatively more effective waste management practices in wealthier countries;
-
Communities living near waste dumps that carry plastic trash and/or waterways polluted with floating plastic debris are especially vulnerable to negative health effects. Waste-pickers, in some countries overwhelmingly women of childbearing age, are especially subjected to potential risks of toxicity during pregnancy. Exposure of plastics to UV radiation in these locations further raises risks of exposure of the community to nano- and microplastics and leachates.
7 Knowledge gaps
Major questions remain concerning the fate and longevity of plastic waste in the environment, and the potential harmful effects of these plastics and their chemical additives on humans and other organisms. Further knowledge is needed on the risks to health and the environment from plastic pollution, and how such risk is impacted by the combined effects of UV radiation and climate change. Despite this knowledge gap, the precautionary principle in environmental science supports preventive action, even where all effects have not been fully quantified.
Weathering experiments in the laboratory have helped to identify the mechanism and rate of breakdown of plastics into micro- and nanoplastics (Sects. 3.1 and 3.4). However, there are still substantial knowledge gaps concerning the spectral sensitivity of UV photo-oxidation and the likelihood of complete plastic mineralisation. Furthermore, oxidation and degradation rates have not been quantified in the natural environment (e.g. in ocean, lakes, soils, and the atmosphere) where mechanical forces (e.g. wave action, wind), biofouling and breakdown by microorganisms modify the effects of solar UV radiation and rates of fragmentation. Thus, the overall fate, and longevity of environmental plastics remain largely unknown. Furthermore, substantial uncertainties persist concerning the quantitative contributions of mechanical fragmentation and biologically mediated fragmentation of plastics relative to that facilitated by photodegradation. Until the various factors that lead to the degradation of plastics are better understood, it is not possible to determine to what degree changes in solar UV radiation, which have been projected to be < 10% over the twenty-first century (Sect. 2), in tandem with climate change, will affect the fragmentation of plastic debris. This is a major knowledge gap that needs to be addressed before the effect of the Montreal Protocol and potential future amendments on the fate of plastics can be more robustly assessed.
8 Towards a more sustainable future
The Montreal Protocol has protected the biosphere from excessive UV-B radiation that would have made the planet inhospitable to many lifeforms. Relentless monitoring and the phase-out of 99% of ozone-depleting substances in most countries, has supported the effort of humankind to preserve the planet for generations to come, evidenced by indications of the recovery of the stratospheric ozone layer.
Stratospheric ozone depletion is considered a challenge that has been brought under control [171]. Climate change and growing pollution, on the other hand, remain critical issues threatening the viability of our planet, exemplified in this assessment by plastic waste in the environment and the role of UV radiation and climate change in its breakdown.
By regulating the production and consumption of ozone-depleting substances the Montreal Protocol has reduced significant detrimental effects of UV radiation on many materials, including plastics. Weathering of materials is, however, a result of combined effects of all weathering agents, including temperature, precipitation, pollutants, freeze–thaw cycles, mechanical stressors, in addition to solar UV radiation. Climate change is raising the global average temperature to which outdoor materials are exposed, and extreme weather events are becoming more frequent, negatively impacting durability of these materials. This highlights the need for innovative new technologies and materials to successfully adapt to climate change. For plastics this would include developing durable materials with a lifetime tailored to their application. Moreover, as climate change is changing the behaviour of people, novel smart materials with high UV protection, UV detection, and/or thermochromic capacity are required to meet the demands of consumers.
Plastics have been intentionally designed for in-use functional performance, but rarely has end-of-life fate of these materials been a dominant consideration in their design. There is a wide variety of grades of plastic resins, additives, and manufacturing that affect performance, weathering, and the lifetime of these materials and their impacts on the environment. Plastic debris persists after its intended life in the air, soil, water, and in living organisms, and there is a need to design novel plastics or plastic alternatives, where weathering and lifetime match the functional life of products, and which can be broken down into harmless substances, thus reducing accumulation of plastic debris in the environment. The design of plastics with tuneable durability can be customised for determining the end-of-life of the product, reducing both microplastic formation and post-use plastic accumulation [172]. For example, PVC, the third most produced plastic, requires long durability when used in construction where it needs to last decades, while in some single-use medical materials a short in-use design life is adequate. Additionally, plastics can be designed for ease of recyclability (chemical, biological, mechanical), depending on their in-use needs, for instance, by designing products that use only a single plastic component. The concept of biodegradable plastics (biological recycling) involves developing plastics that are subject to complete mineralisation to CO2, either in nature or in a commercial facility, within a certain timeframe. This timeframe is dependent on many environmental factors including temperature, UV radiation, and microbe types and needs to include effects of the changing environment. Therefore, many biodegradable plastics may not fully degrade in the environment and will instead end up as small plastics or microplastics for long periods of time [172]. Evolving regulations should consider the desired function intended for the plastic materials as well as end-of-life management, including the recovery and processing in waste treatment systems.
Use of biobased, green, eco-friendly, sustainable materials is of growing interest to industry [173, 174]. Alternative, environmentally sustainable materials can replace plastics in various applications (e.g. transparent wood composite with thermal insulating capability and high UV-blocking properties to replace plastics or glass in energy-efficient buildings and photovoltaic devices [175, 176]). Similarly, novel biodegradable composites based on cellulose and lignin can replace some of the conventional polymer-based products (e.g. tree-sapling shelters) to reduce their contribution to plastic pollution [177, 178]. Such development of eco-friendly alternatives for plastic materials intended for outdoor use is being recognised as a viable strategy in pursuing a sustainable future.
Stabilising additives affording UV-protection for use in coatings and fabrics [179, 180] can also be made of bio-based materials e.g. lignin [181], chitosan [182, 183], plant extracts [182, 184], and low-carbon production processes [185, 186]. Similarly, more sustainable dyes and processes are being developed. For instance, a green tea product was used to dye wool fabrics through laccase-assisted polymerisation [187], while sodium lignosulfonate, an industrial bio-waste, was also successfully used as a dye and UV-protective finish of nylon fabric [188].
Finally, some progress has been made in the development of alternatives to phthalates (a class of chemicals that make plastics more durable) [174] and polybrominated fire retardants [189] used in relatively high-weight fractions in plastics. Overall, to mitigate future risks, every effort should be made to limit the release of hazardous additives from materials degrading with solar UV radiation.
This assessment has detailed some of the complex consequences of the photo-oxidation and weathering of a wide range of traditional plastics, resulting not only in a shortening of the useful lifespan of materials, but also in the release of micro- and nanoparticles, as well as hazardous leachates. Thus, it is essential that the entire cradle-to-grave life cycle of new additives and materials is subject to critical analysis to avoid potential environmental impacts.
9 Conclusions
There is a rapidly increasing awareness of the threat associated with the ubiquitous presence of plastic debris, as well as micro-, and nanoplastic particles in the environment, although the environmental and health effects of plastic pollutants are yet to be fully understood. The precautionary principle encourages preventive action even in the absence of extensive technical data, as expressed in the aim of the UNEP Intergovernmental Negotiating Committee, which aims to develop a comprehensive, legally binding international agreement to end plastic pollution.
This current assessment update has emphasised the importance of UV radiation in plastic degradation through photo-oxidation, and the acceleration of fragmentation into smaller fragments, including micro- and nanoplastics. Overall, UV radiation has negative effects on the fate of plastics; (1) by shortening the lifetime of plastic products and (2) by enhancing the degradation of plastic debris into micro- and nanoplastics. Projected decreases in UV-B radiation as a result of the Montreal Protocol are likely to extend the functional-life of plastic products but also decrease the UV-initiated degradation of plastics and therefore the formation of micro- and nanoparticles. Conversely, plastic debris is likely to have become more resistant to degradation in the environment. As part of a move towards a healthy sustainable planet, UV radiation and climate-mediated impacts on durability, weathering and fragmentation of plastics are key considerations in the design of new, innovative plastics to replace those currently in use. Weathering and lifetime of these novel plastics, or plastic alternatives, will need to match the functional life of materials, while debris will need to be broken down into harmless substances, thus reducing accumulation of plastic in the environment.
Data availability
All data generated or analysed are either included in this published article or part of the analyses of papers cited.
Notes
Gyres are large scale circulation systems in the ocean forming circular patterns of currents that trap floating materials in their central regions. The main ocean gyres are in the North and South Atlantic, the North and South Pacific and in the Indian Ocean.
References
McKenzie, R., Bernhard, G., Liley, B., Disterhoft, P., Rhodes, S., Bais, A., Morgenstern, O., Newman, P., Oman, L., Brogniez, C., & Simic, S. (2019). Success of Montreal Protocol demonstrated by comparing high-quality UV measurements with “world avoided” calculations from two chemistry-climate models. Scientific Reports. https://doi.org/10.1038/s41598-019-48625-z
Madronich, S., Sulzberger, B., Longstreth, J. D., Schikowski, T., Andersen, M. P. S., Solomon, K. R., & Wilson, S. R. (2023). Changes in tropospheric air quality related to the protection of stratospheric ozone in a changing climate. Photochemical & Photobiological Sciences, 22(5), 1129–1176. https://doi.org/10.1007/s43630-023-00369-6
Barnes, P. W., Robson, T. M., Zepp, R. G., Bornman, J. F., Jansen, M. A. K., Ossola, R., Wang, Q. W., Robinson, S. A., Foereid, B., Klekociuk, A. R., Martinez-Abaigar, J., Hou, W. C., Mackenzie, R., & Paul, N. D. (2023). Interactive effects of changes in UV radiation and climate on terrestrial ecosystems, biogeochemical cycles, and feedbacks to the climate system. Photochemical & Photobiological Sciences, 22(5), 1049–1091. https://doi.org/10.1007/s43630-023-00376-7
Neale, P. J., Williamson, C. E., Banaszak, A. T., Häder, D. P., Hylander, S., Ossola, R., Rose, K. C., Wängberg, S. Å., & Zepp, R. (2023). The response of aquatic ecosystems to the interactive effects of stratospheric ozone depletion, UV radiation, and climate change. Photochemical & Photobiological Sciences, 22(5), 1093–1127. https://doi.org/10.1007/s43630-023-00370-z
Nordborg, F. M., Brinkman, D. L., Ricardo, G. F., Agustí, S., & Negri, A. P. (2021). Comparative sensitivity of the early life stages of a coral to heavy fuel oil and UV radiation. The Science of the Total Environment, 781, 146676. https://doi.org/10.1016/j.scitotenv.2021.146676
Freeman, D. H., & Ward, C. P. (2022). Sunlight-driven dissolution is a major fate of oil at sea. Science Advances, 8(7), eabl7605. https://doi.org/10.1126/sciadv.abl7605
Solomon, S., Stone, K., Yu, P., Murphy, D. M., Kinnison, D., Ravishankara, A. R., & Wang, P. (2023). Chlorine activation and enhanced ozone depletion induced by wildfire aerosol. Nature, 615(7951), 259–264. https://doi.org/10.1038/s41586-022-05683-0
Reed, C. (2015). Dawn of the plasticene age. New Scientist (1971), 225(3006), 28–32. https://doi.org/10.1016/S0262-4079(15)60215-9
Plastics Europe. (2022). Plastics—The facts. Plastics Europe AISBL.
Amereh, F., Amjadi, N., Mohseni-Bandpei, A., Isazadeh, S., Mehrabi, Y., Eslami, A., Naeiji, Z., & Rafiee, M. (2022). Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environmental Pollution, 1987(314), 120174–120174. https://doi.org/10.1016/j.envpol.2022.120174
Blackburn, K., & Green, D. (2022). The potential effects of microplastics on human health: what is known and what is unknown. Ambio, 51(3), 518–530. https://doi.org/10.1007/s13280-021-01589-9
Liu, S., Guo, J., Liu, X., Yang, R., Wang, H., Sun, Y., Chen, B., & Dong, R. (2023). Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. The Science of the Total Environment, 854, 158699–158699. https://doi.org/10.1016/j.scitotenv.2022.158699
Bernhard, G. H., Bais, A. F., Aucamp, P. J., Klekociuk, A. R., Liley, J. B., & McKenzie, R. L. (2023). Stratospheric ozone, UV radiation, and climate interactions. Photochemical & Photobiological Sciences, 22(5), 937–989. https://doi.org/10.1007/s43630-023-00371-y
Bais, A. F., Bernhard, G., McKenzie, R. L., Aucamp, P. J., Young, P. J., Ilyas, M., Jockel, P., & Deushi, M. (2019). Ozone-climate interactions and effects on solar ultraviolet radiation. Photochemical & Photobiological Sciences, 18(3), 602–640. https://doi.org/10.1039/c8pp90059k
Lamy, K., Portafaix, T., Josse, B., Brogniez, C., Godin-Beekmann, S., Bencherif, H., Revell, L., Akiyoshi, H., Bekki, S., Hegglin, M. I., Jöckel, P., Kirner, O., Liley, B., Marecal, V., Morgenstern, O., Stenke, A., Zeng, G., Abraham, N. L., Archibald, A. T., … Yoshida, K. (2019). Clear-sky ultraviolet radiation modelling using output from the Chemistry Climate Model Initiative. Atmospheric Chemistry and Physics, 19(15), 10087–10110. https://doi.org/10.5194/acp-19-10087-2019
Bais, A. F., McKenzie, R. L., Bernhard, G., Aucamp, P. J., Ilyas, M., Madronich, S., & Tourpali, K. (2015). Ozone depletion and climate change: impacts on UV radiation. Photochemical & Photobiological Sciences, 14(1), 19–52. https://doi.org/10.1039/c4pp90032d
Andrady, A. L. (2022). Weathering and fragmentation of plastic debris in the ocean environment. Marine Pollution Bulletin, 180, 113761. https://doi.org/10.1016/j.marpolbul.2022.113761
Cao, R., Liu, X., Duan, J., Gao, B., He, X., Nanthi, B., & Li, Y. (2022). Opposite impact of DOM on ROS generation and photoaging of aromatic and aliphatic nano- and micro-plastic particles. Environmental Pollution, 1987(315), 120304–120304. https://doi.org/10.1016/j.envpol.2022.120304
Doğan, M. (2021). Ultraviolet light accelerates the degradation of polyethylene plastics. Microscopy Research and Technique, 84(11), 2774–2783. https://doi.org/10.1002/jemt.23838
Menzel, T., Meides, N., Mauel, A., Mansfeld, U., Kretschmer, W., Kuhn, M., Herzig, E. M., Altstädt, V., Strohriegl, P., Senker, J., & Ruckdäschel, H. (2022). Degradation of low-density polyethylene to nanoplastic particles by accelerated weathering. Science of The Total Environment, 826, 154035. https://doi.org/10.1016/j.scitotenv.2022.154035
Brostow, W., Lu, X., Gencel, O., & Osmanson, A. T. (2020). Effects of UV stabilizers on polypropylene outdoors. Materials (Basel), 13(7), 1626. https://doi.org/10.3390/ma13071626
Meides, N., Mauel, A., Menzel, T., Altstädt, V., Ruckdäschel, H., Senker, J., & Strohriegl, P. (2022). Quantifying the fragmentation of polypropylene upon exposure to accelerated weathering. Microplastics and Nanoplastics. https://doi.org/10.1186/s43591-022-00042-2
Song, Y. K., Hong, S. H., Eo, S., & Shim, W. J. (2023). Fragmentation of nano- and microplastics from virgin- and additive-containing polypropylene by accelerated photooxidation. Environmental Pollution. https://doi.org/10.1016/j.envpol.2023.121590
Wu, X., Zhao, X., Chen, R., Liu, P., Liang, W., Wang, J., Shi, D., Teng, M., Wang, X., & Gao, S. (2023). Size-dependent long-term weathering converting floating polypropylene macro- and microplastics into nanoplastics in coastal seawater environments. Water Research. https://doi.org/10.1016/j.watres.2023.120165
Bai, X., Li, F., Ma, L., & Li, C. (2022). Weathering of geotextiles under ultraviolet exposure: a neglected source of microfibers from coastal reclamation. Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2021.150168
Stapleton, M. J., Ansari, A. J., Ahmed, A., & Hai, F. I. (2023). Change in the chemical, mechanical and physical properties of plastics due to UVA degradation in different water matrices: A study on the recyclability of littered plastics. Environmental Pollution. https://doi.org/10.1016/j.envpol.2023.122226
Born, M. P., Brüll, C., & Schüttrumpf, H. (2023). Implications of a new test facility for fragmentation investigations on virgin (micro)plastics. Environmental Science & Technology, 57(28), 10393–10403. https://doi.org/10.1021/acs.est.3c02189
Chubarenko, I., Efimova, I., Bagaeva, M., Bagaev, A., & Isachenko, I. (2020). On mechanical fragmentation of single-use plastics in the sea swash zone with different types of bottom sediments: Insights from laboratory experiments. Marine Pollution Bulletin, 150, 110726. https://doi.org/10.1016/j.marpolbul.2019.110726
Min, K., Cuiffi, J. D., & Mathers, R. T. (2020). Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nature Communications, 11(1), 727–711. https://doi.org/10.1038/s41467-020-14538-z
Alimi, O. S., Claveau-Mallet, D., Kurusu, R. S., Lapointe, M., Bayen, S., & Tufenkji, N. (2022). Weathering pathways and protocols for environmentally relevant microplastics and nanoplastics: what are we missing? Journal of Hazardous Materials, 423(Pt A), 126955. https://doi.org/10.1016/j.jhazmat.2021.126955
Yousif, E., & Haddad, R. (2013). Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus, 2(1), 398–398. https://doi.org/10.1186/2193-1801-2-398
Andrady, A. L. (1997). Polymer analysis polymer physics (1st ed.). Springer Berlin Heidelberg.
Andrady, A. L., Pegram, J. E., & Searle, N. D. (1996). Wavelength sensitivity of enhanced photodegradable polyethylenes, eco, and ldpe/mx. Journal of Applied Polymer Science, 62(9), 1457–1463. https://doi.org/10.1002/(SICI)1097-4628(19961128)62:9%3c1457::AID-APP15%3e3.0.CO;2-W
Zhenfeng, Z., Xingzhou, H., & Zubo, L. (1996). Wavelength sensitivity of photooxidation of polypropylene. Polymer Degradation and Stability, 51(1), 93–97. https://doi.org/10.1016/0141-3910(95)00210-3
Jeyaraj, J., Baskaralingam, V., Stalin, T., & Muthuvel, I. (2023). Mechanistic vision on polypropylene microplastics degradation by solar radiation using TiO2 nanoparticle as photocatalyst. Environmental Research. https://doi.org/10.1016/j.envres.2023.116366
Julienne, F., Lagarde, F., & Delorme, N. (2019). Influence of the crystalline structure on the fragmentation of weathered polyolefines. Polymer Degradation and Stability. https://doi.org/10.1016/j.polymdegradstab.2019.109012
Chamas, A., Moon, H., Zheng, J., Qiu, Y., Tabassum, T., Jang, J. H., Abu-Omar, M., Scott, S. L., & Suh, S. (2020). Degradation rates of plastics in the environment. ACS Sustainable Chemistry & Engineering, 8(9), 3494–3511. https://doi.org/10.1021/acssuschemeng.9b06635
Jansen, M. A. K., Barnes, P. W., Bornman, J. F., Rose, K. C., Madronich, S., White, C. C., Zepp, R. G., & Andrady, A. L. (2023). The Montreal Protocol and the fate of environmental plastic debris. Photochemical & Photobiological Sciences, 22(5), 1203–1211. https://doi.org/10.1007/s43630-023-00372-x
Khan, D., & Ali, S. A. (2023). On the novel process of pristine microplastic bio-fragmentation by zebrafish (Danio rerio). Archives of Environmental Contamination and Toxicology, 84(3), 299–306. https://doi.org/10.1007/s00244-023-00987-2
Zepp, R. G., Acrey, B., Davis, M. J. B., Andrady, A. L., Locklin, J., Arnold, R., Okungbowa, O., & Commodore, A. (2023). Weathering effects on degradation of low-density polyethylene-nanosilica composite with added pro-oxidant. Journal of polymers and the Environment, 31(10), 4184–4192. https://doi.org/10.1007/s10924-023-02864-4
Alimi, O. S., Claveau-Mallet, D., Lapointe, M., Biu, T., Liu, L., Hernandez, L. M., Bayen, S., & Tufenkji, N. (2023). Effects of weathering on the properties and fate of secondary microplastics from a polystyrene single-use cup. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2023.131855
Binda, G., Costa, M., Supraha, L., Spanu, D., Vogelsang, C., Leu, E., & Nizzetto, L. (2023). Untangling the role of biotic and abiotic ageing of various environmental plastics toward the sorption of metals. Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2023.164807
Berenstein, G., Hughes, E. A., Zalts, A., Basack, S., Bonesi, S. M., & Montserrat, J. M. (2022). Environmental fate of dibutylphthalate in agricultural plastics: Photodegradation, migration and ecotoxicological impact on soil. Chemosphere, 290, 133221. https://doi.org/10.1016/j.chemosphere.2021.133221
Shi, Y., Liu, P., Wu, X., Shi, H., Huang, H., Wang, H., & Gao, S. (2021). Insight into chain scission and release profiles from photodegradation of polycarbonate microplastics. Water Research, 195, 116980. https://doi.org/10.1016/j.watres.2021.116980
Schrank, I., Trotter, B., Dummert, J., Scholz-Böttcher, B. M., Löder, M. G. J., & Laforsch, C. (2019). Effects of microplastic particles and leaching additive on the life history and morphology of daphnia magna. Environmental Pollution, 255(Pt 2), 113233. https://doi.org/10.1016/j.envpol.2019.113233
Kwon, J. H., Chang, S., Hong, S. H., & Shim, W. J. (2017). Microplastics as a vector of hydrophobic contaminants: Importance of hydrophobic additives. Integrated Environmental Assessment and Management, 13(3), 494–499. https://doi.org/10.1002/ieam.1906
Bridson, J. H., Gaugler, E. C., Smith, D. A., Northcott, G. L., & Gaw, S. (2021). Leaching and extraction of additives from plastic pollution to inform environmental risk: A multidisciplinary review of analytical approaches. Journal of Hazardous Materials, 414, 125571. https://doi.org/10.1016/j.jhazmat.2021.125571
Nerin, C., Alfaro, P., Aznar, M., & Domeño, C. (2013). The challenge of identifying non-intentionally added substances from food packaging materials: A review. Analytica Chimica Acta, 775, 14–24. https://doi.org/10.1016/j.aca.2013.02.028
Nash, R., O’Sullivan, J., Murphy, S., Bruen, M., Mahon, A. M., Lally, H., Heerey, L., O’Connor, J., Wang, X., A, K., & I, O. C. (2023). Sources, pathways and environmental fate of microplastics. Epa research (2023). Report n° 430 (pp. 1–70). ISBN: 978-1-80009-092-7.
Rojas, R. R., Arango-Mora, C., Nolorbe-Payahua, C., Medina, M., Vasquez, M., Flores, J., Murayari, F., Vásquez, C., de Almeida, V., Ramos, W., Rios Isern, E., Marapara Del Aguila, J., Castro, J. C., Del Águila, J., Diaz Jarama, F., & Vasconcelos-Souza, M. (2023). Microplastic occurrence in fish species from the Iquitos region in Peru, Western Amazonia. Acta Amazonica, 53(1), 65–72. https://doi.org/10.1590/1809-4392202201212
Soltani, N., Amini-Birami, F., Keshavarzi, B., Moore, F., Busquets, R., Sorooshian, A., Javid, R., & Shahraki, A. R. (2023). Microplastic occurrence in selected aquatic species of the Persian Gulf: No evidence of trophic transfer or effect of diet. Science of The Total Environment, 892, 164685–164685. https://doi.org/10.1016/j.scitotenv.2023.164685
Murano, C., Vaccari, L., Casotti, R., Corsi, I., & Palumbo, A. (2022). Occurrence of microfibres in wild specimens of adult sea urchin Paracentrotus lividus (Lamarck, 1816) from a coastal area of the central Mediterranean Sea. Marine Pollution Bulletin, 176, 113448–113448. https://doi.org/10.1016/j.marpolbul.2022.113448
Choi, S., Kim, J., & Kwon, M. (2022). The effect of the physical and chemical properties of synthetic fabrics on the release of microplastics during washing and drying. Polymers, 14(16), 3384. https://doi.org/10.3390/polym14163384
Cui, H., & Xu, C. Q. (2022). Study on the relationship between textile microplastics shedding and fabric structure. Polymers, 14(23), 5309. https://doi.org/10.3390/polym14235309
Le, L. T., Nguyen, K. Q. N., Nguyen, P. T., Duong, H. C., Bui, X. T., Hoang, N. B., & Nghiem, L. D. (2022). Microfibers in laundry wastewater: problem and solution. Science of the Total Environment, 852, 158412. https://doi.org/10.1016/j.scitotenv.2022.158412
Mahbub, M. S., & Shams, M. (2022). Acrylic fabrics as a source of microplastics from portable washer and dryer: Impact of washing and drying parameters. Science of the Total Environment, 834, 155429. https://doi.org/10.1016/j.scitotenv.2022.155429
Saravanja, A., Pusic, T., & Dekanic, T. (2022). Microplastics in wastewater by washing polyester fabrics. Materials, 15(7), 2683. https://doi.org/10.3390/ma15072683
Pinlova, B., & Nowack, B. (2023). Characterization of fiber fragments released from polyester textiles during UV weathering. Environmental Pollution, 322, 121012. https://doi.org/10.1016/j.envpol.2023.121012
Shi, Y., Zheng, L., Huang, H., Tian, Y.-C., Gong, Z., Liu, P., Wu, X., Li, W.-T., & Gao, S. (2023). Formation of nano- and microplastics and dissolved chemicals during photodegradation of polyester base fabrics with polyurethane coating. Environmental Science & Technology, 57(5), 1894–1906. https://doi.org/10.1021/acs.est.2c05063
Schellenberger, S., Liagkouridis, I., Awad, R., Khan, S., Plassmann, M., Peters, G., Benskin, J. P., & Cousins, I. T. (2022). An outdoor aging study to investigate the release of per- and polyfluoroalkyl substances (PFAS) from functional textiles. Environmental Science & Technology, 56(6), 3471–3479. https://doi.org/10.1021/acs.est.1c06812
Ishmukhametov, I., Batasheva, S., & Fakhrullin, R. (2022). Identification of micro- and nanoplastics released from medical masks using hyperspectral imaging and deep learning. The Analyst, 147(20), 4616–4628. https://doi.org/10.1039/d2an01139e
Pikuda, O., Lapointe, M., Alimi, O. S., Berk, D., & Tufenkji, N. (2022). Fate of microfibres from single-use face masks: release to the environment and removal during wastewater treatment. Journal of Hazardous Materials, 438, 129408. https://doi.org/10.1016/j.jhazmat.2022.129408
de Haan, W. P., Quintana, R., Vilas, C., Cózar, A., Canals, M., Uviedo, O., & Sanchez-Vidal, A. (2023). The dark side of artificial greening: plastic turfs as widespread pollutants of aquatic environments. Environmental Pollution, 334, 122094. https://doi.org/10.1016/j.envpol.2023.122094
Asadi, H., Uhlemann, J., Stranghoener, N., & Ulbricht, M. (2022). Tensile strength deterioration of PVC coated PET woven fabrics under single and multiplied artificial weathering impacts and cyclic loading. Construction and Building Materials, 342, 127843. https://doi.org/10.1016/j.conbuildmat.2022.127843
Rodríguez-Tobías, H., Morales, G., Maldonado-Textle, H., & Grande, D. (2022). Long-term photo-degradation of nanofibrous composites based on poly(3-hydroxybutyrate) electrospun fibers loaded with zinc oxide nanoparticles. Fibers and Polymers, 23(10), 2717–2724. https://doi.org/10.1007/s12221-022-4099-y
Hoque, M. S., & Dolez, P. I. (2023). Aging of high-performance fibers used in firefighters’ protective clothing: State of the knowledge and path forward. Journal of Applied Polymer Science. https://doi.org/10.1002/app.54255
Houshyar, S., Padhye, R., Ranjan, S., Tew, S., & Nayak, R. (2017). The impact of ultraviolet light exposure on the performance of polybenzidimazole and polyaramid fabrics: Prediction of end-of-life performance. Journal of Industrial Textiles, 48(1), 77–86. https://doi.org/10.1177/1528083717725112
Mazari, A., Mazari, F. B., Naeem, J., Havelka, A., & Marahatta, P. (2022). Impact of ultraviolet radiation on thermal protective performance and comfort properties of firefighter protective clothing. Industria Textila, 73(1), 54–61. https://doi.org/10.35530/it.073.01.202116
Shi, Z., Zou, C., Zhou, F., & Zhao, J. (2022). Analysis of the mechanical properties and damage mechanism of carbon fiber/epoxy composites under UV aging. Materials, 15(8), 2919. https://doi.org/10.3390/ma15082919
Bao, Q., Wong, W., Liu, S., & Tao, X. (2022). Accelerated degradation of poly(lactide acid)/poly(hydroxybutyrate) (PLA/PHB) yarns/fabrics by UV and o2 exposure in south china seawater. Polymers, 14(6), 1216. https://doi.org/10.3390/polym14061216
Ramasamy, R., & Subramanian, R. B. (2023). Microfiber mitigation from synthetic textiles—Impact of combined surface modification and finishing process. Environmental Science and Pollution Research, 30(17), 49136–49149. https://doi.org/10.1007/s11356-023-25611-7
Vukoje, M., Kulcar, R., Ivanda, K. I., Bota, J., & Cigula, T. (2022). Improvement in thermochromic offset print UV stability by applying PCL nanocomposite coatings. Polymers, 14(7), 1484. https://doi.org/10.3390/polym14071484
Zhong, Z., Hu, A., Fu, S., & Zhang, L. (2022). High sunlight resistant thermochromic smart textiles based on UV absorbing polymer. Journal of Applied Polymer Science. https://doi.org/10.1002/app.53134
Goehler, L. O., Moruzzi, R. B., Tomazini da Conceição, F., Júnior, A. A. C., Speranza, L. G., Busquets, R., & Campos, L. C. (2022). Relevance of tyre wear particles to the total content of microplastics transported by runoff in a high-imperviousness and intense vehicle traffic urban area. Environmental Pollution. https://doi.org/10.1016/j.envpol.2022.120200
Kole, P. J., Löhr, A. J., Van Belleghem, F., & Ragas, A. (2017). Wear and tear of tyres: A stealthy source of microplastics in the environment. International Journal of Environmental Research and Public Health, 14(10), 1265. https://doi.org/10.3390/ijerph14101265
Varshney, S., Gora, A. H., Siriyappagouder, P., Kiron, V., & Olsvik, P. A. (2022). Toxicological effects of 6 ppd and 6ppd quinone in zebrafish larvae. Journal of Hazardous Materials, 424, 127623. https://doi.org/10.1016/j.jhazmat.2021.127623
Cao, G., Wang, W., Zhang, J., Wu, P., Zhao, X., Yang, Z., Hu, D., & Cai, Z. (2022). New evidence of rubber-derived quinones in water, air, and soil. Environmental Science & Technology, 56(7), 4142–4150. https://doi.org/10.1021/acs.est.1c07376
Fohet, L., Andanson, J.-M., Charbouillot, T., Malosse, L., Leremboure, M., Delor-Jestin, F., & Verney, V. (2023). Time-concentration profiles of tire particle additives and transformation products under natural and artificial aging. Science of The Total Environment, 859, 160150. https://doi.org/10.1016/j.scitotenv.2022.160150
Johannessen, C., Helm, P., & Metcalfe, C. D. (2021). Detection of selected tire wear compounds in urban receiving waters. Environmental Pollution, 287, 117659. https://doi.org/10.1016/j.envpol.2021.117659
(IEA), I. E. A. (2022). Renewables 2022. IEA.
Wieser, R. J., Wang, Y., Fairbrother, A., Napoli, S., Hauser, A. W., Julien, S., Gu, X., O’brien, G. S., Wan, K.-T., Ji, L., Kempe, M. D., Boyce, K. P., & Bruckman, L. S. (2023). Field retrieved photovoltaic backsheet survey from diverse climate zones: Analysis of degradation patterns and phenomena. Solar Energy, 259(C), 49–62. https://doi.org/10.1016/j.solener.2023.04.061
Zhang, J.-W., Deng, W., Ye, Z., Diaham, S., Putson, C., Zhou, X., Hu, J., Yin, Z., & Jia, R. (2023). Aging phenomena of backsheet materials of photovoltaic systems for future zero-carbon energy and the improvement pathway. Journal of Materials Science & Technology, 153, 106–119. https://doi.org/10.1016/j.jmst.2022.12.063
Walsh, A. N., Reddy, C. M., Niles, S. F., McKenna, A. M., Hansel, C. M., & Ward, C. P. (2021). Plastic formulation is an emerging control of its photochemical fate in the ocean. Environmental Science & Technology, 55(18), 12383–12392. https://doi.org/10.1021/acs.est.1c02272
Wu, M., Ma, Y., Xie, H., & Ji, R. (2022). Photodissolution of submillimeter-sized microplastics and its dependences on temperature and light composition. Science of The Total Environment, 848, 157714. https://doi.org/10.1016/j.scitotenv.2022.157714
Zhu, L., Zhao, S., Bittar, T. B., Stubbins, A., & Li, D. (2020). Photochemical dissolution of buoyant microplastics to dissolved organic carbon: Rates and microbial impacts. Journal of Hazardous Materials, 383, 121065. https://doi.org/10.1016/j.jhazmat.2019.121065
Lee, Y. K., He, W., Guo, H., Karanfil, T., & Hur, J. (2023). Effects of organic additives on spectroscopic and molecular-level features of photo-induced dissolved organic matter from microplastics. Water Research, 242, 120272. https://doi.org/10.1016/j.watres.2023.120272
Nabi, I., Bacha, A.-U.-R., Li, K., Cheng, H., Wang, T., Liu, Y., Ajmal, S., Yang, Y., Feng, Y., & Zhang, L. (2020). Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience, 23(7), 101326. https://doi.org/10.1016/j.isci.2020.101326
Yu, Y., Liu, X., Liu, Y., Liu, J., & Li, Y. (2023). Photoaging mechanism of microplastics: A perspective on the effect of dissolved organic matter in natural water. Frontiers of Environmental Science & Engineering. https://doi.org/10.1007/s11783-023-1743-8
Romera-Castillo, C., Birnstiel, S., Álvarez-Salgado, X. A., & Sebastián, M. (2022). Aged plastic leaching of dissolved organic matter is two orders of magnitude higher than virgin plastic leading to a strong uplift in marine microbial activity. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2022.861557
Allen, D., Allen, S., Abbasi, S., Baker, A., Bergmann, M., Brahney, J., Butler, T., Duce, R. A., Eckhardt, S., Evangeliou, N., Jickells, T., Kanakidou, M., Kershaw, P., Laj, P., Levermore, J., Li, D., Liss, P., Liu, K., Mahowald, N., … Wright, S. (2022). Microplastics and nanoplastics in the marine-atmosphere environment. Nature Reviews Earth & Environment, 3(6), 393–405. https://doi.org/10.1038/s43017-022-00292-x
Xiao, C. Q., Lang, M. F., Wu, R. R., Zhang, Z. M., & Guo, X. T. (2023). A review of the distribution, characteristics and environmental fate of microplastics in different environments of china. Reviews of Environmental Contamination and Toxicology. https://doi.org/10.1007/s44169-023-00026-0
Fan, W., Salmond, J. A., Dirks, K. N., Cabedo Sanz, P., Miskelly, G. M., & Rindelaub, J. D. (2022). Evidence and mass quantification of atmospheric microplastics in a coastal New Zealand city. Environmental Science & Technology, 56(24), 17556–17568. https://doi.org/10.1021/acs.est.2c05850
Harb, C., Pokhrel, N., & Foroutan, H. (2023). Quantification of the emission of atmospheric microplastics and nanoplastics via sea spray. Environmental Science & Technology Letters, 10(6), 513–519. https://doi.org/10.1021/acs.estlett.3c00164
Yang, S., Zhang, T., Gan, Y., Lu, X., Chen, H., Chen, J., Yang, X., & Wang, X. (2022). Constraining microplastic particle emission flux from the ocean. Environmental Science & Technology Letters, 9(6), 513–519. https://doi.org/10.1021/acs.estlett.2c00214
Bhatti, Y. A., Revell, L. E., & McDonald, A. J. (2022). Influences of Antarctic ozone depletion on southern ocean aerosols. Journal of Geophysical Research: Atmospheres, 127(18), e2022JD037199. https://doi.org/10.1029/2022JD037199
Brasseur, G., Wang, S., Walters, S., Lichtig, P., & Li, C. (2023). Microplastics in the atmosphere: A global perspective. Research Square. https://doi.org/10.21203/rs.3.rs-3186780/v1
Seinfeld, J. H., & Pandis, S. (1998). Atmospheric chemistry and physics: From air pollution to climate change. Physics Today, 51, 88–90.
Revell, L. E., Kuma, P., Le Ru, E. C., Somerville, W. R. C., & Gaw, S. (2021). Direct radiative effects of airborne microplastics. Nature, 598(7881), 462–467. https://doi.org/10.1038/s41586-021-03864-x
Aeschlimann, M., Li, G., Kanji, Z. A., & Mitrano, D. M. (2022). Potential impacts of atmospheric microplastics and nanoplastics on cloud formation processes. Nature Geoscience, 15(12), 967–975. https://doi.org/10.1038/s41561-022-01051-9
Wang, Y., Okochi, H., Tani, Y., Hayami, H., Minami, Y., Katsumi, N., Takeuchi, M., Sorimachi, A., Fujii, Y., Kajino, M., Adachi, K., Ishihara, Y., Iwamoto, Y., & Niida, Y. (2023). Airborne hydrophilic microplastics in cloud water at high altitudes and their role in cloud formation. Environmental Chemistry Letters, 21(6), 3055–3062. https://doi.org/10.1007/s10311-023-01626-x
Baho, D. L., Bundschuh, M., & Futter, M. N. (2021). Microplastics in terrestrial ecosystems: Moving beyond the state of the art to minimize the risk of ecological surprise. Global Change Biology, 27(17), 3969–3986. https://doi.org/10.1111/gcb.15724
Food and Agriculture Organization (FAO). (2021). Assessment of agricultural plastics and their sustainability: A call for action. FAO.
(UNEP), U. N. E. P. (2022). Plastics in agriculture—An environmental challenge (Vol. Foresight Brief 029). UNEP.
Huang, Y., Liu, Q., Jia, W., Yan, C., & Wang, J. (2020). Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environmental Pollution, 260, 114096. https://doi.org/10.1016/j.envpol.2020.114096
Serrano-Ruiz, H., Martin-Closas, L., & Pelacho, A. M. (2021). Biodegradable plastic mulches: Impact on the agricultural biotic environment. The Science of the Total Environment, 750, 141228–141228. https://doi.org/10.1016/j.scitotenv.2020.141228
Pokhrel, Y., Felfelani, F., Satoh, Y., Boulange, J., Burek, P., Gaedeke, A., Gerten, D., Gosling, S. N., Grillakis, M., Gudmundsson, L., Hanasaki, N., Kim, H., Koutroulis, A., Liu, J., Papadimitriou, L., Schewe, J., Mueller Schmied, H., Stacke, T., Telteu, C.-E., … Wada, Y. (2021). Global terrestrial water storage and drought severity under climate change. Nature Climate Change, 11(3), 226–233. https://doi.org/10.1038/s41558-020-00972-w
El-Beltagi, H. S., Basit, A., Mohamed, H. I., Ali, I., Ullah, S., Kamel, E. A. R., Shalaby, T. A., Ramadan, K. M. A., Alkhateeb, A. A., & Ghazzawy, H. S. (2022). Mulching as a sustainable water and soil saving practice in agriculture: A review. Agronomy, 12(8), 1881.
Nikolaou, G., Neocleous, D., Christou, A., Kitta, E., & Katsoulas, N. (2020). Implementing sustainable irrigation in water-scarce regions under the impact of climate change. Agronomy, 10(8), 1120.
Khalid, N., Aqeel, M., Noman, A., & Fatima Rizvi, Z. (2023). Impact of plastic mulching as a major source of microplastics in agroecosystems. Journal of Hazardous Materials, 445, 130455. https://doi.org/10.1016/j.jhazmat.2022.130455
Qiang, L., Hu, H., Li, G., Xu, J., Cheng, J., Wang, J., & Zhang, R. (2023). Plastic mulching, and occurrence, incorporation, degradation, and impacts of polyethylene microplastics in agroecosystems. Ecotoxicology and Environmental Safety, 263, 115274. https://doi.org/10.1016/j.ecoenv.2023.115274
Zhang, J., Ren, S., Xu, W., Liang, C., Li, J., Zhang, H., Li, Y., Liu, X., Jones, D. L., Chadwick, D. R., Zhang, F., & Wang, K. (2022). Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. Journal of Hazardous Materials, 435, 129065. https://doi.org/10.1016/j.jhazmat.2022.129065
Wang, F., Wang, Q., Adams, C. A., Sun, Y., & Zhang, S. (2022). Effects of microplastics on soil properties: Current knowledge and future perspectives. Journal of Hazardous Materials, 424(Pt C), 127531. https://doi.org/10.1016/j.jhazmat.2021.127531
Hurley, R. R., & Nizzetto, L. (2018). Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Current Opinion in Environmental Science & Health, 1, 6–11. https://doi.org/10.1016/j.coesh.2017.10.006
Wanner, P. (2021). Plastic in agricultural soils—A global risk for groundwater systems and drinking water supplies? A review. Chemosphere, 264(Pt 1), 128453. https://doi.org/10.1016/j.chemosphere.2020.128453
Drechsel, P., Qadir, M., & Wichelns, D. (2015). Wastewater (1st ed.). Springer Netherlands.
Li, Q., Wu, J., Zhao, X., Gu, X., & Ji, R. (2019). Separation and identification of microplastics from soil and sewage sludge. Environmental Pollution, 254(Pt B), 113076. https://doi.org/10.1016/j.envpol.2019.113076
Kumar, M., Xiong, X., He, M., Tsang, D. C. W., Gupta, J., Khan, E., Harrad, S., Hou, D., Ok, Y. S., & Bolan, N. S. (2020). Microplastics as pollutants in agricultural soils. Environmental Pollution, 265(Pt A), 114980. https://doi.org/10.1016/j.envpol.2020.114980
Möller, J. N., Löder, M. G. J., & Laforsch, C. (2020). Finding microplastics in soils: A review of analytical methods. Environmental Science & Technology, 54(4), 2078–2090. https://doi.org/10.1021/acs.est.9b04618
Yang, Y., Li, Z., Yan, C., Chadwick, D., Jones, D. L., Liu, E., Liu, Q., Bai, R., & He, W. (2022). Kinetics of microplastic generation from different types of mulch films in agricultural soil. Science of the Total Environment, 814, 152572. https://doi.org/10.1016/j.scitotenv.2021.152572
O’Connor, D., Pan, S., Shen, Z., Song, Y., Jin, Y., Wu, W. M., & Hou, D. (2019). Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environmental Pollution, 249, 527–534. https://doi.org/10.1016/j.envpol.2019.03.092
Guo, J. J., Huang, X. P., Xiang, L., Wang, Y. Z., Li, Y. W., Li, H., Cai, Q. Y., Mo, C. H., & Wong, M. H. (2020). Source, migration and toxicology of microplastics in soil. Environment International, 137, 105263. https://doi.org/10.1016/j.envint.2019.105263
Huang, D., Wang, X., Yin, L., Chen, S., Tao, J., Zhou, W., Chen, H., Zhang, G., & Xiao, R. (2022). Research progress of microplastics in soil–plant system: Ecological effects and potential risks. Science of the Total Environment, 812, 151487. https://doi.org/10.1016/j.scitotenv.2021.151487
Zhou, W., Wang, Q., Wei, Z., Jiang, J., & Deng, J. (2023). Effects of microplastic type on growth and physiology of soil crops: Implications for farmland yield and food quality. Environmental Pollution. https://doi.org/10.1016/j.envpol.2023.121512
Deng, J., Zhou, L., Zhou, W., Wang, Q., & Yu, D. (2022). Effect of microfibers combined with UV-b and drought on plant community. Chemosphere, 288(Pt 1), 132413. https://doi.org/10.1016/j.chemosphere.2021.132413
Wang, F., Feng, X., Liu, Y., Adams, C. A., Sun, Y., & Zhang, S. (2022). Micro(nano)plastics and terrestrial plants: Up-to-date knowledge on uptake, translocation, and phytotoxicity. Resources, Conservation and Recycling, 185, 106503. https://doi.org/10.1016/j.resconrec.2022.106503
dos Santos, N., Clyde-Smith, D., Qi, Y., Gao, F., Busquets, R., & Campos, L. C. (2023). A study of microfiber phytoremediation in vertical hydroponics. Sustainability. https://doi.org/10.3390/su15042851
Chia, R. W., Lee, J.-Y., Lee, M., Lee, G.-S., & Jeong, C.-D. (2023). Role of soil microplastic pollution in climate change. Science of the Total Environment, 887, 164112. https://doi.org/10.1016/j.scitotenv.2023.164112
Courtene-Jones, W., van Gennip, S., Penicaud, J., Penn, E., & Thompson, R. C. (2022). Synthetic microplastic abundance and composition along a longitudinal gradient traversing the subtropical gyre in the North Atlantic Ocean. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2022.114371
Nava, V., Chandra, S., Aherne, J., Alfonso, M. B., Antão-Geraldes, A. M., Attermeyer, K., Bao, R., Bartrons, M., Berger, S. A., Biernaczyk, M., Bissen, R., Brookes, J. D., Brown, D., Cañedo-Argüelles, M., Canle, M., Capelli, C., Carballeira, R., Cereijo, J. L., Chawchai, S., … Leoni, B. (2023). Plastic debris in lakes and reservoirs. Nature, 619(7969), 317–322. https://doi.org/10.1038/s41586-023-06168-4
Delre, A., Goudriaan, M., Morales, V. H., Vaksmaa, A., Ndhlovu, R. T., Baas, M., Keijzer, E., de Groot, T., Zeghal, E., Egger, M., Röckmann, T., & Niemann, H. (2023). Plastic photodegradation under simulated marine conditions. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2022.114544
Walsh, A. N., Mazzotta, M. G., Nelson, T. F., Reddy, C. M., & Ward, C. P. (2022). Synergy between sunlight, titanium dioxide, and microbes enhances cellulose diacetate degradation in the ocean. Environmental Science & Technology, 56(19), 13810–13819. https://doi.org/10.1021/acs.est.2c04348
Stubbins, A., Zhu, L., Zhao, S., Spencer, R. G. M., & Podgorski, D. C. (2023). Molecular signatures of dissolved organic matter generated from the photodissolution of microplastics in sunlit seawater. Environmental Science & Technology. https://doi.org/10.1021/acs.est.1c03592
Romera-Castillo, C., Pinto, M., Langer, T. M., Álvarez-Salgado, X. A., & Herndl, G. J. (2018). Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nature Communications. https://doi.org/10.1038/s41467-018-03798-5
Sheridan, E. A., Fonvielle, J. A., Cottingham, S., Zhang, Y., Dittmar, T., Aldridge, D. C., & Tanentzap, A. J. (2022). Plastic pollution fosters more microbial growth in lakes than natural organic matter. Nature Communications. https://doi.org/10.1038/s41467-022-31691-9
James, B. D., Karchner, S. I., Walsh, A. N., Aluru, N., Franks, D. G., Sullivan, K. R., Reddy, C. M., Ward, C. P., & Hahn, M. E. (2023). Formulation controls the potential neuromuscular toxicity of polyethylene photoproducts in developing zebrafish. Environmental Science & Technology, 57(21), 7966–7977. https://doi.org/10.1021/acs.est.3c01932
Nelson, T. F., Reddy, C. M., & Ward, C. P. (2021). Product formulation controls the impact of biofouling on consumer plastic photochemical fate in the ocean. Environmental Science & Technology, 55(13), 8898–8907. https://doi.org/10.1021/acs.est.1c02079
Kaiser, D., Kowalski, N., & Waniek, J. J. (2017). Effects of biofouling on the sinking behavior of microplastics. Environmental Research Letters. https://doi.org/10.1088/1748-9326/aa8e8b
Mundhenke, T. F., Li, S. C., & Maurer-Jones, M. A. (2022). Photodegradation of polyolefin thin films in simulated freshwater conditions. Environmental Science: Processes & Impacts, 24(12), 2284–2293. https://doi.org/10.1039/d2em00359g
Peeken, I., Primpke, S., Beyer, B., Gütermann, J., Katlein, C., Krumpen, T., Bergmann, M., Hehemann, L., & Gerdts, G. (2018). Arctic sea ice is an important temporal sink and means of transport for microplastic. Nature Communications. https://doi.org/10.1038/s41467-018-03825-5
Zhang, Y., Gao, T., Kang, S., Allen, D., Wang, Z., Luo, X., Yang, L., Chen, J., Hu, Z., Chen, P., Du, W., & Allen, S. (2023). Cryosphere as a temporal sink and source of microplastics in the arctic region. Geoscience Frontiers. https://doi.org/10.1016/j.gsf.2023.101566
Huserbråten, M. B. O., Hattermann, T., Broms, C., & Albretsen, J. (2022). Trans-polar drift-pathways of riverine european microplastic. Scientific Reports, 12(1), 3016. https://doi.org/10.1038/s41598-022-07080-z
Amini-Birami, F., Keshavarzi, B., Esmaeili, H. R., Moore, F., Busquets, R., Saemi-Komsari, M., Zarei, M., & Zarandian, A. (2023). Microplastics in aquatic species of anzali wetland: An important freshwater biodiversity hotspot in iran. Environmental Pollution, 330, 121762. https://doi.org/10.1016/j.envpol.2023.121762
Saeed, M. S., Halim, S. Z., Fahd, F., Khan, F., Sadiq, R., & Chen, B. (2022). An ecotoxicological risk model for the microplastics in arctic waters. Environmental Pollution. https://doi.org/10.1016/j.envpol.2022.120417
(WHO), W. H. O. (2020). Dietary and inhalation exposure to nano- and microplastic particles and potential implications for human health. WHO.
Nielsen, M. B., Clausen, L. P. W., Cronin, R., Hansen, S. F., Oturai, N. G., & Syberg, K. (2023). Unfolding the science behind policy initiatives targeting plastic pollution. Microplastics and Nanoplastics, 3(1), 3–3. https://doi.org/10.1186/s43591-022-00046-y
Fries, E., & Sühring, R. (2023). The unusual suspects: Screening for persistent, mobile, and toxic plastic additives in plastic leachates. Environmental Pollution, 335, 122263. https://doi.org/10.1016/j.envpol.2023.122263
Savva, K., Borrell, X., Moreno, T., Pérez-Pomeda, I., Barata, C., Llorca, M., & Farré, M. (2023). Cytotoxicity assessment and suspected screening of plastic additives in bioplastics of single-use household items. Chemosphere, 313, 137494. https://doi.org/10.1016/j.chemosphere.2022.137494
Gewert, B., MacLeod, M., & Breitholtz, M. (2021). Variability in toxicity of plastic leachates as a function of weathering and polymer type: A screening study with the copepod nitocra spinipes. The Biological Bulletin (Lancaster), 240(3), 191–199. https://doi.org/10.1086/714506
Dimassi, S. N., Hahladakis, J. N., Yahia, M. N. D., Ahmad, M. I., Sayadi, S., & Al-Ghouti, M. A. (2023). Effect of temperature and sunlight on the leachability potential of BPA and phthalates from plastic litter under marine conditions. Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2023.164954
Fauser, P., Vorkamp, K., & Strand, J. (2022). Residual additives in marine microplastics and their risk assessment—A critical review. Marine Pollution Bulletin, 177, 113467. https://doi.org/10.1016/j.marpolbul.2022.113467
Cheng, F., Zhang, T., Liu, Y., Zhang, Y., & Qu, J. (2021). Non-negligible effects of UV irradiation on transformation and environmental risks of microplastics in the water environment. Journal of Xenobiotics, 12(1), 1–12. https://doi.org/10.3390/jox12010001
Zhang, Y., Xu, C., Zhang, W., Qi, Z., Song, Y., Zhu, L., Dong, C., Chen, J., & Cai, Z. (2021). P-phenylenediamine antioxidants in PM2.5: The underestimated urban air pollutants. Environmental Science & Technology, 56(11), 6914–6921. https://doi.org/10.1021/acs.est.1c04500
Zhang, R., Zhao, S., Liu, X., Tian, L., Mo, Y., Yi, X., Liu, S., Liu, J., Li, J., & Zhang, G. (2023). Aquatic environmental fates and risks of benzotriazoles, benzothiazoles, and p-phenylenediamines in a catchment providing water to a megacity of China. Environmental Research. https://doi.org/10.1016/j.envres.2022.114721
Thomas, M., Cate, B., Garnett, J., Smith, I. J., Vancoppenolle, M., & Halsall, C. (2023). The effect of partial dissolution on sea-ice chemical transport: A combined model–observational study using poly- and perfluoroalkylated substances (pfass). The Cryosphere, 17(8), 3193–3201. https://doi.org/10.5194/tc-17-3193-2023
Guo, C., Wang, L., Lang, D., Qian, Q., Wang, W., Wu, R., & Wang, J. (2023). UV and chemical aging alter the adsorption behavior of microplastics for tetracycline. Environmental Pollution. https://doi.org/10.1016/j.envpol.2022.120859
Hanun, J. N., Hassan, F., Theresia, L., Chao, H.-R., Bu, H. M., Rajendran, S., Kataria, N., Yeh, C.-F., Show, P. L., Khoo, K. S., & Jiang, J.-J. (2023). Weathering effect triggers the sorption enhancement of microplastics against oxybenzone. Environmental Technology & Innovation. https://doi.org/10.1016/j.eti.2023.103112
Ju, H., Yang, X., Osman, R., & Geissen, V. (2023). The role of microplastic aging on chlorpyrifos adsorption-desorption and microplastic bioconcentration. Environmental Pollution. https://doi.org/10.1016/j.envpol.2023.121910
Al Harraq, A., Brahana, P. J., Arcemont, O., Zhang, D., Valsaraj, K. T., & Bharti, B. (2022). Effects of weathering on microplastic dispersibility and pollutant uptake capacity. ACS Environmental Au, 2(6), 549–555. https://doi.org/10.1021/acsenvironau.2c00036
Zafar, R., Bang, T. H., Lee, Y. K., Begum, M. S., Rabani, I., Hong, S., & Hur, J. (2022). Change in adsorption behavior of aquatic humic substances on microplastic through biotic and abiotic aging processes. Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2022.157010
Amaral-Zettler, L. A., Zettler, E. R., Mincer, T. J., Klaassen, M. A., & Gallager, S. M. (2021). Biofouling impacts on polyethylene density and sinking in coastal waters: A macro/micro tipping point? Water Research (Oxford), 201, 117289–117289. https://doi.org/10.1016/j.watres.2021.117289
Khosrovyan, A., & Kahru, A. (2022). Virgin and UV-weathered polyamide microplastics posed no effect on the survival and reproduction of daphnia magna. PeerJ, 10, e13533. https://doi.org/10.7717/peerj.13533
He, S., Jia, M., Xiang, Y., Song, B., Xiong, W., Cao, J., Peng, H., Yang, Y., Wang, W., Yang, Z., & Zeng, G. (2022). Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications. Journal of Hazardous Materials, 424(Pt B), 127286. https://doi.org/10.1016/j.jhazmat.2021.127286
Kiki, C., Qiu, Y., Wang, Q., Ifon, B. E., Qin, D., Chabi, K., Yu, C. P., Zhu, Y. G., & Sun, Q. (2022). Induced aging, structural change, and adsorption behavior modifications of microplastics by microalgae. Environment International, 166, 107382. https://doi.org/10.1016/j.envint.2022.107382
Ziani, K., Ioniță-Mîndrican, C.-B., Mititelu, M., Neacșu, S. M., Negrei, C., Moroșan, E., Drăgănescu, D., & Preda, O.-T. (2023). Microplastics: A real global threat for environment and food safety: A state of the art review. Nutrients, 15(3), 617. https://doi.org/10.3390/nu15030617
Barceló, D., Picó, Y., & Alfarhan, A. H. (2023). Microplastics: Detection in human samples, cell line studies, and health impacts. Environmental Toxicology and Pharmacology, 101, 104204. https://doi.org/10.1016/j.etap.2023.104204
Niu, H., Liu, S., Jiang, Y., Hu, Y., Li, Y., He, L., Xing, M., Li, X., Wu, L., Chen, Z., Wang, X., & Lou, X. (2023). Are microplastics toxic? A review from eco-toxicity to effects on the gut microbiota. Metabolites, 13(6), 739. https://doi.org/10.3390/metabo13060739
Montero, V., Chinchilla, Y., Gómez, L., Flores, A., Medaglia, A., Guillen, R., & Montero, E. (2023). Human health risk assessment for consumption of microplastics and plasticizing substances through marine species. Environmental Research, 237, 116843. https://doi.org/10.1016/j.envres.2023.116843
Kirk, M., Smurthwaite, K. S., Braunig, J., Trevenar, S., D'Este, C., Lucas, R. M., Lal, A., Korda, R. J., Clements, A., Mueller, J., & Armstong, B. K. (2018). The PFAS health study: Systematic literature review, [ANU National Centre for Epidemiology and Population Health (NCEPH). ANU Research Publications (Ed.)].
Vandenburg, J., & Ota, Y. (2022). Ocean nexus equity & marine plastic pollution report 2022: Towards an equitable approach to marine plastic pollution. https://oceannexus.Uw.Edu/2022/11/21/equity-marine-plastic-pollution-report/.
United Nations Environment Programme. (2021). Neglected: Environmental justice impacts of marine litter and plastic pollution. UNEP.
Richardson, K., Steffen, W., Lucht, W., Bendtsen, J., Cornell, S. E., Donges, J. F., Drüke, M., Fetzer, I., Bala, G., von Bloh, W., Feulner, G., Fiedler, S., Gerten, D., Gleeson, T., Hofmann, M., Huiskamp, W., Kummu, M., Mohan, C., Nogués-Bravo, D., … Rockström, J. (2023). Earth beyond six of nine planetary boundaries. Science Advances, 9(37), eadh2458. https://doi.org/10.1126/sciadv.adh2458
Law, K. L., & Narayan, R. (2022). Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nature Reviews. Materials, 7(2), 104–116. https://doi.org/10.1038/s41578-021-00382-0
Lenzi, L., Degli Esposti, M., Braccini, S., Siracusa, C., Quartinello, F., Guebitz, G. M., Puppi, D., Morselli, D., & Fabbri, P. (2023). Further step in the transition from conventional plasticizers to versatile bioplasticizers obtained by the valorization of levulinic acid and glycerol. ACS Sustainable Chemical Engineering, 11(25), 9455–9469. https://doi.org/10.1021/acssuschemeng.3c01536
Ledniowska, K., Nosal-Kovalenko, H., Janik, W., Krasuska, A., Stańczyk, D., Sabura, E., Bartoszewicz, M., & Rybak, A. (2022). Effective, environmentally friendly PVC plasticizers based on succinic acid. Polymers (Basel), 14(7), 1295. https://doi.org/10.3390/polym14071295
Chathoth, A. M., Subba Rao, A. N., Nair, S., Nagarajappa, G. B., & Pandey, K. K. (2023). Luminescent transparent wood from a woody cellulosic template treated with an optical brightener. Journal of Applied Polymer Science. https://doi.org/10.1002/app.54028
Van Hai, L., Cho, S.-W., Kwon, G.-J., Lee, D.-Y., Ma, S.-Y., Bandi, R., Kim, J.-K., Han, S.-Y., Dadigala, R., & Lee, S.-H. (2023). Fabrication of eco-friendly transparent wood for UV-shielding functionality. Industrial Crops and Products, 201, 116918. https://doi.org/10.1016/j.indcrop.2023.116918
Arnold, J. C., & Alston, S. M. (2012). Life cycle assessment of the production and use of polypropylene tree shelters. Journal of Environmental Management, 94(1), 1–12. https://doi.org/10.1016/j.jenvman.2011.09.005
dos Santos, A., Matos, L. C., Mendonça, M. C., do Lago, R. C., dos Santos Muguet, M. C., Damásio, R. A. P., Ponzecchi, A., Soares, J. R., Sanadi, A. R., & Tonoli, G. H. D. (2023). Evaluation of paper coated with cationic starch and carnauba wax mixtures regarding barrier properties. Industrial Crops and Products, 203, 117177. https://doi.org/10.1016/j.indcrop.2023.117177
Gore, A. H., & Prajapat, A. L. (2022). Biopolymer nanocomposites for sustainable UV protective packaging. Frontiers in Materials. https://doi.org/10.3389/fmats.2022.855727
Attia, N. F., Osama, R., Elashery, S. E. A., Kalam, A., Al-Sehemi, A. G., & Algarni, H. (2022). Recent advances of sustainable textile fabric coatings for UV protection properties. Coatings (Basel), 12(10), 1597. https://doi.org/10.3390/coatings12101597
Petkovska, J., Mladenovic, N., Marković, D., Radoičić, M., Vest, N. A., Palen, B., Radetić, M., Grunlan, J. C., & Jordanov, I. (2022). Flame-retardant, antimicrobial, and UV-protective lignin-based multilayer nanocoating. ACS Applied Polymer Materials, 4(6), 4528–4537. https://doi.org/10.1021/acsapm.2c00520
Jia, Y., Jiang, H., Wang, Y., Liu, Z., & Liang, P. (2022). Fabrication of bio-based coloristic and ultraviolet protective cellulosic fabric using chitosan derivative and chestnut shell extract. Fibers and Polymers, 23(10), 2760–2768. https://doi.org/10.1007/s12221-022-4940-3
Patankar, K. C., Biranje, S., Pawar, A., Maiti, S., Shahid, M., More, S., & Adivarekar, R. V. (2022). Fabrication of chitosan-based finishing agent for flame-retardant, UV-protective, and antibacterial cotton fabrics. Materials Today Communications, 33, 104637. https://doi.org/10.1016/j.mtcomm.2022.104637
Luo, X., Ma, Y., Chen, H., Liu, L., Hu, Z., Li, Z., & Yao, J. (2022). Highly fireproof, antimicrobial and UV-resistant cotton fabric functionalized with biomass tannic acid and phytic acid. Journal of Materials Science, 57(30), 14528–14542. https://doi.org/10.1007/s10853-022-07536-7
Porrawatkul, P., Pimsen, R., Kuyyogsuy, A., Teppaya, N., Noypha, A., Chanthai, S., & Nuengmatcha, P. (2022). Microwave-assisted synthesis of ag/zno nanoparticles using averrhoa carambola fruit extract as the reducing agent and their application in cotton fabrics with antibacterial and UV-protection properties. RSC Advances, 12(24), 15008–15019. https://doi.org/10.1039/d2ra01636b
Ye, S., Sun, H., Wu, J., Wan, L., Ni, Y., Wang, R., Xiang, Z., & Deng, X. (2022). Supercritical CO 2 assisted tio 2 preparation to improve the UV resistance properties of cotton fiber. Polymers (Basel), 14(24), 5513.
Garg, H., Singhal, N., Singh, A., Khan, M. D., & Sheikh, J. (2023). Laccase-assisted colouration of wool fabric using green tea extract for imparting antioxidant, antibacterial, and UV protection activities. Environmental Science and Pollution Research International, 30(35), 84386–84396. https://doi.org/10.1007/s11356-023-28287-1
Singh Gangwar, A. K., Shakyawar, D. B., Singh, M. K., Vishnoi, P., & Jose, S. (2022). Optimization of sodium lignosulfonate treatment on nylon fabric using box–behnken response surface design for UV protection. AUTEX Research Journal, 22(2), 248–257. https://doi.org/10.2478/aut-2021-0011
Bifulco, A., Imparato, C., Aronne, A., & Malucelli, G. (2022). Flame retarded polymer systems based on the sol-gel approach: Recent advances and future perspectives. Journal of Sol-Gel Science and Technology. https://doi.org/10.1007/s10971-022-05918-6
Acknowledgements
Generous contributions by UNEP/Ozone Secretariat were provided for the convened author meeting. Anastazia T Banaszak acknowledges funding from the UNEP/Ozone Secretariat. Paul W. Barnes acknowledges funding from the US Global Change Research Program and the Loyola University J.H. Mullahy Endowed Chair in Environmental Biology. Germar H Bernhard acknowledges funding from the US Global Change Research Program. Rosa Busquets acknowledges funding from Kingston University HSSCE Faculty support. Marcel AK Jansen acknowledges funding from the Environmental Protection Agency (Ireland). Samuel Hylander acknowledges funding from the Swedish Environmental Protection Agency and Linnaeus University. Krishna K Pandey acknowledges funding from UNEP/Ozone Secretariat. Laura E Revell acknowledges funding from the Ministry of Business, Innovation & Employment Endeavor Fund (Contract RTVU1906) and the Rutherford Discovery Fellowships from New Zealand Government funding, administered by the Royal Society Te Apārangi. Sharon A Robinson acknowledges funding from the University of Wollongong. T Matthew Robson acknowledges funding from the University of Cumbria Research Fund 1093-23. Kevin C Rose acknowledges funding from the US Global Change Research Program and US National Science Foundation grants 2048031 and 1754265. Antony R Young acknowledges funding from the British Photodermatology Group and the Sunshine Health Foundation. The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and assessment and carried out extensive revisions of content.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jansen, M.A.K., Andrady, A.L., Bornman, J.F. et al. Plastics in the environment in the context of UV radiation, climate change and the Montreal Protocol: UNEP Environmental Effects Assessment Panel, Update 2023. Photochem Photobiol Sci 23, 629–650 (2024). https://doi.org/10.1007/s43630-024-00552-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-024-00552-3