Abstract
Light is an environmental signal that modulates plant defenses against attackers. Recent research has focused on the effects of light on defense hormone signaling; however, the connections between light signaling pathways and the biosynthesis of specialized metabolites involved in plant defense have been relatively unexplored. Here, we show that Arabidopsis BBX29, a protein that belongs to the B-Box transcription factor (TF) family, integrates photomorphogenic signaling with defense responses by promoting flavonoid, sinapate and glucosinolate accumulation in Arabidopsis leaves. AtBBX29 transcript levels were up regulated by light, through photoreceptor signaling pathways. Genetic evidence indicated that AtBBX29 up-regulates MYB12 gene expression, a TF known to induce genes related to flavonoid biosynthesis in a light-dependent manner, and MYB34 and MYB51, which encode TFs involved in the regulation of glucosinolate biosynthesis. Thus, bbx29 knockout mutants displayed low expression levels of key genes of the flavonoid biosynthetic pathway, and the opposite was true in BBX29 overexpression lines. In agreement with the transcriptomic data, bbx29 mutant plants accumulated lower levels of kaempferol glucosides, sinapoyl malate, indol-3-ylmethyl glucosinolate (I3M), 4-methylsulfinylbutyl glucosinolate (4MSOB) and 3-methylthiopropyl glucosinolate (3MSP) in rosette leaves compared to the wild-type, and showed increased susceptibility to the necrotrophic fungus Botrytis cinerea and to the herbivore Spodoptera frugiperda. In contrast, BBX29 overexpressing plants displayed increased resistance to both attackers. In addition, we found that AtBBX29 plays an important role in mediating the effects of ultraviolet-B (UV-B) radiation on plant defense against B. cinerea. Taken together, these results suggest that AtBBX29 orchestrates the accumulation of specific light-induced metabolites and regulates Arabidopsis resistance against pathogens and herbivores.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plants cope with abiotic and biotic stresses by activating adaptive physiological and morphological responses. Some of these plastic responses appear to be effective against multiple stresses, suggesting that different environmental signals can converge in the regulation of similar cellular pathways. The accumulation of specialized metabolites in leaves is a good example of this convergence. For example, anthocyanins, carotenoids, flavonoids and glucosinolates (GS) can be induced by a large number of environmental factors, including low temperature, high light, ultraviolet (UV) radiation, pathogen attack, wounding, and nutritional stress, among others, and can provide protection against multiple stressors [1,2,3].
Light is one of the environmental factors that has a strong effect on the accumulation of specialized metabolites in leaves, acting through photomorphogenetic signaling pathways that are activated by photoreceptors, such as the red- and far-red light-sensing phytochromes, the blue/UV-A-perceiving cryptochromes and phototropins, and the UV-B-sensing photoreceptor UVR8 [4, 5]. The action of photoreceptors depends on a set of TFs that belong to different families, such as bZIPs, MYBs and BBXs, which orchestrate the transcriptional responses to changes in the light environment [6,7,8]. In Arabidopsis seedlings, HY5 (ELONGATED HYPOCOTYL 5) forms a central hub downstream of all photoreceptors, to induce the transcriptional activation of MYB TFs that regulate the biosynthesis of phenolic compounds. These TF include MYB11, MYB12 and MYB111 for flavonol, MYB123 for proanthocyanidin and MYB75/PAP1 for proanthocyanin biosynthesis [9,10,11]. Downstream of HY5 and MYBs, light-related factors establish a coordinated action to activate the transcription of genes of the phenylpropanoid biosynthetic pathway, including CHALCONE SYNTHASE (CHS), FLAVONOL SYNTHASE (FLS), FLAVANONE 3-HYDROXYLASE (F3H) and CHALCONE ISOMERASE (CHI) [12, 13].
The involvement of BBX proteins in the photoregulation of specialized metabolism has been studied in Arabidopsis seedlings, leading to the description of possible models of BBX action. In a general model, BBXs appear as potential partners for HY5 to coordinate and modulate its specificity and activity in the transcriptional regulation of photomorphogenic genes, including some MYB TFs and genes belonging to the flavonoid biosynthetic pathway [14, 15]. For example, AtBBX21 and AtBBX22 interact with HY5 and enhance its biochemical activity leading to increased expression of flavonoid biosynthesis genes and accumulation of anthocyanins [15,16,17]. AtBBX23 plays a positive role in the control of anthocyanin accumulation by binding to the CHS promoter region in a HY5-dependent manner [18]. In contrast, AtBBX24, AtBBX25 and AtBBX32 negatively control anthocyanin accumulation likely by inhibiting HY5 activity and the transcription of HY5-activated genes [19,20,21]. Beyond this model, some BBXs have been reported to directly associate with promoters of various flavonoid biosynthesis genes to alter their expression in an HY5-independent manner. For example, AtBBX21 and AtBBX22 physically bind to the promoter of CHI and activate CHI transcription [15].
In contrast to the situation in seedlings, the mechanisms by which Arabidopsis BBXs regulate the light-induced accumulation of specialized metabolites in rosette leaves is not well established. There is evidence from studies carried out in various crop species that indicate an involvement of BBXs in the photoregulation of flavonoid accumulation. In poplar, PtBBX23 directly binds to the promoter regions of proanthocyanidin and anthocyanin-specific genes to enhance their transcription [22]. In rice, anthocyanin biosynthesis is induced and fine-tuned by OsBBX14 [23]. In pear, PpBBX16 and PpBBX18 antagonistically regulate light-induced anthocyanin accumulation in the fruit [24]. In apple, MdBBX33 and MdBBX37 have been reported to influence anthocyanin accumulation in a light-dependent manner [25, 26]. Heterologous expression of AtBBX21 in potato plants induced the expression of phenylpropanoid biosynthesis genes and showed a higher production of anthocyanins and phenolic compounds [27]. These results suggest a central role of BBX proteins in the regulation of specialized metabolism in green plants.
It has been demonstrated that light-regulated metabolites like flavonoids, sinapates and GSs are functionally important in plant defense against a variety of attackers [28,29,30,31,32]. In several species, low Red/Far-Red ratios (a signal of shading and plant-plant competition) promote plant susceptibility via the inactivation of phytochrome B, which leads to reduced accumulation of phenylpropanoids and GSs in leaves and attenuation of JA-mediated responses [33,34,35]. In contrast, UV-B radiation (which is associated with open habitats and canopy gaps) can enhance plant defense against pathogens and herbivores via JA-dependent and JA-independent mechanisms [36, 37]. In Arabidopsis, physiological doses of UV-B radiation promote the accumulation of phenolic compounds, including flavonoids and sinapates, in a JA-independent manner [30]. Furthermore, at least part of the effect of UV-B radiation increasing plant resistance to the fungus Botrytis cinerea is mediated by the UVR8 photoreceptor via stimulation of sinapate biosynthesis [30]. GSs also play a main role as defensive metabolites in the Brassicaceae family, where the accumulation of GSs negatively affects the growth and development of some herbivores and mediates plant antifungal defenses [31, 38,39,40]. It has been shown that light could modulate the production of GSs vía regulation of GS biosynthetic genes [41, 42] and CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), the key repressor of light signaling, can regulate GS biosynthesis, acting through a mechanism dependent of JA-signaling [32].
Here we show that AtBBX29, a BBX protein that belongs to the structure group V [43] is involved in flavonoid and glucosinolate accumulation in Arabidopsis leaves. AtBBX29 transcription was regulated by light acting through photoreceptors, and the AtBBX29 protein showed nuclear localization. We found that AtBBX29 positively regulated the transcription of several MYB genes and genes encoding enzymes involved in the biosynthesis of specific photoprotective compounds. bbx29 mutant plants were impaired in the accumulation of flavonoids (kaempferol glycosides), sinapates (sinapoyl malate) and some glucosinolates (indol-3-ylmethyl glucosinolate, I3M; 4-methylsulfinylbutyl glucosinolate, 4MSOB and 3-methylthiopropyl glucosinolate 3MSP) in rosette leaves, and this reduced accumulation of defensive metabolites correlated with higher susceptibility to the fungus B. cinerea and to an insect herbivore (Spodoptera frugiperda). In contrast, BBX29 overexpressing plants displayed increased resistance to both attackers. Transcripts of AtBBX29 were also up-regulated by methyl jasmonate (MeJA) treatment in a transient manner, but jasmonate metabolism and response appeared to be functional in bbx29 mutants. Taken together, these results suggest that AtBBX29 is involved in the photoregulation of the biosynthesis of specialized metabolites that play an important role in Arabidopsis defense against attackers.
2 Results
2.1 Red, Blue and UV-B light induce At BBX29 transcript levels through the photomorphogenic signaling pathways
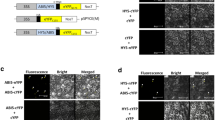
Previous work revealed a molecular role of AtBBX29 in the regulation of seedling photomorphogenesis, being part of a feedback loop with AtBBX28, AtBBX30, AtBBX31 and HY5 to control light-responsive genes at the transcriptional levels [44]. However, how light regulates AtBBX29 is not clear. To gain further insight into the photomorphogenic regulation of AtBBX29 transcription, we performed quantitative PCR (RT-qPCR) experiments with 5-d-old wild-type (Col) etiolated seedlings that were transferred from darkness to either 1 h of Red light (R), Blue light (B), R + B or R + B supplemented with weak UV-B light (Fig. 1a). The expression levels of AtBBX29 were up-regulated by B, R and UV-B light, suggesting that AtBBX29 transcription may be activated by phytochromes, cryptochromes and UVR8. To corroborate this hypothesis, we first measured AtBBX29 transcript abundance in wild-type and quadruple phyA phyB cry1 cry2 mutant seedlings when they were shifted from darkness to R + B light conditions (Fig. 2b); second, we quantified AtBBX29 transcript levels in wild-type and uvr8-6 seedlings grown under continuous white light (WL) with or without 1 h of UV-B supplementation. The induction of AtBBX29 by visible light depended on phytochromes and/or cryptochromes and the effect of additional UV-B radiation required UVR8 (Fig. 1b, c). Given that some BBXs are regulated post-translationally by protein stabilization or degradation [45, 46], we performed an experiment to assess the stability of AtBBX29 under different light conditions. Plants overexpressing AtBBX29 with the HA tag fused to the N terminus (35S::HA-BBX29) in the Col background were cultivated for 5 d under continuous WL and then transferred for 24 h to either WL + weak UV-B or darkness (D). Control seedlings were kept under WL. The results showed that AtBBX29 protein stability was not affected by light under these conditions (Fig. 1d). BBX proteins function as TFs or as cofactors to modulate transcriptional responses in Arabidopsis and, therefore, they are expected to be located in the nucleus to exert their regulatory action. To determine the subcellular localization of the AtBBX29 protein, we generated Arabidopsis transgenic lines tagged with the Yellow Fluorescent Protein (35S::YFP-BBX29). The fluorescence was mainly observed in the nucleus of hypocotyl cells (Fig. 1e).
Photomorphogenic regulation of AtBBX29. a Transcript levels of AtBBX29 relative to UBC in 5-d-old dark-grown Col seedlings after transfer for 1 h to Red (10 μmol m−2 s−1; R), Blue (10 μmol m−2 s−1; B), R + B (10 μmol m−2 s−1) or R + B light plus weak UV-B supplementation. b Transcript levels of AtBBX29 in 5-d old dark-grown seedlings (Ler and phyAphyBcry1cry2) after transfer to 1 h of B + R (10 μmol m−2 s−1) or kept in darkness (control). c Transcript levels of AtBBX29 in 5-days old seedlings (Col and uvr8-6) grown in continuous white light (5 μmol m−2 s−1) were either supplemented with 1 h of 1 μmol m−2 s−1 of UV-B radiation (WL + UVB) or kept in white light (WL). Values relatives to UBC transcript levels. The P-value for the G (Genotype) x T (treatment) interaction term of the ANOVA is shown; different letters indicate significant differences between means (P < 0.05, LSD Fisher test). Each bar represents the mean ± SEM (n = 3 biological replicates). d AtBBX29 protein levels in 5-day-old seedlings kept for 24 h under different light conditions; ACT2 abundance in the same mebrane is used as a loading control. Seedlings were grown under continuous white light (5 μmol m−2 s−1) before the light treatments: WL = continuous WL (5 μmol m−2 s−1); UVB = continuous WL + UV-B (1 μmol m−2 s−1); D = darkness. e YFP fluorescence (green) in transgenic hypocotyls expressing a 35:YFP-BBX29 fusion gene. Wild-type (Col) hypocotyls were used as control. Scale bar: 10 µm. The right panel shows chlorophyll autofluorescence of plastids (purple)
AtBBX29 promotes the accumulation of soluble leaf phenolics. a Quantification of total soluble phenolic compounds in rosette leaves of Col, bbx29-1, bbx29-2 and two independent overexpression lines (BBX29ox). Data were analyzed by one-way ANOVA. The P-value for the genotype term of the ANOVA is shown (pG); different letters indicate significant differences between means (P < 0.05, LSD Fisher test) and each bar represents the mean ± SEM (n = 6 biological replicates). b HPLC quantification of metabolites derived from the phenylpropanoid pathway in rosette leaves (Kaempferol 1, 2 & 3 and sinapoyl malate). Data were analyzed by Student’s t tests, and asterisks indicate a significant difference between Col and mutant lines (*P < 0.05, **P < 0.01, NS, not significant). Each bar represents the mean ± SEM (n = 4 biological replicates)
2.2 AtBBX29 positively regulates the accumulation of soluble phenolic compounds
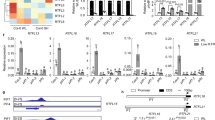
Some Arabidopsis BBXs proteins regulate the light-dependent accumulation of photoprotective flavonoid and phenolic compounds in leaves. In part, this modulation is exerted through the transcriptional regulation of genes involved in flavonoid biosynthesis, such as CHS, CHI, FLS1 and others [24, 27, 47, 48]. To investigate the role of AtBBX29, we quantified the accumulation of total soluble phenolic compounds in leaves of 4-week-old plants of wild-type (Col), a T-DNA knockout mutant (bbx29-1), a T-DNA knockdown mutant (bbx29-2) and two independent overexpression lines (BBX29ox#4 and BBX29ox#8; SI Fig. S1). Plants were grown under controlled environmental conditions (10 h light/14 h dark) with 110 μmol m−2 s−1 of photosynthetically active radiation (PAR). Mutant plants had significantly less total phenolic compounds compared to Col plants, whereas BBX29ox lines presented higher content of total leaf phenolics (Fig. 2a). Next, we analyzed the pool of soluble phenolic compounds by HPLC in bbx29-1 mutants and Col leaves. We found that bbx29-1 leaves had significantly less accumulation of kaempferols and sinapoyl malate than wild-type leaves (Fig. 2b), suggesting that AtBBX29 plays a role in the biosynthesis of these photoprotective metabolites. Key genes of the flavonoid biosynthetic pathway, CHS, FLS1, CHI and F3´H transcripts were up-regulated when AtBBX29 was over-expressed in Arabidopsis, and CHS and FLS were significantly down-regulated in the bbx29-1 knockout mutant compared to Col plants (Fig. 3a).
AtBBX29 is a positive transcriptional regulator of genes involved in flavonoid biosynthesis. a Expression levels of genes involved in flavonoid biosynthesis (CHS, CHI, F3´H and FLS1). b Expression levels of transcription factors known to regulate genes involved in flavonoid biosynthesis (HY5, MYB12). Values are normalized to IPP2 transcript levels and standardized to Col expression levels. The P-value for the genotype term of the ANOVA is shown (pG) and different letters indicate significant differences between means (P < 0.05, LSD Fisher test). Each bar represents the mean ± SEM (n = 3 biological replicates). c Tansient luciferase assay in tobacco leaves. The firefly luciferase gene driven by the MYB12 promoter (pMYB12::LUC) was used as the reporter and AtBBX29 driven by the 35S CaMV promoter was used as the effector. Empty vectors were used for the effector control (see methods). The values represent the ratio between firefly luciferase activity and renilla luciferase activity. Data were analyzed by one-way ANOVA (**P < 0.01, NS, not significant). Each bar represents the mean ± SEM (n ≥ 4 individual plants)
The flavonoid biosynthetic pathway is transcriptionally controlled by a network of transcription factors, where HY5 together with MYB12, MYB11 and MYB111 are involved in the photoregulation of these genes by binding to their promoters through different cis-elements [11, 49, 50]. Some BBX proteins have the ability to bind to the promoter regions of MYBs [24, 48] and HY5 [8, 17, 51] gene to activate their transcription. To investigate whether AtBBX29 plays a role in the regulation of these TFs, we evaluated the expression levels of MYB12, MYB11, MYB111 and HY5 in bbx29-1 mutant, BBX29ox lines and wild-type (Col) adult plants. We found that HY5 transcript levels were not affected by AtBBX29 (Fig. 3b), but MYB12 transcript levels were down-regulated in the bbx29-1 mutant and up-regulated in BBX29ox plants compared to Col plants (Fig. 3B). MYB11 and MYB111 transcripts were only affected in BBX29ox plants compared to Col (SI Fig. S2). These results indicate that AtBBX29 is necessary to modulate MYB12 expression levels. Additionally, we performed a transient expression assay in Nicotiana benthamiana leaves, where the firefly luciferase gene driven by the MYB12 promoter (pMYB12::LUC) was used as the reporter and AtBBX29 driven by the 35S CaMV promoter was used as the effector. We found that the transient expression of AtBBX29 activates the promoter of MYB12 in vivo (Fig. 3c), suggesting that AtBBX29 acts up-stream of MYB12 in the light-responsive mechanisms that control the flavonoid biosynthetic pathway.
2.3 AtBBX29 positively regulates glucosinolate accumulation
Light can modulate the GS accumulation in leaves, contributing to the defensive status of the plant [32, 42, 52, 53]. In Arabidopsis, MYB34, MYB51 and MYB122 TFs play an indispensable role in the regulation of GS biosynthesis genes [54]. Given that AtBBX29 regulates light responsive-MYB transcripts (Fig. 3b; SI Fig. S2) we asked whether the MYB-GS-related genes are affected in bbx29-1 mutant plants and BBX29ox lines. We found that AtBBX29 positively regulates MYB34 and MYB51 transcription (Fig. 4a; SI Fig. S3) in plants grown under our experimental conditions (short days and 110 μmol m−2 s−1 of PAR). To evaluate if AtBBX29 plays a role in GS accumulation, we quantified aliphatic and indolic GSs in Arabidopsis rosette leaves. We found that bbx29-1 knockout mutant plants had lower levels of I3M, 4MSOB and 3MSP than wild-type plants (Fig. 4b). The bbx29-2 knockdown mutants were significantly impaired in I3M accumulation but had normal levels of 4MSOB or 3MSP (SI Fig. S4). Taken together, these results indicate that AtBBX29 positively controls the biosynthesis and accumulation of GSs in Arabidopsis leaves.
AtBBX29 regulates glucosinolate accumulation in Arabidopsis leaves. a Expression levels of genes related to the glucosinolate pathway (MYB34 and MYB51) in Col and bbx29-1 rosette leaves. Values are normalized to IPP2 transcript levels and standardized to Col expression levels. b I3M, 4MSOB and 3MSP accumulation in Col and bbx29-1 rosette leaves. Data were analyzed by Student’s t tests, and asterisks indicate a significant difference between Col and bbx29-1 mutant plants (*P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant). Each bar represents the mean ± SEM (n ≥ 5 biological replicates)
2.4 AtBBX29 is a positive regulator of Arabidopsis defense against Botrytis cinerea and Spodoptera frugiperda
Phenolic and GS compounds play important roles in leaf defense against a variety of attackers. To investigate whether AtBBX29 is involved in defense responses, we performed bioassays with bbx29 mutants, BBX29ox and Col plants challenged with the necrotrophic pathogen B. cinerea and the herbivore S. frugiperda. bbx29-1 and bbx29-2 mutants showed increased susceptibility to the fungus (Fig. 5a) compared to Col plants, whereas BBX29ox lines displayed enhanced resistance (Fig. 5b).
AtBBX29 plays a role in defense against Botrytis cinerea and Spodoptera frugiperda in Arabidopsis leaves. a B.cinerea bioassay with Col, bbx29-1 and bbx29-2 plants. b B. cinerea bioassay with Col and BBX29ox overexpression lines. The bars show the size of the lesions caused by B. cinerea on rosette leaves 2 d after inoculation (see methods). Each bar represents the mean ± SEM (n = 10–20 plants) c S. frugiperda bioassay with Col, bbx29-1 and BBX29ox plants. Bars show the caterpillar mass after 5 day feeding on the indicated genotypes (see methods). Data were analyzed by one-way ANOVA. The P-value for the genotype term of the ANOVA is shown (pG); different letters indicate significant differences between means (P < 0.05, LSD Fisher test). Each bar represents the mean ± SEM (n = 16–22 larvae)
In the Spodoptera bioassay, the caterpillars that were fed on bbx29-1 grew faster than those fed on Col plants, and the opposite was true for the caterpillars that consumed BBX29ox plants (Fig. 5c). These data indicate that AtBBX29 contributes to Arabidopsis defense against B. cinerea and S. frugiperda.
Given that the AtBBX29 protein can physically interact with HY5 [44] and that, at the seedling stage, HY5 TF activity promotes the expression of MYB12 and phenylpropanoid biosynthetic genes in a light-dependent manner [11], we asked whether HY5 has a role modulating defense responses in plants at the rosette stage. In the B. cinerea bioassay, hy5 mutants did not display a susceptibility phenotype compared to their corresponding wild-types (SI Fig. S5). These results suggest that the positive effect of AtBBX29 on Arabidopsis defense is unlikely to be dependent on HY5 activity.
2.5 AtBBX29 does not affect jasmonate levels
Plant defenses against necrotrophic pathogens and chewing insects are controlled by jasmonates, and AtBBX29 transcripts were found to be up-regulated by MeJA in microarray data obtained by our group [53]. To investigate potential connections between AtBBX29 and jasmonic acid (JA) signaling or metabolism, we quantified by qRT-PCR AtBBX29 transcripts in plants treated with MeJA and measured JA pools in bbx29-1 and Col plants at the rosette stage. AtBBX29 transcripts were transiently up-regulated by MeJA (Fig. 6a), which is consistent with our previous microarray data. When Col and bbx29-1 mutant plants were sprayed with MeJA, they responded with the expected increase in JA pools, including JA, JA-Ile and a sulfated derivative (HSO4-JA) whose accumulation has been shown to be regulated by light [53]. The basal levels of these pools and their response to MeJA were not affected in the bbx29-1 mutant (Fig. 6b). In agreement with the metabolic data, the expression levels of JA-marker genes, VSP2 and ST2a, were not affected in bbx29-1 mutant plants (Fig. 6c). Collectively, these results suggest that the function of AtBBX29 in plant resistance against attackers is not related to alterations in JA metabolism or signaling.
MeJA transiently up-regulates AtBBX29 but AtBBX29 does not affect JA metabolism or JA signaling pathway. a Expression levels of AtBBX29 in Col plants sprayed with MeJA (50 µM) and harvested at different time points: 0 h (control), 1, 3 or 5 h. Values are normalized to IPP2 transcript levels and expressed relative to 0 h expression levels. Data were analyzed by Student’s t test, and asterisks indicate significant differences between control (0 h) and other time points (*P < 0.05, **P < 0.01, NS, not significant). Each datum point represents the mean ± SEM (n = 3 biological replicates). b Quantification of jasmonate pools in Col and bbx29-1 plants harvested 6 h after treatment with 200 µM MeJA or mock solutions. Each bar represents the mean ± SEM (n = 4 biological replicates; see methods) and the P values for the relevant terms of the ANOVA are shown (two-way ANOVA, G: Genotype, T: hormonal treatment, GxT interaction). c Expression levels of JA-markers genes (ST2a and VSP2). Values are normalized to IPP2 transcript levels and standardized to Col mock expression levels. Each bar represents the mean ± SEM (n ≥ 3 biological replicates) and the P values for the relevant terms of the ANOVA are shown (two-way ANOVA, G: Genotype, T: hormonal treatment, GxT interaction)
2.6 AtBBX29 is required for the protective effect of UV-B radiation on plant resistance to B. cinerea
Activation of the UV-B photomorphogenic pathway enhances plant defense responses, where one of the key defensive strategies includes the regulation of specialized metabolites [30, 55]. Under these UV-B-enriched light conditions, sinapate metabolites play an important role in increasing plant resistance against B. cinerea [30]. Given that UV-B strongly promotes AtBBX29 transcript accumulation and that bbx29-1 mutant plants show reduced sinapate levels, we asked whether AtBBX29 is involved in the effect of UV-B radiation enhancing Arabidopsis defenses. To this end, we carried out a B. cinerea bioassay with wild-type and bbx29-1 knockout plants cultivated under white light with or without the addition of low doses of UV-B radiation. As expected [30], low doses of UV-B increased the resistance of Col plants to B. cinerea, but this effect was absent in bbx29-1 plants (Fig. 7).
AtBBX29 is required for the effect of UV-B radiation promoting Arabidopsis resistance to B. cinerea. B. cinerea bioassay in Col and bbx29-1 plants grown under WL conditions with or without supplementation with 1 μmol m−2 s−1 of UV-B radiation (see methods). Each bar represents the average size of the lesions caused by B. cinerea on rosette leaves 2 d after inoculation ± SEM (n ≥ 9 individual plants). The P-value for the G (Genotype) x T (UV-B treatment) interaction term of the ANOVA is shown; different letters indicate significant differences between means (P < 0.05, LSD Fisher test)
3 Discussion
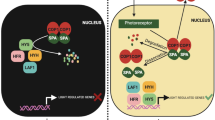
Light signals are known to modulate the accumulation of defense-related specialized metabolites in leaves [35]. Despite considerable advances in recent years, further work is needed to chart the molecular connections between photoreceptor signaling pathways and defense responses. In this context, our results show that AtBBX29, a photomorphogenic and JA-responsive-gene, is a positive regulator of genes involved in the biosynthesis of phenylpropanoids and GSs. Deregulation of AtBBX29 expression levels alters the accumulation of kaempferol, sinapoyl malate and glucosinolates in leaves, affecting Arabidopsis defense responses against the necrotrophic fungus B. cinerea and the insect herbivore S. frugiperda.
Notable progress has been made in characterizing the roles of plant BBX proteins in many biological processes, such as shade avoidance responses, thermo- and photomorphogenesis, and photoperiodic regulation of flowering [27, 56,57,58,59,60], but the role of BBXs in the photoregulation of specialized metabolites with potential effects on plant defense is poorly understood. Here we show that AtBBX29 is a light-regulated TF with a nuclear localization (Fig. 1e). Its photomorphogenic regulation occurs at the transcriptional level (Fig. 1a), downstream of phytochrome, cryptochrome and UVR8 photoreceptors (Fig. 1b, c). Our data indicate that AtBBX29 positively regulates the expression of flavonoid biosynthetic genes (Fig. 3a); however, the observation of normal transcript levels of some flavonoid biosynthetic genes in bbx29-1 mutant plants suggests that other BBX proteins could complement the lack of AtBBX29 for the regulation of these genes. Additionally, AtBBX29 induces MYB12 transcription in Arabidopsis adult plants (Fig. 3b) and the transient expression of AtBBX29 activates the promoter of MYB12 in Nicotiana benthamiana (Fig. 3c). Taken together, these results place AtBBX29 function up-stream of MYB12, with AtBBX29 acting as a positive regulator of flavonoid biosynthetic genes in Arabidopsis leaves. This role of AtBBX29 in adult plants contrasts with the one described for the seedling stage, where it has been proposed that AtBBX29 acts as a negative regulator of photomorphogenesis [44, 61]. Variations in the apparent functionality of BBX proteins have been reported before; for example, AtBBX31 negatively regulates seedling photomorphogenesis under white light, but it promotes the accumulation of specialized metabolites in response to UV-B radiation [47].
Arabidopsis mutants with defects in the biosynthesis or metabolism of soluble phenolic or GS compounds exhibit an enhanced susceptibility to the attack of pathogens and/or herbivores [29, 30]. Here we show that bbx29 mutant plants have reduced accumulation of soluble phenolic compounds in their leaves (Fig. 2a), including reduced levels of kaempferol glucosides and sinapoyl malate (Fig. 2b) compared to Col plants. In addition, the concentration of indolic GSs, represented by I3M, was also reduced in bbx29-1 and bbx29-2 mutant plants in comparison to the wild-type, while 4MSOB or 3MSP accumulation was only significantly reduced in the bbx29-1 null but not in the bbx29-2 knockdown mutant (Fig. 5c; SI Fig.S4). In agreement with the reduced GS levels, the bbx29 mutation negatively affected the expression of MYB34 and MYB51 genes, the main TFs known to regulate indolic GS biosynthesis in Arabidopsis (Fig. 4b). Finally, variations in the accumulation of phenolic compounds and indolic GSs resulting from manipulation of the leaves of AtBBX29 expression were reflected in the bioassays with the B. cinerea and S. frugiperda. bbx29 mutant plants exhibited higher susceptibility to B. cinerea (Fig. 5a) and sustained higher caterpillar growth than wild-type plants (Fig. 5c), whereas the opposite was true for BBX29ox plants (Fig. 5b, c).
There is increasing evidence that besides their photomorphogenic functions, BBX proteins also play integral roles in several hormone signaling pathways in plants [57, 61, 62], Vaishak et al. [63] showed that AtBBX29 integrate photomorphogenesis and brassinosteroid signaling in Arabidopsis seedling development and previous studies suggested that other BBX proteins could play a role in the JA-mediated responses [25, 64]. Here we found that AtBBX29 transcript levels were transiently up-regulated by MeJA (Fig. 6a), but bbx29 mutant plants had wild-type levels of JA metabolites and JA-marker transcript, both under basal conditions or after MeJA treatment (Fig. 6b, c). Taken together, our results suggest that the main function of AtBBX29 in Arabidopsis resistance to attackers is related to its positive effect on the accumulation of defense-related specialized metabolites (kaempferol, sinapates and GSs) in leaves, without interfering with JA metabolism. However, more efforts are needed to characterize the possible roles of AtBBX29 in JA signaling. We also looked for possible homologous of AtBBX29 in other plant species, but AtBBX29 sequence comparisons against green plant genomes did not reveal any orthologous, although paralogs are present in Arabidopsis [43]. It will be a challenge to assess whether (and which) BBX proteins fulfill the role of AtBBX29 in the photomodulation of defenses in other plant species.
UV-B radiation plays an important role in the accumulation of phenolic compounds, which can act as effective sunscreens and also increase plant resistance to a variety of consumer organisms [30, 36, 65,66,67,68]. Considering that UV-B radiation strongly induced AtBBX29 gene expression in a UVR8-dependent manner (Fig. 1a, c), we hypothesized that the increase in resistance against pathogens triggered by low doses of UV-B radiation could be mediated by the action of AtBBX29. The B. cinerea bioassay data presented in Fig. 7 are consistent with this hypothesis and suggest that AtBBX29 plays an important role in integrating UV-B signaling pathways with defense responses triggered by UV-B exposure.
4 Materials and methods
4.1 Plant material
The following Arabidopsis thaliana mutants were used: bbx29-1 (N638034/SALK_138034) and bbx29-2 (N582429/SALK_082429) obtained from the Nottingham Arabidopsis Stock Centre (NASC). Both lines are in Col-0 background and they were genotyped to obtain homozygous lines and finally checked by RT-qPCR (SI Fig. S1A). phyA phyB cry1 cry2 quadruple mutant and uvr8-6 single mutant have been described previously by Favory et al. [69] and Mazzella et al. [70]. To generate transgenic lines over-expressing Arabidopsis BBX29, the coding sequence of BBX29 was amplified by PCR from cDNA using specific primers (Supplemental Table S1). The cDNA was cloned into the pDONR221 plasmid and inserted into the pEarleyGate 201 vector (HA-BBX29 / BBX29ox) or pEarleyGate 104 (YFP-BBX29) under the control of the CaMV 35S promoter using Gateway technology (Invitrogen, http://www.invitrogen.com).
4.2 Growth conditions
Plants were grown under short days (10 h light/14 h dark, 18–20 °C, humidity 50–60%) and 110 μmol m−2 s−1 of photosynthetically active radiation (PAR) provided by LED bulbs (Phillips LED Ecofit T8). For UV-B treatments, white light LED tubes were supplemented for 5 h with UV-B narrowband lamps (Phillips PL-S 9W/01/2P) which delivered photomorphogenic UV-B fluence rates (1 μmol m−2 s−1) during 5 days. Except indicated otherwise, rosette-stage plants of similar age (3–4 weeks old) and size were selected for the experiments and randomly assigned to treatments. For seedlings experiments, sterilized seeds were sown in clear plastic boxes on 0.8% agar with half-strength Murashige and Skoog medium and incubated in darkness at 4 °C to reduce dormancy and homogenize germination. After 3 days, imbibed seeds were exposed to a red light pulse and incubated in darkness for 24 h at 25 °C to induce germination. Then, the boxes were transferred to their corresponding light treatment in the growth chamber (Percival-Scientific) equipped with red, blue and white light LEDs. PAR and UV-B were measured using an SKP215 PAR sensor and SKU430 UV-B sensor, respectively (Skye Instruments Ltd., Powys, UK).
4.3 Gene expression analyses
Total RNA was extracted using a Spectrum Plant Total RNA kit (Sigma Aldrich) and crude RNA samples were treated with Rnase-free Dnase I according to the protocol (Promega, http://www.promega.com). For RT-qPCR analysis, complementary DNAs were obtained with Superscript III Reverse Transcriptase (Invitrogen) following the manufacturer’s instructions. RT-qPCR analysis was performed on an optical 96-well plate using SYBR Green PCR master mix (ROCHE) and an ABI PRISM 7500 real-time PCR system (http://www.appliedbiosystems.com). IPP2 (At3g02780) and UBC (At5g25760) were used to normalize the expression levels for different concentrations of cDNA. The relative expression levels were calculated using the 2–ΔΔCt method (three pools of three individual plants). Specific primer pairs for each gene are listed in Supplemental Table S1.
4.4 Reporter constructs and transcriptional assays
A pMYB12::LUC reporter construct was made by cloning 1387 bp upstream of the start codon of MYB12 with specific primers (Supplemental Table S1) into the pDONR207 plasmid, and finally recombined into the pGreenII 0800-LUC vector [42, 71] using Gateway technology (Invitrogen). We used 35S::HA-BBX29 as an effector (described above). Transient expression in leaves of 3-week-old N. benthamiana was carried out by the infiltration mixtures indicated in Fig. 3c. To prevent silencing, A. tumefaciens C58 carrying a construct that expresses the silencing suppressor P19 was included in all the mixtures. Firefly and the control Renilla–LUC activities were assayed from leaf extracts collected 2 days after infiltration with the Dual-Glo Luciferase Assay System (Promega) and quantified with a GloMax 96 Microplate Luminometer (Promega).
4.5 Confocal
35S::YFP-BBX29 6-d-old seedlings were transferred to glass slides and analyzed with an LSM 780 confocal laser-scanning microscope (Zeiss). Excitation of the Yellow Fluorescent Protein (YFP) was performed at 514 nm and the emission was detected between 525 and 561 nm.
4.6 Protein isolation and Western blot
Total protein was extracted from 6 d-old seedlings with buffer containing 50 mM Tris pH 7.6, 150 mM NaCl, 10% glycerol, 5 mM MgCl2, 0.1% NP-40, 1% DTT, 1% protease inhibitor cocktail (Sigma). The samples were boiled for 10 min in SDS-PAGE buffer, separated by electrophoresis in 10% SDS–polyacrylamide gels and electrophoretically transferred to PVDF membrane, according to the manufacturer’s instructions (Bio-Rad). We used anti-HA (MMS-101R, Covance) and anti-ACT2 (A0480, Sigma) as primary antibodies, and horseradish-peroxidase-conjugated anti-mouse (P0447, Dako) as the secondary antibody. Signal detection was performed using the Amersham ECL Select Western Blotting Detection Reagent (RPN 2235, GE Healthcare) and the Image Quant LAS 4000 mini CCD camera system (GE Healthcare).
4.7 Metabolite determination
For determination of total soluble leaf phenolics, leaf samples (two discs per plant, youngest fully expanded leaves) from 4-week-old plants cultivated under controlled conditions were placed in 1.4 mL of a methanol:HCl solution (99:1, v/v) and allowed to extract for 48 h at − 20 °C. Absorbance of extracts was read in a spectrophotometer at 305 nm (UV-1700 series; Shimadzu). The remaining leaf tissue was freeze-dried and stored in a container with silica gel until HPLC analysis. Individual leaf phenolics were determined by HPLC following the protocol described previously by Demkura et al. [36]. According to Yin et al. [72] we name Kaempferol 1: kaempferol-3-O-[rhamnosyl (1- > 2 glucoside)]—7-O-rhamnoside; Kaempferol 2: kaempferol 3-O-glucoside-7-O-rhamnoside; Kaempferol 3: kaempferol 3-O-rhamnoside-7-O-rhamnoside. For GS determinations we used the youngest fully expanded from 4-week-old Arabidopsis leaves following the protocol described in Cargnel et al. [42].
4.8 MeJA treatments and determination of jasmonate pools
For MeJA treatment we sprayed 4-week-old plants with 50 µmol of MeJA (Sigma) or mock solutions (0.1% ethanol). Plants were harvested at 0 (mock), 1, 3 or 5 h after MeJA treatment for gene expression analysis or at 6 h after treatment for jasmonate determinations. We used four biological replicates (each consisting of three individual rosettes) for each genotype and treatment combination. Jasmonate analysis was performed by LC–MS/MS as described previously in Fernandez-Milmanda et al. [53].
4.9 B. cinerea and S. frugiperda bioassays
Botrytis cinerea (strain B05) was grown, maintained and collected as described by Demkura et al. [30]. Four leaves of 4-week-old rosettes were inoculated on the adaxial surface with a 5 µl droplet of a spore suspension. Plants were kept in cylindrical chambers made of clear polyester to prevent desiccation. After 48 h, infected leaves were collected and scanned with an HP Scanjett 4500c (Hewlett-Packard). Lesion areas were measured using ImageJ software. For Spodoptera frugiperda bioassay, 6-day-old larvae were placed on 4-week-old Arabidopsis under growth chamber conditions as described above. The bioassay was performed with four larvae per plant (10–20 plants per genotype). After 5 days, surviving larvae were collected and weighed. Bioassays were repeated at least three times with similar results. For the bioassays testing the effects of UV-B radiation, the UV-B bulbs were maintained off during the day of inoculation and for the duration of the bioassay.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
D’Auria, J. C., & Gershenzon, J. (2005). The secondary metabolism of Arabidopsis thaliana: Growing like a weed. Current Opinion in Plant Biology, 8(3), 308–316. https://doi.org/10.1016/j.pbi.2005.03.012
Gachon, C. M., Langlois-Meurinne, M., Henry, Y., & Saindrenan, P. (2005). Transcriptional co-regulation of secondary metabolism enzymes in Arabidopsis: Functional and evolutionary implications. Plant Molecular Biology, 58(2). https://doi.org/10.1007/s11103-005-3663-3
Dixon, R. A., & Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. The Plant Cell, 1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Galvao, V. C., & Fankhauser, C. (2015). Sensing the light environment in plants: Photoreceptors and early signaling steps. Current Opinion in Neurobiology., 34, 46–53. https://doi.org/10.1016/j.conb.2015.01.013
Podolec, R., Demarsy, E., & Ulm, R. (2021). Perception and signaling of ultraviolet-B radiation in plants. Annual Review of Plant Biology, 72(1), 793–822. https://doi.org/10.1146/annurev-arplant-050718-095946
Stracke, R., Favory, J. J., Grube, H., Bartelniewoehner, L., Bartels, S., Binkert, M., Funck, M., Weisshaar, B., & Ulm, R. (2010). The Arabidopsis bZIP transcription factor HY5 regulates expression of the pfg1/myb12 gene in response to light and ultraviolet-B radiation. Plant, Cell & Environment. https://doi.org/10.1111/j.1365-3040.2009.02061.x
Takos, A. M., Jaffé, F. W., Jacob, S. R., Bogs, J., Robinson, S. P., & Walker, A. R. (2006). Light-induced expression of a myb gene regulates anthocyanin biosynthesis in red apples. Plant Physiology, 142(3), 1216–1232. https://doi.org/10.1104/pp.106.088104
Xu, D. (2020). COP1 and BBXs-HY5mediated light signal transduction in plants. New Phytologist, 228(6), 1748–1753. https://doi.org/10.1111/nph.16296
Dubos, C., Stracke, R., Grotewold, E., Weisshaar, B., Martin, C., & Lepiniec, L. (2010). MYB transcription factors in Arabidopsis. Trends in Plant Science., 15, 573–581. https://doi.org/10.1016/j.tplants.2010.06.005
Shin, D. H., Choi, M. G., Kim, K., Bang, G., Cho, M., Choi, S.-B., Choi, G., & Park, Y.-I. (2013). HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Letters, 587(10), 1543–1547. https://doi.org/10.1016/j.febslet.2013.03.037
Stracke, R., Werber, M., & Weisshaar, B. (2001). The R2R3-MYB gene family in Arabidopsis thaliana. Current Opinion in Plant Biology, 4(5), 447–456. https://doi.org/10.1016/s1369-5266(00)00199-0
Hartmann, U., Sagasser, M., Mehrtens, F., Stracke, R., & Weisshaar, B. (2005). Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, bZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Molecular Biology, 57(2), 155–171. https://doi.org/10.1007/s11103-004-6910-0
Kaiser, T., & Batschauer, A. (1995). Cis-acting elements of the CHS1 gene from white mustard controlling promoter activity and spatial patterns of expression. Plant Molecular Biology, 28(2), 231–243. https://doi.org/10.1007/bf00020243
Bursch, K., Toledo-Ortiz, G., Pireyre, M., Lohr, M., Braatz, C., & Johansson, H. (2020). Identification of BBX proteins as rate-limiting cofactors of HY5. Nature Plants, 6(8), 921–928. https://doi.org/10.1038/s41477-020-0725-0
Datta, S., Johansson, H., Hettiarachchi, C., Luisa, I. M., Desai, M., Rubio, V., & Holm, M. (2008). LZF1/salt tolerance Homolog3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. The Plant Cell, 20(9), 2324–2338. https://doi.org/10.1105/tpc.108.061747
Datta, S., Hettiarachchi, C., Johansson, H., & Holm, M. (2007). Salt tolerance homolog2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. The Plant Cell, 19(10), 3242–3255. https://doi.org/10.1105/tpc.107.054791
Xu, D., Jiang, Y., Li, J., Lin, F., Holm, M., & Deng, X. W. (2016). BBX21, an Arabidopsis B-box protein, directly activates hy5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proceedings of the National Academy of Sciences, 113(27), 7655–7660. https://doi.org/10.1073/pnas.1607687113
Zhang, X., Huai, J., Shang, F., Xu, G., Tang, W., Jing, Y., & Lin, R. (2017). A PIF1/PIF3-HY5-BBX23 transcription factor cascade affects photomorphogenesis. Plant Physiology, 174(4), 2487–2500. https://doi.org/10.1104/pp.17.00418
Gangappa, S. N., Crocco, C. D., Johansson, H., Datta, S., Hettiarachchi, C., Holm, M., & Botto, J. F. (2013). The Arabidopsis B-box protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. The Plant Cell, 25(4), 1243–1257. https://doi.org/10.1105/tpc.113.109751
Holtan, H. E., Bandong, S., Marion, C. M., Adam, L., Tiwari, S., Shen, Y., Maloof, J. N., Maszle, D. R., & Ohto, M.-aki, Preuss, S., Meister, R., Petracek, M., Repetti, P. P., Reuber, T. L., Ratcliffe, O. J., & Khanna, R. (2011). BBX32, an Arabidopsis B-box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiology, 156(4), 2109–2123. https://doi.org/10.1104/pp.111.177139
Job, N., Yadukrishnan, P., Bursch, K., Datta, S., & Johansson, H. (2018). Two B-box proteins regulate photomorphogenesis by oppositely modulating HY5 through their diverse C-terminal domains. Plant Physiology, 176(4), 2963–2976. https://doi.org/10.1104/pp.17.00856
Li, C., Pei, J., Yan, X., Cui, X., Tsuruta, M., Liu, Y., & Lian, C. (2021). A poplar b-box protein PtrBBX23 modulates the accumulation of anthocyanins and proanthocyanidins in response to high light. Plant, Cell & Environment, 44(9), 3015–3033. https://doi.org/10.1111/pce.14127
Kim, D. H., Park, S., Lee, J. Y., Ha, S. H., Lee, J. G., & Lim, S. H. (2018). A rice B-box protein, OSBBX14, finely regulates anthocyanin biosynthesis in rice. International Journal of Molecular Sciences, 19(8), 2190. https://doi.org/10.3390/ijms19082190
Bai, S., Tao, R., Tang, Y., Yin, L., Ma, Y., Ni, J., Yan, X., Yang, Q., Wu, Z., Zeng, Y., & Teng, Y. (2019). BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in Red Pear. Plant Biotechnology Journal, 17(10), 1985–1997. https://doi.org/10.1111/pbi.13114
An, J. P., Wang, X. F., Zhang, X. W., You, C. X., & Hao, Y. J. (2021). Apple B-box protein BBX37 regulates jasmonic acid mediated cold tolerance through the JAZ-BBX37-ICE1-CBF pathway and undergoes MIEL1-mediated ubiquitination and degradation. New Phytologist., 229, 2707–2729. https://doi.org/10.1111/nph.17050
Bai, S., Saito, T., Honda, C., Hatsuyama, Y., Ito, A., & Moriguchi, T. (2014). An Apple B-box protein, MDCOL11, is involved in UV-B- and temperature-induced anthocyanin biosynthesis. Planta, 240(5), 1051–1062. https://doi.org/10.1007/s00425-014-2129-8
Crocco, C. D., Ocampo, G. G., Ploschuk, E. L., Mantese, A., & Botto, J. F. (2018). Heterologous expression of AtBBX21 enhances the rate of photosynthesis and alleviates photoinhibition in Solanum tuberosum. Plant Physiology, 177(1), 369–380. https://doi.org/10.1104/pp.17.01417
Treutter, D. (2005). Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biology, 7(6), 581–591. https://doi.org/10.1055/s-2005-873009
Kliebenstein, D. J., Rowe, H. C., & Denby, K. J. (2005). Secondary metabolites influence Arabidopsis/Botrytis interactions: Variation in host production and pathogen sensitivity. The Plant Journal, 44(1), 25–36. https://doi.org/10.1111/j.1365-313x.2005.02508.x
Demkura, P. V., & Ballaré, C. L. (2012). UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Molecular Plant, 5(3), 642–652. https://doi.org/10.1093/mp/sss025
Bednarek, P., Mariola, P.-B., & Svatoš Aleš, Schneider, B., Doubský Jan, Mansurova, M., Humphry, M., Consonni, C., Panstruga, R., Sanchez-Vallet, A., Molina, A., & Schulze-Lefert, P. (2009). A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science, 323(5910), 101–106. https://doi.org/10.1126/science.1163732
Frerigmann, H., Hoecker, U., & Gigolashvili, T. (2021). New insights on the regulation of glucosinolate biosynthesis via COP1 and DELLA proteins in Arabidopsis thaliana. Frontiers in Plant Science, 12. https://doi.org/10.3389/fpls.2021.680255
Mazza, C. A., & Ballaré, C. L. (2015). Photoreceptors UVR8 and phytochrome B cooperate to optimize plant growth and defense in patchy canopies. New Phytologist, 207(1), 4–9. https://doi.org/10.1111/nph.13332
Pierik, R., & Ballaré, C. L. (2021). Control of plant growth and defense by photoreceptors: From mechanisms to opportunities in agriculture. Molecular Plant, 14(1), 61–76. https://doi.org/10.1016/j.molp.2020.11.021
Ballaré, C. L. (2014). Light regulation of plant defense. Annual Review of Plant Biology, 65(1), 335–363. https://doi.org/10.1146/annurev-arplant-050213-040145
Demkura, P. V., Abdala, G., Baldwin, I. T., & Ballaré, C. L. (2010). Jasmonate-dependent and -independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiology, 152(2), 1084–1095. https://doi.org/10.1104/pp.109.148999
Escobar-Bravo, R., Chen, G., Kim, H. K., Grosser, K., van Dam, N. M., Leiss, K. A., & Klinkhamer, P. G. L. (2019). Ultraviolet radiation exposure time and intensity modulate tomato resistance to herbivory through activation of jasmonic acid signaling. Journal of Experimental Botany, 70(1), 315–327. https://doi.org/10.1093/jxb/ery347
Mewis, I., Schreiner, M., Nguyen, C. N., Krumbein, A., Ulrichs, C., Lohse, M., & Zrenner, R. (2012). UV-B irradiation changes specifically the secondary metabolite profile in Broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant and Cell Physiology, 53(9), 1546–1560. https://doi.org/10.1093/pcp/pcs096
Halkier, B. A., & Gershenzon, J. (2006). Biology and biochemistry of glucosinolates. Annual Review of Plant Biology, 57(1), 303–333. https://doi.org/10.1146/annurev.arplant.57.032905.105228
Winde, I., & Wittstock, U. (2011). Insect herbivore counteradaptations to the plant glucosinolate–myrosinase system. Phytochemistry, 72(13), 1566–1575. https://doi.org/10.1016/j.phytochem.2011.01.016
Huseby, S., Koprivova, A., Lee, B. R., Saha, S., Mithen, R., Wold, A. B., Bengtsson, G. B., & Kopriva, S. (2013). Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. Journal of Experimental Botany, 64(4), 1039–1048. https://doi.org/10.1093/jxb/ers378
Cargnel, M. D., Demkura, P. V., & Ballaré, C. L. (2014). Linking phytochrome to Plant Immunity: Low red: Far-red ratios increase Arabidopsis susceptibility to Botrytis cinerea by reducing the biosynthesis of indolic glucosinolates and camalexin. New Phytologist, 204(2), 342–354. https://doi.org/10.1111/nph.13032
Crocco, C. D., & Botto, J. F. (2013). BBX proteins in green plants: Insights into their evolution, structure, feature and functional diversification. Gene, 531(1), 44–52. https://doi.org/10.1016/j.gene.2013.08.037
Song, Z., Yan, T., Liu, J., Bian, Y., Heng, Y., Lin, F., Jiang, Y., Wang Deng, X., & Xu, D. (2020). BBX28/BBX29, HY5 and BBX30/31 form a feedback loop to fine-tune photomorphogenic development. The Plant Journal, 104(2), 377–390. https://doi.org/10.1111/tpj.14929
Chang, C. S. J., Maloof, J. N., & Wu, S. H. (2011). COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiology, 156(1), 228–239. https://doi.org/10.1104/pp.111.175042
Wei, C. Q., Chien, C. W., Ai, L. F., Zhao, J., Zhang, Z., Li, K. H., Burlingame, A. L., Sun, Y., & Wang, Z.-Y. (2016). The Arabidopsis B-box protein BZS1/BBX20 interacts with HY5 and mediates strigolactone regulation of photomorphogenesis. Journal of Genetics and Genomics, 43(9), 555–563. https://doi.org/10.1016/j.jgg.2016.05.007
Yadav, A., Bakshi, S., Yadukrishnan, P., Lingwan, M., Dolde, U., Wenkel, S., Masakapalli, S. K., & Datta, S. (2019). The B-box-containing microprotein MIP1A/BBX31 regulates photomorphogenesis and UV-B protection. Plant Physiology, 179(4), 1876–1892. https://doi.org/10.1104/pp.18.01258
Fang, H., Dong, Y., Yue, X., Hu, J., Jiang, S., Xu, H., Wang, Y., Su, M., Zhang, J., Zhang, Z., Wang, N., & Chen, X. (2019). The b-box zinc finger protein MDBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant, Cell & Environment, 42(7), 2090–2104. https://doi.org/10.1111/pce.13552
Lee, J., He, K., Stolc, V., Lee, H., Figueroa, P., Gao, Y., Tongprasit, W., Zhao, H., Lee, I., & Deng, X. W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. The Plant Cell, 19(3), 731–749. https://doi.org/10.1105/tpc.106.047688
Mehrtens, F., Kranz, H., Bednarek, P., & Weisshaar, B. (2005). The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiology, 138, 1083–1096. https://doi.org/10.1104/pp.104.058032
Zhao, X., Heng, Y., Wang, X., Deng, X. W., & Xu, D. (2020). A positive feedback loop of BBX11–BBX21–HY5 promotes photomorphogenic development in Arabidopsis. Plant Communications, 1(5), 100045. https://doi.org/10.1016/j.xplc.2020.100045
Cerrudo, I., Caliri-Ortiz, M. E., Keller, M. M., Degano, M. E., Demkura, P. V., & Ballaré, C. L. (2017). Exploring growth-defence trade-offs in Arabidopsis: Phytochrome B inactivation requires JAZ10 to suppress plant immunity but not to trigger shade-avoidance responses. Plant, Cell & Environment, 40(5), 635–644. https://doi.org/10.1111/pce.12877
Fernández-Milmanda, G. L., Crocco, C. D., Reichelt, M., Mazza, C. A., Köllner, T. G., Zhang, T., Cargnel, M. D., Lichy, M. Z., Fiorucci, A.-S., Fankhauser, C., Koo, A. J., Austin, A. T., Gershenzon, J., & Ballaré, C. L. (2020). A light-dependent molecular link between competition cues and defence responses in plants. Nature Plants, 6(3), 223–230. https://doi.org/10.1038/s41477-020-0604-8
Frerigmann, H., & Gigolashvili, T. (2014). MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Molecular Plant, 7(5), 814–828. https://doi.org/10.1093/mp/ssu004
Vandenbussche, F., Yu, N., Li, W., Vanhaelewyn, L., Hamshou, M., Van Der Straeten, D., & Smagghe, G. (2018). An ultraviolet B condition that affects growth and defense in Arabidopsis. Plant Science, 268, 54–63. https://doi.org/10.1016/j.plantsci.2017.12.005
Crocco, C. D., Holm, M., Yanovsky, M. J., & Botto, J. F. (2010). ATBBX21 and COP1 genetically interact in the regulation of shade avoidance. The Plant Journal, 64(4), 551–562. https://doi.org/10.1111/j.1365-313x.2010.04360.x
Crocco, C. D., Locascio, A., Escudero, C. M., Alabadí, D., Blázquez, M. A., & Botto, J. F. (2015). The transcriptional regulator BBX24 impairs DELLA activity to promote shade avoidance in Arabidopsis thaliana. Nature Communications, 6(1). https://doi.org/10.1038/ncomms7202
Ding, L., Wang, S., Song, Z.-T., Jiang, Y., Han, J.-J., Lu, S.-J., Li, L., & Liu, J.-X. (2018). Two B-box domain proteins, BBX18 and BBX23, interact with ELF3 and regulate thermomorphogenesis in Arabidopsis. Cell Reports, 25(7). https://doi.org/10.1016/j.celrep.2018.10.060
Podolec, R., Wagnon, T. B., Leonardelli, M., Johansson, H., & Ulm, R. (2022). Arabidopsis B-box transcription factors BBX20 -22 promote UVR8 photoreceptor-mediated UV-B responses. The Plant Journal, 111(2), 422–439. https://doi.org/10.1111/tpj.15806
Tripathi, P., Carvallo, M., Hamilton, E. E., Preuss, S., & Kay, S. A. (2016). Arabidopsis B-BOX32 interacts with CONSTANS-LIKE3 to regulate flowering. Proceedings of the National Academy of Sciences, 114, 172–177. https://doi.org/10.1073/pnas.1616459114
Cao, J., Liang, Y., Yan, T., Wang, X., Zhou, H., Chen, C., Zhang, Y., Zhang, B., Zhang, S., Liao, J., Cheng, S., Chu, J., Huang, X., Xu, D., Li, J., Deng, X. W., & Lin, F. (2022). The photomorphogenic repressors BBX28 and BBX29 integrate light and brassinosteroid signaling to inhibit seedling development in Arabidopsis. The Plant Cell, 34(6), 2266–2285. https://doi.org/10.1093/plcell/koac092
Zhang, Z., Ji, R., Li, H., Zhao, T., Liu, J., Lin, C., & Liu, B. (2014). Constans-like 7 (COL7) is involved in phytochrome B (phyB)-mediated light-quality regulation of auxin homeostasis. Molecular Plant, 7(9), 1429–1440. https://doi.org/10.1093/mp/ssu058
Vaishak, K. P., Yadukrishnan, P., Bakshi, S., Kushwaha, A. K., Ramachandran, H., Job, N., Babu, D., & Datta, S. (2019). The B-box bridge between light and hormones in plants. Journal of Photochemistry and Photobiology B, 191, 164–174. https://doi.org/10.1016/j.jphotobiol.2018.12.021
Zhang, H., Zhang, Q., Zhai, H., Gao, S., Yang, L., Wang, Z., Xu, Y., Huo, J., Ren, Z., Zhao, N., Wang, X., Li, J., Liu, Q., & He, S. (2020). IBBBX24 promotes the jasmonic acid pathway and enhances fusarium wilt resistance in sweet potato. The Plant Cell, 32(4), 1102–1123. https://doi.org/10.1105/tpc.19.00641
Caputo, C., Rutitzky, M., & Ballaré, C. L. (2006). Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): Impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia, 149(1), 81–90. https://doi.org/10.1007/s00442-006-0422-3
Izaguirre, M. M., Mazza, C. A., SvatoS, A., Baldwin, I. T., & Ballaré, C. L. (2007). Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Annals of Botany, 99(1), 103–109. https://doi.org/10.1093/aob/mcl226
Vanhaelewyn, L., Van Der Straeten, D., De Coninck, B., & Vandenbussche, F. (2020). Ultraviolet radiation from a plant perspective: The plant-microorganism context. Frontiers in Plant Science, 11. https://doi.org/10.3389/fpls.2020.597642
Zavala, J., Mazza, C. A., Dillon, F. M., Chludil, H. D., & Ballaré, C. L. (2015). Soybean resistance to stink bugs (Nezara viridulaandpiezodorus guildinii) increases with exposure to solar UV-B radiation and correlates with isoflavonoid content in pods under field conditions. Plant, Cell & Environment, 38(5), 920–928. https://doi.org/10.1111/pce.12368
Favory, J. J., Stec, A., Gruber, H., Rizzini, L., Oravecz, A., Funk, M., Albert, A., Cloix, C., Jenkins, G. I., Oakeley, E. J., Seidlitz, H. K., Nagy, F., & Ulm, R. (2009). Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. The EMBO Journal, 28(5), 591–601. https://doi.org/10.1038/emboj.2009.4
Mazzella, M. A., Cerdán, P. D., Staneloni, R. J., & Casal, J. J. (2001). Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsis development. Development, 128(12), 2291–2299. https://doi.org/10.1242/dev.128.12.2291
Hellens, R. P., Allan, A. C., Friel, E. N., Bolitho, K., Grafton, K., Templeton, M. D., Karunairetnam, S., Gleave, A. P., & Laing, W. A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods, 1, 13. https://doi.org/10.1186/1746-4811-1-13
Yin, R., Han, K., Heller, W., Albert, A., Dobrev, P. I., Zažímalová, E., & Schäffner, A. R. (2013). Kaempferol 3- o -rhamnoside-7- o -rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in arabidopsis shoots. New Phytologist, 201(2), 466–475. https://doi.org/10.1111/nph.12558
Acknowledgements
We thanks Michael Reichelt and Guadalupe Fernández-Milmanda for their help with jasmonate determinations and to Roman Ulm for contributing genetic and analytical resources and critically reading the manuscript.
Funding
Open access funding provided by University of Geneva. This wok was supported by grants from the Agencia Nacional de Promoción de la Investigación, el Desarollo Tecnológico y la Innovación of Argentina to CDC (PICT-2016-4490 and PICT-2018-4407) and CLB (PICT-2016-1711 and PICT-2018-02483). Work in Geneva was supported by Swiss National Science Foundation grants no. 31003A_132902 and CRSII3_154438 to Roman Ulm (University of Geneva, Switzerland).
Author information
Authors and Affiliations
Contributions
CDC designed the experiments. CDC, ALMF, LAC and MZL performed and analyzed the experiments. PVD contributed to the measurement of specialized metabolites by HPLC. JG provided analytical tools and expertise for phytohormone determinations. CDC wrote and CLB contributed to the discussion of the results and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This publication is dedicated to Prof. Silvia E. Braslavsky, a pioneer in photobiology and photobiophysics, on the occasion of her 80th birthday.
Supplementary Information
Below is the link to the electronic supplementary material.
Additional file 1: Figure S1.
A) Transcript levels of AtBBX29 in Col, bbx29-1 and bbx29-2 mutant plants. B) Transcript levels of AtBBX29 in Col and two independent overexpression lines (BBX29ox#4 and BBX29ox#8). Values are normalized to IPP2 transcript levels and standardized to Col expression levels. Each bar represents the mean ± SEM (n ≥ 3 biological replicates). Data were analyzed by Student’s t tests, and asterisks indicate significant differences between Col and mutants or transgenic lines (**P < 0.01, ***P < 0.001). Figure S2. Transcript levels of MYB11 and MYB111 in rosette leaves of Col, bbx29-1 and BBX29ox overexpression lines. Values are normalized to IPP2 transcript levels and standardized to Col expression levels. Each bar represents the mean ± SEM (n ≥ 3 biological replicates). Data were analyzed by Student’s t tests, and asterisks indicate significant differences between Col and bbx29-1 or BBX29ox transgenic lines (*P < 0.05, **P < 0.01, NS, not significant). Figure S3. Transcript levels of genes involved in the GS biosynthetic pathway (MYB14 and MYB51) in Col and BBX29ox overexpression lines. Values are normalized to IPP2 transcript levels and standardized to Col expression levels. Each bar represents the mean ± SEM (n ≥ 4 biological replicates). Data were analyzed by Student’s t tests, and asterisks indicate significant differences between Col and transgenic lines (**P < 0.01). Figure S4. Glucosinolate (I3M, 3MSP and 4MSOB) accumulation in rosette leaves of Col and bbx29-2 knockdown mutant plants. Each bar represents the mean ± SEM (n ≥ 4 biological replicates). Data were analyzed by Student’s t tests, and asterisks indicate significant differences between Col and bbx29-2 mutant plants (*P < 0.05, NS, not significant). Figure S5. B. cinerea bioassay in Col, single mutant hy5-215 (Col background), Ws and double mutant hy5-ks50/hyh (Ws background) plants. Values are means ± SEM (n ≥ 25 individual plants). Data were analyzed by Student’s t tests, and asterisks indicate significant differences between Wild-types (Col or Ws) and mutant lines (**P < 0.01); NS, not significant.
Additional file 2: Table S1.
List of primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Medina-Fraga, A.L., Chinen, L.A., Demkura, P.V. et al. AtBBX29 integrates photomorphogenesis and defense responses in Arabidopsis. Photochem Photobiol Sci 22, 1475–1489 (2023). https://doi.org/10.1007/s43630-023-00391-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00391-8