Abstract
Photo-Fenton processes activated by biodegradable Fe(III)–EDDS complexes have attracted huge attention from the scientific community, but the operative mechanism of the photo-activation of H2O2 in the presence of Fe(III)–EDDS has not been fully clarified yet. The application of the Fe(III)–EDDS complex in Fenton and photo-Fenton (mainly under UV-B light) processes, using 4-chlorophenol (4-CP) as a model pollutant was explored to give insights into the operative mechanism. Furthermore, the potential synergistic contribution of soybean peroxidase (SBP) was investigated, since it has been reported that upon irradiation of Fe(III)–EDDS the production of H2O2 can occur. SBP did not boost the 4-CP degradation, suggesting that the possibly produced H2O2 reacts immediately with the Fe(II) ion with a quick kinetics that does not allow the diffusion of H2O2 into the bulk of the solution (i.e., outside the solvent cage of the complex). So, a concerted mechanism in which the photochemically produced H2O2 and Fe(II) react inside the hydration sphere of the Fe(III)–EDDS complex is proposed.
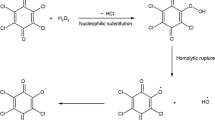
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The more and more intensive exploitation of hydric resources, due to the increase of global population, urbanization and industrialization, is causing a depletion of both their quantity and quality [1]. Moreover, climate change is worsening the shortage of water due to the altered precipitations and the droughts. Beside this, water quality has been deteriorating because of the presence of toxic and bio-refractory compounds not efficiently removed by the traditional wastewater treatment plants. Among these, contaminants of emerging concern (CECs) are increasingly being detected in surface water, groundwater, wastewater, and drinking water in quantities ranging from ng/L to few μg/L, and are raising great concern due to their high biopersistence [2,3,4]. Neither strictly regulated nor routinely monitored, CECs are a heterogeneous group of substances which comprises newly developed chemicals as well as known compounds whose toxicity has been discovered or re-evaluated (e.g., pharmaceuticals, personal care products, pesticides, microplastics, and antibiotic resistant bacteria and genes).
As CECs can threaten aquatic and terrestrial organisms and humans [5, 6], their abatement has become a crucial issue in preserving water quality, not only to meet the increasing worldwide water demand, but also to implement the reuse of reclaimed water, thus contributing to tackle water scarcity. Unfortunately, the biological degradation, adopted as secondary treatment in most wastewater treatment plants (WWTPs), cannot always ensure the efficient complete removal of any organic substrate, rather depending on its biorecalcitrant feature; in some cases, even CECs transformation products with an enhanced eco-toxicity have been detected [7].
The Directive 2013/39/EU promotes a preventive action toward the emission of CECs in the environment, upgrades the watch list of substances that must be monitored as potential sources of contamination, and encourages the development of innovative water treatment technologies [8] by WWTPs. The goal is to attain the complete removal of the toxic compounds, or at least their degradation into less harmful by-products, thus minimizing the discharge of pollutants into the receiving water body.
When considering innovative wastewater treatments for CECs removal, in the last decades increasing attention has been devoted to the potentiality of advanced oxidation processes (AOP), previously proposed by Glaze et al. as effective tools for the degradation of organic compounds from water [9] through the generation of highly reactive radical species. Among these, hydroxyl radical is a very strong oxidant (E0 = 2.8 V vs NHE) and acts unselectively against a wide variety of substances [10]. The generation of radical species can occur through different pathways, which allows to distinguish AOPs in a variety of subcategories [11]; among them, Fenton and photo-Fenton processes, based on the activation of H2O2 by means of Fe(II) (and eventually light) to generate ·OH radicals and/or other reactive species (e.g., ferryl ion), are receiving an increasing interest also for CECs removal [12,13,14].
One of the main drawbacks of (photo) Fenton processes is the optimum working pH around ≈ 2.8. In more recent times, numerous studies have assessed the ability of Fe(III) complexes to widen the range of the operating pH, maintaining low costs, and even fastening the degradative reactions [15].
Ethylenediamine-N,N′-disuccinic acid (EDDS) is a structural isomer of ethylenediaminetetraacetic acid (EDTA), highly biodegradable in different environmental compartments [16], and a strong iron chelating agent [17]. Several studies have demonstrated its efficiency in the photo-Fenton degradation of some CECs including endocrine disruptors [18], plastics precursors [19], pathogens [20], and microcontaminants [21]: Fe(III) is complexed by EDDS with a 1:1 ratio and, under UV irradiation, is easily photolyzed and generates ·OH radicals in a pH range between 3 and 9. However, the mechanism of photo-activation of H2O2 during the process has not been fully clarified yet.
On the other hand, chemical processes are not the only valuable methods for the removal of emerging contaminants; biological processes employing microorganisms and enzymes have been studied for the same objective and one recent approach for wastewater treatment relies on the coupling of AOPs and biological methods [22].
Soybean peroxidase (SBP: EC 1.11.1.7) is an oxidoreductase that can be isolated from soybean (Glycine max, L.) seed hulls; it has shown a good ability to degrade compounds like phenols and chlorophenols [23] and synthetic dyes [24]. Moreover, the great chemical and thermal stability make this enzyme interesting for possible application in wastewater treatment on a larger scale.
Calza et al. made the first attempt to couple SBP with an AOP (i.e., photocatalysis with TiO2) and observed an enhanced degradation of the target substrates compared to the use of SBP and TiO2 alone [25].
It is noteworthy that soybean, today, represents one of the major industrial and food crops grown in every continent, with a global production of 368.12 million metric tons in 2020–2021 [26]: SBP from soybean seed hulls is then widely available and relatively cheap and its exploitation in wastewater treatment could represent both an economical advantage, and a valuable example of waste recovery and reuse.
Though a synergy between SBP and Fe(II) salts has been tested already [27], as far as we know, the effects of the co-presence of the enzyme and Fe(III)–EDDS have never been investigated before.
Based on the above premises, the present work aims (i) to give insights on the Fe(EDDS) working mechanism in photo-Fenton reaction, by considering in detail the role of pH, concentration of reagents, and presence of O2, and (ii) to investigate the effect of the contemporary presence of SBP and Fe(III)–EDDS on the removal of 4-chlorophenol (4-CP) from water solution, exploring for the first time the possibility of a synergy between the two systems. Furthermore, the photo-reactivity of Fe(III)–EDDS in the presence of SBP may offer additional information on the mechanistic functioning of the complex, especially on the availability and reactivity of H2O2, which is hypothesized to be photo-produced during the photo-Fenton process but has never been measured in an effective way.
2 Materials and methods
2.1 Chemicals
Iron (III) chloride hexahydrate (FeCl3‧6H2O), ferric perchlorate (Fe(ClO4)3·9H2O), S,S′-ethylenediamine-N,N′-disuccinic acid trisodium salt (EDDS, 35% in water), hydrogen peroxide (H2O2), 4-chlorophenol (4-CP), propan-2-ol (2-prop), acetonitrile, phosphoric acid (H3PO4), methanol, and potassium iodide (KI) were obtained from Sigma Aldrich. Ammonium heptamolybdate ((NH4)6Mo7O24·H2O) and potassium hydrogen phthalate (C8H5KO5) were obtained from Merck. Sodium hydroxide (NaOH) was from Alfa Aesar. Soybean peroxidase (SBP) was obtained from BioResearch Products. All the chemical reagents were used as received. Suspensions and standard solutions were prepared in Milli-Q® water.
The Fe(III)−EDDS complex solution was prepared by mixing appropriate amounts of FeCl3 and EDDS salt solution with 1:1 stoichiometry. The complex was freshly prepared before each experiment.
2.2 Irradiation setup and degradation procedure
For the degradation of 4-CP via photo-Fenton process, a fluorescent lamp (TL 20 W/01, Philips, Netherlands) with a total flux of 7.54 W m−2 in the 295–400 nm range was employed. Experiments were performed in closed Pyrex glass cells filled with 5 mL of solution under magnetic stirring and irradiated for different times (0 to 120 min). After every sampling, made at fixed intervals of time, 1.0 mL of methanol was added to the 5 mL of the suspension to quench the Fenton reaction. The initial concentration of 4-CP was 0.1 mM. All the experiments were carried out at room temperature (293 ± 2 K) and, except for the experiments at varying pH values, the pH was adjusted at a value of ≈ 5.5 using H3PO4 and NaOH. pH values of the solutions were measured using a 913 Metrohm pH meter.
2.3 Analysis
The concentration of 4-chlorophenol remaining in the aqueous solution was measured through a YL9300 HPLC system equipped with a YL9330 Column Compartment and a YL9150 autosampler. The column was a RP C18 column (LiChroCART®, Merck, 12.5 cm × 0.4 cm; 5 μm packing). 4-chlorophenol was analyzed in isocratic mode, using a 30:70% v/v acetonitrile: phosphoric acid solution (1 × 10–2 M) as mobile phase. The experiments were performed by UV detection at 220 nm, the flow rate was of 1 mL min−1. In these conditions, the retention time of 4-CP was 8.8 min.
The decomposition of H2O2 was monitored using the method outlined by Klassen et al. [28], which is accurate to H2O2 concentrations as low as 1 μM. This method employs an ammonium molybdate catalyzed reaction between H2O2 and I− to form I2 (iodine). I2 then reacts with free I− ions in solution to form the I3− ion which can be measured using optical absorption at λ = 350 nm. The spectrophotometric analyses were performed using a Varian CARY 100 Scan double-beam UV–Vis spectrophotometer, using glass cuvettes with 1 cm path length.
The repetition of degradation experiments in the same conditions gave an overall relative standard deviation (RSD) for the concentration of 4-CP and H2O2 of roughly 10%.
3 Results and discussion
The initial concentrations of 4-CP (0.1 mM), Fe(III)–EDDS (0.1 mM), and H2O2 (0.1–0.2 mM) were chosen in agreement with previous works [29, 30]. The choice of an UV-B irradiation, despite the previously investigated UV-A, was carried out not only to test different conditions, but also to maximize the overlap between the emission spectrum of the used lamp and the absorption spectrum of Fe(III)–EDDS (Fig. S1 of the SI).
3.1 Degradation kinetics of 4-CP in photo-Fenton processes
Figure 1 shows the effects of the presence of Fe(III)–EDDS, H2O2 and the role of UV-B irradiation on the concentration of 4-CP. The direct photolysis of 4-CP under irradiation was also evaluated and, as expected from the absence of significant absorption at λ > 300 nm, a limited degradation of 4-CP was observed (≈ 18% after 120 min of irradiation). Furthermore, in the dark the complex Fe(III)–EDDS was not able to activate H2O2 and to promote the removal of the substrate, as manifested from the stability of 4-CP in the presence of both Fe(III)–EDDS and H2O2. On the contrary, the UV-B irradiation of Fe(III)–EDDS activated the production of reactive species (e.g., ·OH and HO2·/O2·−) also in the absence of additional oxidants (i.e., H2O2), in agreement with the reactions (R1–R5 [29, 30]).
The addition of H2O2 increased the degradation of 4-CP up to 59% after 120 min, in agreement with the fast reaction between the added H2O2 and the Fe(II) photochemically generated from Fe(III)–EDDS photolysis (R1).
The profiles of 4-CP concentration under irradiation gave some additional insights. The UV-B irradiation activated a fast initial degradation (tirr < 15 min) and a following step in which a slower (at least null) degradation was observed. This behavior deserved to be investigated, so experiments at different doses of both H2O2 and Fe(III)–EDDS were carried out (vide infra).
3.2 Effect of H2O2 concentration under irradiated Fe(III)–EDDS
Figure 2a shows the degradation of 4-CP under irradiation and in the presence of different concentrations of hydrogen peroxide (from 0 to 1 mM). Figure 2b summarizes the % removal after 120 min as a function of the initial [H2O2]. The same concentration profiles previously commented were observed, with most of the degradation taking place in the first minutes of the irradiation time (< 10–15 min). The addition of an increasing amount of H2O2 resulted in a rising % of 4-CP removal with saturating behavior (see Fig. 2b), indicating that H2O2 could be the limiting reagent for the process in the explored conditions. In this light, the effect of sequential additions of H2O2 was studied (see Fig. 2b inset) and a profile with 3 sequential drops of the 4-CP was observed. Note that during the addition of H2O2, no further Fe(III)–EDDS was added. The effect of H2O2 addition was less effective after the first step, with a decrease of the degradation in absolute value after the following additions. This highlighted that (i) H2O2 played as a limiting reagent for the process and (ii) the catalytic properties of Fe(III)–EDDS faded as a consequence of the prolonged treatment (in agreement with a limited catalytic role for Fe(III)–EDDS in the explored experimental conditions).
a Photodegradation of 4-CP at different concentrations of H2O2; b % removal of 4-CP after 120 min of irradiation. Inset: 4-CP degradation after 3 consecutive additions (every 20 min) of H2O2 0.1 mM. Phototransformation of 4-CP c and H2O2 d at different concentrations of H2O2 (tirr ≤ 5 min). Conditions: [4-CP]0 = 0.1 mM, [Fe(III)–EDDS] = 0.1 mM, [H2O2] = 0–1 mM, initial pH = 5.5
Note that under UV-B, H2O2 can undergo direct photolysis (H2O2 + hν → 2 ·OH) as a consequence of its small absorption tail at λ > 300 nm. The 4-CP degradation in the presence of H2O2 0.1 mM and in the absence of the iron complex was investigated. After 120 min, a 40% removal of 4-CP was reached (data not reported). In the same conditions but with the addition of Fe(III)–EDDS 0.1 mM, the removal was 55%. In the presence of the iron complex, this latter was the major photon absorber as the molar absorption coefficient of Fe(III)–EDDS at 313 nm is at least 3 orders of magnitude higher than that of H2O2. As a consequence, the role of the direct photolysis of H2O2 in the presence of Fe(III)–EDDS was negligible.
To give insights into the role of H2O2, the concentration of 4-CP and H2O2 was monitored at short irradiation time (≤ 5 min) in experiments at the same concentration of 4-CP (0.1 mM) and increasing amounts of H2O2 (from 0.1 to 1 mM). Figure 2c, d show the 4-CP and H2O2 concentration profiles, respectively. At the lowest concentration of oxidant (0.1 and 0.2 mM) H2O2 was consumed within 1 min of irradiation, while at the highest concentration (1 mM) a residual quantity of H2O2 was observed even after 5 min of irradiation.
From the comparison of the two profiles, it can be manifested that (i) the complete disappearance of H2O2 did not coincide with the stop of the 4-CP degradation, that continued also in the absence of H2O2, and (ii) at the maximum concentration of H2O2, 4-CP reached its plateau value in the presence of a residual amount of H2O2 that was not able to further promote its degradation. These experimental results highlighted the essential role of H2O2 in activating the first and fast step of 4-CP degradation (and that higher is the concentration of oxidant, higher is the % 4-CP removal). However, the decline of the degradation ability of the system might be ascribed also to the faded catalytic properties of the Fe(III)–EDDS complex (which was not recycled or was only partially recycled after the activation of H2O2).
3.3 Effect of Fe(III)–EDDS concentration
Figure 3 shows the degradation profiles and the residual fraction of 4-CP after 120 min of irradiation in the presence of increasing concentrations of Fe(III)–EDDS without and with H2O2 0.1 mM. With the rising concentration of the iron complex–both with and without H2O2–an increase of the 4-CP degradation was observed. At higher complex concentration, an increase of the photo-formed Fe(II) and consequently of ·OH (or alternative reactive species) production was observed. In all the cases, the process slowed down after the first step of degradation and the 4-CP concentration reached a plateau. Without H2O2 (i) the 4-CP residual fraction was–at all the explored Fe(III)–EDDS concentrations– ≈ 30% lower than in the presence of H2O2; (ii) the degradation rate of the initial step was significantly lower in the absence of hydrogen peroxide than in the presence–being H2O2 not immediately at disposal of the photo-produced Fe(II).
a Photodegradation of 4-CP at different concentrations of Fe(III)–EDDS; b % removal of 4-CP after 120 min of irradiation. Inset: 4-CP degradation after 3 consecutive additions (every 20 min) of Fe(III)–EDDS 0.1 mM. Conditions: [4-CP]0 = 0.1 mM, [Fe(III)–EDDS] = 0.1–0.5 mM, [H2O2] = 0.1 mM, initial pH = 5.5
The inset of Fig. 3b shows the effect of successive addition of Fe(III)–EDDS 0.1 mM (every 20 min). Note that the second addition was carried out in a solution in which H2O2 concentration was null (see Fig. 2d). The addition of the sole Fe(III)–EDDS gave effects similar to those observed with the additions of H2O2 alone: a further, but less evident, 4-CP degradation after each addition. Lastly, a test with sequential additions of both H2O2 0.1 mM and Fe(III)–EDDS 0.1 mM was carried out and compared with the profiles measured with sequential additions of H2O2 and Fe(III)–EDDS alone (see Fig. S3). The simultaneous addition of both reactants gave the best results, suggesting that the real active reagent in the UV-B activated photo-Fenton process (with EDDS as ligand for Fe+3) is the couple iron complex + H2O2, and that catalytic cycles able to regenerate a photoactive catalyst are scarcely operative. The limited (but not null) effect of the third addition of H2O2 + Fe(III)–EDDS can be explained with the progressive accumulation of organic by-products (e.g., products of Fe(III)–EDDS UV-B photolysis and 4-CP degradation) competing with 4-CP for the same oxidative reactive species.
To summarize the results of the experiments of Paragraph 3.2 and 3.3, we argue that the initial sharp degradation of 4-CP has to be attributed to radical species as ·OH, HO2· or O2·− resulting from the photolysis of Fe(III)–EDDS, rather than to the direct photolysis of H2O2. These species can be formed even in the absence of H2O2, as previously reported in other studies [19, 30]. The main role of Fe(III)–EDDS in the process is supported by its higher molar absorption coefficient in the UV-B (λmax = 313 nm). Nevertheless, the H2O2 that rapidly reacts with the Fe(II) is crucial in increasing the % of 4-CP degradation.
3.4 Reactive species involved in the photo-Fenton process: role of hydroxyl radicals
With the aim to highlight the role of ·OH, the degradation of 4-CP in the presence of Fe(III)–EDDS and H2O2 was investigated with increasing concentrations of propan-2-ol. This compound is an efficient scavenger for ·OH (k2-prop, HO· = 1.9 × 109 M−1 s−1 [31]).
Figure 4 shows the effect of propan-2-ol addition on the 4-CP degradation profiles (Fig. 4a) and the % removal (tirr = 120 min) as a function of the propan-2-ol concentration (Fig. 4b). Propan-2-ol had a clear inhibitory effect on the maximum % removal reached at the end of the irradiation and on the initial rate of 4-CP transformation in the first minutes of irradiation. This is a clear evidence of the predominant role of the ·OH radicals in the 4-CP degradation under UV-B and in the presence of Fe(III)–EDDS and H2O2. Furthermore, Fig. 4 shows that the reactivity with ·OH cannot totally explain the substrate removal: with the increase of the scavenger concentration, the % removal decreased monotonically down to a plateau value equal to ≈ 15%. This is in agreement with an operative role of alternative (even if minor) reactive species (e.g., ferryl ion) that were not totally scavenged by propan-2-ol. In similar experimental conditions but in the presence of chloroform, Huang et al. [29] demonstrated an important role of HO2·/O2·– not as reactive species involved in the removal of the organic substrate, but as reductants for Fe(III)–EDDS responsible for the indirect generation of ·OH.
The degradation of 4-CP with Fe(III)–EDDS/H2O2/UV-B at different pH values (from 3 to 8) and the role of the dissolved oxygen were also investigated. The results (reported in paragraphs 2.1 and 2.2 of SI) were in agreement with the previous reports about the photo-Fenton process with Fe(III)–EDDS [30].
3.5 Effect of SBP addition
The catalytic activity of SBP for the oxidation of phenolic compounds with H2O2 has been documented [32], together with its potential application for the removal of organic pollutants, both in homogeneous conditions [33] and as supported catalyst [34]. Furthermore, synergistic effects between advanced oxidation processes where H2O2 is produced (e.g., heterogeneous photocatalysis under irradiated semiconductors) and the presence of SBP have been previously reported [25]. As mentioned in Paragraph 3.1, from the existing literature on Fenton processes it is hypothesized that H2O2 could be photo-produced during the photo-Fenton reaction (R1–R4): the radical EDDS· generated by the photolysis of Fe(III)–EDDS complex would react with the dissolved oxygen and generate O2·−, which is able to disproportionate (R4a–R4b) and to produce further H2O2 and O2. The H2O2 can then react with the Fe(II) resulting from the same photolysis of Fe(III)–EDDS and start a Fenton reaction that would enhance the final total degradation of the substrate. In this light, the effect of SBP on the removal of 4-CP in the presence of Fe(III)–EDDS (both in dark and under irradiation) has been investigated with the aim to understand if the H2O2 possibly produced under the irradiation of the Fe(III)–EDDS complex can be efficiently used by SBP to boost the transformation rate of 4-CP. Further goal of the work was to give insights into the mechanism of transformation of organic compounds in photo-Fenton processes activated by Fe(III)–EDDS.
3.5.1 Activity of SBP in dark
In the dark, 4-CP was efficiently removed through the SBP catalyzed reaction with H2O2; after 120 min of treatment with SBP 0.01 µM and H2O2 0.1 mM ([H2O2]/[SBP] ratio = 10,000), the remaining concentration of 4-CP in solution was ≈ 10% of its initial concentration (Fig. 5a). When the same experiment was carried out adding Fe(III)–EDDS 0.1 mM, a significant decrease in the overall removal of 4-CP was observed. Indeed, after 120 min, only a degradation efficiency of ≈ 50% was reached. On the other hand, changing the concentration of SBP ([H2O2]/[SBP] ratio from 100,000 to 1000), both the initial degradation rate and the C/C0 value at the end of the reaction changed as a function of SBP concentration (Fig. 5a). Moreover, at the highest concentration of SBP an almost quantitative removal of 4-CP was reached in 5 min from the addition of H2O2, also in the presence of the Fe(III)–EDDS complex.
a Concentration of 4-CP as a function of the treatment time in dark conditions in the presence of Fe(III)–EDDS (0.1 mM, if present), H2O2 (0.1 mM) and SBP (0.001, 0.01 and 0.1 μM); b Concentration of 4-CP as a function of the treatment time under UV-B irradiation in the presence of different concentrations of Fe(III)–EDDS and SBP, all the experiments were carried out without H2O2; c Concentration of 4-CP as a function of the treatment time under UV-B and in the dark with 0.1 mM initial H2O2 concentration; d % removal of 4-CP after 120 min of reaction. Composition of each solution reported in the figure’s legend
These results did not indicate a cooperative effect between SBP and the Fe(III)–EDDS complex in the dark, but rather a possible inhibition of SBP by Fe(III)–EDDS, or a competition between SBP and Fe(III)–EDDS for H2O2.
3.5.2 Activity of SBP under irradiation
The experiments under UV-B irradiation reported in Fig. 5b were carried out to better clarify the results obtained in the dark and investigate if the H2O2 potentially formed under irradiation of Fe(III)–EDDS could be effectively used by SBP for boosting the 4-CP degradation. For this reason, no H2O2 was initially added to the solution. Furthermore, the concentration of H2O2 was also monitored since the first minutes of irradiation through the fluorimetric method reported by Lazrus et al. [35]. The measured concentrations were always lower than the LOD of the applied analytical method (1.2 × 10–8 M [35]).
Figure 5b shows the 4-CP concentration as a function of the treatment time under UV-B in the presence of different concentrations of Fe(III)–EDDS and SBP. Comparing the degradation profiles in the presence of Fe(III)–EDDS 0.1 mM alone and in the presence of SBP (0.01 µM), no significant difference was manifest and an overall final degradation of ≈ 40% was observed. This can be explained considering that the Fe(II) generated by the photolysis of Fe(III)–EDDS is immediately used by the photo-produced H2O2, giving hydroxyl radicals. In this light, there is a competition between Fe(II) and SBP for the photo-produced H2O2 that, on the basis of this experimental evidence, is almost totally shifted toward the Fe(II) ion, thus limiting the SBP action. When SBP concentration was increased (0.1 µM), the overall degradation of 4-CP slightly increased from 35 to 40%, whereas a more evident increment in the degradation of 4-CP was observed by increasing the Fe(III)–EDDS concentration from 0.1 to 0.5 mM at the same SBP concentration (0.01 µM), giving a clear indication that the role of Fe(III)–EDDS on the overall degradation mechanism was higher–in the tested experimental conditions–than that of SBP.
Further experiments with SBP were carried out under irradiation with a fixed initial concentration of H2O2 (Fig. 5c). In this case, the degradation performed by the system Fe(III)–EDDS/SBP/H2O2 under UV-B was higher than in the dark (removal equal to 62 and 52%, respectively), but not so high to be explained as the sum of the two distinguished contributions (reactivity of SBP in dark and photo-reactivity of Fe(III)–EDDS). Furthermore, removing the Fe(III)–EDDS a higher 4-CP degradation was observed, even if slightly lower than the degradation observed in the correspondent dark conditions (removal equal to 81 and 92%, respectively). So, the detrimental effect of Fe(III)–EDDS on the SBP activity was effective under irradiation too and a partial photo-deactivation of SBP under UV-B was observed. To limit the photo-deactivation of the SBP, the degradation of 4-CP in the presence of Fe(III)–EDDS alone and with Fe(III)–EDDS + SBP was also monitored under UV-A irradiation (data not shown). As with UV-B, no significant difference was observed: the absence of a synergistic effect between Fe(III)–EDDS and SBP cannot therefore be attributed to a detrimental effect of the UV-B on the structure of the enzyme.
The experimental evidence reported above gives some important mechanistic information. Considering that (i) the second order kinetic constant for the reaction between SBP and H2O2 is quite high (\(k_{{\text{SBP}},{\text{H}}_2 {\text{O}}_2 }\) = 2·107 M−1 s−1 [36]) compared to the constant between Fe(II) and H2O2 (\(k_{{\text{Fe}}\left( {{\text{II}}} \right),{\text{H}}_{2} {\text{O}}_{2} }\) = 76 M−1 s−1 [37]), (ii) SBP is an effective catalyst for H2O2 in the oxidation of diverse organic substrates and (iii) no synergistic effect was observed between Fe(III)–EDDS complex and SBP under UV-B irradiation, it is possible to conclude that the photolysis of Fe(III)–EDDS is unlikely to release significant amounts of H2O2 in the bulk of the solution. This could be not in contrast with the hypothesis that the Fe(II) ion produced from the photolysis of Fe(III)–EDDS would react with H2O2 (photochemically produced from the reduction of the dissolved oxygen under the photolysis of the iron complex) and generate hydroxyl radicals (or other species with similar reactivity, e.g., ferryl ion [38]). However, all these reactive steps (reactions R1–R4) are concerted and the photochemically generated species (i.e., Fe(II) and H2O2) react immediately after their production, as an example inside the solvent cage. This prevents their diffusion in the bulk of the solution, where they could react with SBP. The experimental data suggest that the quantity of hydrogen peroxide produced by Fe(III)–EDDS irradiation in the bulk of the solution is too low to be effectively used by the peroxidase.
4 Conclusions
The photolysis of the Fe(III)–EDDS complex is an efficient tool for the removal of 4-CP under UV-B, especially in the presence of H2O2. Increasing the concentration of both H2O2 and Fe(III)–EDDS, we observed an increase of 4-CP degradation due to the increment in the production of hydroxyl radicals, the main reactive species in this process (as proved by the experiments with propan-2-ol as selective ·OH radical scavenger). Despite of the classic Fenton process, the UV-B-activated Fe(III)–EDDS photo-Fenton is operative even at neutral or slightly basic pH. The presence of dissolved oxygen is essential to allow significant 4-CP degradation. From the photoreduction of O2, the superoxide radical anion (O2·–) is produced and this has a paramount role in the process not only to promote the production of H2O2 (likely blocked in the solvent cage and consequently immediately used for the production of reactive species before its diffusion in the bulk), but also to reduce Fe(III) to Fe(II).
The addition of SBP did not show the desired synergistic effect with the photo-Fenton system based on the UV-B irradiation of the Fe(III)–EDDS complex, but gives us some clear indication of a concerted mechanism where the possibly produced H2O2 reacts immediately with the Fe(II) ion with so fast kinetics that the diffusion of hydrogen peroxide into the bulk of the solution (i.e., outside of the solvent cage of the complex) cannot compete with the reaction inside the hydration sphere of the photo-reactive complex.
The experiments with SBP and H2O2 only show a significant effect on 4-CP degradation, both under irradiation and in dark, reaching up to the 80%–90% of removal. In general, this is consistent with the results of previous studies elucidating the efficacy of the two reagents against different compounds [34, 39]. Furthermore, a recent study reports a degradation of approximatively 40% of pentachlorophenol in similar conditions [27], while higher degradations were obtained when the enzyme was associated to other materials such as TiO2 or ZnO [40]. These promising results suggest that the efficiency of peroxidases and H2O2 in degrading organic compounds may be case specific and encourage further investigations on their functioning and effectiveness in water treatment.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akinsete, E., Apostolaki, S., Chatzistamoulou, N., Koundouri, P., & Tsani, S. (2019). The link between ecosystem services and human wellbeing in the implementation of the european water framework directive: Assessing four River Basins in Europe. Water, 11(3), 508. https://doi.org/10.3390/w11030508
Rizzo, L., Malato, S., Antakyali, D., Beretsou, V. G., Đolić, M. B., Gernjak, W., Heath, E., Ivancev-Tumbas, I., Karaolia, P., Lado Ribeiro, A. R., Mascolo, G., McArdell, C. S., Schaar, H., Silva, A. M. T., & Fatta-Kassinos, D. (2019). Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Science of the Total Environment, 655, 986–1008. https://doi.org/10.1016/j.scitotenv.2018.11.265
Pastorino, P., & Ginebreda, A. (2021). Contaminants of emerging concern (CECs): Occurrence and fate in aquatic ecosystems. International Journal of Environmental Research and Public Health, 18(24), 13401. https://doi.org/10.3390/ijerph182413401
Fairbairn, D. J., Karpuzcu, M. E., Arnold, W. A., Barber, B. L., Kaufenberg, E. F., Koskinen, W. C., Novak, P. J., Rice, P. J., & Swackhamer, D. L. (2016). Sources and transport of contaminants of emerging concern: A two-year study of occurrence and spatiotemporal variation in a mixed land use watershed. Science of the Total Environment, 551–552, 605–613. https://doi.org/10.1016/j.scitotenv.2016.02.056
Fent, K., Weston, A., & Caminada, D. (2006). Ecotoxicology of human pharmaceuticals. Aquatic Toxicology, 76(2), 122–159. https://doi.org/10.1016/j.aquatox.2005.09.009
Hossain, K. A., & Roy, K. (2018). Chemometric modeling of aquatic toxicity of contaminants of emerging concern (CECs) in Dugesia japonica and its interspecies correlation with daphnia and fish: QSTR and QSTTR approaches. Ecotoxicology and Environmental Safety, 166, 92–101. https://doi.org/10.1016/j.ecoenv.2018.09.068
Schlüter-Vorberg, L., Prasse, C., Ternes, T. A., Mückter, H., & Coors, A. (2015). Toxification by transformation in conventional and advanced wastewater treatment: The antiviral drug acyclovir. Environmental Science & Technology Letters, 2(12), 342–346. https://doi.org/10.1021/acs.estlett.5b00291
Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013. Official Journal of the European Union (2013).
Glaze, W. H., Kang, J.-W., & Chapin, J.-W. (1987). The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone: Science & Engineering, 9(4), 335–352. https://doi.org/10.1080/01919518708552148
Pera-Titus, M., García-Molina, V., Baños, M. A., Giménez, J., & Esplugas, S. (2004). Degradation of chlorophenols by means of advanced oxidation processes: A general review. Applied Catalysis B, 47(4), 219–256. https://doi.org/10.1016/j.apcatb.2003.09.010
Andreozzi, R., Caprio, V., Insola, A., & Marotta, R. (1999). Advanced oxidation processes (AOP) for water purification and recovery. Catalysis Today, 53(1), 51–59. https://doi.org/10.1016/S0920-5861(99)00102-9
Barbeni, M., Minero, C., Pelizzetti, E., Borgarello, E., & Serpone, N. (1987). Chemical degradation of chlorophenols with Fenton’s reagent (Fe2+ + H2O2). Chemosphere, 16(10–12), 2225–2237. https://doi.org/10.1016/0045-6535(87)90281-5
Miralles-Cuevas, S., Oller, I., Pérez, J. A. S., & Malato, S. (2015). Application of solar photo-Fenton at circumneutral pH to nanofiltration concentrates for removal of pharmaceuticals in MWTP effluents. Environmental Science and Pollution Research, 22(2), 846–855. https://doi.org/10.1007/s11356-014-2871-2
Umar, M., Aziz, H. A., & Yusoff, M. S. (2010). Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Management, 30(11), 2113–2121. https://doi.org/10.1016/j.wasman.2010.07.003
Pignatello, J. J., Oliveros, E., & MacKay, A. (2006). Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Critical Reviews in Environmental Science and Technology, 36(1), 1–84. https://doi.org/10.1080/10643380500326564
Orama, M., Hyvönen, H., Saarinen, H., & Aksela, R. (2002). Complexation of [S, S] and mixed stereoisomers of N,N′-ethylenediaminedisuccinic acid (EDDS) with Fe(III), Cu(II), Zn(II) and Mn(II) ions in aqueous solution. Journal of the Chemical Society Dalton Transactions, 24, 4644–4648. https://doi.org/10.1039/B207777A
Schowanek, D., Feijtel, T. C. J., Perkins, C. M., Hartman, F. A., Federle, T. W., & Larson, R. J. (1997). Biodegradation of [S,S], [R,R] and mixed stereoisomers of Ethylene Diamine Disuccinic Acid (EDDS), a transition metal chelator. Chemosphere, 34(11), 2375–2391. https://doi.org/10.1016/S0045-6535(97)00082-9
Li, J., Mailhot, G., Wu, F., & Deng, N. (2010). Photochemical efficiency of Fe(III)–EDDS complex: OH radical production and 17β-estradiol degradation. Journal of Photochemistry and Photobiology A: Chemistry, 212(1), 1–7. https://doi.org/10.1016/j.jphotochem.2010.03.001
Huang, W., Brigante, M., Wu, F., Hanna, K., & Mailhot, G. (2012). Development of a new homogenous photo-Fenton process using Fe(III)–EDDS complexes. Journal of Photochemistry and Photobiology A: Chemistry, 239, 17–23. https://doi.org/10.1016/j.jphotochem.2012.04.018
García-Fernández, I., Miralles-Cuevas, S., Oller, I., Malato, S., Fernández-Ibáñez, P., & Polo-López, M. I. (2019). Inactivation of E. coli and E. faecalis by solar photo-Fenton with EDDS complex at neutral pH in municipal wastewater effluents. Journal of Hazardous Materials, 372, 85–93. https://doi.org/10.1016/j.jhazmat.2018.07.037
Miralles-Cuevas, S., Oller, I., Ruíz-Delgado, A., Cabrera-Reina, A., Cornejo-Ponce, L., & Malato, S. (2019). EDDS as complexing agent for enhancing solar advanced oxidation processes in natural water: Effect of iron species and different oxidants. Journal of Hazardous Materials, 372, 129–136. https://doi.org/10.1016/j.jhazmat.2018.03.018
Castro, E., Avellaneda, A., & Marco, P. (2014). Combination of advanced oxidation processes and biological treatment for the removal of benzidine-derived dyes. Environmental Progress & Sustainable Energy, 33(3), 873–885. https://doi.org/10.1002/ep.11865
Bassi, A., Geng, Z., & Gijzen, M. (2004). Enzymatic removal of phenol and chlorophenols using soybean seed hulls. Engineering in Life Scieces, 4(2), 125–130. https://doi.org/10.1002/elsc.200420021
Marchis, T., Avetta, P., Bianco Prevot, A., Fabbri, D., Viscardi, G., & Laurenti, E. (2011). Oxidative degradation of Remazol Turquoise Blue G 133 by soybean peroxidase. Journal of Inorganic Biochemistry, 105(2), 321–327. https://doi.org/10.1016/j.jinorgbio.2010.11.009
Calza, P., Avetta, P., Rubulotta, G., Sangermano, M., & Laurenti, E. (2014). TiO2-soybean peroxidase composite materials as a new photocatalytic system. Chemical Engineering Journal, 239, 87–92. https://doi.org/10.1016/j.cej.2013.10.098
https://ipad.fas.usda.gov/, Jun. 06, 2022.
Tolardo, V., García-Ballesteros, S., Santos-Juanes, L., Vercher, R., Amat, A. M., Arques, A., & Laurenti, E. (2019). Pentachlorophenol removal from water by soybean peroxidase and iron(II) salts concerted action. Water, Air & Soil Pollution, 230(6), 140. https://doi.org/10.1007/s11270-019-4189-7
Klassen, N. V., Marchington, D., & McGowan, H. C. E. (1994). H2O2 determination by the I3- method and by KMnO4 titration. Analytical Chemistry, 66(18), 2921–2925. https://doi.org/10.1021/ac00090a020
Huang, W., Brigante, M., Wu, F., Mousty, C., Hanna, K., & Mailhot, G. (2013). Assessment of the Fe(III)–EDDS complex in Fenton-like processes: From the radical formation to the degradation of bisphenol A. Environmental Science & Technology, 47(4), 1952–1959. https://doi.org/10.1021/es304502y
Wu, Y., Passananti, M., Brigante, M., Dong, W., & Mailhot, G. (2014). Fe(III)–EDDS complex in Fenton and photo-Fenton processes: From the radical formation to the degradation of a target compound. Environmental Science and Pollution Research, 21(21), 12154–12162. https://doi.org/10.1007/s11356-014-2945-1
Buxton, G. V., Greenstock, C. L., Helman, W. P., & Ross, A. B. (1988). Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O−) in Aqueous Solution. Journal of Physical and Chemical Reference Data, 17, 513–886. https://doi.org/10.1063/1.555805
McEldoon, J. P., Pokora, A. R., & Dordick, J. S. (1995). Lignin peroxidase-type activity of soybean peroxidase. Enzyme and Microbial Technology, 17(4), 359–365. https://doi.org/10.1016/0141-0229(94)00060-3
Caza, N., Bewtra, J. K., Biswas, N., & Taylor, K. E. (1999). Removal of phenolic compounds from synthetic wastewater using soybean peroxidase. Water Research, 33(13), 3012–3018. https://doi.org/10.1016/S0043-1354(98)00525-9
Calza, P., Zacchigna, D., & Laurenti, E. (2016). Degradation of orange dyes and carbamazepine by soybean peroxidase immobilized on silica monoliths and titanium dioxide. Environmental Science and Pollution Research, 23(23), 23742–23749. https://doi.org/10.1007/s11356-016-7399-1
Lazrus, A. L., Kok, G. L., Gitlin, S. N., Lind, J. A., & McLaren, S. E. (1985). Automated fluorimetric method for hydrogen peroxide in atmospheric precipitation. Analytical Chemistry, 57(4), 917–922. https://doi.org/10.1021/ac00281a031
Nissum, M., Schiødt, C. B., & Welinder, K. G. (2001). Reactions of soybean peroxidase and hydrogen peroxide pH 2.4–12.0, and veratryl alcohol at pH 2.4. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology, 1545(1–2), 339–348. https://doi.org/10.1016/S0167-4838(00)00295-8
Barb, W. G., Baxendale, J. H., George, P., & Hargrave, K. R. (1951). Reactions of ferrous and ferric ions with hydrogen peroxide. Part II. - The ferric ion reaction. Transactions of the Faraday Society, 47, 591–616. https://doi.org/10.1039/TF9514700591
Farinelli, G., Minella, M., Pazzi, M., Giannakis, S., Pulgarin, C., Vione, D., & Tiraferri, A. (2020). Natural iron ligands promote a metal-based oxidation mechanism for the Fenton reaction in water environments. Journal of Hazardous Materials, 393, 122413. https://doi.org/10.1016/j.jhazmat.2020.122413
Al-Maqdi, K. A., Hisaindee, S., Rauf, M. A., & Ashraf, S. S. (2018). Detoxification and degradation of sulfamethoxazole by soybean peroxidase and UV + H2O2 remediation approaches. Chemical Engineering Journal, 352, 450–458. https://doi.org/10.1016/j.cej.2018.07.036
Sarro, M., Gule, N. P., Laurenti, E., Gamberini, R., Paganini, M. C., Mallon, P. E., & Calza, P. (2018). ZnO-based materials and enzymes hybrid systems as highly efficient catalysts for recalcitrant pollutants abatement. Chemical Engineering Journal, 334, 2530–2538. https://doi.org/10.1016/j.cej.2017.11.146
Acknowledgements
MM, EL and ABP are grateful for the financial support from Università di Torino (Ricerca Locale).
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bertolotti, S., Minella, M., Laurenti, E. et al. Application of Fe(III)–EDDS complexes and soybean peroxidase in photo-Fenton processes for organic pollutant removal: insights into possible synergistic effects. Photochem Photobiol Sci 22, 603–613 (2023). https://doi.org/10.1007/s43630-022-00339-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00339-4