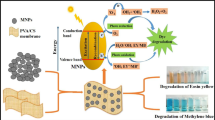
Abstract
Flavin mononucleotide (FMN) is a dye belonging to the flavin family. These dyes produce photosensitized degradation of organic compounds via reaction with the excited states of the dye or with reactive oxygen species photogenerated from the triplet of the dye. This article presents a new polymeric dye (FMN–CS) composed of the photosensitizer FMN covalently bonded to chitosan polysaccharide (CS). FMN–CS obtained has a molecular weight of 230 × 103 g mol−1 and a deacetylation degree of 74.8%. The polymeric dye is an environmentally friendly polymer with spectroscopic and physicochemical properties similar to those of FMN and CS, respectively. Moreover, under sunlight, it is capable of generating 1O2 with a quantum yield of 0.31. FMN–CS, like CS, is insoluble in basic media. This allows easy recovery of the polymeric dye once the photosensitized process has been carried out and makes FMN–CS a suitable photosensitizer for the degradation of pollutants in contaminated waters. To evaluate whether FMN–CS may be used for pollutant degradation, the photosensitized degradation of two trihydroxybenzenes by FMN–CS was studied.
Graphical abstract









Similar content being viewed by others
References
Marin, M. L., Santos-Juanes, L., Arques, A., Amat, A. M., & Miranda, M. A. (2012). Organic photocatalysts for the oxidation of pollutants and model compounds. Chemical Reviews, 112, 1710–1750. https://doi.org/10.1021/cr2000543
Cabezuelo, O., Martinez-Haya, R., Montes, N., Bosca, F., & Marin, M. L. (2021). Heterogeneous riboflavin-based photocatalyst for pollutant oxidation through electron transfer processes. Applied Catalysis B: Environmental, 298, 120497. https://doi.org/10.1016/j.apcatb.2021.120497
Barbieri, Y., Massad, W. A., Díaz, D. J., Sanz, J., Amat-Guerri, F., & Garcia, N. A. (2008). Photodegradation of bisphenol A and related compounds under natural-like conditions in the presence of riboflavin: kinetics, mechanism and photoproducts. Chemosphere, 73, 564–571. https://doi.org/10.1016/j.chemosphere.2008.06.013
Ferrari, G. V., Andrada, M. E., Natera, J., Muñoz, V. A., Paulina Montãna, M., Gambetta, C., Boiero, M. L., Montenegro, M. A., Massad, W. A., & García, N. A. (2014). The employment of a removable chitosan-derivatized polymeric sensitizer in the photooxidation of polyhydroxylated water-pollutants. Photochemistry and Photobiology, 90, 1251–1256. https://doi.org/10.1111/php.12350
Tobin, J. M., McCabe, T. J. D., Prentice, A. W., Holzer, S., Lloyd, G. O., Paterson, M. J., Arrighi, V., Cormack, P. A. G., & Vilela, F. (2017). Polymer-supported photosensitizers for oxidative organic transformations in flow and under visible light irradiation. ACS Catalysis, 7, 4602–4612. https://doi.org/10.1021/acscatal.7b00888
Blacha-Grzechnik, A., Drewniak, A., Walczak, K. Z., Szindler, M., & Ledwon, P. (2020). Efficient generation of singlet oxygen by perylene diimide photosensitizers covalently bound to conjugate polymers. Journal of Photochemistry and Photobiology A: Chemistry, 388, 112161. https://doi.org/10.1016/j.jphotochem.2019.112161
Wondraczek, H., Kotiaho, A., Fardim, P., & Heinze, T. (2011). Photoactive polysaccharides. Carbohydrate Polymers, 83, 1048–1061. https://doi.org/10.1016/j.carbpol.2010.10.014
Escalada, J. P., Pajares, A., Bregliani, M., Biasutti, A., Criado, S., Molina, P., Massad, W., & García, N. A. (2014). Kinetic aspects of the photodegradation of phenolic and lactonic biocides under natural and artificial conditions. Advanced Oxidation Technologies - Sustainable Solutions for Environmental Treatments, 9, 59–80.
Natera, J., Gatica, E., Challier, C., Possetto, D., Massad, W., Miskoski, S., Pajares, A., & García, N. A. (2015). On the photooxidation of the multifunctional drug niclosamide. A kinetic study in the presence of vitamin B2 and visible light. Redox Report, 20, 259–266. https://doi.org/10.1179/1351000215Y.0000000010
Krishna, C. M., Uppuluri, S., Riesz, P., Zigler Jr, J. S., & Balasubramanian, D. (1991). A study of the photodynamic efficiencies of some eye lens constituents. Photochemistry and Photobiology, 54, 51–58. https://doi.org/10.1111/j.1751-1097.1991.tb01984.x
Liang, J.-Y., Yuann, J.-M.P., Cheng, C.-W., Jian, H.-L., Lin, C.-C., & Chen, L.-Y. (2013). Blue light induced free radicals from riboflavin on E. coli DNA damage. Journal of Photochemistry and Photobiology B: Biology, 119, 60–64. https://doi.org/10.1016/j.jphotobiol.2012.12.007
Waiman, C. V., Natera, J., Massad, W. A., & Zanini, G. P. (2020). Novel hybrid materials based on alginate-boehmite-riboflavin for photogeneration of reactive oxygen species in aqueous media. Potential environmental implications. Dyes and Pigments, 177, 108281. https://doi.org/10.1016/j.dyepig.2020.108281
Ray, C., Caillau, M., Jonin, C., Benichou, E., Moulin, C., Salmon, E., Maldonado, M. E., Gomes, A. S. L., Monnier, V., Laurenceau, E., Leclercq, J.-L., Chevolot, Y., Delair, T., & Brevet, P.-F. (2018). Quadratic nonlinear optics to assess the morphology of riboflavin doped chitosan for eco-friendly lithography. Optical Materials, 80, 30–36. https://doi.org/10.1016/j.optmat.2018.04.007
Ronzani, F., Saint-Cricq, P., Arzoumanian, E., Pigot, T., Blanc, S., Oelgemöller, M., Oliveros, E., Richard, C., & Lacombe, S. (2014). Immobilized organic photosensitizers with versatile reactivity for various visible-light applications. Photochemistry and Photobiology, 90, 358–368. https://doi.org/10.1111/php.12166
Renault, F., Sancey, B., Badot, P.-M., & Crini, G. (2009). Chitosan for coagulation/flocculation processes—An eco-friendly approach. European Polymer Journal, 45, 1337–1348. https://doi.org/10.1016/j.eurpolymj.2008.12.027
Bonnett, R., Krysteva, M. A., Lalov, I. G., & Artarsky, S. V. (2006). Water disinfection using photosensitizers immobilized on chitosan. Water Research, 40, 1269–1275.
Gmurek, M., Foszpańczyk, M., Olak-Kucharczyk, M., Gryglik, D., & Ledakowicz, S. (2017). Photosensitive chitosan for visible-light water pollutant degradation. Chemical Engineering Journal, 318, 240–246. https://doi.org/10.1016/j.cej.2016.06.125
Dibona-Villanueva, L., & Fuentealba, D. (2021). Novel chitosan-riboflavin conjugate with visible light-enhanced antifungal properties against Penicillium digitatum. Journal of Agriculture and Food Chemistry, 69, 945–954. https://doi.org/10.1021/acs.jafc.0c08154
Garcia, N. A. (1992). Environmental significance of singlet molecular oxygen-mediated degradation of phenolic aquatic pollutants. Journal of Photochemistry and Photobiology B, 14, 381–383.
Pardeshi, S. K., & Patil, A. B. (2009). Solar photocatalytic degradation of resorcinol a model endocrine disrupter in water using zinc oxide. Journal of Hazardous Materials, 163, 403–409. https://doi.org/10.1016/j.jhazmat.2008.06.111
Hanafy, A. I., Hassan, A. M., El-Rahman, N. M. A., Al-Sayed, M. M., Hanafy, A. I., Hassan, A. M., El-Rahman, N. M. A., & Al-Sayed, M. M. (2012). Oxidation of polyphenol trihydroxybenzene using environment friendly catalyst Copper(II) complex of 4-methoxyphenyl benzopyran oxidation of polyphenol trihydroxybenzene using environment friendly catalyst Copper(II) complex of 4-methoxyphenyl. Journal of American Science, 8, 22–27. https://doi.org/10.7537/marsjas081012.05
Choi, S. H., Collins, J. N. R., Smith, S. A., Davis-Harrison, R. L., Rienstra, C. M., & Morrissey, J. H. (2010). Phosphoramidate end labeling of inorganic polyphosphates: Facile manipulation of polyphosphate for investigating and modulating its biological activities. Biochemistry, 49, 9935–9941. https://doi.org/10.1021/bi1014437
Salehi, E., & Farahani, A. (2017). Macroporous chitosan/polyvinyl alcohol composite adsorbents based on activated carbon substrate. Journal of Porous Materials, 24, 1197–1207. https://doi.org/10.1007/s10934-016-0359-9
Knaul, J. Z., Bui, V. T., Creber, K. A. M., & Kasaai, M. R. (1998). Characterization of deacetylated chitosan and chitosan molecular weight review. Canadian Journal of Chemistry, 76, 1699–1706. https://doi.org/10.1139/cjc-76-11-1699
Roy, B., Depaix, A., Périgaud, C., & Peyrottes, S. (2016). Recent trends in nucleotide synthesis. Chemical Reviews, 116, 7854–7897. https://doi.org/10.1021/acs.chemrev.6b00174
James, T. L. (1985). Phosphorus-31 NMR as a probe for phosphoprotein. Critical Reviews in Biochemistry, 18, 1–30. https://doi.org/10.3109/10409238509082538
Domszy, J. G., & Roberts, G. A. F. (1985). Evaluation of infrared spectroscopic techniques for analysing chitosan. Makromolekulare Chemie, 186, 1671–1677. https://doi.org/10.1002/macp.1985.021860815
Morales, G., Pajares, A., Natera, J., Escalada, J. P., Massad, W., & García, N. A. (2017). The riboflavin-photosensitized degradation of the UV-absorbing azo dye-metabolites benzidine and o-tolidine. Kinetic and mechanistic aspects. Journal of Photochemistry and Photobiology A: Chemistry, 344, 49–55. https://doi.org/10.1016/j.jphotochem.2017.04.035
Gambetta, C., Natera, J., Massad, W. A., & García, N. A. (2013). Methyl anthranilate as generator and quencher of reactive oxygen species: A photochemical study. Journal of Photochemistry and Photobiology A: Chemistry, 269, 27–33. https://doi.org/10.1016/j.jphotochem.2013.06.013
Miskoski, S., & García, N. A. (1991). Dark and photoinduced interactions between riboflavin and indole auxins. Collection of Czechoslovak Chemical Communications, 56, 1838–1849. https://doi.org/10.1135/cccc19911838
Massad, W. A., Barbieri, Y., Romero, M., & Garcia, N. A. (2008). Vitamin B2-sensitized photo-oxidation of dopamine. Photochemistry and Photobiology, 84, 1201–1208.
Hermanson, G. T. (2008). Chapter 1—Functional targets. In G. T. Hermanson (Ed.), Bioconjugate techniques (2nd ed., pp. 1–168). Academic Press. https://doi.org/10.1016/B978-0-12-370501-3.00001-1
Thuillier, G., Floyd, L., Woods, T. N., Cebula, R., Hilsenrath, E., Hersé, M., & Labs, D. (2004). Solar irradiance reference spectra. Solar variability and its effects on climate (pp. 171–194). American Geophysical Union (AGU). https://doi.org/10.1029/141GM13
Losi, A., & Gärtner, W. (2011). Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochemistry and Photobiology, 87, 491–510. https://doi.org/10.1111/j.1751-1097.2011.00913.x
Nyquist, R. A. (1963). Correlations between infrared spectra and structure: phosphoramides and related compounds. Spectrochimica Acta, 19, 713–729. https://doi.org/10.1016/0371-1951(63)80137-X
Larkin, P. J. (2018). General outline for IR and Raman spectral interpretation. Infrared and Raman spectroscopy (pp. 135–151). Elsevier. https://doi.org/10.1016/B978-0-12-804162-8.00007-0
Iuliano, J. N., French, J. B., & Tonge, P. J. (2019). Vibrational spectroscopy of flavoproteins. Methods in enzymology (Vol. 620, pp. 189–214). Elsevier. https://doi.org/10.1016/bs.mie.2019.03.011
Spexard, M., Immeln, D., Thöing, C., & Kottke, T. (2011). Infrared spectrum and absorption coefficient of the cofactor flavin in water. Vibrational Spectroscopy, 57, 282–287. https://doi.org/10.1016/j.vibspec.2011.09.002
Pawlak, A., & Mucha, M. (2003). Thermogravimetric and FTIR studies of chitosan blends. Thermochimica Acta, 396, 153–166. https://doi.org/10.1016/S0040-6031(02)00523-3
Edmondson, D. E., & James, T. L. (1979). Covalently bound non-coenzyme phosphorus residues in flavoproteins: 31P nuclear magnetic resonance studies of Azotobacter flavodoxin. Proceedings of the National Academy of Sciences, 76, 3786–3789. https://doi.org/10.1073/pnas.76.8.3786
Corazzari, I., Nisticò, R., Turci, F., Faga, M. G., Franzoso, F., Tabasso, S., & Magnacca, G. (2015). Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polymer Degradation and Stability, 112, 1–9. https://doi.org/10.1016/j.polymdegradstab.2014.12.006
Kim, S., Jang, M., Park, M., Park, N.-H., & Ju, S.-Y. (2017). A self-assembled flavin protective coating enhances the oxidative thermal stability of multi-walled carbon nanotubes. Carbon, 117, 220–227. https://doi.org/10.1016/j.carbon.2017.02.098
Heelis, P. F. (1982). The photophysical and photochemical properties of flavins (isoalloxazines). Chemical Society Reviews, 11, 15–39. https://doi.org/10.1039/CS9821100015
Grajek, H. (2011). Review—Flavins as photoreceptors of blue light and their spectroscopic properties. Current Topics in Biophysics, 34, 53–65. https://doi.org/10.2478/v10214-011-0008-z
Drössler, P., Holzer, W., Penzkofer, A., & Hegemann, P. (2002). pH dependence of the absorption and emission behaviour of riboflavin in aqueous solution. Chemical Physics, 282, 429–439. https://doi.org/10.1016/S0301-0104(02)00731-0
Görner, H. (2007). Oxygen uptake after electron transfer from amines, amino acids and ascorbic acid to triplet flavins in air-saturated aqueous solution. Journal of Photochemistry and Photobiology B: Biology, 87, 73–80. https://doi.org/10.1016/j.jphotobiol.2007.02.003
Barbieri, Y. (2008). Photodegradation of bisphenol A and related compounds under natural-like conditions in the presence of riboflavin: kinetics, mechanism and photoproducts. Chemosphere, 73, 564–571.
Haggi, E., Bertolotti, S., Miskoski, S., Amat-Guerri, F., & García, N. A. (2002). Environmental photodegradation of pyrimidine fungicides—Kinetics of the visible-light-promoted interactions between riboflavin and 2-amino-4-hydroxy-6-methylpyrimidine. Canadian Journal of Chemistry, 80, 62–67. https://doi.org/10.1139/v01-192
Gambetta, C., Massad, W. A., Nesci, A. V., & García, N. A. (2015). Vitamin B2-sensitized degradation of the multifunctional drug Evernyl, in the presence of visible light—microbiological implications. Pure and Applied Chemistry, 87, 997–1010. https://doi.org/10.1515/pac-2015-0407
Chacon, J. N., McLearie, J., & Sinclair, R. S. (1988). Singlet oxygen yields and radical contributions in the dye-sensitised photo-oxidation in methanol of esters of polyunsaturated fatty acids (oleic, linoleic, linolenic and arachidonic). Photochemistry and Photobiology, 47, 647–656. https://doi.org/10.1111/j.1751-1097.1988.tb02760.x
Holzer, W., Shirdel, J., Zirak, P., Penzkofer, A., Hegemann, P., Deutzmann, R., & Hochmuth, E. (2005). Photo-induced degradation of some flavins in aqueous solution. Chemical Physics, 308, 69–78.
Vanden Braber, N. L., Paredes, A. J., Rossi, Y. E., Porporatto, C., Allemandi, D. A., Borsarelli, C. D., Correa, S. G., & Montenegro, M. A. (2018). Controlled release and antioxidant activity of chitosan or its glucosamine water-soluble derivative microcapsules loaded with quercetin. International Journal of Biological Macromolecules, 112, 399–404. https://doi.org/10.1016/j.ijbiomac.2018.01.085
Yen, M.-T., Yang, J.-H., & Mau, J.-L. (2008). Antioxidant properties of chitosan from crab shells. Carbohydrate Polymers, 74, 840–844. https://doi.org/10.1016/j.carbpol.2008.05.003
Gutiérrez, M. I., Soltermann, A. T., Amat-Guerri, F., & Garcı́a, N. A. (2000). Kinetics of the dye-sensitized photooxidation of trihydroxybenzenes. Journal of Photochemistry and Photobiology A: Chemistry, 136, 67–71. https://doi.org/10.1016/S1010-6030(00)00309-9
Zhang, R., Wu, C., Tong, L., Tang, B., & Xu, Q.-H. (2009). Multifunctional core−shell nanoparticles as highly efficient imaging and photosensitizing agents. Langmuir, 25, 10153–10158. https://doi.org/10.1021/la902235d
Acknowledgements
We are grateful to Secretaría de Ciencia y Técnica of Universidad Nacional de Río Cuarto (SECyT-UNRC), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), all from Argentina, for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sacchetto, J., Gutierrez, E., Reta, G.F. et al. A novel eco-friendly polymeric photosensitizer based on chitosan and flavin mononucleotide. Photochem Photobiol Sci 22, 2827–2837 (2023). https://doi.org/10.1007/s43630-023-00489-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00489-z