Abstract
A comparison study examines six different metal oxides (CuO, ZnO, Fe3O4, Co3O4, NiO, and α-MnO2) for the degradation of malachite green dye using four distinct processes. These processes are as follows: sonocatalysis (US/metal oxide), sonocatalysis under ultra-violet irradiation (US/metal oxide/UV), sonocatalysis in the presence of hydrogen peroxide (US/metal oxide/H2O2), and a combination of all these processes (US/metal oxide/UV/H2O2). The effective operating parameters, such as the dosage of metal oxide nanoparticles (MONPs), the type of the process, and the metal oxides’ efficiency order, were studied. At the same reaction conditions, the sonophotocatalytic is the best process for all six MOsNPs, CuO was the better metal oxide than other MOsNPs, and at the sonocatalysis process, ZnO was the best metal oxide in other processes. It was found that the metal oxide order for sonocatalytic process is CuO > α-MnO2 ≥ ZnO > NiO ≥ Fe3O4 ≥ Co3O4 within 15–45 min. The order of (US/metal oxide/UV) process is ZnO ≥ NiO ≥ α-MnO2 > Fe3O4 ≥ CuO ≥ Co3O4 within 5–40 min. The order of (US/ MOsNPs/ H2O2) process is ZnO ≥ CuO ≥ α-MnO2 ≥ NiO > Co3O4 > Fe3O4 within 5–20 min. The maximum removal efficiency order of the sonophotocatalytic process is ZnO ≥ CuO > α-MnO2 > NiO > Fe3O4 ≥ Co3O4 within 2–8 min. The four processes degradation efficiency was in the order US/MOsNPs ˂ US/MOsNPs/UV ˂ US/MOsNPs/H2O2 ˂ (UV/Ultrasonic/MOsNPs/H2O2). Complete degradation of MG was obtained at 0.05 g/L MONPs and 1 mM of H2O2 using 296 W/L ultrasonic power and 15 W ultra-violet lamp (UV-C) within a reaction time of 8 min according to the MOsNPs type at the same sonophotocatalytic/H2O2 reaction conditions. The US/metal oxide/UV/H2O2 process is inexpensive, highly reusable, and efficient for degrading dyes in colored wastewater.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Human health and the environment are at risk due to increased organic pollutants in wastewater. Malachite green (MG) has long been used as a representative dye for dyeing wool, silk, paper, textiles, and leather [20]. Its task of removing industrial effluents is critical [55, 56].
Due to secondary pollution, chemical treatment of wastewater containing dyes is inappropriate. To more effectively remove hazardous dyes from wastewater, additional novel research is required [22, 56]. Coagulation–flocculation, ozonation, membrane filtration, electrolysis, oxidation, active sludge biochemical processes, bio-decolorization, and other physical and chemical techniques have been widely used to remove dyes from wastewater [9, 24, 27, 28, 43, 45]. These well-established technologies are frequently incapable of lowering the dye concentration to the desired effective level.
Among the advanced oxidation processes (AOPs), photocatalysis by semiconducting materials has become a leading technology in wastewater purification because of these materials' high stability and activity [6, 10, 26, 47, 54]. The heterogeneous photocatalytic process is a legitimate method for oxidizing organic pollutants to carbon dioxide and water in an aqueous system. On the other hand, the UV/H2O2-based advanced oxidation processes are important because of the formation of hydroxyl radicals (OH•). These radicals act as a non-selective oxidizing agent with a high oxidation capacity to degrade or oxidize most organic contaminants in water streams [35]. The H2O2 can react with the surfaces of metal oxides via catalytic decomposition and redox reactions, where the.OH radicals are stabilized by forming bonds between their unpaired electrons and the oxide surface [32].
Ultrasound (US) energy creates positive holes that can mineralize various organic pollutants. This technique has several advantages, including simplicity, safety, and environmental friendliness. It is based on the acoustic cavitation phenomenon, which involves the growth, nucleation, and violent collapse of microbubbles, resulting in sonoluminescence (SL) emission and the generation of hot spots at extremely high temperatures and pressures [34]. It produces OH radicals due to water dissociation, which oxidize organic pollutants into CO2 and H2O [21, 41].
The combination of the ultrasonic field with ultra-violet radiation and a semiconducting material was thus developed as sonophotocatalysis (SPC). The SPC has attracted attention to removing hazardous organic pollutants from wastewater. Under an ultrasonic field, the growth and collapse of gas bubbles improve mass transfer and surface cleaning due to its synergistic effect in the sonophotocatalytic process [3, 4, 48]. Both processes act together where the sonolysis can degrade the hydrophobic materials while the photocatalysis can degrade the hydrophilic ones. In the practical application of dye-wastewater treatment with ultrasonic, photocatalytic, and sonophotocatalytic, there is a need to determine the optimal conditions of experimental parameters [44]. Little information in the literature was found on the sonocatalysis, sonophotocatalysis, and sonophotocatalysis/H2O2 processes with different metal oxides. Recent studies focused on the most preferred conventional semiconductor (TiO2), widely investigated as sonocatalyst due to its low-cost, good chemical and thermal stability, excellent photo-thermal-catalytic properties, and environmental integrity [5, 8, 23, 38, 51, 54].
This paper reports a benchmark survey of the first six transition metal oxides of 3d elements for the first time. These metal oxides were evaluated to remove MG dye as a model of an organic pollutant in combination with the two ultrairradiation systems, ultrasonic and ultra-violet, with and without H2O2. The metal oxide(s)/H2O2 system act as a one-pot powerful and facile process for the degradation of MG. Suitable low concentrations of H2O2 and small metal oxide dose were used to achieve the highest efficiency with the ultra-MONPs (Ultrasonic/UV/Metal Oxide/H2o2). Different six types of metal oxides have been synthesized. These metal oxides are ZnO, CuO, α-MnO2, NiO, Fe3O4, and Co3O4. They have been used under optimum reaction conditions to reach the desirable degradation efficiency of MG.
2 Materials and methods
2.1 Materials
Malachite green dye (MG) is purchased from Aldrich and used as received, Scheme (S1). Zinc nitrate hexahydrate Zn(NO3)2.6H20 (99.5%), acetic anhydride (CH3CO)2O (99.9%), copper nitrate Cu(NO3)2.3H2O (99%), potassium permanganate KMnO4, and Cobalt (II) chloride CoCl2 6H2O (99%) from Universal lab. Company, Mumbai. Urea CH4N2O (99%) and sodium hydroxide NaOH (99%) from Bio. Chem Company, Egypt. Hydrogen peroxide H2O2 (Assay 50%), Pharaonia Lab. Company, Egypt. Iron (II) sulfate heptahydrate FeSO4.7H2O L.R., Nice Company, India. Nickel chloride NiCl2, Shido Company, Spain. All other chemicals were of analytical grade reagents and used without further purification.
2.2 Instruments
A Shimadzu 2100-S UV/Vis double-beam recording spectrophotometer (Japan). Ultrasonic cleaner set (Volts 230, Watts 296, distilled water was used all over the entire work) and constant frequency 60 Hz (WUC-A03H, Korea), and ultra-violet lamp (PHILIPS UV-C 15 W) were used. Fourier Transform Infrared (FT-IR) spectra were recorded on (JASCO FT-IR-4100) spectrophotometer using KBr pellets with a spectrum of wavenumber ranges from 4000 to 200 cm−1 with an accuracy of 2 cm−1. The crystal structure of the synthesized oxides nanoparticles was analyzed by X-ray powder diffraction (XRD; Regaku D/max-250) with Cu Ka radiation (λ = 1.540598 Å) and 2θ range from 10° to 90° at a step size of 0.020 (2θ) and scanning step 0.2/2θ. The operational voltage and current were kept at 40 kV and 300 mA, respectively. The band gap was measured by spectrometer/data system-Jasco Corp., V-570, rev. 1.00. The size and morphology of the nanoparticles were characterized using the scanning electron microscope (SEM; JEOL JSM-7000F) and transmission electron microscope (TEM; The JEM-2100 operated at 200 kV).
2.3 MOsNPs’ preparations
2.3.1 ZnO synthesis
500 mL of 0.2 M zinc acetate anhydrous (S1) were stirred for 15 min in a 4000 mL round flask held at 60 ºC. After 15 min, 500 mL of 0.5 M NaOH solution heated separately at 60 ºC were added slowly to the zinc acetate solution. The reaction time was 60 min, and the reaction mixture was allowed to cool to room temperature without stirring. The suspension was further ultrasonicated and then centrifuged. It was washed with water, followed by centrifugation. After the four cycles of washing and centrifuging, it was dried at 80 °C and grinded [40]. Scheme S2 illustrates the chemical reactions for the synthesis of the six metal oxides’ catalysts.
2.3.2 CuO synthesis
400 mL of Cu(NO3)2.3H2O (0.08 M) were slowly added to 400 mL of NaOH (0.5 M) solution in the round flask at 78 °C ± 2 with a rate of 20 mL/15 min. 40 mL of distilled water were added with a ratio of 20 mL/15 min. The resultant product was aged together at the same temperature for 16 h. The CuO nanocrystals were washed with water repeatedly, separated by centrifugation at a speed of 6000 rpm for 10 min, and finally dried overnight [12].
2.3.3 α-MnO2 synthesis
α-MnO2 was prepared by dissolving 4.74 g of KMnO4 in 300 mL of water and stirring. 150 mL of ethanol (99%) were added, and the solution was heated at 50 °C for 30 min. The ethanol acts as a reducing agent for the KMnO4 into Mn+4. The brown precipitate formed was separated by centrifugation, washed several times with water, and dried at 90 °C. α-MnO2 was obtained after calcination at 500 °C for 5 h [15].
2.3.4 NiO synthesis
300 ml of 0.1 M NiCl2 solution was added dropwise to 300 ml of 0.8 M NaOH solution in a round flask at 80 °C. This mixture was refluxed with simultaneous stirring at 80 ± 5 °C for 6 h. The green precipitate formed was thoroughly washed with water, centrifuged, and dried overnight. The dried green Ni(OH)2 precursor was crushed into powder and calcined at 600 °C for 5 h [37].
2.3.5 Fe3O4 synthesis
The magnetite (Fe3O4) was synthesized by the sonochemical method. 9.24 g of FeSO4 0.7H2O were dissolved in 360 ml of water, stirred, and sonicated for 75 min. 36 ml of 3 M NaOH were added 15 min from the start of ultrasonication. The precipitate was collected with an external magnet, washed several times with water, and dried overnight at 80 ºC. The magnetite nanoparticles were then calcined at 400 ºC for 2 h [1].
2.3.6 Co3O4 synthesis
Cobalt hydroxide was synthesized using urea as the precipitating agent by homogeneous precipitation of Co2+ ions. An aqueous solution of CoCl2.6H2O (6 g in 500 mL water) was added to a conical flask containing 50 g of urea. The contents were refluxed at 80 ºC for 6 h under constant stirring. The precipitate was filtered, washed with water, and dried at 80 ºC overnight. The dried precipitate was heated at 350 ºC for 3 h to obtain the Co3O4 nanoparticles [29].
2.4 Kinetics measurements
MG stock solutions were prepared in distilled H2O and were diluted to the desired concentrations. Equation (1) was used to find the degradation efficiency
where C0 and Ct represent the initial and residual MG concentrations, respectively. The stock solution of MG was prepared in distilled water that was almost unaffected by the ultrasonic and the ultra-violet Lamp-C irradiation. At room temperature (24 ± 1 °C), 20 mL of MG stock solution were sonicated in a series of beakers containing 20 mL of water (100 mL). The beakers were immersed in the sonicator by maintaining a higher water level in the sonicator than in the reaction level, which was precisely 15 cm below the UV-C lamp. The water in the sonicator was replaced to avoid temperature-related effects. The mixture was sonicated (US/MOsNPs) after adding a suitable amount of metal oxide nanoparticles (MONPs). The experiment was conducted in the presence of H2O2 (US/MOsNPs/H2O2). It was again repeated under the UV irradiation (US/MOsNPs/UV), (US/MOsNPs/H2O2/UV), Scheme (1). At definite time intervals, 10 mL samples were withdrawn from the reaction mixture and immediately centrifuged at 7000 rpm for 5 min to separate the MONPs and obtain a clear solution for measuring the absorbance of the unreacted dye.
3 Results and discussion
3.1 Crystal size and structure
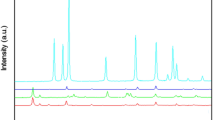
The X-ray diffractograms of the six metal oxide samples are depicted in Fig. (1). The diffraction peaks of these metal oxides are fully indexed to their pure phase in this study. The XRD peaks of ZnO indicate the formation of the pure phase of hexagonal cell structure for ZnO (JCPDS card No. 36–1451, hexagonal phase, space group P63mc, and unit cell a = 3.2490 Å, c = 5.206 Å) with high crystallinity. The crystallite size was obtained from Scherer (S1). The crystallite size based on the peak at 31° corresponding to the (100) plane is 19 nm. The typical XRD pattern for CuO nanoparticles is pure tenorite CuO with a space group C2/c and unit cell lattice parameters a = 4.65, b = 3.41, and c = 5.11 Å. It corresponds to the characteristic diffractions of the monoclinic phase CuO (JCPDS card no. 41–0254), verifying that the CuO obtained is phase-pure. In particular, the low Miller-indexed (002) and (200) reflections are among the strongest, which indicates that they are preferential crystal planes of the nanorods with an average crystallite size of 11 nm. XRD pattern of α-MnO2 corresponds to the pure tetragonal cryptomelane phase (card number 29–1020, space group I4/m, with unit cell parameters a = b = 9.815 Å and c = 2.847 Å and average crystallite size 18 ± 3 nm.
The XRD peaks of NiO appeared at 2θ = 37.64, 43.65, 63.24, 75.67, and 79.73 with cubic crystal system, space group Fm-3 m (225), and unit cell a = 4.1700 Å is, corresponding to the standard JCPDS card no. 04–0835 indicates that the NiO particles are crystalline with a face center cubic structure. The average crystallite size calculated by Scherer from the NiO XRD pattern showed that the particles' average diameter is 20 ± 2 nm.
The sharp and strong peaks obtained from the XRD of Fe3O4 data confirmed the cubic inverse spinel structure of the magnetite with a space group Fd-3 m and unit cell a = 8.3811 Å. They correspond to the standard JCPDS card no. 01–076-7159. No peaks of any other phases are observed in the pattern, thus indicating the high purity of the magnetite with an average crystallite size of 23 nm.
The XRD pattern of Co3O4 shows peaks at 2θ = 18.56, 43.65, 63.24, 75.67, and 79.73 with cubic crystal system, space group Fd-3 m, and unit cell a = 8.1484 Å. They correspond to the characteristic diffractions of (JCPDS file no. 96–900-5892). The crystallite size of Co3O4 nanoparticles is 14 nm as calculated using the average reflection by Scherrer equation.
3.2 FT-IR study
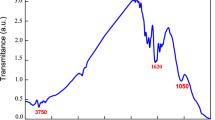
FT-IR spectra of the six metal oxides are shown in Fig. (S1). The spectrum of pure ZnO nanoparticles was recorded in 300–4000 cm−1, Fig. (S1-a). Predictably, the characteristic band of wurtzite ZnO in the range of 400–500 cm–1 is the strongest in the case of ZnO pure phase. Other marked absorption bands corresponding to the O–H bending and stretching [39]. Figure (S1-b), shows the FT-IR spectrum of the CuO nanocrystals. The peaks around 413, 509, and 620 cm−1 are due to the stretching of the Cu–O bond along the [101] direction. The peak at ca. 1623 cm−1 is due to the bending vibrations of physically adsorbed water, whereas the OH in-plane bending is at 1367 cm−1. The strong presence of the O–H stretching band at 3403 cm−1 is mainly due to water residues [14]. In Fig. (S1-c), the absorption peaks at 461, 525, and 700 cm−1 are characteristics of Mn − O vibrations in FT-IR spectrum of α-MnO2. The strong absorption band around 413 cm−1 in Fig. (S1-d) was assigned to the Ni–O-stretching vibration, indicating pure inorganic NiO species in finer particles. The Fe3O4 nanoparticles spectrum is given in Fig. (S1-e). It depicts major absorption bands at about 383 and 574 cm−1 due to the FeB–O- and FeA–O-stretching vibration. These could be assigned to the tetrahedral and octahedral Fe–O covalent structural bond environment of the Fe3O4 nanoparticles [2]. The FT-IR spectrum of the Co3O4 nanoparticles in Fig. (S1-f) shows characteristic bands at 557 and 668 cm−1. The band at about 557 cm−1 is assigned to the Co–O stretching where the Co3+ ions are octahedrally coordinated. The band at 668 cm−1 is attributed to the Co–O stretching, where Co2+ ions are tetrahedrally coordinated [29, 46].
3.3 Morphology
The SEM and TEM images of ZnO are shown in Fig. 2 (A-B) and Fig. 3 (A-B). The most narrowly sized particles dominated spherical morphology with step-edged surfaces along the side with an average particle size of 20 ± 2 nm. The CuO morphology shows aggregated nanorods Fig. 2 (C-D) and Fig. 3(C-D). The average nanorods width is 11 nm, and the average length is 64 nm. The α-MnO2 morphology is depicted in Fig. 2(E–F) and Fig. 3(E–F). α-MnO2 shows relatively long and thin nanorods. The nanorods have an average length of 82 nm. The selected area electron diffraction (SAED) of nanorods, insets of panels of Fig. 3(E), showed that the oxide is highly crystalline. The fast Fourier transform (FFT) of nanorods shows lattice fringes with an interplanar distance of 4 nm Fig. 3(F). The NiO morphology exhibits nanocrystals of short nanorods morphology Fig. 2(G-H) and Fig. 4(G-H). The SEM and TEM images of NiO show uniform nanorods with a small particle size of 8 ± 2 nm. The SAED shows that the NiO particles are crystalline.
The fast Fourier transform (FFT) shows that the interplanar distance between the lattice fringes of NiO is 2 nm. The SEM and TEM observed for Fe3O4 in Fig. 2 (K-L) and Fig. 4(K-L) reveal hexagonal-plate like particles with little agglomeration. The electron diffraction pattern is shown in the same TEM image as the inset, where the hexagonal-plate shape with high crystallinity is revealed. This is consistent with the sharp and stronger peaks obtained from the magnetite XRD data, which confirm the cubic inverse spinel structure of the magnetite.
The SEM images of Co3O4 nanoparticles given in Fig. 2(M–N) show a petal-like morphology. Typical TEM images of the Co3O4 nanoparticles with SAED inset are shown in Fig. 4(M–N). They indicate the presence of porous petal-like nanoparticles with inter-connected nanoparticles and high crystallinity.
3.4 UV–Vis spectroscopy
The UV–Vis diffuse reflectance spectra show the optical comparison of the six prepared metal oxide nanoparticles, Figs. (5, 6). ZnO nanoparticles show higher UV-light absorption ability than others, with a band gap of 3.31 eV. It was noticed that α-MnO2, Fe3O4, NiO, and Co3O4 are equal in their UV-light absorption ability with approximately a band gap of 3.2 eV, while CuO nanoparticles show the highest visible light absorption with a band gap of 2.81 eV.
3.5 Catalytic degradation of MG
3.5.1 Comparison of the efficiency of alternative processes
The catalytic degradation of MG was investigated by four different processes, sonocatalysis (US/MOsNPs), sonocatalysis under ultra-violet irradiation (US/MOsNPs/UV), sonocatalysis in the presence of hydrogen peroxide (US/MOsNPs/H2O2), and a combination of all processes (US/MOsNPs/UV/H2O2), Scheme (S1). The kinetics of the six metal oxides through the four degradation processes was studied under the same dose of catalyst (0.05 g/L) and the results are depicted in Fig. (7). The same study was also performed at different doses of metal oxides (0.15 and 0.3 g/L) as given in Fig. (S2) and Fig. (S3). The degradation efficiency was in the order US/MOsNPs ˂ US/MOsNPs/UV ˂ US/MOsNPs/H2O2 ˂ (UV/Ultrasonic/MOsNPs/H2O2) for all types and weights of metal oxides within 2–8 min.
Comparison of different processes as Ultrasonic, Ultrasonic + UV, Ultrasonic + H2O2, and Ultrasonic + UV + H2O2 at same metal oxides dose 0.05 gm/L on the decolorization of MG concentration of 3 × 10–5 M, H2O2 concentration of 1 mM, Ultrasonic power of 296 W, UV-C lamp (15 W). a ZnO, b CuO, c NiO, d Fe3O4, e Co3O4, and f α-MnO2
The US/MOsNPs process leads to more energy generation and increases the pulsation and collapse of bubbles. Thus, hydroxyl radicals are generated by breaking the bond between the hydrogen and hydroxyl ions of water. They increase with the increase in the number of the transient collapse of the cavitation bubble [41, 42]. The MG degradation enhancement was also observed by applying the US/MOsNPs/UV and US/MOsNPs/H2O2 systems, where an excess of OH• radicals is generated. The complete degradation of MG was achieved by the synergetic effect between the ultrasonic and the heterogeneous photocatalytic oxidation process (US/UV/MOsNPs/H2O2). MG's high activity of this sonophotocatalytic oxidation may be due to the acoustic microstreaming that cleans, sweeps the catalyst surface, and generates more active sites on the catalyst surface. In solution, the mass transport of reactants and products increases by the facilitated transport via shockwave propagation and by ultrasonic fragmentation. In the presence of H2O2, H2O, and this catalyst, the concentration of OH• radicals increases, and subsequently, their reaction with the target pollutants increases [16, 38, 44].
3.5.2 Comparison between MOsNPs’ efficiencies
To evaluate the efficiency of the metal oxides under investigation for degradation of MG, all metal oxides were tested under each process at the same conditions. Figure (8) displays a comparative study between the six metal oxides at each specific process at constant dose (0.05 g/L). Similar study was performed at different doses of metal oxides, 0.15 and 0.3 g/L. The results are given in Fig. (S10) and Fig. (S11), respectively. For the US/MOsNPs process the results are depicted in Fig. 8(a), with dose 0.05 gm/L, while for the doses 0.15, and 0.3 g/L, the results are shown in Fig. S4(a) and Fig. S5(a), respectively. Generally, the degradation rate increased with increasing the metal oxide dose, as revealed from Fig. S4(a) and Fig. S5(a). The removal percentage of MG reached 80% within 15–45 min depending on the types of metal oxide with the order: CuO > α-MnO2 ≥ ZnO > NiO ≥ Fe3O4 ≥ Co3O4.
In the US/MOsNPs/UV process, MG degradation rate is given in Fig. 8(b) with a metal oxide dose 0.05 g/L. The results of 0.15 and 0.3 g/L doses are shown in Fig. S4 (b) and Fig. S5 (b), respectively. The removal percentage of MG was 80% within 5–40 min according to the metal oxide type which follows the order ZnO ≥ NiO ≥ α-MnO2 > Fe3O4 ≥ CuO ≥ Co3O4. This order is matching with band-gap values of these oxides, Fig. (5) and Fig. (6). Figure 8(c) depicts the results obtained from the use of the US/ MOsNPs/ H2O2 process at the same metal oxides dose 0.05 gm/L, 0.15 gm/L, and 0.3 gm/L, as shown in Fig. S4 (c) and Fig. S5 (c), respectively. The degradation percentage of MG reached 80% within 5–20 min depending on the metal oxide according to the order ZnO ≥ CuO ≥ α-MnO2 ≥ NiO > Co3O4 > Fe3O4. The US/ MOsNPs/ H2O2 process is more active than US/MOsNPs/UV. In Fig. 8(d), the metal oxide dose was 0.05 g/L, but 0.15 g/L and 0.3 g/L of metal oxide are shown in Fig. S4 (d) and Fig. S5 (d), respectively. The maximum removal efficiency reached 90% within 2–8 min, depending on the activity of each metal oxide that follows the order ZnO ≥ CuO > α-MnO2 > NiO > Fe3O4 ≥ Co3O4. Figure (9) shows the full comparison of six metal oxides, and at the same time, the four alternative processes. It was observed that the sonophotocatalytic process has the highest degradation efficiency of other processes to be considered the best in all MOsNPs types. The US/MOsNPs/H2O2 process is the most competitive to the sonophotocatalytic process. The two processes efficiencies were nearly equal for ZnO, CuO, and α-MnO2. The four processes efficiencies are similar and equal for α-MnO2 and Co3O4. However, a large gap was noticed between the sonophotocatalytic process and the other processes with the lowest efficiency for Fe3O4 and all MOsNPs processes. The sonocatalytic process has high removal efficiency for α-MnO2 than NiO. The US/MOsNPs/UV process efficiency was nearly equal for ZnO, NiO, and α-MnO2.
3.5.3 Catalyst dose effect
Several experiments were performed to find the dosage effect of all six catalysts. (MOsNPs) ranging from 0.05 to 0.3 g/L on the degradation of MG from an aqueous solution. The critical parameter in the Ultra-MOsNPs like oxidation processes was hydrogen peroxide concentration, as a dominant source of OH•. However, an excessive H2O2 dosage has a significant negative effect that scavenges the hydroxyl radicals by forming the hydroperoxy radicals (HO2•) (Eq. (2). Hydroperoxy radicals (HO2•) have lower oxidation potential than OH• and compete with the MG molecules to occupy the adsorption sites on the surface of MOsNPs, which reduces the degradation efficiency of MG in this heterogeneous catalytic process; consequently; it raises the treatment cost [49]. Herein, we avoided the formation of hydroperoxy radicals (HO2•) using the lowest hydrogen peroxide concentration (1 mM) to achieve high efficiency with the sonophotocatalytic process.
The catalyst dose in catalytic degradation reactions is a critical factor that should be optimized to reduce wastewater treatment costs. This effect was investigated and the results are shown in Figs. (S6-S11) for ZnO, CuO, NiO, Fe3O4, Co3O4, and α-MnO2, respectively. All figures show an enhancement in the degradation efficiency with increasing the metal oxides dose from 0.05 to 0.3 g/L. The highest removal percentage was observed at the catalyst dose of 0.3 g/L. There was also no significant difference in removal efficiency on going from 0.05 to 0.15 g/L for ZnO, CuO, α-MnO2, and NiO. Increasing the dose of ZnO did not affect the degradation efficiency which was relatively high at each of the four reaction processes. Increasing the dose of CuO, α-MnO2, and NiO increased the degradation efficiency along with the four reaction processes. This may be attributed to the less effect of the ultrasonic dispersion turbidity, which facilitates the UV-light penetration in the solution to produce more hydroxyl radicals. This finding can also be explained by the fact that MOsNPs act as a peroxidase-like catalyst. Therefore, an increase in the catalyst dose provides more active sites, accelerating the decomposition of H2O2 to generate more reactive OH• radicals [52]. The low degradation efficiency of MG is principally derived from the insufficient production of hydroxyl radicals in solution with increasing the metal oxides amount more than 0.5 g/L, which causes an increase in solution turbidity by ultrasonic dispersion that reduces the penetration of the UV radiation into the Fe3O4 and Co3O4 suspension. Table (S1) includes different catalysts in the field of color removal.
3.6 Structure and morphology effect
3.6.1 Effect of zinc oxide
To investigate the best oxidation system for degradation of MG, the role of US/ZnO, US/ZnO/UV, US/ZnO/H2O2, and US/ZnO/H2O2/UV processes were studied, respectively, under the same conditions. ZnO is considered the more stable photocatalyst due to its high electron mobility (~ 100 cm2 V−1 s−1). The effect of the sonophotocatalysis increased the surface area of zinc oxide for photocatalytic activity and increased the cavitation activity by providing additional nucleation growth. The combined effects of US and UV increased the catalyst particles' fragmentation; thus, their surface area and activity increased [18, 30]. Figure 6(a) shows the higher ability of ZnO in UV-light absorption with a band gap of 3.31 eV. The hydroxyl radical population increased using the US/UV/ZnO system and the suitable particle size and morphology of ZnO led to a higher degradation of MG. Results of MG degradation in the presence of ZnO are shown in Fig. (8), where the improvement of the reaction time of the four systems was observed. The US/ZnO system took 25 min to complete MG degradation. The US/UV/ZnO system took 10 min. US/ZnO/H2O2 system completes MG degradation in 5 min by adding a suitable amount of H2O2. US/UV/ZnO/H2O2 system degrades the MG in 2 min at the same conditions. Table (1) summarizes some of ZnO results reported in the literature.
3.6.2 Effect of copper oxide
Copper oxide can be considered the most effective oxidation catalyst for ultrasonic cavitation events. During the sonication, tiny amounts of copper oxide particles are further fragmented into smaller particles and act as nuclei sources to create an additional source to increase the cavitation activity [30]. Thus, CuO nanorods are compatible with the spherical ZnO nanoparticles for MG degradation in all processes US/CuO, US/CuO/H2O2, and US/CuO/H2O2/UV except the US/CuO /UV process Fig. (8). The synergetic effect of the US/UV system in CuO nanorods was not observed, Fig. 8(b). The lower MG degradation by US/CuO/UV may be due to the low ability to UV-light absorption with band gap 2.81 eV, Fig. 6(b), and may also be due to the scavenging effects of H2O2 or other less active radicals that formed from the hydroxyl radicals’ combination [18]. The active sites of the CuO may be blocked by adsorption of the dye, decreasing the photocatalytic efficiency. Overall, it can be considered that CuO is a suitable catalyst for the US compared to the US/UV process [19, 33]. Table (1) shows the CuO results improvement in both US/CuO and US/CuO/UV. The US/CuO/H2O2 and US/CuO/H2O2/UV were studied individually and their efficiencies were higher than the efficiencies of other systems.
3.6.3 Effect of α-MnO2
Manganese oxide (α-MnO2) nanorods show high crystallinity with high surface area. It was the strongest competitor metal oxide prepared compared with the previous results for ZnO (20 nm) and CuO (11 nm) in all different processes US/α-MnO2, US/α-MnO2/H2O2, US/α-MnO2/UV, and US/α-MnO2/H2O2/UV for MG degradation at the same conditions as in Fig. 8. The US/α-MnO2 is more active than other metal oxides’ systems where MnO2 splits H2O2 to form ·OH and HO2· radicals [31]. The MnO2 systems were competitors in the degradation of MG by increasing the α-MnO2 dose and due to the higher ability of α-MnO2 in UV-irradiation with band gap 3.27 eV, Fig. 6(c). α-MnO2 accelerates the decomposition of H2O2 to produce an excess of reactive OH• radicals which attack the MG molecules to form degradation products, Table (1) shows little information in the literature for all α-MnO2 processes. The US/α-MnO2/H2O2, US/α-MnO2/UV, and US/α-MnO2/H2O2/UV systems were studied individually and approved their efficiency.
3.6.4 Effect of NiO
Figure 8 shows the increase of MG degradation efficiency by increasing the NiO dose in all processes US/NiO, US/NiO/H2O2, US/NiO/UV, and US/NiO/H2O2/UV under the same conditions during 45, 30, 30, and 5 min, respectively. This may be due to the higher ability in the UV-light absorption, where its band gap is 3.21 eV, Fig. 6(d). The higher surface area and increasing number of cavities and radicals at higher concentrations led to a higher removal rate. Improving the mass transfer from the bulk to the catalyst surface by shock waves’ sonication is also a reason for the high production of OH• radicals from the pyrolysis and sonication of H2O and H2O2 which participate in MG degradation [53]. According to our knowledge, no information was found by a critical search in the literature on NiO/four processes under investigation for MG degradation. All the investigated processes US/NiO, US/NiO/H2O2, US/NiO/UV, and US/NiO/H2O2/UV for the degradation of MG are used for the first time.
3.6.5 Effect of magnetite (Fe3O4)
The solution turbidity was observed using the ultrasonic dispersion technique, which reduces the ultra-violet light penetration at the higher dose of magnetite 0.15–0.3 g/l in the four processes of US/Fe3O4, US/Fe3O4/H2O2, US/Fe3O4/UV, and US/Fe3O4/H2O2/UV for MG degradation at the same conditions, Fig. (8). The best results were obtained using the US/Fe3O4/UV and US/Fe3O4/H2O2/UV (Sono-Fenton-like) processes at a lower dosage 0.05 g/l. This dose facilitates the ultra-violet light to pass into the solution to help the MG degradation within 25 and 8 min, respectively. The higher activity of the magnetite of band gap 3.27 eV comes from the absorption of the UV-light, Fig. 6(e). Although it is highly effective than sonicator dispersion which makes the solution turbid, it can easily be collected and separated by a simple magnet. Therefore, it is clear that the sonophotocatalysis processes with magnetite of high activity can be used as an efficient method for degrading recalcitrant contaminants. Table (1) depicts little information found in the literature for the US/Fe3O4, US/Fe3O4/H2O2 processes and the US/ Fe3O4/UV and US/ Fe3O4/H2O2/UV processes were applied individually.
3.6.6 Effect of Co3O4 nanoparticles
Figure (8) shows that Co3O4 was affected by ultrasonic dispersion. Thus, the lowest dose is preferable for MG degradation by all processes US/Co3O4, US/Co3O4/UV, US/Co3O4/H2O2, and US/Co3O4/H2O2/UV under the same conditions at 45, 45, 25, and 10 min, respectively. The results indicate the preferable appendance of H2O2 (US/Co3O4/H2O2) and (US/ Co3O4 /H2O2/UV) in the reaction for increasing production of OH• radicals to complete MG degradation in 25 and 10 min, respectively. Generally, the sonophotocatalytic process is the most efficient for all metal oxides investigated. No information was found on the use of Co3O4 through the four processes US/Co3O4, US/Co3O4/UV, US/Co3O4/H2O2, and US/Co3O4/H2O2/UV investigated for the degradation of MG.
Figure (9) shows the full comparison of six metal oxides and, at the same time, the four alternative processes. It was observed that the sonophotocatalytic process has the highest degradation efficiency of other processes to be considered the best in all MOsNPs types. The US/MOsNPs/H2O2 process is the most competitive to the sonophotocatalytic process. The two processes' efficiency were nearly equal for ZnO, CuO, and α-MnO2. The four processes' efficiency is similar and equal for α-MnO2 and Co3O4. However, a large gap was noticed between the sonophotocatalytic process and the other processes with the lowest efficiency for Fe3O4 and all MOsNPs’ processes. The sonocatalytic process has high removal efficiency for α-MnO2 than NiO. The US/MOsNPs/UV process efficiency was nearly equal for ZnO, NiO, and α-MnO2.
4 MOsNPs’ reusability
The reusability of the MOsNPs was determined by recovering the oxide from the solution using a centrifuge (6000 rpm/ 4 min), and washing it three times with water. Then, it was reused for the degradation of MG. The reusability results for the six metal oxides are shown in Fig. (S1) and Fig. (S12). The XRD peaks’ sharpness almost decreased for all recycled metal oxides. The peak intensity decreased in FT-IR bands for all recycled metal oxides. This is due to the formation of MG particles on the surface of metal oxide, as an example in the recycled ZnO at 3435 cm−1 (stretching) and 1431 cm−1 to 1638 cm−1 (bending) indicates the presence of hydroxyl residue by atmospheric moisture [36]. After five regeneration cycles, the sonophotocatalytic process was still relatively high, Fig. (S13). In the sonophotocatalysis process (US/MOsNPs/H2O2/UV), the activity oxides’ order is (ZnO > CuO > α-MnO2 > NiO > Fe3O4 > Co3O4). The complete degradation of MG in the first cycle was achieved after 2, 2, 4, 5, 8, and 10 min, while after the fifth cycle, it was 3, 4, 5, 8, 10, and 12 min, respectively. Thus, MOsNPs showed perfect reusability and can be used at least for five reaction cycles without noticeable activity changes.
5 Conclusion
In this study, all six competitive metal oxide nanoparticles were synthesized using the co-precipitation method. These nanoparticles were then used as catalysts in the four different processes involving MG degradation. The sonocatalytic process (US/MOsNPs), (US/MOsNPs/UV), (US/MOsNPs/H2O2), and (US/MOsNPs/H2O2/UV) were each studied individually to determine which of them is the most active toward the degradation of the dye under the same conditions. It was found that the activities of these processes are in the order: (US/MOsNPs), 15–45 min > (US/MOsNPs/UV), 5–40 min > (US/ MOsNPs/ H2O2), 5–20 min > (US/MOsNPs/H2O2/UV), 2–10 min. On the other hand, the activity of the oxides applied under the sonophotocatalysis process (US/MOsNPs/H2O2/UV) is (ZnO ≥ CuO > α-MnO2 > NiO > Fe3O4 ≥ Co3O4) in 2, 2, 4, 5, 8, and 10 min, respectively. It was also found that the (US/MONPs/H2O2/UV) process is the most efficient for wastewater treatment.
Data availability
The data that support the findings of this study are available in the supporting information of this article.
References
Abbas, M., Takahashi, M., & Kim, C. (2013). Facile sonochemical synthesis of high-moment magnetite (Fe3o4) nanocube. Journal Of Nanoparticle Research, 15, 1–12.
Abbas, B. M., Parvatheeswara Rao, S. M., Naga, M. T., & Kim, CheolGi. (2013). Synthesis of high magnetization hydrophilic magnetite (fe3o4) nanoparticles in single reaction—surfactantless polyol process. Ceramics International, 39, 7605–7611.
Al-Bsoul, A., Al-Shannag, M., Tawalbeh, M., Al-Taani, A. A., Lafi, W. K., Al-Othman, A., & Alsheyab, M. (2020). Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Science of The Total Environment, 700, 134576.
Al-Musawi, T. J., McKay, G., Rajiv, P., Mengelizadeh, N., & Balarak, D. (2022). Efficient sonophotocatalytic degradation of acid blue 113 dye using a hybrid nanocomposite of cofe2o4 nanoparticles loaded on multi-walled carbon nanotubes. Journal of Photochemistry and Photobiology A: Chemistry, 424, 113617.
Al-Musawi, T. J., Rajiv, P., Mengelizadeh, N., Mohammed, I. A., & Balarak, D. (2021). Development of sonophotocatalytic process for degradation of acid orange 7 dye by using titanium dioxide nanoparticles/graphene oxide nanocomposite as a catalyst. Journal of environmental management, 292, 112777.
Al-Musawi, T., Zaidan, H., Saloot, M. K., Shahbaksh, S., & Balarak, D. (2022). Photocatalytic Degradation of Acid Red 88 Dye Using Pd@ Tmu-16 Metal Organic Framework. International Journal of Environmental Analytical Chemistry. https://doi.org/10.1080/03067319.2022.2063721
Amirian, P., Bazrafshan, E., Payandeh, A. (2017). Optimisation of chemical oxygen demand removal from landfill leachate by sonocatalytic degradation in the presence of cupric oxide nanoparticles. Waste Management & Research, 35, 636–646.
Ayare, S. D., & Gogate, P. R. (2020). Sonophotocatalytic oxidation based treatment of phthalocyanine pigment containing industrial wastewater intensified using oxidising agents. Separation and Purification Technology, 233, 115979.
Barzegar, G., Jorfi, S., Soltani, R. D. C., Ahmadi, M., Saeedi, R., Abtahi, M., Ramavandi, B., & Baboli, Z. (2017). Enhanced sono-fenton-like oxidation of pah-contaminated soil using nano-sized magnetite as catalyst: optimization with response surface methodology. Soil and Sediment Contamination: An International Journal, 26, 538–557.
Barzegar, M. H., Ghaedi, M., Avargani, V. M., Sabzehmeidani, M. M., Sadeghfar, F., & Jannesar, R. (2019). Electrochemical synthesis of zn: zno/ni2p and efficient photocatalytic degradation of auramine o in aqueous solution under multi-variable experimental design optimization. Polyhedron, 165, 1–8.
Bokhale, N.B., Bomble, S.D., Dalbhanjan, R.R., Mahale, D.D., Hinge, S.P., Banerjee, B.S., Mohod, A.V., Gogate, P.R. (2014). Sonocatalytic and sonophotocatalytic degradation of rhodamine 6g containing wastewaters. Ultrasonics sonochemistry, 21, 1797–1804.
Chang, Yu., & Zeng, H. C. (2004). Controlled synthesis and self-assembly of single-crystalline cuo nanorods and nanoribbons. Crystal Growth & Design Crystal Growth & Design, 4, 397–402.
Chauhan, M., Kaur, N., Bansal, P., Kumar, R., Srinivasan, S., Chaudhary, G.R. (2020). Proficient Photocatalytic and Sonocatalytic Degradation of Organic Pollutants Using Cuo Nanoparticles. Journal of Nanomaterials, 2020.
David Dodoo-Arhin, M., & Leoni, and P Scardi,. (2012). Microemulsion Synthesis of Copper Oxide Nanorod-Like Structures. Molecular Crystals and Liquid Crystals, 555, 17–31.
El-Sawy, A. M., Cecil, K., & King’ondu, Chung-Hao Kuo, David A Kriz, Curtis J Guild, Yongtao Meng, Samuel J Frueh,. (2014). Saminda dharmarathna, steven n ehrlich, and steven l suib, “x-ray absorption spectroscopic study of a highly thermally stable manganese oxide octahedral molecular sieve (oms-2) with high oxygen reduction reaction activity.” Chemistry of Materials, 26, 5752–5760.
Eren, Z. (2012). “Degradation of an Azo dye with homogeneous and heterogeneous catalysts by sonophotolysis”, clean–soil, air. Water, 40, 1284–1289.
Eren, Z., Acar, F.N., Tekin, D. (2012). Degradation Ci reactive yellow 81 by Us/Uv/Zno heterogeneous oxidation process: Effect of system parameters. Fresenius Environmental Bulletin, 21, 3400–3406.
Ertugay, N., & Acar, F. N. (2014). The degradation of direct blue 71 by sono, photo and sonophotocatalytic oxidation in the presence of zno nanocatalyst. Applied Surface Science, 318, 121–126.
Gole, V. L., Priya, A., & Danao, S. P. (2017). Decolorization of brilliant green dye using immersed lamp sonophotocatalytic reactor. Applied Water Science, 7, 4237–4245.
Peter Gregory, 'Dyes and Dye Intermediates', 2009 Kirk‐Othmer Encyclopedia of Chemical Technology (), 1–66.
Hapeshi, E., Fotiou, I., & Fatta-Kassinos, D. (2013). Sonophotocatalytic treatment of ofloxacin in secondary treated effluent and elucidation of its transformation products. Chemical engineering journal, 224, 96–105.
Hasan, I., Bassi, A., Alharbi, K. H., BinSharfan, I. I., Khan, R. A., & Alslame, A. (2020). Sonophotocatalytic degradation of malachite green by nanocrystalline chitosan-ascorbic acid@ nife2o4 spinel ferrite. Coatings, 10, 1200.
Hinge, S. P., Orpe, M. S., Sathe, K. V., Tikhe, G. D., Pandey, N. S., Bawankar, K. N., Bagal, M. V., Mohod, A. V., & Gogate, P. R. (2016). Combined removal of rhodamine b and rhodamine 6g from wastewater using novel treatment approaches based on ultrasonic and ultraviolet irradiations. Desalination and Water Treatment, 57, 23927–23939.
Jaafarzadeh, N., Barzegar, G., & Ghanbari, F. (2017). Photo assisted electro-peroxone to degrade 2, 4-d herbicide: the effects of supporting electrolytes and determining mechanism. Process Safety and Environmental Protection, 111, 520–528.
Jaafarzadeh, N., Takdastan, A., Jorfi, S., Ghanbari, F., Ahmadi, M., Barzegar, G. (2018). The performance study on ultrasonic/Fe3O4/H2O2 for degradation of azo dye and real textile wastewater treatment. Journal of molecular liquids, 256, 462–470.
Jiang, T., Poyraz, A. S., Iyer, A., Zhang, Y., Luo, Z., Zhong, W., Miao, R., El-Sawy, A. M., Guild, C. J., & Sun, Yu. (2015). Synthesis of mesoporous iron oxides by an inverse micelle method and their application in the degradation of orange ii under visible light at neutral ph. The Journal of Physical Chemistry C, 119, 10454–10468.
Jorfi, S., Samaei, M. R., Soltani, R. D. C., Khozani, A. T., Ahmadi, M., Barzegar, G., Reshadatian, N., & Mehrabi, N. (2017). Enhancement of the bioremediation of pyrene-contaminated soils using a hematite nanoparticle-based modified fenton oxidation in a sequenced approach. Soil and Sediment Contamination: An International Journal, 26, 141–156.
Khataee, A., Gholami, P., & Vahid, B. (2016). Heterogeneous sono-fenton-like process using nanostructured pyrite prepared by ar glow discharge plasma for treatment of a textile dye. Ultrasonics sonochemistry, 29, 213–225.
Kishore, P. N. R., & Jeevanandam, P. (2013). Synthesis of cobalt oxide nanoparticles via homogeneous precipitation using different synthetic conditions. J nanosci nanotechnol Journal of Nanoscience and Nanotechnology, 13, 2908–2916.
Kumar, R., Girish Kumar, M. S., & Akhtar, and Ahmad Umar,. (2015). Sonophotocatalytic Degradation of Methyl Orange Using Zno Nano-Aggregates. Journal of Alloys and Compounds, 629, 167–172.
Lekshmi, K. P. V., Yesodharan, S., & Yesodharan, E. P. (2018). Mno2 efficiently removes indigo carmine dyes from polluted water. Heliyon, 4, e00897.
Lousada, C. M., Yang, M., Nilsson, K., & Jonsson, M. (2013). Catalytic decomposition of hydrogen peroxide on transition metal and lanthanide oxides. Journal of Molecular Catalysis A: Chemical, 379, 178–184.
Marković, D., Šaponjić, Z., Radoičić, M., Radetić, T., Vodnik, V., Potkonjak, B., & Radetić, M. (2015). Sonophotocatalytic degradation of dye ci acid orange 7 by tio2 and ag nanoparticles immobilized on corona pretreated polypropylene non-woven fabric. Ultrasonics sonochemistry, 24, 221–229.
Meroni, D., Jiménez-Salcedo, M., Falletta, E., Bresolin, B. M., Kait, C. F., Boffito, D. C., Bianchi, C. L., & Pirola, C. (2020). Sonophotocatalytic degradation of sodium diclofenac using low power ultrasound and micro sized Tio2. Ultrasonics sonochemistry, 67, 105123.
Miklos, D. B., Wang, W.-L., Linden, K. G., Drewes, J. E., & Hübner, U. (2019). Comparison of Uv-Aops (Uv/H2o2, Uv/Pds and Uv/Chlorine) for Torc removal from municipal wastewater effluent and optical surrogate model evaluation. Chemical Engineering Journal, 362, 537–547.
Nagaraju, G., Prashanth, S. A., Shastri, M., Yathish, K. V., Anupama, C., & Rangappa, D. (2017). Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of ag–zno nanomaterial. Materials Research Bulletin, 94, 54–63.
Nasseri, M. A., Ahrari, F., & Zakerinasab, B. (2015). Nickel oxide nanoparticles: a green and recyclable catalytic system for the synthesis of diindolyloxindole derivatives in aqueous medium. RA RSC Advances, 5, 13901–13905.
Panda, D., & Manickam, S. (2017). Recent advancements in the sonophotocatalysis (spc) and doped-sonophotocatalysis (dspc) for the treatment of recalcitrant hazardous organic water pollutants. Ultrasonics sonochemistry, 36, 481–496.
Pholnak, C., Sirisathitkul, C., Suwanboon, S., & Harding, D. J. (2014). Effects of precursor concentration and reaction time on sonochemically synthesized zno nanoparticles. Materials Research, 17, 405–411.
Pourrahimi, A. M., Liu, D., Pallon, L. K. H., Andersson, R. L., Martínez Abad, A., Lagarón, J. M., Hedenqvist, M. S., Ström, V., Gedde, U. W., & Olsson, R. T. (2014). Water-based synthesis and cleaning methods for high purity zno nanoparticles comparing acetate, chloride, sulphate and nitrate zinc salt precursors. RSC Adv. RSC Adv., 4, 35568–35577.
Qiu, P., Park, B., Choi, J., Thokchom, B., Pandit, A. B., & Khim, J. (2018). A review on heterogeneous sonocatalyst for treatment of organic pollutants in aqueous phase based on catalytic mechanism. Ultrasonics sonochemistry, 45, 29–49.
Reddy, D. R., Kumaravel Dinesh, G., Anandan, S., & Sivasankar, T. (2016). Sonophotocatalytic treatment of naphthol blue black dye and real textile wastewater using synthesized Fe doped Tio2. Chemical Engineering and Processing: Process Intensification, 99, 10–18.
Saad, M., Tahir, H., Khan, J., Hameed, U., & Saud, A. (2017). Synthesis of polyaniline nanoparticles and their application for the removal of crystal violet dye by ultrasonicated adsorption process based on response surface methodology. Ultrasonics sonochemistry, 34, 600–608.
Sabzehmeidani, M. M., Karimi, H., & Ghaedi, M. (2019). Sonophotocatalytic treatment of rhodamine b using visible-light-driven ceo 2/ag 2 cro 4 composite in a batch mode based on ribbon-like ceo 2 nanofibers via electrospinning. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-019-04253-8
Salam, M. A., Kosa, S. A., & Al-Beladi, A. A. (2017). Application of Nanoclay for the Adsorptive Removal of Orange G Dye from Aqueous Solution. Journal of Molecular Liquids, 241, 469–477.
Salavati-Niasari, M., Davar, F., Mazaheri, M., & Shaterian, M. (2008). preparation of cobalt nanoparticles from [bis (salicylidene) cobalt (ii)]–oleylamine complex by thermal decomposition. Journal of Magnetism and Magnetic Materials, 320, 575–578.
Sharafeldin, M. (2017). Gregory W bishop, snehasis bhakta, abdelhamid el-sawy, steven l suib, and james f rusling, “fe3o4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins.” Biosensors and Bioelectronics, 91, 359–366.
Theerthagiri, J., Lee, S. J., Karuppasamy, K., Arulmani, S., Veeralakshmi, S., Ashokkumar, M., & Choi, M. Y. (2021). Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. Journal of Hazardous Materials, 412, 125245.
Wang, N., Zheng, T., Zhang, G., & Wang, P. (2016). A review on fenton-like processes for organic wastewater treatment. Journal of Environmental Chemical Engineering, 4, 762–787.
Wang, N., Zhu, L., Wang, M., Wang, D., Tang, H. (2010). Sono-Enhanced degradation of dye pollutants with the use of H2o2 activated by Fe3o4 magnetic nanoparticles as peroxidase mimetic. Ultrasonics sonochemistry, 17, 78–83.
Wei, H., Hasibur, M. R., Jujiao, Z., Dayan, L., & Jun, Z. (2021). Hydrogen Peroxide Enhanced Sonophotocatalytic Degradation of Acid Orange in Aqueous Solution: Optimization by Box-Behnken Design. Journal of Chemical Technology & Biotechnology., 96(9), 2647–2658.
Wei, H., Da, Hu., Jie, Su., & Li, K. (2015). Intensification of levofloxacin sono-degradation in a us/h2o2 system with fe3o4 magnetic nanoparticles. Chinese Journal of Chemical Engineering, 23, 296–302.
Yehia, F. Z., Badawi, A. M., Eshaq, Gh., & Dimitry, O. I. H. (2015). Investigation on the sonocatalytic degradation of nitrobenzene using heterogeneous nanostructured catalysts in absence and presence of surfactant. Egyptian Journal of Petroleum, 24, 265–276.
Yilmaz, M., & Mengelizadeh, N. (2022). Morteza khodadadi Saloot, and Davoud Balarak, ’facile synthesis of fe3o4/zno/go photocatalysts for decolorization of acid blue 113 under Solar. Visible and Uv Lights’, Materials Science in Semiconductor Processing, 144, 106593.
Zhang, H., Lv, Y., Liu, F., & Zhang, D. (2008). Degradation of Ci acid orange 7 by ultrasound enhanced ozonation in a rectangular air-lift Reactor. Chemical Engineering Journal, 138, 231–238.
Zhou, X.-J., Guo, W.-Q., Yang, S.-S., & Ren, N.-Q. (2012). A rapid and low energy consumption method to decolorize the high concentration triphenylmethane dye wastewater: Operational parameters optimization for the ultrasonic-assisted ozone oxidation process. Bioresource technology, 105, 40–47.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sawy, A.M., Salem, M.A., Salem, I.A. et al. Sonophotocatalytic degradation of malachite green in aqueous solution using six competitive metal oxides as a benchmark. Photochem Photobiol Sci 22, 579–594 (2023). https://doi.org/10.1007/s43630-022-00336-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00336-7