Abstract
Natural antioxidants have attracted attention for their therapeutic use as photochemopreventive agents. Inga edulis leaves extract and its purified fraction have high polyphenolic content and high antioxidant capacity. In addition, they presented UV photostability and low citotoxicity in fibroblast cells. In this context, this study first aimed at development of topical formulation containing purified fraction of I. edulis extract and the evaluation of skin penetration of the compounds. Moreover, the photoprotective/photochemopreventive potential of the formulation containing I. edulis purified fraction were investigated in vitro and in vivo. The topical formulation containing 1% of the purified fraction of I. edulis increased the endogenous antioxidant potential of the skin, and vicenin-2 and myricetin compounds were able to penetrate the epidermis and dermis. Additionally, the purified fraction (25 and 50 mg/mL) showed a photoprotective effect against UVA and UVB radiation in L929 fibroblast cells. In vivo studies have shown that the formulation added with purified fraction provided an anti-inflammatory effect on the skin of animals after UVB exposure, since it was observed a reduction in MPO activity, IL-1β and TNF-α cytokines, and CXCL1/KC chemokine concentrations. In conclusion, the purified fraction of I. edulis, rich in phenolic compounds, when incorporated in topical formulation, appears as an alternative to prevent skin damages induced by UV radiation, due to its antioxidant and anti-inflammatory properties.
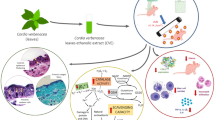
Graphical abstract








Similar content being viewed by others
Data availability
Data will be available on demand.
References
Lai-Cheong, J. E., & McGrath, J. A. (2009). Structure and function of skin, hair and nails. Medicine, 37(5), 223–226. https://doi.org/10.1016/j.mpmed.2009.03.002
Sharma, R. R., Deep, A., & Abdullah, S. T. (2021). Herbal products as skincare therapeutic agents against ultraviolet radiation-induced skin disorders. Journal of Ayurveda and Integrative Medicine, 13(1), 100500. https://doi.org/10.1016/j.jaim.2021.07.016
Holick, M. F. (2016). Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Research, 36(3), 1345–1356.
de Oca, M. K. M., Pearlman, R. L., McClees, S. F., Strickland, R., & Afaq, F. (2017). Phytochemicals for the prevention of photocarcinogenesis. Photochemistry Photobiology, 93, 956–974. https://doi.org/10.1111/php.12711
Figueiredo, S. A., de Moraes, D. C., Vilela, F. M. P., de Faria, A. N., dos Santos, M. H., & Fonseca, M. J. V. (2018). A novel research model for evaluating sunscreen protection in the UV-A1. Journal of Photochemistry and Photobiology, B: Biology, 178, 61–68. https://doi.org/10.1016/j.jphotobiol.2017.10.031
Divya, S. P., Wang, X., Pratheeshkumar, P., Son, Y.-O., Roy, R. V., Kim, D., Dai, J., Hitron, J. A., Wang, L., Asha, P., Shi, X., & Zhang, Z. (2015). Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicology and Applied Pharmacology, 284(1), 92–99. https://doi.org/10.1016/j.taap.2015.02.003
Figueiredo, S. A., Vilela, F. M. P., da Silva, C. A., Cunha, T. M., dos Santos, M. H., & Fonseca, M. J. V. (2014). In vitro and in vivo photoprotective/photochemopreventive potential of Garcinia brasiliensis epicarp extract. Journal of Photochemistry and Photobiology B: Biology, 131, 65–73. https://doi.org/10.1016/j.jphotobiol.2014.01.004
Afaq, F., & Mukhtar, H. (2006). Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Experimental Dermatology, 15, 678–684. https://doi.org/10.1111/j.1600-0625.2006.00466.x
Radhiga, T., Agilan, B., Muzaffer, U., Karthikeyan, R., Kanimozhi, G., Paul, V. I., & Prasad, N. R. (2016). Phytochemicals as modulators of ultraviolet-b radiation induced cellular and molecular events: A review. Journal of Radiation and Cancer Research, 7(1), 2–12. https://doi.org/10.4103/0973-0168.184607
Alves, G. A. D., da Silva, D. F., Teixeira, T. V., de Souza, R. O., Rogez, H., & Fonseca, M. J. V. (2020). Obtainment of an enriched fraction of Inga edulis: Identification using UPLC-DAD-MS/MS and photochemopreventive screening. Preparative Biochemistry & Biotechnology, 50(1), 28–36. https://doi.org/10.1080/10826068.2019.1658118
Souza, J. N. S., Silva, E. M., da Silva, M. N., Arruda, M. S. P., Larondelle, Y., & Rogez, H. (2007). Identification and antioxidant activity of several flavonoids of Inga edulis leaves. Journal of the Brazilian Chemical Society, 18, 1276–1280. https://doi.org/10.1590/S0103-50532007000600025
Figueiredo, S. A., Vilela, M. F. P., dos Anjos, T. N., de Pádua, A. N. F., & Fonseca, M. J. V. (2021). Evaluation of cell biomarkers as in vitro photoprotective assays for sunscreen formulations. Journal of Basic and Applied Pharmaceutical Sciences, 42, e713. https://doi.org/10.4322/2179-443X.0713
Laporte, A., Lortz, S., Schaal, C., Lenzen, S., & Elsner, M. (2020). Hydrogen peroxide permeability of cellular membranes in insulin-producing cell. BBB-Biomembranes, 1862(2), 183096. https://doi.org/10.1016/j.bbamem.2019.183096
Yu, D., Zha, Y., Zhong, Z., Ruan, Y., Li, Z., Sun, L., & Hou, S. (2021). Improved detection of reactive oxygen species by DCFH-DA: New insight into self-amplification of fluorescence signal by light irradiation. Sensors and Actuators B: Chemical, 339, 129878. https://doi.org/10.1016/j.snb.2021.129878
Wang, H., & Joseph, J. A. (1999). Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biology & Medicine, 27, 612–616. https://doi.org/10.1016/S0891-5849(99)00107-0
Silva, D. F. (2012). Extract and medium polarity fraction of Inga edulis: Physico chemical, functional, cytotoxic studies and penetration/retention evaluation of cutaneous topical formulations (p. 119). Dissertation (master). Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil
Vilela, F. M. P., Fonseca, Y. M., Jabor, J. R., Vicentini, F. T. M. C., & Fonseca, M. J. V. (2012). Effect of ultraviolet filters on skin superoxide dismutase activity in hairless mice after a single dose of ultraviolet radiation. European Journal of Pharmaceutics and Biopharmaceutics, 80, 387–392. https://doi.org/10.1016/j.ejpb.2011.10.005
Girotti, S., Fini, F., Ferri, E., Budini, R., Piazzi, S., & Cantagalli, S. (2000). Determination of superoxide dismutase in erytrocytes by a chemiluminescent assay. Talanta, 51, 685–692. https://doi.org/10.1016/S0039-9140(99)00332-X
Georgetti, S. R., Casagrande, R., Di Mambro, M. V., Azzolini, A. E. C. S., & Fonseca, M. J. V. (2003). Evaluation of the antioxidant activity of different flavonoids by the chemiluminescent method. AAPS PharmSci, 5, 210–214. https://doi.org/10.1208/ps050216
Cayrol, C., Sarraute, J., Tarroux, R., Redoules, D., Charveron, M., & Gall, Y. (1999). A mineral sunscreen affords genomic protection against ultraviolet (UV) B and UVA radiation: in vitro and in situ assays. British Journal of Dermatology, 141(2), 250–258. https://doi.org/10.1046/j.1365-2133.1999.02973.x
Chrétien, M. N., Heafey, E., & Scaiano, J. C. (2010). Reducing adverse effects from UV sunscreens by zeolite encapsulation: Comparison of oxybenzone in solution and in zeolites. Photochemistry and Photobiology, 86, 153–161. https://doi.org/10.1111/j.1751-1097.2009.00644.x
Yoshimoto, S., Kohara, N., Sato, N., Ando, H., & Ichihashi, M. (2020). Riboflavin plays a pivotal role in the UVA-induced cytotoxicity of fibroblasts as a key molecule in the production of H2O2 by UVA radiation in collaboration with amino acids and vitamins. International Journal of Molecular Sciences, 21(2), 554. https://doi.org/10.3390/ijms21020554
Vicentini, F. T. M. C., Simi, T. R. M., Del Ciampo, J. O., Wolga, N. O., Pitol, D. L., Iyomasa, M. M., Bentley, M. V. L. B., & Fonseca, M. J. V. (2008). Quercetin in w/o microemulsion: In vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. European Journal of Pharmaceutics and Biopharmaceutics, 69(3), 948–957. https://doi.org/10.1016/j.ejpb.2008.01.012
Vilela, F. M. P., Fonseca, Y. M., Vicentini, F. T. M. C., & Fonseca, M. J. V. (2013). Sunscreen protection against ultraviolet-induced oxidative stress: Evaluation of reduced glutathione levels, metalloproteinase secretion, and myeloperoxidase activity. Die Pharmazie - An International Journal of Pharmaceutical Sciences, 68(11), 872–876. https://doi.org/10.1691/ph.2013.3045
Vilela, F. M. P., Oliveira, F. M., Vicentini, F. T. M. C., Casagrande, R., Verri, W. A., Jr., Cunha, T. M., & Fonseca, M. J. V. (2016). Commercial sunscreen formulations: UVB irradiation stability and effect on UVB irradiation-induced skin oxidative stress and inflammation. Journal of Photochemistry and Photobiology, B: Biology, 163, 413–420. https://doi.org/10.1016/j.jphotobiol.2016.09.007
Bradley, P. P., Priebat, D. A., Christensen, R. D., & Rothstein, G. (1982). Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology, 78, 206–209. https://doi.org/10.1111/1523-1747.EP12506462
Casagrande, R., Georgetti, S. R., Verri, W. A., Jr., Dorta, D. J., dos Santos, A. C., & Fonseca, M. J. V. (2006). Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. Journal of Photochemistry and Photobiology B: Biology, 84(1), 21–27. https://doi.org/10.1016/j.jphotobiol.2006.01.006
Zou, B., Xiao, G., Xu, Y., Wu, J., Yu, Y., & Fu, M. (2018). Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chemistry, 255, 23–30. https://doi.org/10.1016/j.foodchem.2018.02.028
Domitrović, R., Rashed, K., Cvijanović, O., Vladimir-Knežević, S., Škoda, M., & Višnić, A. (2015). Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chemico-Biological Interactions, 230, 21–29. https://doi.org/10.1016/j.cbi.2015.01.030
Petruk, G., Illiano, A., Giudice, R. D., Raiola, A., Amoresano, A., Rigano, M. M., Piccoli, R., & Monti, D. M. (2017). Malvidin and cyanidin derivatives from açai fruit (Euterpe oleracea Mart.) counteract UV-A-induced oxidative stress in immortalized fibroblasts. Journal of Photochemistry and Photobiology B: Biology, 172, 42–51. https://doi.org/10.1016/j.jphotobiol.2017.05.013
Ryšava, A., Čižkova, K., Frankova, J., Roubalova, L., Ulrichova, J., Vostalovaa, J., Vrba, J., Zalešak, B., & Svobodova, A. R. (2020). Effect of UVA radiation on the Nrf2 signaling pathway in human skin cells. Journal of Photochemistry & Photobiology, B: Biology, 209, 111948. https://doi.org/10.1016/j.jphotobiol.2020.111948
Jiang, S. J., Chen, J. Y., Lu, Z. F., Yao, J., Che, F. D., & Zhou, X. J. (2006). Biophysical and morphological changes in the stratum corneum lipids induced by UVB irradiation. Journal of Dermatological Science, 44, 29–36. https://doi.org/10.1016/j.jdermsci.2006.05.012
Budai, M., Reynaud-Angelin, A., Szabo, Z., Tóth, S., Rontó, G., Sage, E., & Gróf, P. (2004). Effect of UVA radiation on membrane fluidity and radical decay in human fibroblasts as detected by spin labeled stearic acids. Journal of Photochemistry and Photobiology B: Biology, 77, 27–38. https://doi.org/10.1016/j.jphotobiol.2004.08.003
Moser, K., Kriwet, K., Naik, A., Kalia, Y. N., & Guy, R. H. (2001). Passive skin penetration enhancement and its quantification in vitro. European Journal of Pharmaceutics and Biopharmaceutics, 54(2), 103–112. https://doi.org/10.1016/S0939-6411(01)00166-7
Getie, M., Gebre-Mariam, T., Rietz, R., & Neubert, R. H. H. (2002). Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae). Pharmazie, 57, 320–322.
Wester, C. R. & Maibach, H. L. (1990). In vitro testing of topical pharmaceutical formulations. In: D. W. Osborne & A. H. Amamnn, Topical drug delivery formulations (pp. 213–220). Marcel Dekker
Harborne, J. B., & Williams, C. A. (2000). Advances in flavonoid research since 1992. Phytochemistry, 55, 481–504. https://doi.org/10.1016/S0031-9422(00)00235-1
Liakoura, V., Bornean, J. F., & Karabourniotis, G. (2003). The ability of abaxial and adaxial epiderms of sun and shade leaves to attenuate UV-A and UV-B radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiology, 122, 117–125. https://doi.org/10.1034/j.1399-3054.2003.1170104.x
Merzlyak, M. N., Solovchenko, A. E., Smagin, A. I., & Gitelson, A. A. (2005). Apple flavonols during fruit adaptation to solar radiation: spectral features and technique for non-destructive assessment. Journal of Plant Physiology, 162, 151–160. https://doi.org/10.1016/j.jplph.2004.07.002
Halliday, G. M., Norval, M., Byrne, S. N., Huang, X. X., & Wolf, P. (2008). The effects of sunlight on the skin. Drug Discovery Today: Disease Mechanisms/Common skin conditions and disorders, 5(2), e201–e209. https://doi.org/10.1016/j.ddmec.2008.04.005
Svobodova, A., Walterova, D., & Vostalova, J. (2006). Ultraviolet light induced alteration to the skin. Biomedical papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia, 150(1), 25–38. https://doi.org/10.5507/bp.2006.003
Luster, A. D., Alon, R., & Von Adrian, U. H. (2005). Immune cell migration in inflammation: present and future therapeutic targets. Nature Immunology, 6(12), 1182–1190.
Khan, A. A., Alsahli, M. A., & Rahmani, A. H. (2018). Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Medical Sciences (Basel, Switzerland), 6(2), 33. https://doi.org/10.3390/medsci6020033
Lee, B.-S., Yang, S., Lee, C., Ku, S.-K., & Bae, J.-S. (2020). Renal protective effects of vicenin-2 and scolymoside in a mouse model of sepsis. Brazilian Journal of Pharmaceutical Sciences, 56, e18636. https://doi.org/10.1590/s2175-97902019000418636
Zhang, X., Zhang, K., Wang, Y., & Ma, R. (2020). Effects of myricitrin and relevant mechanisms. Current Stem Cell Research & Therapy, 15, 11–17. https://doi.org/10.2174/1574888X14666181126103338
Müller, K., & Meineke, V. (2007). Radiation-induced alterations in cytokine production by skin cells. Experimental Hematology, 35, 96–104. https://doi.org/10.1016/j.exphem.2007.01.017
Baggiolini, M., Dewald, B., & Moser, B. (1994). Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Advances in Immunology, 55, 97–179. https://doi.org/10.1016/S0065-2776(08)60509-X
Murphy, P. M. (1997). Neutrophil receptors for interleukin-8 and related CXC chemokines. Seminars in Hematology, 34(4), 311–318.
Cassatella, M. A. (1999). Neutrophil-derived proteins: selling cytokines by the pound. Advances in Immunology, 73, 369–509. https://doi.org/10.1016/s0065-2776(08)60791-9
Yin, Y., Ye, L., Niu, Z., & Fang, W. (2019). Anti-inflammatory effects of vicenin-2 on dextran sulfate sodium-induced colitis in mice. Drug Development Research, 80, 546–555. https://doi.org/10.1002/ddr.21529
Kan, X., Liu, B., Guo, W., Wei, L., Lin, Y., Guo, Y., Gong, Q., Li, Y., Xu, D., Cao, Y., Huang, B., Dong, A., Ma, H., Fu, S., & Liu, J. (2019). Myricetin relieves LPS-induced mastitis by inhibiting inflammatory response and repairing the blood-milk barrier. Journal of Cellular Physiology, 234, 16252–16262. https://doi.org/10.1002/jcp.28288
Mao, M. T., & Huang, M. Y. (2017). Myricetin attenuates lung inflammation and provides protection against lipopolysaccharide-induced acute lung injury by inhibition of NF-κB pathway in rats. Tropical Journal of Pharmaceutical Research, 16(11), 2585–2593. https://doi.org/10.4314/tjpr.v16i11.3
Song, X., Tan, L., Wang, M., Ren, C., Guo, C., Yang, B., Ren, Y., Cao, Z., Li, Y., & Pei, J. (2021). Myricetin: A review of the most recente research. Biomedicine & Pharmacotherapy, 134, 111017. https://doi.org/10.1016/j.biopha.2020.111017
Lee, D., & Lee, C. S. (2016). Flavonoid myricetin inhibits TNF-α-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κβ pathways in human keratinocytes. European Journal of Pharmacology, 784, 164–172. https://doi.org/10.1016/j.ejphar.2016.05.025
Xie, J., & Zheng, Y. (2017). Myricetin protects keratinocyte damage induced by UV through IκB/NFκb signaling pathway. Journal of Cosmetic Dermatology, 16(4), 444–449. https://doi.org/10.1111/jocd.12399
Acknowledgements
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support. The authors also thank Professor Hervé Louis Ghislain Rogez from Federal University of Pará - Brazil for kindly providing the purified fraction of Inga edulis extract.
Funding
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil [grant number 166315/2013-3] and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, Brazil [grant number 001].
Author information
Authors and Affiliations
Contributions
KCC performed the experiments, collected the data and evaluated the results; FMPV and SAF performed the evaluation and interpretation of the data and the writing of the article; CHFC performed the evaluation and interpretation of the data; MJVF designed the study and performed the interpretation of the data and wrote the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-fnancial interests to disclose.
Ethical approval
The Ethics Committee of the Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, approved the study with the use of animals under Protocol # 13.1.525.53.1.
Consent of publication
All authors agree with the publication of this manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Costa, K.C., Cuelho, C.H.F., Figueiredo, S.A. et al. Photochemoprevention of topical formulation containing purified fraction of Inga edulis leaves extract. Photochem Photobiol Sci 22, 2105–2120 (2023). https://doi.org/10.1007/s43630-023-00433-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00433-1