Abstract
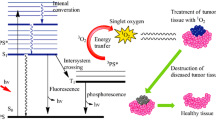
In this work, screening studies of the cytotoxic effect of chlorins with fragments of di-, tri-, and pentaethylene glycol at the macrocycle periphery in relation to HeLa, A549, and HT29 cells were performed. It is shown that, despite different hydrophobicity, all the compounds studied have a comparable photodynamic effect. The conjugate of chlorin e6 with pentaethylene glycol, which has the lowest tendency to association among the studied compounds with tropism for low density lipoproteins and the best characteristics of the formation of molecular complexes with Tween 80, has a significant difference in dark and photoinduced toxicity (ratio IC50(dark)/IC50(photo) approximately 2 orders of magnitude for all cell lines), which allows to hope for a sufficiently large "therapeutic window". A study of the interaction of this compound with HeLa cells shows that the substance penetrates the cell and, after red light irradiation induces ROS appearance inside the cell, associated, apparently, with the photogeneration of singlet oxygen. These data indicate that photoinduced toxic effects are caused by damage to intracellular structures as a result of oxidative stress. Programmed type of cell death characterized with caspase-3 induction is prevailing. So, the conjugate of chlorin e6 with pentaethylene glycol is a promising antitumor PS that can be successfully solubilized with Tween 80, which makes it suitable for further in vivo studies.
Graphical abstract






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Malatesti, N., Munitic, I., & Jurak, I. (2017). Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents. Biophysical Reviews, 9, 149–168.
Abrahamse, H., & Hamblin, M. R. (2016). New photosensitizers for photodynamic therapy. Biochemical Journal, 473, 347–364.
Imran, M., Ramzan, M., Qureshi, A., Khan, M., & Tariq, M. (2018). Emerging applications of porphyrins and metalloporphyrins in biomedicine and diagnostic magnetic resonance imaging. Biosensors, 8, 95.
Koifman, O. I., Ageeva, T. A., Kuzmina, N. S., Otvagin, V. F., Nyuchev, A. V., Fedorov, AYu., Belykh, D. V., Lebedeva, NSh., Yurina, E. S., Syrbu, S. A., Koifman, M. O., & Gubarev, Y. A. (2022). Synthesis strategy of tetrapyrrolic photosensitizers for their practical application in photodynamic therapy. MHC, 15, 207–304.
Hamblin, M. R. (2016). Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Current Opinion in Microbiology, 33, 67–73.
Kustov, A. V., Kustova, T. V., Belykh, D. V., Khudyaeva, I. S., & Berezin, D. B. (2020). Synthesis and investigation of novel chlorin sensitizers containing the myristic acid residue for antimicrobial photodynamic therapy. Dyes and Pigments, 173, 107948.
Pylina, Y., Shadrin, D., Shevchenko, O., Startseva, O., Velegzhaninov, I., Belykh, D., & Velegzhaninov, I. (2017). Dark and photoinduced cytotoxic activity of the new chlorophyll-a derivatives with oligoethylene glycol substituents on the periphery of their macrocycles. IJMS, 18, 103.
Aggarwal, A., Samaroo, D., Jovanovic, I. R., Singh, S., Tuz, M. P., & Mackiewicz, M. R. (2019). Porphyrinoid-based photosensitizers for diagnostic and therapeutic applications: An update. Journal of Porphyrins and Phthalocyanines, 23, 729–765.
Grin, M., Suvorov, N., Ostroverkhov, P., Pogorilyy, V., Kirin, N., Popov, A., Sazonova, A., & Filonenko, E. (2022). Advantages of combined photodynamic therapy in the treatment of oncological diseases. Biophysical Reviews, 14, 941–963.
Singh, S., Aggarwal, A., Bhupathiraju, N. V. S. D. K., Arianna, G., Tiwari, K., & Drain, C. M. (2015). Glycosylated porphyrins, phthalocyanines, and other porphyrinoids for diagnostics and therapeutics. Chemical Reviews, 115, 10261–10306.
Koifman, O. I., Ageeva, T. A., Beletskaya, I. P., Averin, A. D., Yakushev, A. A., Tomilova, L. G., Dubinina, T. V., Tsivadze, AYu., Gorbunova, Y. G., Martynov, A. G., Konarev, D. V., Khasanov, S. S., Lyubovskaya, R. N., Lomova, T. N., Korolev, V. V., Zenkevich, E. I., Blaudeck, T., Von Borczyskowski, C., Zahn, D. R. T., … Yurina, E. S. (2020). Macroheterocyclic compounds—A key building block in new functional materials and molecular devices. Macroheterocycles, 13, 311–467.
Lebedeva, NSh., Gubarev, Y. A., Koifman, M. O., & Koifman, O. I. (2020). The application of porphyrins and their analogues for inactivation of viruses. Molecules, 25, 4368.
Kustov, A. V., Smirnova, N. L., Privalov, O. A., Moryganova, T. M., Strelnikov, A. I., Morshnev, P. K., Koifman, O. I., Lyubimtsev, A. V., Kustova, T. V., & Berezin, D. B. (2021). Transurethral resection of non-muscle invasive bladder tumors combined with fluorescence diagnosis and photodynamic therapy with chlorin e6-type photosensitizers. JCM, 11, 233.
Otvagin, V. F., Kuzmina, N. S., Kudriashova, E. S., Nyuchev, A. V., Gavryushin, A. E., & Fedorov, AYu. (2022). Conjugates of porphyrinoid-based photosensitizers with cytotoxic drugs: Current progress and future directions toward selective photodynamic therapy. Journal of Medicinal Chemistry, 65, 1695–1734.
Suvorov, N., Pogorilyy, V., Diachkova, E., Vasil’ev, Y., Mironov, A., & Grin, M. (2021). Derivatives of natural chlorophylls as agents for antimicrobial photodynamic therapy. International Journal of Molecular Sciences, 22, 6392.
Pylina, Y. I., Khudyaeva, I. S., & Belykh, D. V. (2022). Dark and photoinduced cytotoxicity of chlorophyll a derivatives and their analogues towards HeLa Cells: Some structure—Activity relationships. MHC, 15, 25–33.
Pylina, Y. I., Khudyaeva, I. S., Startseva, O. M., Shadrin, D. M., Shevchenko, O. G., Velegzhaninov, I. O., Kukushkina, N. V., Berezin, D. B., & Belykh, D. V. (2021). Dark and photoinduced cytotoxicity of cationic chlorin e6 derivatives with different numbers of charged groups. MHC, 14, 317–322.
Berezin, D. B., Solodukhin, T. N., Shukhto, O. V., Belykh, D. V., Startseva, O. M., Khudyaeva, I. S., & Kustov, A. V. (2018). Association of hydrophilic derivatives of chlorophyll a in ethanol–water and ethanol–water–solubilizer systems. Russian Chemical Bulletin, 67, 1273–1279.
Belykh, D. V., Startseva, O. M., & Patov, S. A. (2014). Novel pH-independent amphiphilic chlorophyll a derivatives with oligoethyleneglycol substituents as a hydrophilic part: Synthesis and hydrophilicity estimation. MHC, 7, 401–413.
Tarabukina, I. S., Startseva, O. M., Patov, S. A., & Belykh, D. V. (2015). Novel Dicationic chlorin e6 derivatives. MHC, 8, 168–176.
Shukhto, O. V., Berezin, D. B., Khudyaeva, I. S., & Belykh, D. V. (2021). Aggregation of hydrophobic chlorins with fragments of antimicrobial drugs in aqueous solutions of ethanol and tween 80. ChemChemTech, 64, 86–96.
Berezin, D. B., Kustov, A. V., Krest’yaninov, M. A., Shukhto, O. V., Batov, D. V., & Kukushkina, N. V. (2019). The behavior of monocationic chlorin in water and aqueous solutions of non-ionic surfactant Tween 80 and potassium iodide. Journal of Molecular Liquids, 283, 532–536.
Gerola, A. P., Tsubone, T. M., Santana, A., De Oliveira, H. P. M., Hioka, N., & Caetano, W. (2011). Properties of chlorophyll and derivatives in homogeneous and microheterogeneous systems. The Journal of Physical Chemistry B, 115, 7364–7373.
Lindhagen, E., Nygren, P., & Larsson, R. (2008). The fluorometric microculture cytotoxicity assay. Nature Protocols, 3, 1364–1369.
Lyublinskaya, O. G., Antonov, S. A., Gorokhovtsev, S. G., Pugovkina, N. A., Kornienko, J. S., Ivanova, J. S., Shatrova, A. N., Aksenov, N. D., Zenin, V. V., & Nikolsky, N. N. (2018). Flow cytometric HyPer-based assay for hydrogen peroxide. Free Radical Biology and Medicine, 128, 40–49.
Lyublinskaya, O. G., Ivanova, J. S., Pugovkina, N. A., Kozhukharova, I. V., Kovaleva, Z. V., Shatrova, A. N., Aksenov, N. D., Zenin, V. V., Kaulin, Y. A., Gamaley, I. A., & Nikolsky, N. N. (2017). Redox environment in stem and differentiated cells: A quantitative approach. Redox Biology, 12, 758–769.
Shcherbo, D., Souslova, E. A., Goedhart, J., Chepurnykh, T. V., Gaintzeva, A., Shemiakina, I. I., Gadella, T. W., Lukyanov, S., & Chudakov, D. M. (2009). Practical and reliable FRET/FLIM pair of fluorescent proteins. BMC Biotechnology, 9, 24.
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Ritz, C., Baty, F., Streibig, J. C., & Gerhard, D. (2015). Dose-response analysis using R. PLoS ONE, 10, e0146021.
Agostinis, P., Berg, K., Cengel, K. A., Foster, T. H., Girotti, A. W., Gollnick, S. O., Hahn, S. M., Hamblin, M. R., Juzeniene, A., Kessel, D., Korbelik, M., Moan, J., Mroz, P., Nowis, D., Piette, J., Wilson, B. C., & Golab, J. (2011). Photodynamic therapy of cancer: An update. CA: A Cancer Journal for Clinicians, 61, 250–281.
Van Straten, D., Mashayekhi, V., De Bruijn, H., Oliveira, S., & Robinson, D. (2017). Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers, 9, 19.
D. A. Smith, H. van de Waterbeemd, D. K. Walker, R. Mannhold, H. Kubinyi and H. Timmerman. (2001). In Methods and Principles in Medicinal Chemistry, Mannhold R., Kubinyi H, Timmerman H, editors. Weinheim, Wiley–VCH Verlag. p. 141.
Kustov, A. V., Berezin, D. B., Zorin, V. P., Morshnev, P. K., Kukushkina, N. V., Krestyaninov, M. A., Kustova, T. V., Strelnikov, A. I., Lyalyakina, E. V., Zorina, T. E., Abramova, O. B., & Kozlovtseva, E. A. (2022). Monocationic chlorin as a promising photosensitizer for antitumor and antimicrobial photodynamic therapy. Pharmaceutics, 15, 61.
Kustov, A. V., Belykh, D. V., Smirnova, N. L., Venediktov, E. A., Kudayarova, T. V., Kruchin, S. O., Khudyaeva, I. S., & Berezin, D. B. (2018). Synthesis and investigation of water-soluble chlorophyll pigments for antimicrobial photodynamic therapy. Dyes and Pigments, 149, 553–559.
Kustov, A. V., & Belykh, D. V. (2016). New sensitizers developed on a methylpheophorbide a platform for photodynamic therapy: Synthesis, singlet oxygen generation and modeling of passive membrane transport. Pharmaceutica Analytica Acta. https://doi.org/10.4172/2153-2435.1000480
Dąbrowski, J. M. (2017). Advances in inorganic chemistry (Vol. 70, pp. 343–394). Amsterdam: Elsevier.
Castano, A. P., Demidova, T. N., & Hamblin, M. R. (2004). Mechanisms in photodynamic therapy: Part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis and Photodynamic Therapy, 1, 279–293.
Yakavets, I., Millard, M., Zorin, V., Lassalle, H.-P., & Bezdetnaya, L. (2019). Current state of the nanoscale delivery systems for temoporfin-based photodynamic therapy: Advanced delivery strategies. Journal of Controlled Release, 304, 268–287.
Zeinali, M., Abbaspour-Ravasjani, S., Ghorbani, M., Babazadeh, A., Soltanfam, T., Santos, A. C., Hamishehkar, H., & Hamblin, M. R. (2020). Nanovehicles for co-delivery of anticancer agents. Drug Discovery Today, 25, 1416–1430.
Kustov, A. V., Morshnev, P. K., Kukushkina, N. V., Smirnova, N. L., Berezin, D. B., Karimov, D. R., Shukhto, O. V., Kustova, T. V., Belykh, D. V., Mal’shakova, M. V., Zorin, V. P., & Zorina, T. E. (2022). Solvation, cancer cell photoinactivation and the interaction of chlorin photosensitizers with a potential passive carrier non-ionic surfactant tween 80. International Journal of Molecular Sciences, 23, 5294.
Kustov, A. V., Berezin, D. B., Kruchin, S. O., & Batov, D. V. (2022). Interaction of macrocyclic dicationic photosensitizers with tween 80. Russian Journal of Physical Chemistry, 96, 793–799.
Jori, G. (2007). Ciba foundation symposium 146—Photosensitizing compounds: Their chemistry, biology and clinical use (pp. 78–94). Chichester: Wiley.
Khludeev, I. I., Kozyr’, L. A., Zorina, T. E., & Zorin, V. P. (2015). pH-dependent changes in the mechanisms of transport of chlorine e6 and its derivatives in the blood. Bulletin of Experimental Biology and Medicine, 160, 208–212.
Mishchenko, T., Balalaeva, I., Gorokhova, A., Vedunova, M., & Krysko, D. V. (2022). Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death & Disease, 13, 455.
Acknowledgements
This work was financially supported by the Russian Science Foundation (project No. 21-13-00398). The compounds synthesized were analyzed using the equipment of the Center of Collective Usage (CCU) «Chemistry» at the Institute of Chemistry of the Komi Scientific Centre of the Ural Branch RAS. The studies of biological activity were carried out using the equipment of the Center for Collective Use «Molecular Biology» at the Institute of Biology of the Komi Scientific Centre of the Ural Branch RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest exist.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belykh, D.V., Pylina, Y.I., Kustov, A.V. et al. Photosensitizing effects and physicochemical properties of chlorophyll a derivatives with hydrophilic oligoethylene glycol fragments at the macrocycle periphery. Photochem Photobiol Sci 23, 409–420 (2024). https://doi.org/10.1007/s43630-023-00527-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00527-w